Abstract
The Malpighian tubule is the main organ for excretion and osmoregulation in most insects. During a short period of embryonic development the tubules of Drosophila are shaped, undergo differentiation and become precisely positioned in the body cavity, so they become fully functional at the time of larval hatching a few hours later. In this review I explore three developmental events on the path to physiological maturation. First, I examine the molecular and cellular mechanisms that generate organ shape, focusing on the process of cell intercalation that drives tubule elongation, the roles of the cytoskeleton, the extracellular matrix and how intercalation is coordinated at the tissue level. Second, I look at the genetic networks that control the physiological differentiation of tubule cells and consider how distinctive physiological domains in the tubule are patterned. Finally, I explore how the organ is positioned within the body cavity and consider the relationship between organ position and function.
Keywords: convergent-extension, Drosophila, insect excretory system, Malpighian tubule, morphogenesis, organogenesis, renal tubule, physiological differentiation, tubulogenesis
Introduction
The fastest fluid-secreting cell known belongs to an insect. Remarkably this cell, one of a few hundred that make up the renal or Malpighian tubule (MpT) of the blood-sucking bug Rhodnius, can secrete a volume of fluid equal to its own volume every 15 sec.1 The hard-working MpT cells allow these bugs to eliminate urine at a rate equivalent to their original body weight every 20–30 min. This impressive feat is necessary because the prodigious blood meals the insects —sometimes in quantities of up to 12 times their own body weight—leaves them with surplus fluid and ions that must be excreted quickly to allow them to return to a more comfortable state. Bumblebee MpTs are equally industrious. The high water content of the nectar used to fuel flight coupled with the exertion of flying mean that bee tubules are required to work flat out to produce a continuous stream of urine to rid them of surplus fluid.2 These extreme examples underscore the important homeostatic functions performed by the MpTs. In much the same way that our kidneys do for us, the insect’s MpTs maintain water, ion and acid/base balance and rid the body of foreign and metabolic toxins to maintain a constant internal environment that is necessary for wellbeing, health and survival.
The development of the insect MpT has been studied extensively in Drosophila. This fly manages to assemble a set of fully functional MpTs from a group of undifferentiated cells in a little over 12 h. During this short period, the MpTs are specified, they grow and are shaped, tubule cells differentiate and the organ is positioned precisely within the body to achieve optimal function. In this article I consider MpT development focusing on three aspects that contribute directly to organ function: how tubule shape is sculpted; how cells within it specialize to produce different physiological cell types that underlie function, and; how the organ is positioned within the body cavity, with respect to other organs and tissues.
There are several reasons why research into insect MpTs is important. Insects as disease vectors and agents of pestilence are widespread and have considerable associated health and economic costs. Elucidation of the mechanisms of MpT epithelial transport and toxin clearance, and the ways in which they are regulated might lead to specific strategies for control. Besides this however, there is another more fundamental reason why studying MpTs is important: as a model for organogenesis. Many of our own tissues and organs, such as the kidney, vascular system, lungs and gut, to name only a few, are composed entirely or partly from epithelial or endothelial tubes some of which have transporting functions. Breakdown in tubular structure or function in these organs is often associated with human diseases such as polycystic kidney disease or cystic fibrosis. As MpTs are an extreme example of a transporting epithelium1 they represent a particularly attractive model to study the process of epithelial transport and the mechanisms that underpin the development of transporting epithelia. Lessons learnt from this simple MpT model are likely to have wide relevance for other organs, including our own. I pinpoint key questions that still remain in the field and discuss the powerful tools that exist in the fly that will enable us to tackle fundamental questions about how organs develop.
The Insect Malpighian Tubule
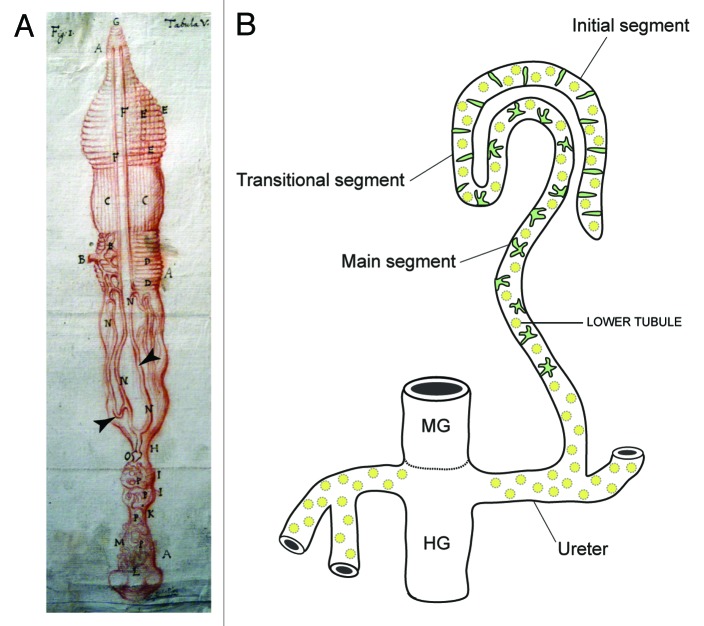
MpTs are found in all insects apart from Thysanura and aphids, they are also found in other terrestrial arthropods—the spiders and myriapods (millipedes and centipedes). The first description of an insect Malpighian tubule was of the silkmoth Bombyx (Fig. 1A) by Signor Marcello Malpighi in the seventeenth century (whose manuscripts are held in the library of the Royal Society in London).3,4 Their size, length and number can vary substantially between insect species, but their general form is similar (Fig. 1B). They are simple, single-cell layered epithelial tubes, which insert into the gut at the midgut-hindgut boundary. They float freely in the body cavity, bathed by the fluid they act upon—the insect blood or hemolymph. Primary urine is generated in the distal portion of the tubule by a secretory mechanism (see below), and subjected to modification as it is conveyed down the lumen toward the gut. Excreta are passed into the hindgut (where further modification such as retrieval of additional water can take place) before being expelled from the animal. In addition to these excretory roles, the tubules can mount an immune response and thus serve to protect the animal against pathogenic insult.5,6 Still more exotic activities have also been shown for tubules in some species, such as the production of light or of silk that are used to attract mates, make cocoons and capture prey.7-9
Figure 1. Insect Malpighian tubules (A), Malpighi’s original drawing of the MpTs (and gut) of the silkmoth Bombyx mori (Malpighi, 1669). The MpTs are indicated with arrowheads. (B) Schematic representation of the MpTs of Drosophila. Drosophila, has four MpTs, a longer anterior pair and a shorter posterior pair (a single anterior tubule is depicted). Each tubule is divided into four regions based on differences in cell structure and physiology along the proximodistal axis: initial, transitional, main segment and ureter. Two main physiologically distinctive cell types are known, principal cells (yellow) and stellate/bar cells (green). Midgut (MG) and hindgut (HG) are indicated. Image in A reproduced courtesy of the Royal Society of London.
An Overview of MpT Development in Drosophila
Assembling the building blocks for organogenesis
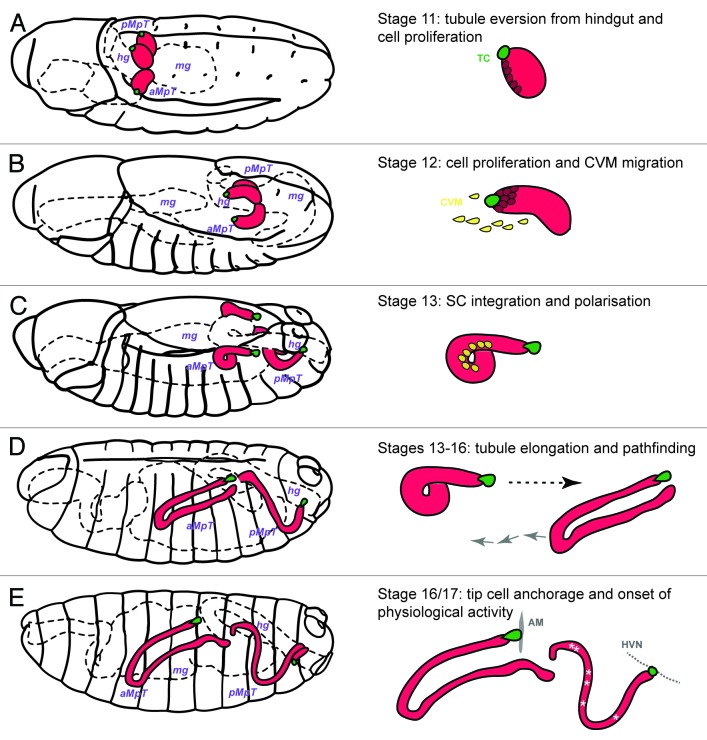
A good place to start when considering how an organ develops is to ask where the cells used to build the organ originate. Potential sources of cells for organ building include: (1) allocation of organ primordial cells, (2) cell proliferation within this population and (3) recruitment of cells from other sources. All three mechanisms are employed in the development of Drosophila MpTs. As these aspects of tubule development have been covered in detail elsewhere10,11 I provide only an outline here. The tubules arise from an ectodermal primordium shared with the hindgut. At least three important signals are important for tubule cell specification and eversion: the Wnt (Wingless), and BMP (Decapentaplegic) proteins and an as yet unidentified signal from the midgut.12 These signals converge and lead to the specification of four small clusters of about 20 cells, each marked by the expression of two transcription factors Cut and Krüppel both of which are important for early tubule development. The clusters evaginate as four little cylindrical buds and increase in size by cell proliferation (Fig. 2A andB). Tubule cell proliferation is controlled sequentially by the Wingless (Fig. 2A), then EGF signaling pathways (Fig. 2B), the later driven by EGF ligand secretion from the distal-most pair of cells – the tip cell and its sibling cell.13-16 By mid-embryogenesis cell proliferation is over, however the number of tubule cells is fortified by recruitment of mesodermal cells from the caudal visceral mesoderm (which also form the founder cells of the midgut visceral muscle). These mesodermal cells migrate to the tubule, undergo a mesenchymal-to-epithelial transition and integrate into the epithelium, developing full apico-basal polarity (Fig. 2C).17,18 They begin to express the transcription factor called teashirt and ultimately differentiate into a physiologically distinctive subset of tubule cells known as stellate cells (SCs, see below). It has been shown that the basolateral and apical membranes and the adherens junctions of the ectodermally-derived principal cells (PCs), that make up the tubule epithelium, act as cues to establish and then stabilize SC polarity.18
Figure 2. Embryonic development of Drosophila Malpighian tubules A, Stage 11 (5h15–7h15) MpTs grow out as two then four small buds. Cell division occurs synchronously in all tubule cells and then in a subset of tubule cells regulated by Wingless. (B) Stage 12 (7h15–9h15) cell proliferation continues in a subset of tubule cell regulated by the EGF secretion from the tip cell (green cell) and sibling cell (not highlighted). The caudal visceral mesoderm (yellow cells) migrate toward the tubule. (C) Stage 13 (9h15–10h15) Cell division is complete. Stellate cells integrate into the tubule epithelium and develop apico-basal polarity. (D) Stages 13–16 (10h15–13h) Tubule elongation takes place by cell intercalation. MpTs migrate through the body cavity following highly stereotypical routes. (E) Stages 16/17 (13h-22h) the tip cell locates and anchors to its final position [alary muscle (AM) for anterior tubules and hindgut visceral nerve (HVN) for posterior tubules]. The onset of physiological activity becomes apparent as white crystals of uric acid appear in the lumen of the posterior MpTs.
Tubule morphogenesis, pathfinding and physiological maturation
The processes of tubule morphogenesis, pathfinding and physiological maturation that occur during stages 13–17 (Fig. 2D and E) are the main topics of this paper and are discussed in the following sections.
Post-embryonic development
The larva hatches after ~22 h of embryonic development, further growth of the tubule during larval stages occurs by an increase in cell size coupled with endoreduplication of DNA. Differences in the appearance and structure of tubule cells allow four domains to be recognized: a distal initial domain, a transitional domain, the main tubule segment and the lower tubule (Fig. 1B). These differences reflect functional differences, e.g., the main segment is secretory whereas the lower tubule is reabsorptive.19 Unlike most larval tissues the MpTs survive metamorphosis and are retained in the adult fly. During pupal development the tubule shrinks to half its former length with the initial segment expanding to form a bulb. The diameter of the tubule and its lumen also decreases.20 Changes in cell shape also occur during pupation. For example, during the last 12 h of pupal development SCs transform from their cuboidal larval shape to adopt “bar-shaped” morphologies (in the initial and transitional segments) and “stellate-shaped” morphologies (in the main segment, Fig. 1B and 3B) characteristic of the adult tubule (Nan Hu, personal communication). After hatching, adult tubule domains resemble those of the larva, with the exception that the initial segment of the anterior tubule is much smaller than in the larva.20
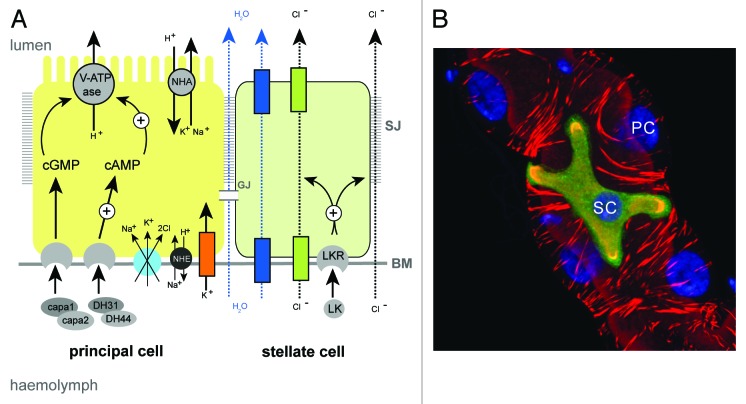
Figure 3. Principal and stellate cells (A), Drawing to show the major physiological activities performed by PCs (yellow) and SCs (green) in adult Drosophila tubules. Ion transport is driven by the activity of a H+-transporting vacuolar-ATPase (V-ATPase) located on the luminal membrane of principal cells. The V-ATPase, coupled with a cation/H+ antiporter (NHA), constitutes a cation pump that transports cations from the cytoplasm to the tubule lumen. Chloride ions (black dashed arrows) move into the lumen down an electrochemical gradient possibly through as yet unidentified chloride permeable channels (green) in SCs and via paracellular routes. Water (blue dashed arrows) follows by osmosis through water channels (dark blue) in SCs and via paracellular routes. Possible routes for basolateral cation entry include: K+ channels (orange), cation coupled Cl- cotransporters (light blue), and Na/H exchangers (NHE, black). Movement of ions between cells may occur through gap junctions (GJ). capa, capability peptides 1 and 2; DH, diuretic hormone; LK, leucokinin; LKR, leucokinin receptor; SJ, septate junction; BM, basement membrane. (B) Small section of an adult Drosophila MpT depicting a SC (green) and PCs (unlabelled). PCs and SC are indicated, the tubule is counterstained with phalloidin (red) to highlight the actin cytoskeleton and DAPI (blue) to highlight nuclei.
Malpighian Tubule Morphogenesis: Sculpting a Tubular Organ
Organs come in different shapes and sizes, tailored for particular physiological functions. In tubular organs like the MpTs or the vertebrate nephron it is important that appropriate tube dimensions are established during development and maintained throughout life for normal function. In circumstances where this fails, as it does in human polycystic kidney diseases, where nephron diameters are grossly enlarged,21 physiological function is severely compromised and this often leads to organ failure. However, the ways in which tubular epithelial are modeled in development are generally poorly understood. Here I describe the mechanisms that shape MpTs during development and discuss how this might be controlled at the tissue level.
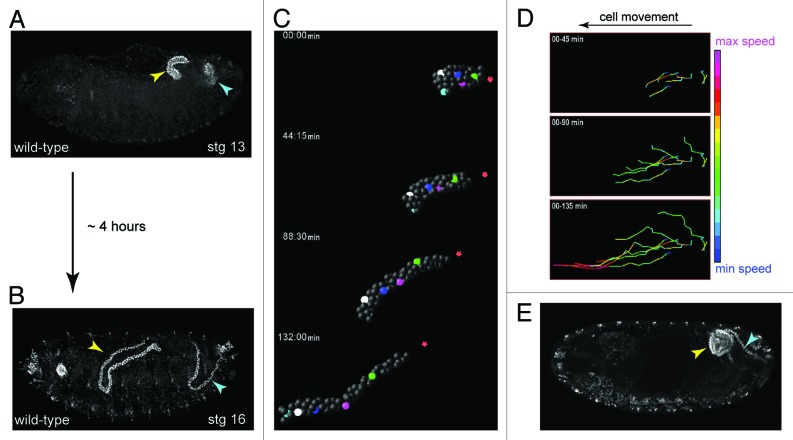
Embryonic development, the period prior to the first larval stage, takes approximately 22 h at 25°C in Drosophila. From mid-embryogenesis, over the course of approximately 4 h the MpTs undergo a remarkable change in shape—increasing 4-fold in length along their proximo-distal (P-D) axis, while reducing circumferential cell number (the number of cells surrounding the lumen) from ~8–12 cells to just two (Figs. 2C−E, 4A and B). This transformation occurs in the absence of cell division and is underpinned by an organized pattern of cell intercalation. It represents a classic example of convergent-extension (C-E), where a tissue narrows (the cells converge) in one axis, and elongates (extends) in the orthogonal axis.22,23 What mechanisms underlie C-E in the tubules? We know that MpTs grown in isolated culture elongate normally (S. Bunt, personal communication)24,25 revealing that C-E is driven by internal forces and is independent of other tissues. As ordered cell intercalation lies at the heart of C-E, this is the process we would like to understand. In very simple terms we can break this down to ask: (1) what are the motile cell behaviors that drive intercalation, and (2) what is the nature of the global cues that control these behaviors?
Figure 4. Shaping of the Malpighian tubule (A and B), During a period of about four hours MpTs undergo a dramatic change in shape that is driven by orderly cell intercalation, transforming from short, stubby tubules in mid-embryogenesis stage 13, (A) to long, elongated tubules in late embryogenesis stage 16 (B). (C) SIMI-Biocell assisted 3-D reconstruction of the distal portion of an anterior MpT. Five cells are marked in different colors and their positions tracked in XYZ over time. The distal-most tip cell is marked with red star. Such tracking can be used to quantify individual cell behaviors during tubule elongation (D). (D) Quantification of cell speed during intercalation. Cell speeds for the 5 cells marked in C are shown. The lines show cell tracks and color indicates cell speed. Net direction of cell movement is indicated by the arrow. (E) Orderly cell intercalation fails in crossveinless-c mutants. Anterior and posterior MpTs are indicated by yellow and cyan arrowheads in A, B and E. Images in C and D courtesy of Aditya Saxena.
Cell behaviors during intercalation
Genetic screens designed to isolate mutants where tubule elongation fails have been particularly fruitful in revealing the molecular mechanisms that underpin MpT C-E movements. In mutants for the gene crossveinless-c (cv-c) tubule elongation fails completely.26 In cv-c mutant tubules, cell intercalation does not initiate, so rather than undergoing P-D elongation and circumferential narrowing to produce four long thin tubules, the tissue coalesces into a single sack-like blister (A. Saxena, personal communication; Fig. 4E).26 Cv-c encodes a RhoGAP (GTPase Activating Protein) that catalyzes the transition of Rho-family GTPases (Rho, Rac and cdc42) from their active to inactive state. In the tubules Rho appears to be the primary target for cv-c. Rho-family GTPase have established roles in regulating both actin and myosin, and consistent with this cortical actin is strongly disrupted in cv-c mutants during C-E movements.26 Interestingly, elongation defects similar to those of cv-c are found in tubules mutant for zipper, the gene encoding the non-muscle myosin heavy chain, and ribbon and raw, two genes required for non-muscle myosin subcellular localization.27-29 These data indicate that an actin-myosin process underlies C-E movements. It has been shown that the Cv-c protein localizes to basolateral cortex and RhoGEF64 (a guanine exchange factor that has opposing, activating effects on RhoGTPases) to the apical cortex.30 Together this suggests a model where subcellular differences in Rho activity, underpinned by the differential cellular distribution of its regulators, control polarized actomyosin-mediated processes in tubule cells to bring about orderly intercalation and tubule elonagtion.
The precise cellular mechanisms that bring about C-E in the tubules are not known. Two principal mechanisms that underpin C-E in other tissues have been described, and these may be informative to C-E in tubules: (1) local remodelling of cell junctions and (ii2 formation of lamelliform cellular protrusions used by cells to “walk” or “crawl.” During germband extension, which describes the developmental elongation of the Drosophila embryo, ordered cell intercalation proceeds through remodelling of the adherens junctions. Here, junctions are spatially reorganized within the plane of the epithelium following an ordered pattern of junction disassembly and reassembly. Junctional disassembly is dependent upon myosin II, and its planar polarized localization ensures directional cell intercalation to bring about axis elongation.31,32 Embryonic MpT cells are linked by adherens junctions and these are remodelled during elongation, however it is not known whether junctional remodelling via myosin II action is the driving force behind intercalation in this tissue. A requirement for actin, myosin and their regulators described above could be viewed as evidence for this mechanism.
An alternative mechanism, perhaps best characterized during mediolateral intercalation in frog mesodermal cells and the ascidian notochord,33-36 involves the formation of lamellipodial protrusions. These protrusions contact and exert traction on neighboring cells so that cells effectively crawl over one another. Because the protrusions are planar polarized–emanating from the lateral sides of the cell only—cell intercalation is directional. Similarly, during elongation in the C. elegans embryo dorsal hypodermal cells intercalate using medially-directed lamelliform protrusions.37 These protrusions, which are positioned on the basal side of the cell, may use the extracellular matrix (ECM) in addition to neighboring cells as a substrate upon which traction is gained. Active lamellipodial-like basal ruffles are found in tubule cells during elongation.38 Furthermore, an ECM is deposited on the basal side of the tubules and is in place in time for the onset of tubule elongation,38 suggesting a mechanism where tubule cells walk or crawl using the basement membrane as their substrate. In support of this hypothesis, work from our laboratory indicates that ECM components and their receptors, such as the integrins, are required for tubule elongation (Tarun Kumar, personal communication). Interestingly a similar mechanism may underlie the morphogenesis of the pronephros in zebrafish, where polarized basal lamellipodial protrusions from pronephric epithelial cells are used to walk along the basement membrane during the collective cell migrations that sculpt morphogenesis in the pronephric tubule.39
Additional roles for the ECM in tubule morphogenesis
The ECM may be more than just a passive substrate to be walked or crawled upon. There is evidence the ECM plays an active and instructive role in the morphogenesis of some tissues. It has recently been discovered that the transformation of the Drosophila egg chamber from its initially near-spherical shape to an ellipsoid is dependent upon the surrounding ECM.40 Here the ECM acts like a “molecular corset” restricting growth to produce the characteristic elongated shape of the mature chamber. A tightening corset of ECM around the tubule might also act to sculpt morphology during development. It is also possible that the ECM polymers and fibers have an intrinsic polarity that guide tubule cells as they intercalate, rather like the way railway tracks guide the movement of a train.
In addition to the basal ECM, there are also indications that apical or luminal ECM may contribute to tubule elongation. In tubules mutant for genes that support the formation of apical ECM such as mummy/cystic and krotzkopf verkehrt, which encode enzymes involved in the catalysis of chitin, the tubules are malformed, distended and fail to elongate.41,42 Clues to the function of these genes in tubulogenesis are revealed in studies of Drosophila tracheal development. mummy/cystic and krotzkopf verkehrt are both required for apical ECM formation and serve to regulate normal tracheal tube dimensions.43-46 It has been suggested that chitin is secreted into the tracheal lumen where it forms an expanding cylinder that coordinates the behavior of surrounding tracheal cells to ensure uniform tube expansion.45 Furthermore, a general requirement for apical ECM in the morphogenesis of tracheal tubes and other epithelia is beginning to emerge.44,47-51 Could a luminal chitin or apical ECM cylinder regulate MpT morphogenesis? If it does the cylinder would need to begin wide and then contract (i.e., in the opposite direction to that hypothosized for the trachea), because the MpT lumen normally narrows during the elongation process. The presence of extracellular material in the lumen of elongating tubules supports this hypothesis.52 However, any role for lumenal ECM in tubule C-E would have to be transient because it is known that mature MpTs are not cuticularized.
A more revealing picture of the cell biology underlying C-E in the tubules is within our grasp by using live-imaging techniques (Fig. 4C). This will allow us to catalog the precise pattern of intercalation, and to quantify individual cell behaviors during the process (Fig. 4D). By following actinomyosin dynamics, cell membranes, junctions and ECM in real-time during elongation we will be able to visualize the dynamic cell behaviors that drive cell intercalation. These analyses can also be performed in mutant backgrounds, such as cv-c, to probe the molecular networks that underpin these cell behaviors during intercalation.
Orchestration of cell intercalation
MpT cell intercalation during C-E proceeds in a highly stereotypical fashion (Aditya Saxena, personal communication), suggesting there is a global signal that orchestrates the process. Recent evidence from our lab indicates that the EGF pathway is important in this respect (Denholm et al., in preparation). Briefly, an EGF signal is secreted from the distal tip of the tubule polarizing the tissue within the plane of the epithelium. When EGF signaling is perturbed the tissue does not polarize and C-E movements fail. It remains to be seen how this planar information is ‘read’ or acted on by cells to bring about orderly intercalation, however polarized remodelling of cell junctions like those that occur during GBE31,53 or the formation of directed cell protrusions like those that occur during C-E in the frog mesoderm and ascidian notocord34,36 are viable possibilities.
Planar cell polarity (PCP) is known to be indispensable for the morphogenesis and elongation of many different vertebrate tissues and tubular structures including the kidney tubule and the gut.54,55 Recently, Chung and colleagues (2009) have shown some of the core PCP proteins and the apically enriched PCP regulator, Serrano, are important in controlling tracheal tube size and elongation.56 This study provides an example in flies where planar polarization regulated by PCP proteins is necessary for tubulogenesis. It is presently unclear if, as in the case of vertebrates, PCP is a general prerequisite for the elongation and morphogenesis of Drosophila tubular organs. Rigorous testing for planar polarization and its genetic regulation during morphogenesis of different fly epithelial tubes is required to address this issue. The MpTs can serve as one such organ system to test these ideas.
A genetic network for organ morphogenesis
Several other mutants have been described where MpT C-E fails. These include the transcription factors Buttonhead41 and Trachealess;42 the nuclear protein Lines;41 a cell adhesion molecule of the immunoglobulin superfamily called Faint Sausage;42,57 and Crooked neck (also known as Yok) a protein with a tetratricopeptide repeat protein interaction motif.42 As several of these are required for the morphogenesis of other tissues including salivary glands, tracheal system, heart, head, hindgut and dorsal epidermis it suggests that common genetic networks might operate to shape diverse tissues and organs. There is good evidence to suggest that a genetic network linking trachealess, EGF signaling and cv-c is common in shaping both the MpTs and trachea. Trachealess is required for normal MpT morphogenesis42 and for an early phase of tracheal development where the tracheal pits invaginate into the embryo by a two-part process involving apical constriction of invaginating cells and tissue remodelling. In the trachea, tkh links EGF-signaling and cv-c which are in turn both required for modifying the cytoskeleton to bring about pit invagination.58 It is tempting to speculate that this represents a genetic module that is utilized repeatedly during the morphogenesis of different organs. The challenge for the future will be to establish links between transcription factors, signaling pathways and the intracellular effectors of the cytoskeleton that bring about three-dimensional tissue shaping.
Physiological Maturation
The Drosophila MpT can be divided into several morphologically distinct domains the initial, transitional, main and lower segments (Fig. 1B) with distinct functional roles.19,20 Furthermore, physiologically distinctive cell types can be found within each domain (Fig. 1B). Based on the premise that differential gene expression equates to differential function, the work of Sozen and colleagues suggest that, in a tubule with average size of ~150 cells, there are at least 10 functionally different cell types.59 How these cells differ, how their differentiation is controlled, and how they specifically align with respect to each other to ensure normal excretory function are important questions that will be explored below. To set the scene for this I first give a brief outline of insect tubule physiology.
Malpighian tubule physiology
Primary urine is generated by a secretory mechanism and this is coupled with active transport of nitrogenous wastes and specific toxins such as cardine glycosides, in addition to the excretion of novel toxins by diffusion down paracellular routes.60 MpT physiology has been studied in detail for many insect species and the transport processes that underlie urine production and its hormonal regulation are known in detail (Fig. 3A). Briefly, primary urine production is energised by a vacuolar-type H+ ATPase (V-ATPase) located at the apical brush border of tubule principal cells (PCs).61-64 This pump maintains a proton gradient across the apical membrane that drives the movement of alkali metal cations (Na+ or K+, depending on the insect species) into the lumen through Na+/K+/H+ exchange via members of the monovalent cation proton antiporter (CPA) transporter family. Rheault and colleagues characterized a member of the CPA2 family called NHA1 and show that it is expressed in MpTs of the Anopheles mosquito.65 Further, the two Drosophila orthologs (NHA1 and NHA2) are co-expressed with the V-ATPase on the apical membrane of MpTs, and overexpression of NHA1 was shown to stimulate basal fluid secretion in tubules.66 Together, this provides strong evidence that cation transport in insect MpT occurs by the action of a V-ATPase working in association with a NHA. Possible routes for cation movement across the basolateral membrane of PCs from the hemolymph include, potassium channels,67-69 cation coupled Cl- cotransporters,67 and Na/H exchangers (NHE).70 The rates of cation transport are regulated by the diuretic hormones DH31/44 and capa1/2, which act via cGMP and cAMP pathways to stimulate V-ATPase activity.71-74
Active cation transport establishes a favorable electrochemical gradient for the movement of chloride ions across the epithelium. The route for chloride ions across the epithelium might be different depending on circumstance or species. In many, but possibly not all, insect species75 chloride ion movement takes place across a distinct cell-type called the stellate cell (SC, also known as a type-II cell).76 SCs are morphologically distinct and are interspersed between PCs along the main segments of the tubules (Fig. 1B and 3B), where they facilitate the transepithelial movement of chloride ions from the hemolymph into the lumen. The Anopheles and Aedes aegypti SLC4, a Cl-/HCO3- exchanger expressed in SCs, may be the route by which Cl- enters the cell from the hemolymph.77,78 Evidence for apical Cl- channels comes from patch-clamp experiments performed on the luminal surface of unstimulated Ades MpTs.79 This study found evidence for two types of Cl- conductance channel: type I channels with intermediate conductance and slow kinetics and type II with low conductance and fast kinetics that operate in unstimulated MpTs. The molecular identity of these channels is unknown. However, two channels of the ClC family (ClC-a and ClC-c) have been identified in Drosophila as being enriched in MpTs,80 and of these ClC-a is expressed highly in SCs in the embryo and adult,81 providing two promising candidates.
In addition to the transcellular passage of chloride ions, there is strong evidence for movement of chloride ions between cells in some insect species such as the mosquito Aedes aegypti, particularly under conditions of kinin-activated diuresis.82 This paracellular pathway is in effect a “transepithelial shortcut” bypassing cells of the epithelium to promote maximal transport under conditions of diuresis. The effects of leucokinin are extremely quick and reversible and it is thought that the post-transcriptional modification of one or more components of the septate junction—a ladder-like structure that adheres cells together and confers some permeability properties on the tubule83—underpins the rate of transport through the paracellular pathway.82 Septate junctions consist of transmembrane proteins and cytoplasmic scaffolding proteins, linked into the actin/spectrin cytoskeleton. It has been hypothesized that modification to scaffold and cytoskeletal proteins results in the “unzipping” of the septate junction paracellular strands to bring about increased paracellular Cl- conductance.84 In support of this, actin, adducin and actin-depolymerising factors are mobilised after kinin stimulation.84-86
Similarly, movement of water occurs through both transcellular and paracellular routes (Fig. 3A).87-90 Transcellular movement occurs through water channels, with aquaporin water channels identified in MpTs of Rhodnius, the house cricket and Drosophila.87,91-93 Of note, the aquaporin channels drip and AQP1 are expressed specifically in the SCs of Drosophila and Anopheles respectively, indicating a further important function for SCs in some species.87,94
SCs have been found in tubules from a phylogenetically wide range of insects including Diptera, Lepidoptera, Coleoptera and Orthoptera, suggesting they are an ancestral feature of insect MpTs (reviewed in Dow, 2012).75 However, they are not found in all insects. Rhodnius (Hemipterna) lack SCs, as do tsetse flies (a fairly close Dipteran relative of Drosophila).95 In these species it has been suggested that cation and anion transport may occur through the same cell. The presence of SCs in the tubules of some insects but not others is curious and raises some interesting questions: What are the precise functions of SCs, and are they functionally equivalent in all insects? Are SCs a derived or ancestral character of insect tubules? Do those species without SCs have unrecognized cell physiological specializations, or does a single cell-type carry out all secretory functions in these insects? A wider sampling of species to determine the phylogenetic spread of SCs in insect tubules, in combination with functional analyses using RNAi will shed light on these questions.
Physiological differentiation of stellate cells
Work from our laboratory has begun to address the developmental mechanisms that underpin physiological maturation of SCs. We have recently found that the closely related Teashirt/Tiptop class of transcription factors are key regulators of SC differentiation in the tubules of the fly and other insects.81 In Drosophila, teashirt is expressed from mid-embryogenesis throughout the lifetime of the animal in SCs but not in PCs. Teashirt activity in SCs is required to regulate the expression of several genes associated with the mature physiological function of the SC including an aquaporin water channel, a chloride ion channel and a hormone receptor. Furthermore, teashirt is required for the regulation of cell shape. SC differentiation in the pupa is associated with a dramatic change in shape, where cuboidal larval SCs transform to adopt “bar-shaped” (in the initial and transitional segments) and “stellate-shaped” (in the main segment) morphologies characteristic of the adult tubule (Fig. 1B). The significance of these striking cell morphologies in the adult have not been explored but could be a mechanism to maximize contact with neighboring PCs. In the absence of teashirt SCs remain cuboidal, with a morphology similar to that found for larval SCs. These data indicate that teashirt controls multiple aspects of the SC phenotype and suggest that it occupies a pivotal position in the SC differentiation hierarchy. The role of teashirt/tiptop in SCs appears to be conserved in other insects because tiptop is expressed in the SCs of Coleopterans (the beetle, Tribolium castaneum) and the Othopterans (the cricket, Gryllus bimaculatus).81,96 These species are distantly related to Drosophila (a Dipteran), sharing a common ancestor approximately 280 (beetle) and 360 (cricket) million years ago, indicating that the activities of teashirt/tiptop in SCs are ancient and widespread. The genetic network operating downstream to teashirt is not known yet. However, mouse Teashirt-3 regulates the expression of an aquaporin channel in the smooth muscle layer of the ureter in the mouse kidney, raising the intriguing possibility that teashirt targets may be conserved between widely divergent species.81
Physiological differentiation of principal cells
Less is known about the physiological maturation of PCs. The transcription factors Cut and Krüppel are expressed in PCs from early embryonic stages and are required for tubule morphogenesis and PC differentiation.97,98 In Kr mutants tubule eversion from the shared hindgut/Malpighian tubule primordium fails, and in cut mutants the tubules do not elongate; instead they form a multilayered cyst. Despite this, these mutant “tubule cells” still transport urates (a diagnostic feature for differentiated tubule cell function) revealing that PC differentiation can still occur.12 However, in the absence of both cut and Kr urate transport is abolished, suggesting that both factors are required, perhaps redundantly, for PC physiological differentiation.12 Although we know the repertoire of channels, pumps and receptors that define PC function (Fig. 3A), it is not yet known how these are linked with cell specification by transcription factors such as Cut and Kr.
The proximo-distal patterning of physiological activities
Domains with distinct physiological activities exist along the proximo-distal axis of the tubule. For example: the initial segment is geared toward calcium homeostasis and excretion;20,99,100 the transitional/main segments are secretory whereas the lower tubule is reabsorptive.19 Tubule cells with distinct physiological functions need to be aligned with respect to one another to ensure normal secretory function. For example, the interplay between PCs and SCs is key to the production of primary urine in some species, and their interspersed pattern is likely to be important for this. Therefore, tissue patterning and cell differentiation must take place to establish the correct arrangement of physiologically distinctive cell-types within the organ. Our understanding of these developmental mechanisms is limited. Distinct distal-proximal domains of gene expression arise during the specification of the tip cell lineage.15,101 During this process a cluster of cells expressing the proneural genes is established at the distal end of each tubule. Lateral inhibition, mediated by the neurogenic genes, selects a single cell—the tip mother cell (TMC)—in which proneural gene expression strengthens. In the remaining cells of the cluster proneural gene expression is dampened, however these low levels of expression are still sufficient to activate seven up, a mediator of the EGF pathway. In effect, this generates domains of differential gene expression along the distal-proximal axis: a distal tip cell with high levels of the proneural genes; a group of intermediate cells expressing seven up; and finally, a more proximal cell population that lack both proneural and seven-up gene expression.15 It is conceivable that these ‘tiers’ of gene expression lead to the differentiation of specialized cell-types along the distal-proximal axis of the tubule. A further role for the tip cell could be envisaged in which it secretes a morphogen to generate a morphogenetic field. In this scenario tubule cells would differentiate according to their distal-proximal position within this field.
In addition to these spatial mechanisms, there may be temporal control of differentiation. One could imagine a mechanism whereby cells adopt different physiological fates according to their “birth order” in much the same way that neurons differentiate according to neuroblast birth sequence.102 The pattern of cell proliferation within the tubule is tightly controlled and to a large extent invariant;103 the consequence of this is distal cells are born later in development than their counterparts in more proximal regions. This temporal birth order could be transformed into distinct spatial patterns of gene expression that go on to control physiological differentiation.
Structural differentiation
The subcellular structural changes that accompany maturation of physiological competence have been closely examined in the Malpighian tubule cells of the blood-sucking bug Rhodnius.104 In these insects, tubule development occurs over a longer period making it easier to correlate changes in cell architecture with the development of physiological competence brought about by these changes and, by virtue of their size, it is possible to carry out in vitro physiology measurements in first instar MpTs. In Rhodnius there are two periods of functional maturation: a period beginning 3–4 d prior to hatching, where the transport of organic solutes such as urates and p-aminohippuric acid progressively increase in the tubule; and a second period, 1–8 d after hatching characterized by the onset of urine secretion. These phases in physiological maturation are accompanied by underlying changes in cell and tissue architecture. During the first phase, approximately 4 d before hatching septate junctions are assembled.104 One possible function of septate junctions is to lower paracellular permeability by increasing the effective path length from the hemolymph to luminal sides of the tubule, and thereby to reduce bulk fluid flow between cells.83 Further, the septate junction is an important structure that controls Cl- conductance in Aedes MpTs.82 An increase in paracellular Cl- conductance is expected to increase the transcellular secretion of cations with the effect of increasing transepithelial water flow. Lumen formation begins around this stage. At 4 d prior to hatching the tubules have no obvious lumen, however 2 d later a lumen is apparent, and continues to expand until approximately 8 d post-hatching. Lumen formation correlates with the onset of organic solute transport, and provides a space for its deposition.
Structural changes continue to occur until the eighth day after hatching. These changes include a striking amplification of cell membranes, apically to produce a carpet of microvilli, and basally to produce complex, interdigitating basal infoldings.105 The amplification of the membranes provides increased surface area and is likely to be accompanied by their specialization, through the localization of specific receptor proteins, ion pumps and channels. At the same time microvilli and basal infoldings become highly populated with mitochondria, presumably to generate the energy needed to facilitate active transport. These changes correlate with the onset of urine secretion and together equip the animal for the prodigies of excretion called for by the immense size of their first blood meal. Structural and/or physiological maturation of MpTs may continue after eclosion in other insects. For example, Schepel and colleagues observe that responsiveness of Aedes MpTs to kinin stimulation sometimes develops only a few days after the adult hatches.106 The authors suggest this delay may represent the continued development of the MpT after eclosion and/or be linked nutritional history of the mosquito in the larval stage.
The area of research at the interface between developmental biology and physiology is not yet fully explored for the majority of organs. Our exquisitely detailed knowledge of insect renal tubule physiology, and the ease by which physiological activity can be measured on the one hand, coupled with our considerable understanding of tubule development (particularly for Drosophila) on the other, provide fertile ground for unpicking the mechanisms leading to physiological maturation. The resources to carry this out already exist and include: a complete transcriptome of Malpighian tubules80 along with tools that allow cell specific gene knock-down, cell-specific transcriptional profiling and in vivo binding-site profiling.107 Furthermore, the use of simple screens that reflect tubule physiological performance (e.g., uric acid deposition or osmoregulation in the intact animal) coupled with tubule-specific gene knockdown could be employed to uncover genetic mechanisms that underpin physiological maturation. Mechanisms uncovered in insect MpTs are likely to have wide significance for transporting epithelia more generally.
Organ Positioning Within the Body
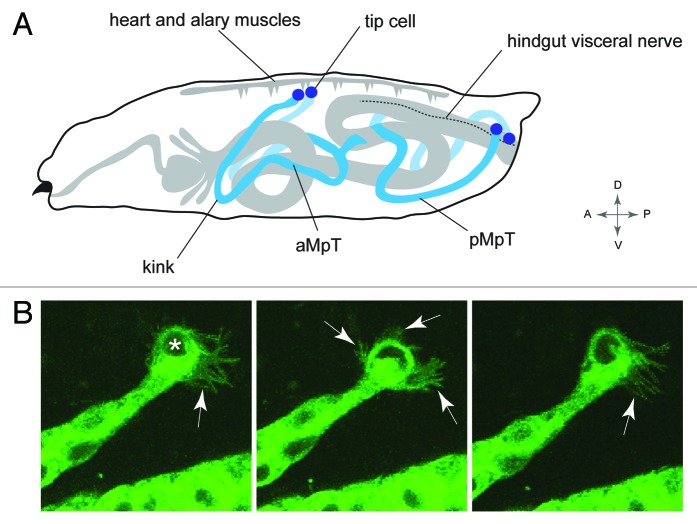
Insects have an open circulatory system and effective excretion is dependent upon the tubules having wide coverage to remove waste. Accordingly, the mature tubules tend to adopt highly stereotypical positions within the body to maximize hemolymph sampling (Fig. 5A).38,108 In Drosophila one pair of tubules extend forward toward the head, and a second pair projects to the posterior. Further, one tubule of each pair lies to the right and one to the left within body cavity. This stereotypical arrangement is developmentally controlled: as tubules elongate through C-E movements they follow a highly reproducible course through the body cavity. The anterior tubules bend back on themselves so the apex of the bend “the elbow” or “kink region” extends forward, and this kink region leads the anterior tubules toward the head. Toward the end of embryogenesis the kink region changes course and dips ventrally.38 The posterior tubules grow toward the tail of the embryo where, at the end of embryogenesis, their distal ends switch sides by crossing over the posterior region of the hindgut. Ultimately the distal-most cell in each tubule—the tip cell—makes specific contact with alary muscles of the heart for the anterior tubules, and a hindgut visceral nerve for the posterior tubules (Helen Weavers, personal communication).101 These movements can be seen in a movie (www.zoo.cam.ac.uk/zoostaff/skaer/links.htm).
Figure 5. Positioning the tubule in the body cavity (A), Drawing of a Drosophila larva showing the final position of the MpTs relative to other internal structures. Anterior and posterior MpTs (aMpT and pMpT) are indicated, tip cells are shown in purple, gut and heart are shown in gray, the hindgut visceral nerve is depicted by a dashed line. Anterior, posterior, dorsal, ventral coordinates are given. (B) Still images from MpT movie showing the dynamic filopodial extensions (arrows) of the tip cell (indicated in first panel by asterisk). The time interval between images is 5 min. Images in B courtesy of Helen Weavers.
The stereotypic navigation path taken by the tubules suggest that “guidepost signals” promote or inhibit extension into particular domains. These guidepost signals have been uncovered for anterior tubules to reveal a complex but fascinating picture with the involvement of multiple tissues and signaling cues.38 The process is initiated by tubule cells through their secretion of PDGF/VEGF ligands to attract migrating blood cells known as hemocytes. Upon arrival the hemocytes begin to deposit the basement membrane component collagen IV that envelopes the growing tubules forming an extracellular collagen sheath. Here, as shown previously for other developing tissues in Drosophila,109 collagen IV modulates BMP pathway activity, sensitizing a subset of tubule cells to local sources of the BMP ligand decapentaplegic (dpp), which act as attractant guidepost cues that propel the tubules forward. At least three dpp guidepost signals (from the dorsal epidermis, midgut visceral mesoderm and gastric cecal visceral mesoderm) are sequentially encountered to steer the tubules during their outgrowth. In the absence of dpp signaling, in mutants for the Dpp receptor punt or pathway-induced transcriptional activator schnurri, the tubules locate abnormally in the body cavity.38,42 Further, an ectopic source of Dpp is sufficient to misroute tubule migration indicating that that Dpp is an instructive guidepost signal.38 The ultimate position of the anterior tubules is likely to include additional signals. For example the ventral dip taken by the kink region during late development may be mediated by attractive cues from ventral tissues, or by repulsive cues emanating from dorsal regions.
Each tubule is capped at the distal end by a tip cell and its sibling cell. These cells also contribute important but distinct roles in tubule positioning. In tubules with two tip cells but no sibling cell, or two sibling cells and no tip cell the tubules do not course through the body cavity in the highly stereotypical fashion typical of wild-type embryos. Instead they appear to lose their way, with the distal ends randomly arranged within the body.110 During the migration phase the tip cell is replete with highly dynamic filopodia suggesting an active role in tubule pathfinding (Helen Weavers, personal communication; Fig. 5B). These studies demonstrate that positioning of tissues and organs within the body does not take place in a vacuum, but involve multiple signaling pathways and a complex interplay between several different cells and tissues, highlighting the importance of studying organogenesis within its normal developmental context.
Functional significance of organ position
The functional significance of tubule positioning is clear in some cases. A so-called “cryptonephridial” arrangement is common in most Lepidopteran larvae (butterflies and moths) and many Coleoptera (beetles) where the distal ends of the tubule associate intimately with the rectum, with which they are enclosed in a common membrane and sheath.111,112 Such an arrangement permits maximum water reabsorption from the hindgut and is often associated with insects living in particularly arid environments. In Drosophila the posterior pair of tubules also have intimate contact with the hindgut. The tubules loop over the lower region of the hindgut and the tip cell makes contact with the a9 abdominal ganglion nerve that runs up on either side of the hindgut.101 The significance of this is not known, but the authors speculate that tip cells make synaptic connections with a9 neurons on the surface of the hindgut. In support of this, a number of synaptic proteins such as Synaptotagmin; Synaptophysin and the Cysteine-string protein stain the tip cells of late stage embryos.101 Interestingly, these neurons have been shown to control MpT physiology via release of the neuropeptide—pigment-dispersing factor (PDF).113 PDF is secreted from hindgut neurons and acts on the visceral muscle of the ureter to stimulate increased contractility. Another observation that may be significant shows that the hindgut of the crane fly (but apparently not of Drosophila) is innervated by leucokinin-positive efferent neurons.114 These observations suggest renal tubule physiology is under control of local circuits involving multiple tissues and cell-types. Further work will be necessary to characterize the details and directionality of these interactions.
Although anterior and posterior tubules in Drosophila have the same domain structure (Fig. 1B) and the rate of fluid secretion does not differ between them115 it is possible that the two pairs serve different functions by virtue of their position within the animal. A recent systematic investigation into the functional significance of tubule positioning has been performed using a transcriptomic approach suggests this may be the case.100 In this study a comparison between gene expression in anterior vs. posterior tubules revealed that the expression of some genes were enriched in one pair relative to the other. These positional asymmetries appear to reflect physiological adaptations in the insect. For example the anterior tubules adjacent to the midgut are enriched for genes equipping them to deal with fluxes in overabundant ions (particularly calcium), xenobiotics and toxins that they encounter as a function of their proximity to the highly permeable midgut.100 By contrast the posterior tubules, which lie close to the hindgut, are enriched with genes allowing them to handle and be protected from elevated levels of ammonia from nitrogenous excretion. Some of the genes that pattern anterior vs. posterior tubule identity are known.12 By manipulating these genes it would be interesting to generate animals containing only anterior or only posterior tubules and to determine how this affects the physiology of the animal.
The Insect Malpighian Tubule: An Experimental Toolkit and Future Perspectives
In this review I argue the value of the insect MpT as a model for organogenesis. The success of this model is rooted in its experimental tractability, particularly in vivo genetic analysis. Here I highlight some of the tools that have been so instrumental to its success:
Domain and cell-type specific expression
The Gal4-UAS system116 is an extremely powerful tool in the fly biologists toolkit. The system exploits the yeast transcriptional activator, Gal4 and its cognate binding sites known as Upstream Activation Sequences (UAS). Gal4 “driver lines” are created by linking the Gal4 gene to tissue- or cell-specific promoters. Gal4 expression on its own has no phenotype. However, when this fly is crossed to another stock that has a gene of interest cloned downstream to UAS the progeny containing both elements will allow Gal4-mediated activation of the gene of interest only in those cells expressing Gal4. A large collection of driver lines with different expression patterns is available. Further temporal and spatial control of gene expression can be gained by using a temperature sensitive Gal80, an inhibitor of Gal4-mediated transcriptional activation.117,118 A large collection of Gal4 driver lines with tissue-, domain- or cell-specific expression patterns allows us to manipulate gene expression in the MpT with considerable resolution. For example there are lines that specifically drive in secretory regions or reabsorbptive regions, there are other lines that drive in only PCs or SCs.59
Gene knock-down using UAS-dsRNA
One of the most powerful tools to dissect gene function that has emerged in recent years is a genome-wide transgenic RNAi library for conditional gene inactivation.119 This is a UAS-GAL4 based system that allows gene knock-down for any gene in any tissue or cell. This can be performed in the intact animal or in organ culture (see secretory assay below) allowing the investigator to determine the physiological effects of gene function in specific cells/tissues at whole-animal and whole-organ levels.
Live imaging
The advent of live imaging has heralded a new era in developmental biology. Drosophila embryos are small and transparent, and it is possible to image the entirety of MpT embryonic development at high temporal and spatial resolution (Figs. 2C−E and 3B). Several lines that fluorescently label cell membranes, junctions, cytoskeleton, ECM can be used to illuminate the cell and molecular basis of organ development.
Organ transcriptome
The MpT transcriptome is known.80,120 This identifies those genes that are likely to be best studied in the tubule by virtue of their high enrichment. It also provides an important resource to target relevant genes when devising reverse-genetic screening approaches. Interestingly, many human disease-causing genes are highly enriched in the tubule, suggesting that the MpT may be the best tissue to dissect the function of these genes.121,122
Assay to directly measure organ function
The MpT secretion or “Ramsay” assay was pioneered by Alfred Ramsay in the 1950s.123 In this simple set-up a tubule is placed in a little drop of saline, and covered with mineral oil. The proximal end of the tubule is teased out and anchored in place using a fine pin. Fluid secreted by the tubule emerges from a small nick in wall of the tubule or from the ureter into the oil. This can be measured over time to establish secretory rate. Hormones, drugs etc. can be added to the saline drop to monitor their effects on secretion. This assay has been highly informative in determining the mechanisms of fluid secretion and its hormonal control. This simple assay can now be combined with gene knock-down technology described above to provide a powerful tool to dissect the genes and genetic networks that underpin physiological function.
Simple genetic screens to assay physiology
Very simple screening methods exist to directly assess tubule function. For example, urate transport can be visualized in embryonic and larval tubules by simply looking down the microscope,26 and osmoregulation can be assessed in intact adult animals by determining if they retain or lose too much water. These easily assessed phenotypes can be used to rapidly screen for: (1) genes encoding products directly involved in tubule physiology (channels, transporter and receptors); and (2) genes that underpin the developmental networks that establish physiological competence (transcription factors, signaling pathway molecules).
Uncovering the genetic networks of physiological differentiation
With the advent of next-generation sequencing technology, such as RNaseq, cell-specific transcriptional profiling is within grasp. This will allow us to determine exactly which genes are expressed in physiologically distinctive cell types, and how this is modified under conditions of genetic perturbation. In vivo tissue- and cell-specific binding-site profiling using DamID107 or ChIP-chip allow transcription factors targets to be identified. Together these techniques will allow us to build a picture of the genetic networks that lead to physiological differentiation.
The combined application of these tools to the MpT model will continue to illuminate our understanding of organ development and function. For example, guided by MpT transcriptome data sets and using tubule- and tubule-cell-specific RNAi genetic knockdown, simple screens can be performed for genes effecting organismal physiology. Screens of this kind have the potential to reveal the genetic networks underpinning the development and physiology of this organ. Of particular interest will be those mechanisms that also have relevance for other organs in other species, especially those pertinent to mammalian organogenesis, human disease and regenerative medicine. It is worth considering the paths by which studies of MpTs can be translated to other systems. The techniques of in vitro culture of intact mammalian kidney cells and embryonic kidney rudiments are key techniques in the study of mammalian renal development. These techniques may provide one such route.
Grobstein,124 and later Saxen,125,126 pioneered the kidney in vitro culture assay. In these assays explanted embryonic kidneys or kidney rudiments, when incubated on a filter above appropriate media, will grow for several days, during which time the early phases of kidney development such as ureteric bud (UB) branching and nephrogenesis take place in a manner closely approximating in vivo development. Experiments of this kind in which the UB was physically separated from the metanephric mesenchyme (MM) revealed the important reciprocal interactions that occur between the MM and UB during kidney development.124-126 More recently, this organ culture system has been coupled with live imaging and transgenic mouse strains expressing GFP in specific tissues. For example, using specific promoters to light up the UB, this approach was used to determine the pattern of UB branching, and to reveal the cellular and molecular mechanisms that underpin branching.127-130 Further innovations using viral vectors131,132 or small interfering RNAs (siRNA)133,134 for efficient gene knockdown allow functional analyses to performed rapidly.
Taking advantage of a simple experimental system such as this, one can envisage testing candidate genes discovered in the “fly MpT model” to rapidly assess their relevance for mammalian kidney development. For example it would be interesting to determine whether conserved developmental programmes underlie tubular branching and tubular elongation in both MpTs and the mammalian kidney. The existence of deeply conserved cellular mechanisms for epithelial morphogenesis is suggested by a recent study showing that tubular elongation in the vertebrate kidney is driven by a myosin-dependent, multicellular rosette-based mechanism, similar to the mechanism driving GBE in flies.32,135 Using live imaging to follow: (1) developing MpTs in flies and (2) in vitro mammalian kidney explants, would reveal the extent of similarities between the cellular and molecular mechanisms underpinning tubule elongation across animal phyla.
The work of Caubit and collagues suggest the “fly MpT to kidney explant” will be fruitful approach.136 They set out to investigate a potential role for the Teashirt-family proteins in mammalian renal development, based on an earlier study highlighting a role for tsh in the fly MpT.17 They found that Teashirt-3 (Tshz3) is required for smooth muscle differentiation in the mouse ureter, and by studying wild-type and Tshz3 mutant explanted embryonic ureters where able to demonstrate a functional requirement for Tshz3 in ureter peristalsis.136
Concluding Remarks
The insect MpTs have provided valuable insight into the development of tubular epithelia. However, there are large gaps in our knowledge and understanding about the development of this organ, and indeed of the more complex organs of our own bodies. An important function of this review is to emphasize these shortcomings. This said, the genetic and experimental tractability afforded by the fly, coupled with the recognition that common molecular and cellular processes often underpin development in diverse organs, means that future studies of MpT development will continue to illuminate the important and fundamental processes by which organs are generated.
Acknowledgments
I am very grateful to Helen Skaer and members of the lab for useful discussions and comments on a draft of this review. I would like to thank especially Aditya Saxena and Helen Weavers for kindly providing images. This work was supported by Kidney Research UK.
Glossary
Abbreviations:
- AM
alary muscle
- BMP
bone morphogenetic protein
- C-E
convergent extension
- ChIP
chromatin immunoprecipitation
- CPA
cation proton antiporter
- CVM
caudal visceral mesoderm
- DamID
DNA adenine methyltransferase identification
- DH
diuretic hormone
- ECM
extracellular matrix
- EGF
epidermal growth factor
- GAP
GTPase activating protein
- GBE
germband extension
- GFP
green fluorescent protein
- HG
hindgut
- HVN
hindgut visceral nerve
- MG
midgut
- MM
metanephric mesenchyme
- MpT
Malpighian tubule
- NHA
Na+/H+ antiporter
- NHE
Na+/H+ exchanger
- PC
principal cell
- PCP
planar cell polarity
- P-D
proximodistal
- PDGF/VEGF
platelet-derived growth factor/vascular endothelial growth factor
- RNAi
RNA interference
- RNAseq
whole transcriptome shotgun RNA sequencing
- SC
stellate cell
- SLC
solute carrier
- TMC
tip mother cell
- UAS
upstream activating sequence
- UB
ureteric bud
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/24107
References
- 1.Maddrell SHP. The Fastest Fluid-secreting Cell Known: The Upper Malpighian Tubule Cell of Rhodnius. Bioessays. 1991;13:357–62. doi: 10.1002/bies.950130710. [DOI] [Google Scholar]
- 2.Bertsch A. Foraging in male bumblebess (Bombus lucorum L.): maximizing energy or minimizing water load? Oecologia. 1984;62:325–36. doi: 10.1007/BF00384264. [DOI] [PubMed] [Google Scholar]
- 3.Malpighi M. Dissertatio Epistolica De Bombyce London, 1669. [Google Scholar]
- 4.Cobb M. Malpighi, Swammerdam and the colourful silkworm: replication and visual representation in early modern science. Ann Sci. 2002;59:111–7. doi: 10.1080/00033790110050759. [DOI] [PubMed] [Google Scholar]
- 5.McGettigan J, McLennan RK, Broderick KE, Kean L, Allan AK, Cabrero P, et al. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect Biochem Mol Biol. 2005;35:741–54. doi: 10.1016/j.ibmb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Davies SA, Overend G, Sebastian S, Cundall M, Cabrero P, Dow JA, et al. Immune and stress response ‘cross-talk’ in the Drosophila Malpighian tubule. J Insect Physiol. 2012;58:488–97. doi: 10.1016/j.jinsphys.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Craig CL. Evolution of arthropod silks. Annu Rev Entomol. 1997;42:231–67. doi: 10.1146/annurev.ento.42.1.231. [DOI] [PubMed] [Google Scholar]
- 8.Green LF. The fine structure of the light organ of the New Zealand glow-worm Arachnocampa luminosa (Diptera: Mycetophilidae) Tissue Cell. 1979;11:457–65. doi: 10.1016/0040-8166(79)90056-9. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland TD, Young JH, Weisman S, Hayashi CY, Merritt DJ. Insect silk: one name, many materials. Annu Rev Entomol. 2010;55:171–88. doi: 10.1146/annurev-ento-112408-085401. [DOI] [PubMed] [Google Scholar]
- 10.Denholm B, Skaer H. Development of Malpighian tubules in Insects. Oxford, UK: Elseveir, 2005. [Google Scholar]
- 11.Beyenbach KW, Skaer H, Dow JA. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol. 2010;55:351–74. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- 12.Hatton-Ellis E, Ainsworth C, Sushama Y, Wan S, VijayRaghavan K, Skaer H. Genetic regulation of patterned tubular branching in Drosophila. Proc Natl Acad Sci U S A. 2007;104:169–74. doi: 10.1073/pnas.0606933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skaer H, Martinez Arias A. The wingless product is required for cell proliferation in the Malpighian tubule anlage of Drosophila melanogaster. Development. 1992;116:745–54. [Google Scholar]
- 14.Wan S, Cato AM, Skaer H. Multiple signalling pathways establish cell fate and cell number in Drosophila malpighian tubules. Dev Biol. 2000;217:153–65. doi: 10.1006/dbio.1999.9499. [DOI] [PubMed] [Google Scholar]
- 15.Sudarsan V, Pasalodos-Sanchez S, Wan S, Gampel A, Skaer H. A genetic hierarchy establishes mitogenic signalling and mitotic competence in the renal tubules of Drosophila. Development. 2002;129:935–44. doi: 10.1242/dev.129.4.935. [DOI] [PubMed] [Google Scholar]
- 16.Kerber B, Fellert S, Hoch M. Seven-up, the Drosophila homolog of the COUP-TF orphan receptors, controls cell proliferation in the insect kidney. Genes Dev. 1998;12:1781–6. doi: 10.1101/gad.12.12.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denholm B, Sudarsan V, Pasalodos-Sanchez S, Artero R, Lawrence P, Maddrell S, et al. Dual origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr Biol. 2003;13:1052–7. doi: 10.1016/S0960-9822(03)00375-0. [DOI] [PubMed] [Google Scholar]
- 18.Campbell K, Casanova J, Skaer H. Mesenchymal-to-epithelial transition of intercalating cells in Drosophila renal tubules depends on polarity cues from epithelial neighbours. Mech Dev. 2010;127:345–57. doi: 10.1016/j.mod.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donnell MJ, Maddrell SH. Fluid reabsorption and ion transport by the lower Malpighian tubules of adult female Drosophila. J Exp Biol. 1995;198:1647–53. doi: 10.1242/jeb.198.8.1647. [DOI] [PubMed] [Google Scholar]
- 20.Wessing A, Eichelberg D. Malpighian tubules, rectal papillae and excretion. London: Academic Press, 1978. [Google Scholar]
- 21.Piersol GM. Polycystic Disease of the Kidney. Trans Am Climatol Clin Assoc. 1927;43:221–31. [PMC free article] [PubMed] [Google Scholar]
- 22.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 23.Keller R. Mechanisms of elongation in embryogenesis. Development. 2006;133:2291–302. doi: 10.1242/dev.02406. [DOI] [PubMed] [Google Scholar]
- 24.Skaer H. Cell division in Malpighian tubule development in D. melanogaster is regulated by a single tip cell. Nature. 1989;342:566–9. doi: 10.1038/342566a0. [DOI] [Google Scholar]
- 25.Broadie K, Skaer H, Bate M. Whole-embryo culture of Drosophila: development of embryonic tissues in vitro. Rouxs Arch Dev Biol. 1992;201:364–75. doi: 10.1007/BF00365124. [DOI] [PubMed] [Google Scholar]
- 26.Denholm B, Brown S, Ray RP, Ruiz-Gómez M, Skaer H, Hombría JC. crossveinless-c is a RhoGAP required for actin reorganisation during morphogenesis. Development. 2005;132:2389–400. doi: 10.1242/dev.01829. [DOI] [PubMed] [Google Scholar]
- 27.Blake KJ, Myette G, Jack J. The products of ribbon and raw are necessary for proper cell shape and cellular localization of nonmuscle myosin in Drosophila. Dev Biol. 1998;203:177–88. doi: 10.1006/dbio.1998.9036. [DOI] [PubMed] [Google Scholar]
- 28.Blake KJ, Myette G, Jack J. ribbon, raw, and zipper have distinct functions in reshaping the Drosophila cytoskeleton. Dev Genes Evol. 1999;209:555–9. doi: 10.1007/s004270050288. [DOI] [PubMed] [Google Scholar]
- 29.Jack J, Myette G. The genes raw and ribbon are required for proper shape of tubular epithelial tissues in Drosophila. Genetics. 1997;147:243–53. doi: 10.1093/genetics/147.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simões S, Denholm B, Azevedo D, Sotillos S, Martin P, Skaer H, et al. Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development. 2006;133:4257–67. doi: 10.1242/dev.02588. [DOI] [PubMed] [Google Scholar]
- 31.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 32.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–70. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Shih J, Keller R. Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis. Development. 1992;116:915–30. doi: 10.1242/dev.116.4.915. [DOI] [PubMed] [Google Scholar]
- 34.Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–14. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- 35.Keller R, Tibbetts P. Mediolateral cell intercalation in the dorsal, axial mesoderm of Xenopus laevis. Dev Biol. 1989;131:539–49. doi: 10.1016/S0012-1606(89)80024-7. [DOI] [PubMed] [Google Scholar]
- 36.Munro EM, Odell GM. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development. 2002;129:13–24. doi: 10.1242/dev.129.1.13. [DOI] [PubMed] [Google Scholar]
- 37.Williams-Masson EM, Heid PJ, Lavin CA, Hardin J. The cellular mechanism of epithelial rearrangement during morphogenesis of the Caenorhabditis elegans dorsal hypodermis. Dev Biol. 1998;204:263–76. doi: 10.1006/dbio.1998.9048. [DOI] [PubMed] [Google Scholar]
- 38.Bunt S, Hooley C, Hu N, Scahill C, Weavers H, Skaer H. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev Cell. 2010;19:296–306. doi: 10.1016/j.devcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasilyev A, Liu Y, Mudumana S, Mangos S, Lam PY, Majumdar A, et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 2009;7:e9. doi: 10.1371/journal.pbio.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–4. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harbecke R, Lengyel JA. Genes controlling posterior gut development in the Drosophila embryo. Rouxs Arch Dev Biol. 1995;204:308–29. doi: 10.1007/BF02179500. [DOI] [PubMed] [Google Scholar]
- 42.Jack J, Myette G. Mutations that alter the morphology of the malpighian tubules in Drosophila. Dev Genes Evol. 1999;209:546–54. doi: 10.1007/s004270050287. [DOI] [PubMed] [Google Scholar]
- 43.Araújo SJ, Aslam H, Tear G, Casanova J. mummy/cystic encodes an enzyme required for chitin and glycan synthesis, involved in trachea, embryonic cuticle and CNS development--analysis of its role in Drosophila tracheal morphogenesis. Dev Biol. 2005;288:179–93. doi: 10.1016/j.ydbio.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 44.Tonning A, Hemphälä J, Tång E, Nannmark U, Samakovlis C, Uv A. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev Cell. 2005;9:423–30. doi: 10.1016/j.devcel.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Devine WP, Lubarsky B, Shaw K, Luschnig S, Messina L, Krasnow MA. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc Natl Acad Sci U S A. 2005;102:17014–9. doi: 10.1073/pnas.0506676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tonning A, Helms S, Schwarz H, Uv AE, Moussian B. Hormonal regulation of mummy is needed for apical extracellular matrix formation and epithelial morphogenesis in Drosophila. Development. 2006;133:331–41. doi: 10.1242/dev.02206. [DOI] [PubMed] [Google Scholar]
- 47.Fernandes I, Chanut-Delalande H, Ferrer P, Latapie Y, Waltzer L, Affolter M, et al. Zona pellucida domain proteins remodel the apical compartment for localized cell shape changes. Dev Cell. 2010;18:64–76. doi: 10.1016/j.devcel.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Luschnig S, Bätz T, Armbruster K, Krasnow MA. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr Biol. 2006;16:186–94. doi: 10.1016/j.cub.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 49.Bökel C, Prokop A, Brown NH. Papillote and Piopio: Drosophila ZP-domain proteins required for cell adhesion to the apical extracellular matrix and microtubule organization. J Cell Sci. 2005;118:633–42. doi: 10.1242/jcs.01619. [DOI] [PubMed] [Google Scholar]
- 50.Jaźwińska A, Ribeiro C, Affolter M. Epithelial tube morphogenesis during Drosophila tracheal development requires Piopio, a luminal ZP protein. Nat Cell Biol. 2003;5:895–901. doi: 10.1038/ncb1049. [DOI] [PubMed] [Google Scholar]
- 51.Wang S, Jayaram SA, Hemphälä J, Senti KA, Tsarouhas V, Jin H, et al. Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr Biol. 2006;16:180–5. doi: 10.1016/j.cub.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 52.Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161:563–96. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- 53.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–55. doi: 10.1016/S1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 54.Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–7. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- 55.Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–53. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 56.Chung S, Vining MS, Bradley PL, Chan CC, Wharton KA, Jr., Andrew DJ. Serrano (sano) functions with the planar cell polarity genes to control tracheal tube length. PLoS Genet. 2009;5:e1000746. doi: 10.1371/journal.pgen.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lekven AC, Tepass U, Keshmeshian M, Hartenstein V. faint sausage encodes a novel extracellular protein of the immunoglobulin superfamily required for cell migration and the establishment of normal axonal pathways in the Drosophila nervous system. Development. 1998;125:2747–58. doi: 10.1242/dev.125.14.2747. [DOI] [PubMed] [Google Scholar]
- 58.Brodu V, Casanova J. The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination. Genes Dev. 2006;20:1817–28. doi: 10.1101/gad.375706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sözen MA, Armstrong JD, Yang M, Kaiser K, Dow JA. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci U S A. 1997;94:5207–12. doi: 10.1073/pnas.94.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maddrell SHP. The Functional Design of the Insect Excretory System. J Exp Biol. 1981;90:1–15. [Google Scholar]
- 61.Du J, Kean L, Allan AK, Southall TD, Davies SA, McInerny CJ, et al. The SzA mutations of the B subunit of the Drosophila vacuolar H+ ATPase identify conserved residues essential for function in fly and yeast. J Cell Sci. 2006;119:2542–51. doi: 10.1242/jcs.02983. [DOI] [PubMed] [Google Scholar]
- 62.Weng XH, Huss M, Wieczorek H, Beyenbach KW. The V-type H(+)-ATPase in Malpighian tubules of Aedes aegypti: localization and activity. J Exp Biol. 2003;206:2211–9. doi: 10.1242/jeb.00385. [DOI] [PubMed] [Google Scholar]
- 63.Davies SA, Goodwin SF, Kelly DC, Wang Z, Sözen MA, Kaiser K, et al. Analysis and inactivation of vha55, the gene encoding the vacuolar ATPase B-subunit in Drosophila melanogaster reveals a larval lethal phenotype. J Biol Chem. 1996;271:30677–84. doi: 10.1074/jbc.271.48.30677. [DOI] [PubMed] [Google Scholar]
- 64.Bertram G, Shleithoff L, Zimmermann P, Wessing A. Bafilomycin A1 is a potent inhibitor of urine formation by Malpighian tubules of Drosophila hydei - is a vacuolar-type ATPase involved in ion and fluid secretion? J Insect Physiol. 1991;37:201–9. doi: 10.1016/0022-1910(91)90070-G. [DOI] [Google Scholar]
- 65.Rheault MR, Okech BA, Keen SB, Miller MM, Meleshkevitch EA, Linser PJ, et al. Molecular cloning, phylogeny and localization of AgNHA1: the first Na+/H+ antiporter (NHA) from a metazoan, Anopheles gambiae. J Exp Biol. 2007;210:3848–61. doi: 10.1242/jeb.007872. [DOI] [PubMed] [Google Scholar]
- 66.Day JP, Wan S, Allan AK, Kean L, Davies SA, Gray JV, et al. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J Cell Sci. 2008;121:2612–9. doi: 10.1242/jcs.033084. [DOI] [PubMed] [Google Scholar]
- 67.Scott BN, Yu MJ, Lee LW, Beyenbach KW. Mechanisms of K+ transport across basolateral membranes of principal cells in Malpighian tubules of the yellow fever mosquito, Aedes aegypti. J Exp Biol. 2004;207:1655–63. doi: 10.1242/jeb.00932. [DOI] [PubMed] [Google Scholar]
- 68.Evans JM, Allan AK, Davies SA, Dow JA. Sulphonylurea sensitivity and enriched expression implicate inward rectifier K+ channels in Drosophila melanogaster renal function. J Exp Biol. 2005;208:3771–83. doi: 10.1242/jeb.01829. [DOI] [PubMed] [Google Scholar]
- 69.Piermarini PM, Rouhier MF, Schepel M, Kosse C, Beyenbach KW. Cloning and functional characterization of inward-rectifying potassium (Kir) channels from Malpighian tubules of the mosquito Aedes aegypti. Insect Biochem Mol Biol. 2013;43:75–90. doi: 10.1016/j.ibmb.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pullikuth AK, Aimanova K, Kang’ethe W, Sanders HR, Gill SS. Molecular characterization of sodium/proton exchanger 3 (NHE3) from the yellow fever vector, Aedes aegypti. J Exp Biol. 2006;209:3529–44. doi: 10.1242/jeb.02419. [DOI] [PubMed] [Google Scholar]
- 71.Coast GM, Webster SG, Schegg KM, Tobe SS, Schooley DA. The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J Exp Biol. 2001;204:1795–804. doi: 10.1242/jeb.204.10.1795. [DOI] [PubMed] [Google Scholar]
- 72.Johnson EC, Shafer OT, Trigg JS, Park J, Schooley DA, Dow JA, et al. A novel diuretic hormone receptor in Drosophila: evidence for conservation of CGRP signaling. J Exp Biol. 2005;208:1239–46. doi: 10.1242/jeb.01529. [DOI] [PubMed] [Google Scholar]
- 73.Kean L, Cazenave W, Costes L, Broderick KE, Graham S, Pollock VP, et al. Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1297–307. doi: 10.1152/ajpregu.00584.2001. [DOI] [PubMed] [Google Scholar]
- 74.Cabrero P, Radford JC, Broderick KE, Costes L, Veenstra JA, Spana EP, et al. The Dh gene of Drosophila melanogaster encodes a diuretic peptide that acts through cyclic AMP. J Exp Biol. 2002;205:3799–807. doi: 10.1242/jeb.205.24.3799. [DOI] [PubMed] [Google Scholar]
- 75.Dow JA. The versatile stellate cell - more than just a space-filler. J Insect Physiol. 2012;58:467–72. doi: 10.1016/j.jinsphys.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 76.O’Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SH, et al. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol. 1998;274:R1039–49. doi: 10.1152/ajpregu.1998.274.4.R1039. [DOI] [PubMed] [Google Scholar]
- 77.Piermarini PM, Grogan LF, Lau K, Wang L, Beyenbach KWA. A SLC4-like anion exchanger from renal tubules of the mosquito (Aedes aegypti): evidence for a novel role of stellate cells in diuretic fluid secretion. Am J Physiol Regul Integr Comp Physiol. 2010;298:R642–60. doi: 10.1152/ajpregu.00729.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Linser PJ, Neira Oviedo M, Hirata T, Seron TJ, Smith KE, Piermarini PM, et al. Slc4-like anion transporters of the larval mosquito alimentary canal. J Insect Physiol. 2012;58:551–62. doi: 10.1016/j.jinsphys.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Connor KR, Beyenbach KW. Chloride channels in apical membrane patches of stellate cells of Malpighian tubules of Aedes aegypti. J Exp Biol. 2001;204:367–78. doi: 10.1242/jeb.204.2.367. [DOI] [PubMed] [Google Scholar]
- 80.Wang J, Kean L, Yang J, Allan AK, Davies SA, Herzyk P, et al. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 2004;5:R69. doi: 10.1186/gb-2004-5-9-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Denholm B, Hu N, Fauquier T, Caubit X, Fasano L, Skaer H. The tiptop/teashirt genes regulate cell differentiation and renal physiology in Drosophila. Development. 2013;140:1100–10. doi: 10.1242/dev.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pannabecker TL, Hayes TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J Membr Biol. 1993;132:63–76. doi: 10.1007/BF00233052. [DOI] [PubMed] [Google Scholar]
- 83.Skaer HB, Maddrell SH, Harrison JB. The permeability properties of septate junctions in Malpighian tubules of Rhodnius. J Cell Sci. 1987;88:251–65. doi: 10.1242/jcs.88.2.251. [DOI] [PubMed] [Google Scholar]
- 84.Beyenbach KW. A dynamic paracellular pathway serves diuresis in mosquito Malpighian tubules. Ann N Y Acad Sci. 2012;1258:166–76. doi: 10.1111/j.1749-6632.2012.06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beyenbach KW, Baumgart S, Lau K, Piermarini PM, Zhang S. Signaling to the apical membrane and to the paracellular pathway: changes in the cytosolic proteome of Aedes Malpighian tubules. J Exp Biol. 2009;212:329–40. doi: 10.1242/jeb.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyauchi JT, Piermarini PM, Yang JD, Gilligan DM, Beyenbach KW. Roles of PKC and phospho-adducin in transepithelial fluid secretion by Malpighian tubules of the yellow fever mosquito. Tissue Barriers. 2013;11:1–14. doi: 10.4161/tisb.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaufmann N, Mathai JC, Hill WG, Dow JA, Zeidel ML, Brodsky JL. Developmental expression and biophysical characterization of a Drosophila melanogaster aquaporin. Am J Physiol Cell Physiol. 2005;289:C397–407. doi: 10.1152/ajpcell.00612.2004. [DOI] [PubMed] [Google Scholar]
- 88.O’Donnell MJ, Maddrell SH. Paracellular and transcellular routes for water and solute movements across insect epithelia. J Exp Biol. 1983;106:231–53. doi: 10.1242/jeb.106.1.231. [DOI] [PubMed] [Google Scholar]
- 89.O'Donnell MJ, Aldis GK, Maddrell SHP. Measurements of osmotic permeability in the Malpighian tubules of an insect Rhodnius prolixus. Proc R Soc Lond B Biol Sci. 1982;216:267–78. doi: 10.1098/rspb.1982.0074. [DOI] [Google Scholar]
- 90.Sofía Hernández C, González E, Whittembury G. The paracellular channel for water secretion in the upper segment of the Malpighian tubule of Rhodnius prolixus. J Membr Biol. 1995;148:233–42. doi: 10.1007/BF00235041. [DOI] [PubMed] [Google Scholar]
- 91.Spring JH, Robichaux SR, Kaufmann N, Brodsky JL. Localization of a Drosophila DRIP-like aquaporin in the Malpighian tubules of the house cricket, Acheta domesticus. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:92–100. doi: 10.1016/j.cbpa.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 92.Echevarría M, Ramírez-Lorca R, Hernández CS, Gutiérrez A, Méndez-Ferrer S, González E, et al. Identification of a new water channel (Rp-MIP) in the Malpighian tubules of the insect Rhodnius prolixus. Pflugers Arch. 2001;442:27–34. doi: 10.1007/s004240000494. [DOI] [PubMed] [Google Scholar]
- 93.Martini SV, Goldenberg RC, Fortes FS, Campos-de-Carvalho AC, Falkenstein D, Morales MM. Rhodnius prolixus Malpighian tubule’s aquaporin expression is modulated by 5-hydroxytryptamine. Arch Insect Biochem Physiol. 2004;57:133–41. doi: 10.1002/arch.20017. [DOI] [PubMed] [Google Scholar]
- 94.Liu K, Tsujimoto H, Cha S-J, Agre P, Rasgon JL. Aquaporin water channel AgAQP1 in the malaria vector mosquito Anopheles gambiae during blood feeding and humidity adaptation. Proc Natl Acad Sci U S A. 2011;108:6062–6. doi: 10.1073/pnas.1102629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cabrero P, Pollock VP, Davies SA, Dow JA. A conserved domain of alkaline phosphatase expression in the Malpighian tubules of dipteran insects. J Exp Biol. 2004;207:3299–305. doi: 10.1242/jeb.01156. [DOI] [PubMed] [Google Scholar]
- 96.Shippy TD, Tomoyasu Y, Nie W, Brown SJ, Denell RE. Do teashirt family genes specify trunk identity? Insights from the single tiptop/teashirt homolog of Tribolium castaneum. Dev Genes Evol. 2008;218:141–52. doi: 10.1007/s00427-008-0212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gloor H. Schädigungsmuster eines Letalfaktors (Kr) in Drosophila melanogaster. Arch Julius Klaus-Stift Vererb Sozialanhropol Rassenhyg. 1950;25:38–44. [Google Scholar]
- 98.Liu S, Jack J. Regulatory interactions and role in cell type specification of the Malpighian tubules by the cut, Krüppel, and caudal genes of Drosophila. Dev Biol. 1992;150:133–43. doi: 10.1016/0012-1606(92)90013-7. [DOI] [PubMed] [Google Scholar]
- 99.Dube K, McDonald DG, O’Donnell MJ. Calcium transport by isolated anterior and posterior Malpighian tubules of Drosophila melanogaster: roles of sequestration and secretion. J Insect Physiol. 2000;46:1449–60. doi: 10.1016/S0022-1910(00)00069-X. [DOI] [PubMed] [Google Scholar]
- 100.Chintapalli VR, Terhzaz S, Wang J, Al Bratty M, Watson DG, Herzyk P, et al. Functional correlates of positional and gender-specific renal asymmetry in Drosophila. PLoS One. 2012;7:e32577. doi: 10.1371/journal.pone.0032577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoch M, Broadie K, Jäckle H, Skaer H. Sequential fates in a single cell are established by the neurogenic cascade in the Malpighian tubules of Drosophila. Development. 1994;120:3439–50. doi: 10.1242/dev.120.12.3439. [DOI] [PubMed] [Google Scholar]
- 102.Isshiki T, Pearson B, Holbrook S, Doe CQ. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell. 2001;106:511–21. doi: 10.1016/S0092-8674(01)00465-2. [DOI] [PubMed] [Google Scholar]
- 103.Skaer H. Cell proliferation and development of the Malpighian tubules in Drosophila melanogaster. Exp Nephrol. 1996;4:119–26. [PubMed] [Google Scholar]
- 104.Skaer H, Harrison JB, Maddrell SHP. Physiological and structural maturation of a polarised epithelium: the malpighian tubules of a blood sucking insect, Rhodnius prolixus. J Cell Sci. 1990;96:537–47. [Google Scholar]
- 105.O’donnell MJ, Maddrell SH, le B Skaer H, Harrison JB. Elaborations of the basal surface of the cells of the Malpighian tubules of an insect. Tissue Cell. 1985;17:865–81. doi: 10.1016/0040-8166(85)90042-4. [DOI] [PubMed] [Google Scholar]
- 106.Schepel SA, Fox AJ, Miyauchi JT, Sou T, Yang JD, Lau K, et al. The single kinin receptor signals to separate and independent physiological pathways in Malpighian tubules of the yellow fever mosquito. Am J Physiol Regul Integr Comp Physiol. 2010;299:R612–22. doi: 10.1152/ajpregu.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choksi SP, Southall TD, Bossing T, Edoff K, de Wit E, Fischer BE, et al. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev Cell. 2006;11:775–89. doi: 10.1016/j.devcel.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 108.Sirodot S. Malpighian tubules: anatomy and chemistry. Ann Sci Nat Zool. 1858;10:141–89. [Google Scholar]
- 109.Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–7. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 110.Ainsworth C, Wan S, Skaer H. Coordinating cell fate and morphogenesis in Drosophila renal tubules. Philos Trans R Soc Lond B Biol Sci. 2000;355:931–7. doi: 10.1098/rstb.2000.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishimori AC. Relation of Malpighian tubes to rectum: Lepidoptera larvae. Ann Entomol Soc Am. 1924;17:75–86. [Google Scholar]
- 112.Ramsay JA. The rectal complex of the mealworm Tenebrio molitor, L. (Coleoptera, Tenebrionidae). Philosophical Transactions of the Royal Society B. Biological Sciences. 1964;248:279–314. doi: 10.1098/rstb.1964.0013. [DOI] [Google Scholar]
- 113.Talsma AD, Christov CP, Terriente-Felix A, Linneweber GA, Perea D, Wayland M, et al. Remote control of renal physiology by the intestinal neuropeptide pigment-dispersing factor in Drosophila. Proc Natl Acad Sci U S A. 2012;109:12177–82. doi: 10.1073/pnas.1200247109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cantera R, Nässel DR. Segmental peptidergic innervation of abdominal targets in larval and adult dipteran insects revealed with an antiserum against leucokinin I. Cell Tissue Res. 1992;269:459–71. doi: 10.1007/BF00353901. [DOI] [PubMed] [Google Scholar]
- 115.Dow JA, Maddrell SH, Görtz A, Skaer NJ, Brogan S, Kaiser K. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol. 1994;197:421–8. doi: 10.1242/jeb.197.1.421. [DOI] [PubMed] [Google Scholar]
- 116.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 117.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–8. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 118.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 119.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–6. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 120.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–20. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 121.Dow JA, Romero MF. Drosophila provides rapid modeling of renal development, function, and disease. Am J Physiol Renal Physiol. 2010;299:F1237–44. doi: 10.1152/ajprenal.00521.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hirata T, Cabrero P, Berkholz DS, Bondeson DP, Ritman EL, Thompson JR, et al. In vivo Drosophilia genetic model for calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol. 2012;303:F1555–62. doi: 10.1152/ajprenal.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramsay JA. Active transport of water by the Malpighian tubules of the stick insect, Dixippus morosus (Orthoptera, Phasmidae) J Exp Biol. 1954;31:104–13. [Google Scholar]
- 124.Grobstein C. Trans-filter induction of tubules in mouse metanephrogenic mesenchyme. Exp Cell Res. 1956;10:424–40. doi: 10.1016/0014-4827(56)90016-7. [DOI] [PubMed] [Google Scholar]
- 125.Saxén L, Lehtonen E. Transfilter induction of kidney tubules as a function of the extent and duration of intercellular contacts. J Embryol Exp Morphol. 1978;47:97–109. [PubMed] [Google Scholar]
- 126.Saxen L. Organogenesis of the kidney. Cambridge: Cambridge University Press, 1987. [Google Scholar]
- 127.Srinivas S, Goldberg MR, Watanabe T, D’Agati V, al-Awqati Q, Costantini F. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev Genet. 1999;24:241–51. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<241::AID-DVG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 128.Watanabe T, Costantini F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol. 2004;271:98–108. doi: 10.1016/j.ydbio.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 129.Costantini F, Watanabe T, Lu B, Chi X, Srinivas S. Imaging kidney development. Cold Spring Harbor protocols. 2011;2011:109. doi: 10.1101/pdb.top109. [DOI] [PubMed] [Google Scholar]
- 130.Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009;17:199–209. doi: 10.1016/j.devcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li X, Hyink DP, Polgar K, Gusella GL, Wilson PD, Burrow CR. Protein kinase X activates ureteric bud branching morphogenesis in developing mouse metanephric kidney. J Am Soc Nephrol. 2005;16:3543–52. doi: 10.1681/ASN.2005030240. [DOI] [PubMed] [Google Scholar]
- 132.Polgar K, Burrow CR, Hyink DP, Fernandez H, Thornton K, Li X, et al. Disruption of polycystin-1 function interferes with branching morphogenesis of the ureteric bud in developing mouse kidneys. Dev Biol. 2005;286:16–30. doi: 10.1016/j.ydbio.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 133.Davies JA, Ladomery M, Hohenstein P, Michael L, Shafe A, Spraggon L, et al. Development of an siRNA-based method for repressing specific genes in renal organ culture and its use to show that the Wt1 tumour suppressor is required for nephron differentiation. Hum Mol Genet. 2004;13:235–46. doi: 10.1093/hmg/ddh015. [DOI] [PubMed] [Google Scholar]
- 134.Maeshima A, Vaughn DA, Choi Y, Nigam SK. Activin A is an endogenous inhibitor of ureteric bud outgrowth from the Wolffian duct. Dev Biol. 2006;295:473–85. doi: 10.1016/j.ydbio.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 135.Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, et al. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat Genet. 2012;44:1382–7. doi: 10.1038/ng.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Caubit X, Lye CM, Martin E, Coré N, Long DA, Vola C, et al. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development. 2008;135:3301–10. doi: 10.1242/dev.022442. [DOI] [PubMed] [Google Scholar]