Abstract
Posttranslational histone modifications define chromatin structure and function. In recent years, a number of studies have characterized many of the enzymatic activities and diverse regulatory components required for monomethylation of histone H3 lysine 4 (H3K4me1) and the expression of specific genes. The challenge now is to understand how this specific chemical modification is written and the Set7 methyltransferase has emerged as a key regulatory enzyme mediating methylation of lysine residues of histone and non-histone proteins. In this review, we comprehensively explore the regulatory proteins modified by Set7 and highlight mechanisms of specific co-recruitment of the enzyme to activating promoters. With a focus on signaling and transcriptional control in disease we discuss recent experimental data emphasizing specific components of diverse regulatory complexes that mediate chromatin modification and reinterpretation of Set7-mediated gene expression.
Keywords: Set7, Set9, histone modification, epigenetics, chromatin, gene expression, lysine methylation, metabolic memory, p65
Introduction
Transcription of genomic sequences is primarily regulated by the degree of chromatin accessibility to core transcriptional machinery and transcription factors (TFs).1 Nucleosomes are fundamental structural units of chromatin composed of histone proteins. Combinatory patterns of posttranslational modifications (PTMs) including acetylation, methylation and phosphorylation on N-terminal histone tails play a major role in the propagation of a regulatory code.2-4 In essence, collaborative histone modifications significantly amplify the information potential of the genetic code through mechanisms that ultimately culminate in the modulation of chromatin structure and function.
The major effects on higher order chromatin structure and transcriptional modulation by histone PTMs have been attributed to (1) charge disruption of histone tails by acetylation/deacetylation which alters affinity for adjacent histones and DNA and, (2) establishment of high-affinity binding sites for recruitment of non-histone effector proteins and complexes responsible for the local remodeling of chromatin.5,6 A multitude of histone PTMs and their general associations to transcriptional states have been described; however, the specific consequences for chromatin function remain largely undetermined for many modifications. Several features of histone PTMs, such as the intricacies associated with various degrees of modification (e.g., mono-, di- and tri-methylation) at single residues, as well as inter- and intra-nucleosomal PTM cross-talk, increase the complexity surrounding precise distillation of the effects on gene expression.
Distinct roles for deregulated PTMs have recently emerged as mechanistic links between environmental signaling and human diseases, such as the chronic complications associated with diabetes.7 Moreover, characterization of these processes has greatly advanced the overall understanding of the molecular mechanisms that govern gene expression. Enzymes responsible for the transfer and removal of histone modification marks exhibit strong specificity toward particular amino acid positions within histone tails.8 Recent advances in high-throughput sequencing technologies have significantly contributed to the identification and characterization of individual histone modifications and associated enzymes in the context of gene expression in health and disease.9,10
Methylation of specific histone residues appears particularly influential and understanding the functional significance of these modifications is the focus of an increasing number of studies. Across eukaryotic genomes, lysine methylation at position 4 of histone H3 (H3K4) is commonly associated with transcriptional activation.11,12 Several HMTs that mediate the highly methylated states of this residue have been described, and recent experimental evidence now shows that Set7 methyltransferase is not alone in writing monomethylation of H3K4.7,13 In addition to this chromatin-modifying role, numerous non-histone substrates including TFs have recently been described for Set7. Although the in vivo function of Set7 remains largely undetermined, experimental observations suggest participation in transcriptional activation across several discrete loci by a number of distinct, non-exclusive mechanisms. In this review we highlight reported examples of Set7-mediated transcriptional regulation by modification of the chromatin template and address concerns regarding the physiological validity of H3 histones as methylation substrates. We evaluate the role of Set7 in non-histone methylation, and focus on how such events contribute to transcriptional regulation. Furthermore, experimental observations that consolidate TF methylation, Set7 co-recruitment and H3K4 methylation at discrete loci are explored. Finally, we discuss current approaches to expand the compendium of characterized Set7 substrates.
The Histone Methylation Modification
Nearly 50 years since the first reports of methylation of calf thymus histones,14 the importance of posttranslational methylation of lysine and arginine residues predominantly at H3 and H4 histone tails is increasingly investigated. Catalyzed by histone methyltransferases (HMTs), these modifications define and direct distinct chromatin conformations and subsequent transcriptional competency. Histone lysine methylation was traditionally considered a stable mark involved in the maintenance of genome expression, consistent with a role in long-term epigenetic memory.15 However, this modification is dynamically regulated during transcriptional activation.16 To this end, lysine specific demethylases have been described,17,18 highlighting the importance of methyl-writing and -erasing enzymes to modification of the chromatin template. Contrasting histone acetylation, methylated lysine and arginine residues are associated with both gene activation and repression, depending on the residue modified and the number of methyl groups covalently assigned.19
H3 lysine methylation modifications are diverse
Distinct transcriptional outcomes are strongly associated with posttranslational methylation of specific residues of H3 histone tails. For instance, lysine 9 (H3K9) and 27 (H3K27) methylation by SET-domain HMTs such as Suv39h1 and G9a correlates with structurally condensed and transcriptionally incompetent regions of chromatin.16 In addition to a role in the recruitment of regulatory factors associated with chromatin remodeling, this repressive histone modification co-localizes with methylated CpG residues predominately through recruitment of H3K9 methyltransferase activity by the methyl-CpG-binding protein MeCP2.20 Furthermore, the methyltransferase reaction may compete for lysine substrates with transcriptionally permissive acetylation. In striking contrast, immunofluorescent analyses of human chromosomes demonstrate specific localization of H3K4 methylation at transcriptionally active chromatin.21 Analysis of immunoprecipitated chromatin similarly revealed specific enrichment of this modification at euchromatic loci.11,12
The three methylated states of H3K4 appear to play distinct roles in transcription as they are differentially distributed throughout actively transcribed chromatin. Small-scale studies22,23 revealed patterns of H3K4 dimethylation (H3K4me2) and H3K4 trimethylation (H3K4me3) at 5′ ends of actively expressed genes. Comprehensive analysis of non-repetitive regions on human chromosomes 21 and 22 further clarified that H3K4me3 is predominantly localized to histones immediately flanking transcription start sites (TSS). Contrasting the small-scale studies that focused on a restricted subset of genes, the H3K4me2 modification was predominately observed within introns of highly expressed genes.24 Indeed, progressive transition from 5′ H3K4me3 enrichment to H3K4me2 and then to H3K4me1 within coding regions of the yeast genome25,26 holds true for higher eukaryotes as demonstrated by high-resolution global methylation analyses.27,28 A bimodal distribution flanking the TSS of active human promoters is commonly observed for all H3K4 methylation states, although this pattern is generally less pronounced with regard to H3K4me1.27,28
While several studies have demonstrated participation of H3K4me1 promoter enrichment in transcriptional activation,29-38 the precise role of this modification remains poorly understood. Deletion of the yeast serine 2 C-terminal domain kinase for RNAP II, Ctk1, resulted in near complete genome-wide loss of H3K4me1, most likely through the regulation of HMT binding. Interestingly, a significant increase in H3K4me2 and H3K4me3 enrichment was observed to spread 3′ into the coding regions of genes.26 This finding suggests that H3K4me1 may act to restrict intragenic 3′ expansion of H3K4me3, and therefore maintain the normal chromatin signature of transcribed genes. Proximal, intergenic, and distal regulatory regions exhibit remarkable enrichment for H3 histones methylated at K4.28 To this end, distinct H3K4me1 signatures were used to accurately predict the location and activity of multiple functionally validated enhancers, suggesting a role in tissue-specific transcriptional regulation.27
The Set7 Lysine Methyltransferase Catalyzes H3K4me1
Originally isolated from HeLa nuclear extracts,39 Set7 (also Set940 or Set7/941) is a 41 kDa lysine-specific SET-domain methyltransferase encoded by a gene conserved in vertebrates.42 Embryonic lethality for a significant proportion of Set7 knockout mice43 indicates an important role in development. Transcriptional activation of the SETD7 promoter mediated through an islet-specific enhancer element was shown to be PDX1-dependent in β-cells.33 Similarly, Set7 expression is activated for skeletal muscle differentiation.38 Thus, Set7 may function in development through cellular differentiation and tissue-specific gene activation. Specific H3K4 methyltransferase activity was demonstrated by preclusion of the reaction by mutation of this residue from lysine to arginine32,39 and by Edman degradation of H3 histones labeled with 3H-S-adenosylmethionine (3H-SAM) by Set7.40 Recently, developed specific bisubstrate-type inhibitors of Set7 exhibit potent antagonism toward the H3K4me1 in vitro using recombinant Set7 and H3 peptides.44
Structural and biochemical studies indicate that Set7 catalyzes transfer of a single methyl group to H3K4.45 Monomethylation of this residue may provide a site for further rounds of modification; however, several findings discount involvement of Set7 in the H3K4me2 or H3K4me3 reactions. Methyltransferase activity toward previously monomethylated H3 peptide is largely undetectable in vitro.40,41,46 Similarly, peptide array analysis revealed inhibition of Set7 activity toward methylated H3K4.46 This is further supported by crystal structure analysis that revealed the methyl group transferred to K4 interferes with the formation of a water channel to this residue. Absence of this channel prevents further methylation events, excluding subsequent rounds of methylation by Set7 on its enzymatic product. Furthermore, molecular simulations report that disruption of the enzymatic machinery of Set7 upon binding monomethylated lysine is due to opening of the SAM binding channel.47 A single base mutation from a conserved histidine to alanine residue within the SET-domain impairs the methyltransferase activity of the enzyme.39,40 Interestingly, mutagenesis of conserved tyrosine to phenylalanine residues at positions 245 and 305 of the active site alters the enzymatic product of Set7 to trimethylation48 and dimethylation respectively.49 These active site mutants allow formation of a water channel between the enzyme and monomethylated K4, permitting the transfer of additional methyl groups.48,50
Despite initial characterization of Set7 as a histone H3-specific lysine methyltransferase, the physiological validity of histone H3 as a biochemical substrate has been justifiably questioned. The robust methyltransferase activity toward core histone H3 is markedly weakened when nucleosomal histones are the substrate.39,40 Since nucleosomal histones are in direct association with chromatin, the role for Set7-mediated H3K4me1 in transcriptional activation was uncertain. These observations may be explained by the inaccessibility of the H3 histone N-terminal tails in nucleosomal complexes. However, Suv39h1, which modifies the same histone tail, displays comparable enzymatic activity toward core and nucleosomal histones.40 In accordance with the in vitro data, recent observations suggest that RNAi-mediated Set7 knockdown36,51 or somatic Set7 knockout does not affect global nucleosomal H3K4me1.52 These experimental observations contrast reports that show global H3K4me1 depletion in rat mesangial cells following Set7 knockdown by transfection.35
While cell-specific differences cannot be discounted, the discordance is most likely attributable to the method of histone preparation exemplified by distinguishable H3K4me1 patterns across distinct nucleosomal and non-chromatinized histone fractions purified from human endothelial cells.53 Importantly these findings are consistent with a role for Set7-mediated transcriptional regulation by enrichment of H3K4me1 at discrete loci identified by chromatin immunoprecipitation (ChIP) across a range of human31,32,36,53 and animal cell types.33,35,38 Several studies simultaneously exclude Set7 as a global regulator of this modification and raise the possibility of another uncharacterized HMT that maintains nucleosomal H3K4me1 patterns. Indeed, Mll3, the mammalian homolog of Drosophila Trr H3K4-monomethylase was recently associated with this modification pattern in embryonic fibroblasts derived from mice. Knockout of Mll3 resulted in significant global reduction of H3K4me1 enrichment at distal regulatory sites including enhancer elements of the Hoxd gene cluster.13 While MLL3 exhibits H3K4 methyltransferase activity, direct H3K4me1 enrichment by this enzyme was not definitively demonstrated and secondary effects on Set7 expression and activity cannot be discounted. Alternatively H3K4me1 could be regulated through reduction of the highly methylated forms (H3K4me2 and H3K4me3) by specific lysine demethylases.17
Alternative histone substrates
Recent experimental findings indicate that the histone methyltransferase activity of Set7 is not restricted to H3. Set7 methylated recombinant full-length H2A and H2B peptides at comparable levels to H3 peptides in vitro.46 H2A appears to harbor several methylation sites. By contrast, H2B is methylated exclusively at K15. Transcriptional regulation by acetylation of H2A and H2B N-terminal domains has been characterized at several residues in yeast and mammals;54 however, little is known of the potential role of lysine methylation on these histones. More recently, PARP1-mediated ADP-ribosylation of H3 histones was shown to promote Set7-dependent methylation of linker histone variant H1.4. Interestingly, ADP-ribosylation of H3 and H1.4 inhibited Set7-mediated methylation of those substrates respectively, suggesting that this PTM modulates Set7 histone substrate preference. Methylation by Set7 was observed at six distinct lysines of H1.4 in vitro.55 Set7-mediated methylation of these alternative histone substrates is yet to be demonstrated in vivo; thus, the physiological consequences remain unresolved.
Transcriptional Regulation by Modification of the Chromatin Template
Early studies that revealed the ability for Set7 to stimulate GAL4-VP16-activated luciferase expression in cancer cells40 provided some of the first evidence that this enzyme may potentiate positive transcriptional events. Experimental observations indicate that Set7-mediated H3K4me1 can maintain a state of transcriptionally active chromatin by preventing NuRD chromatin remodeling and deacetylase complex-mediated transcriptional silencing,56 as well as inhibiting Suv39h1-mediated H3K9 methylation and subsequent HP1-dependent repression.40 Similar cross talk facilitates subsequent activation-associated acetylation of both H3 and H4 histones by p300.40
Studies in cell culture demonstrating transcriptional regulation by Set7-mediated H3K4 methylation are described in Table 1. H3K4me1 enrichment and transcriptional activation through co-recruitment of Set7 as a constituent of a co-activator complex to discrete promoters may necessitate disruption of chromatinized nucleosomes.40 Subsequently Set7 could methylate dissociated core H3 histones prior to re-association of the nucleosome.57 Co-recruitment interactions potentially explain the mechanisms of promoter-specific Set7 enrichment. Indeed, co-recruitment of Set7 to activating promoters via interaction with TFs has been observed for MyoD-dependent skeletal muscle differentiation,38,58 NFκB-p65 binding at inflammatory gene promoters of TNF-α-stimulated monocytes,30 PDX-1-mediated insulin expression by pancreatic β-cells37 and androgen-responsive transcriptional activation via the androgen receptor (AR).34,36 Co-activational roles of Set7 may extend beyond interaction with a single TF, as experiments suggest participation in multi-component assemblies such as the pre-initiation complex required for transcriptional activation of MMP1.29
Table 1. Cell culture studies demonstrating transcriptional regulation by Set7-mediated chromatin modification.
| Cell type | Experimental conditions | Target promoter(s) | Reference |
|---|---|---|---|
| Human T98G glioblastoma cells |
Serum stimulation |
MMP1 |
29 |
| LNCaP human prostate cancer cells |
Androgen stimulation |
PSA PSA, NKX3–1 |
36 34 |
| Human THP-1 monocytes |
TNF-α stimulation |
MCP-1, IL8, TNF |
30 |
| Human and bovine endothelial cells |
Transient hyperglycemia |
RELA |
31,32 |
| Human microvascular endothelial cells |
Hyperglycemia |
IL8 |
53 |
| Murine βTC3 β-cells |
Basal growth |
INS1, INS2, SLC2A2 |
33,37 |
| Murine C2C12 myoblasts |
Serum-induced differentiation |
MYOD1, MYOG, MCK, MHC |
38 |
| Rat mesangial cells | TGF-β stimulation | COL1A1, CTGF, PAI-1 | 35 |
Metabolic signaling to histone methylation
Epigenetic processes at the chromatin template have been recently explored for their role in sensitizing transcriptional and phenotypic outcomes to environmental signaling. Epigenetic components of multifactorial diseases such as cancer and diabetes are beginning to emerge. Several well-characterized examples of Set7-dependent gene expression were revealed under experimental conditions that mimic the diabetic milieu. Involvement of Set7 in glucose metabolism was demonstrated at a subset of glucose responsive promoters in murine β-cells and primary islets in vitro.33 Curiously, Set7-depletion by siRNA transfection reduced H3K4me2 enrichment at the INS1, INS2 and SLC2A2 promoters, while H3K4me1 enrichment remained stable. It was noted that this observation could reflect differences across cell types or the existence of a closely linked, uncharacterized HMT that completes the di-methylation of H3K4.33 Knockdown of PDX-1 diminished insulin expression by reducing H3K4 methylation at promoter and intragenic regions. Furthermore, co-immunoprecipitation and co-transfection experiments confirm that Set7 is recruited to the activating promoter via specific interaction with PDX-1.37
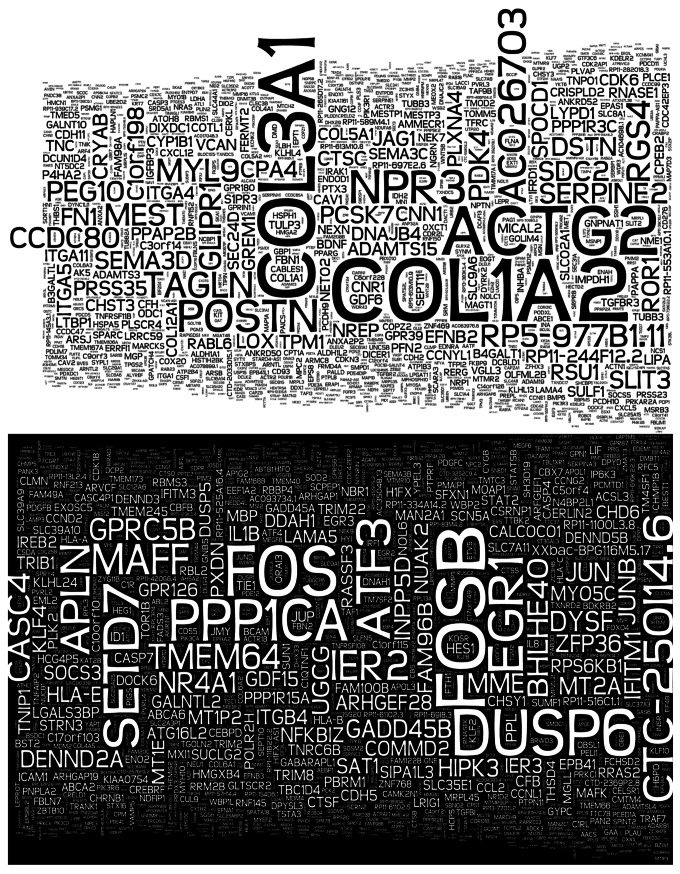
In studies that directly associate glucose signaling to Set7-mediated chromatin modification, inhibition of H3K4me1 enrichment and concomitant transcriptional activation of RELA was detected following Set7 knockdown in cell culture models of hyperglycemic variability.31,32 These observations implicate Set7 in the clinical phenomenon of metabolic memory originally employed to describe the persistent progression of chronic vascular complications in diabetic patients despite implementation of strict glycemic control regimens.59,60 RELA encodes the NFκB-p65 subunit and its expression is accordingly linked with inflammatory pathway activation.61 Transient high glucose induced Set7-mediated H3K4m1 enrichment paralleling persistent transcriptional activation of RELA and downstream NFκB-dependent inflammatory genes in human and bovine vascular endothelial cells. This response was maintained for 6 d in culture despite restoration of physiological glucose conditions, indicating a metabolic memory of the original glucose stimulus. Furthermore, sustained reduction of H3K9 methylation following hyperglycemia was reported to be dependent on Set7 activity.31,32 ChIP analysis of aortas isolated from a rodent model of hyperglycemic variability revealed similarly sustained enrichment for H3K4me1 at the CXCL2 promoter (mouse IL8 homolog), paralleling gene expression that persisted for 7 d beyond exposure to high glucose.53 In accordance with these data, recent studies report high glucose-stimulated nuclear localization of endothelial Set7.53 Thus, Set7 appears to operate as a sensor of vascular glucose stimulation, and nuclear translocation may potentiate the H3K4m1 modification at numerous discrete, activating loci. Recent transcriptome profiling by high-throughput mRNA sequencing of human cells of vascular origin depleted of Set7 by shRNA revealed widespread transcriptional deregulation under normal culture conditions (Fig. 1, unpublished data). Ontology analysis of deregulated gene sets revealed enrichment of upregulated genes associated with cell cycle regulation. Apoptotic and p53 signaling pathways were significantly enriched among genes repressed by Set7 knockdown (Keating and El-Osta, personal communication). These observations suggest that Set7 may regulate the expression of numerous genes expressed by the vascular endothelium and possibly other cell types.
Figure 1. Wordcloud depiction of transcriptional changes exhibited by human microvascular endothelial cells depleted of Set7. According to mRNA sequencing, gene symbols in the upper panel were upregulated and gene symbols in the lower panel were downregulated compared with control samples. The size of the gene symbol indicates relative p value strength.
Set7 knockdown of monocytes compromised TNF-α-activated transcription of a subset of NFκB p65-dependent inflammatory genes by a distinct mechanism. Specific enrichment of p65 at MCP-1 and TNF promoters was shown to be Set7-dependent, and facilitated co-recruitment of Set7 and H3K4me1 enrichment.30 The similar level of p65 protein detected by western blot across control and Set7 deficient cells indicates that the observed transcriptional effects on downstream targets were not the result of changes in RELA transcriptional regulation. In fact Set7 appears to co-activate a subset of p65-regulated TNF-α-sensitive genes.30 In addition to potential cell type differences, it is possible that Set7 functions differently in the context of distinct metabolic stimuli.
TGF-β is considered a central mediator of the deleterious effects of the diabetic milieu in the kidney.62 This growth factor was shown to stimulate Set7 and H3K4me1-dependent transcriptional activation of fibrotic genes in rat mesangial cells under diabetic conditions.35 High glucose-stimulated expression of COLL1A1, CTGF and PAI-1 and concomitant H3K4 methylation by Set7 was inhibited by pre-treatment with a TGF-β-specific antibody and targeted Set7 disruption by siRNA transfection. Interestingly, Set7 expression was increased upon TGF-β exposure.35
The Broader Substrate Specificity of Set7
In addition to chromatin-associated protein substrates, exposed amino acid residues of non-histone proteins present as potential sites of modification. Numerous proteins are subject to a variety of PTMs, including phosphorylation, acetylation, sumoylation ubiquitination and methylation.63-68 These modifications contribute predominantly to the regulation of protein activity, stability and protein-protein interactions depending on the substrate and type of modification.69-71 Notably lysine methylation has emerged as a common modification involved in regulating protein stability and activity.51,72-74 Reported examples include modification of tumor suppressors and TFs.51,75,76 SET-domain proteins have been implicated in such events, and in some instances demonstrate broad substrate specificity.77,78 Recent investigation of Set7 target specificity revealed numerous non-histone substrates and additional mechanisms of transcriptional regulation by modification of specific transcription-associated proteins. Unexpectedly dimethylation by Set7 is reported in the case of two novel substrates: Msx2-interacting protein (MINT)46 and signal transducer and activator of transcription 3 (STAT3),79 further questioning the classification of Set7 as exclusively a monomethylase. Moreover, STAT3 and MINT are both dimethylated by Set7 in vitro reconstitution in the absence of other HMTs, confirming that Set7 directly catalyzes the dimethylation of these substrates.46,79
Regulation of protein stability
The stability and degradation of several recently described non-histone substrates are reportedly regulated by Set7-mediated lysine methylation, and serve as potential mechanisms of indirect transcriptional regulation. Monomethylation of p53 at K372 was found to be Set7-dependent and a positive regulator of p53 nuclear retention and stability in human cancer cell lines73,80 and several observations indicate a critical role for this modification in the p53 response to DNA damage. Overexpression of Set7 significantly increased p53-dependent p21WAF/CIP expression and exacerbated the transcriptional response of this gene to genotoxic stress.73 Similarly, Set7 depletion by shRNA inhibited Adriamycin (Adr)-induced p21WAF/CIP transcriptional activation.80 Interplay of PTMs appears central to Set7-mediated p53 activation. In accordance with increased acetylation of methylated p53,43,80 Tip60 acetyltransferase binding was enhanced following p53 methylation by Set7 in mouse and human cells.43 By contrast, methylation at K372 inhibits the transcriptionally repressive methylation at K370 by SMYD2.81 Interaction between the SIRT1 deacetylase and Set7 facilitates dissociation of SIRT1 from p53. Consequently, p53 acetylation and transactivation of p21WAF/CIP are enhanced in response to DNA damage.82
Paralleling observations from cell lines, embryonic fibroblasts isolated from mice lacking Set7 expression and consequently p53 methylation exhibited impaired cell cycle arrest and p53 target gene expression following DNA damage. These cells are more susceptible to oncogenic transformation, suggesting a role for Set7 as a tumor suppressor through p53 stabilization and activation.43 By contrast, fibroblasts from independently generated Set7 knockout mouse models exhibited normal p53 acetylation83 and transactivational response to DNA damage in the absence of Set7. Furthermore, Adr-induced proliferative arrest and p53-dependent apoptosis were preserved.52,83 Reasons for the discordance across these in vivo studies are currently unclear.
In a study that analyzed p53-deficient cancer cells, the opposing activities of Set7 and LSD1 were found to regulate E2F1 stability and downstream p27 gene expression in response to DNA-damaging agents.84 Specifically, E2F1 was destabilized by methylation at K185 by Set7. DNA damage induced removal of this modification by LSD1 leading to increased cellular E2F1 protein levels. Similarly, regulation of DNA (cytosine-5)-methyltransferase 1 (DNMT1) stability by methylation at K142 was found to be Set7 and LSD1-dependent. Targeted Set7 disruption by RNAi stabilized cellular DNMT1 levels, while overexpression of Set7 correlated with a decrease in DNMT1 protein by facilitating polyubiquitination and subsequent proteasome-mediated degradation.74 DNMT1 is a well-characterized maintenance DNA methyltransferase that specifically methylates DNA at CpG residues. Overexpression of Set7 in HeLa cells led to an approximate 10% reduction of global DNA methylation.74 LSD1 knockdown and subsequent impairment of DNMT1 demethylation markedly reduced DNMT1 protein levels and global CpG methylation in mouse ES cells.85 Certainly, it appears that DNMT1 protein stability is dynamically regulated by the opposing activity of these two enzymes, and may represent a subtle mechanism responsible for the fine-tuning of DNA methylation.
A further example of Set7-regulated protein stability is estrogen receptor α (ERα) methylation at K302. The ERα protein is stabilized by this modification, with detectable downstream effects on estrogen-induced gene activation. Breast cancer cells depleted of Set7 exhibited decreased ERα protein levels and attenuated transcriptional induction of the PS2 and progesterone receptor (PgR) genes in response to estrogen stimulation.86
Set7 modulates transcription factor activity
Several recently identified Set7 substrates display modified biological activity following methylation by Set7, exclusive of changes to cellular protein levels. Further implicating Set7 in the regulation of NFκB-dependent pathways, K37 of the NFκB subunit p65 was also recently identified as a novel substrate for Set7.51 Like p53, methylation dependent regulation of p65 may represent an integral control point with regard to the expression of a wide variety of pathways including the inflammatory response,61 apoptotic signaling87 and proliferation.88 In unstimulated cells, NFκB is sequestered in the cytoplasm through binding IkB proteins. Upon stimulation by a variety of signaling events, IkB is ultimately degraded and NFκB translocates to the nucleus to regulate inducible gene expression.89 Contrasting the unmodified protein, p65 methylated at K37 is restricted to the nucleus and enriched at a subset of NFκB-dependent promoters in response to TNF-α.51 By contrast, Set7-dependent methylation of additional sites at K315 and K316 of p65 can result in negative regulation of p65 transactivity.90 Kinetic analyses suggest that K37 is the preferential methylation site for Set7;51 however, the precise role of these modifications is unclear.
Another important example of Set7-mediated transcriptional repression is methylation of two separate lysine residues at K810 and K873 of the retinoblastoma tumor suppressor protein (pRb). The pRb protein physically interacts with and regulates the activity of the cell cycle associated E2F family TFs.91 Set7 knockdown by transfection weakened the association of pRb and HP1. ChIP analyses revealed reduced pRb and HP1 enrichment at E2F-target promoters of cancer cells under conditions of growth arrest.76,92 Analysis of the pRb peptide implicates methylation of K873 by Set7 as a regulator of this process and therefore further associates Set7 with cell cycle regulation.76 Furthermore, the K810 modification site is located within a CDK phosphorylation site of pRb, where methylation impedes pRb phosphorylation to antagonize CDK-dependent cell cycle progression.93 Accordingly pRb protein mutated at K810 cannot be methylated, and cells expressing this mutant display deficient cell cycle arrest.92 Both methylation sites harbor the amino residues proline (P) and leucine (L) immediately preceding the target lysines, suggesting that they might be bound and methylated by Set7 with similar efficiency. Given their close proximity, these lysine residues are perhaps modified by Set7 sequentially.
Methylation-dependent co-recruitment of Set7 to activating promoters
Further expansion of the catalog of substrates may in fact reveal the mechanisms behind Set7-mediated H3K4 methylation at specific promoters. Recent experimental evidence suggests that posttranslational TF methylation by Set7 can regulate promoter-specific recruitment of the HMT. For instance, methylation of the TAF10 component of the TFIID complex at K189 by Set7 increased the affinity for RNAP II and enhanced transcription of a subset of TAF10-dependent genes, suggesting a role in preinitiation complex formation.94 Retinoic acid induced expression of ERF1 and ERA1 genes was attenuated in cells expressing recombinant TAF10 mutated at K189; however, expression of HPRT and CCNE1 was unaffected. Paralleling these observations, selective Set7 enrichment at ERF1 and ERA1, but not HPRT and CCNE1 promoters was revealed by ChIP.94 Such observations argue for promoter-specific recruitment of Set7 via this interaction with TAF10, however the subsequent effects on H3K4 methylation remain unresolved. Similar promoter-specific TF occupancy was observed following methylation of STAT3 at K140 by Set7 under IL-6 stimulation. The SOCS3 promoter displays increased STAT3 enrichment and concomitant transcriptional upregulation following Set7 knockdown in cancer cells. Interestingly, Set7 recruitment to SOCS3 was found to follow STAT3 recruitment, and possibly clears STAT3 from the activating promoter to potentiate H3K4 methylation.79 While sequential Set7 recruitment to SOCS3 was observed, the downstream effects on H3K4 methylation remain uncharacterized.
Similar methylation-dependent interaction between Set7 and AR is important for AR activation and recruitment to androgen-regulated promoters. Methylation of the receptor at K632 by Set7 is required for normal downstream target gene expression in prostate and kidney cancer cell lines independent of changes to AR protein stability or cytoplasmic-nuclear translocation.36 Analysis of the lysine substrate by an independent research group indicates K630 to be the target of Set7 methylation.34 Despite the discordance, both studies demonstrated that loss of AR methylation following Set7 knockdown of cancer cells results in significant H3K4me1 depletion at androgen response element (ARE) regulatory regions of the PSA34 and NKX3-1 promoters.34,36
Expanding the Substrate Compendium of Set7
Further characterization of TF substrates of Set7 will contribute toward a greater understanding of its role in transcriptional regulation with particular regard to mechanisms of Set7 enrichment at discrete loci. Following early observations of non-histone Set7 substrates, a consensus recognition motif was proposed to account for the selective methyltransferase activity. The amino acid sequence motif [K/R]-[S/T/A] preceding the modified lysine is in accordance with the sequence targeted on confirmed Set7 substrates; TAF10,94 DNMT174 H3K4, p53 and ERα and was successfully used to identify TAF7 as an in vitro substrate.95 Using an optimized recognition sequence: [G/R/H/K/P/S/T]–[K > R]–[S > K/Y/A/R/T/P/N]–K–[Q/N]–[A/Q/G/M/S/P/T/Y/V] recent peptide array methylation experiments have revealed a multitude of novel Set7 substrates with numerous targets confirmed in vivo,46 and significantly expanded the list of characterized Set7 substrates (Table 2). With K representing the target lysine at position 0, previously identified and novel substrates follow the formula at positions -1 and -2 and usually -3. The -1 position favors serine (S), alternatively this position can be occupied by a number of basic and aromatic residues. Basic amino acids residues predominate the latter positions of many Set7 methylation sites. Accordingly, the substrate-binding pocket of Set7 is flanked by several acidic amino acid residues, indicating a general preference for positively charged substrates. While the amino acids at positions +1 and +2 to some extent appear redundant in the binding of Set7,46 several verified substrates display polar residues at this site as predicted by the optimized formula.
Table 2. Set7 substrates and target motifs characterized in vitro and in vivo.
| Substrate | Methylation site | Experiment | ||
|---|---|---|---|---|
| Histones |
H3 |
K4 |
A R T K Q T A R K S |
in vitro,39 in vivo29-38 |
| H2A |
unknown |
in vitro peptide assay46 |
||
| H2B |
K15 |
A P K K G S K K A V T K A |
in vitro peptide assay46 |
|
| H1.4 |
K121 |
E A K P K A K K A G A A K |
in vitro55 |
|
| K129 |
A G A A K A K K P A G A A |
|||
| K159 |
K T P K K A K K P A A A A |
|||
| K171 |
A G A K K A K S P K K A K |
|||
| K177 |
K S P K K A K A A K P K K |
|||
| K192 |
K S P A K A K A V K P K A |
|||
| Chromatin-associated |
MeCP2 |
K347 |
S P G R K S K E S S P K G |
in vivo46 |
| PPARBP |
K1006 |
S N D G K S K D K P P K R |
in vitro46 |
|
| Transcription factors |
p53 |
K372 |
S S H L K S K K G Q S T S |
in vivo43,73,80 |
| E2F1 |
K185 |
L I A K K S K N H I Q W L |
in vivo84 |
|
| MINT |
K2076 |
E K N S K S K R G R S R N |
in vivo46 |
|
| IRF1 |
K126 |
R K E R K S K S S R D A K |
in vivo46 |
|
| ERα |
K302 |
L M I K R S K K N S L A L |
in vivo86 |
|
| TAF7 |
K5 |
M S K S K D D A P H E |
in vitro |
|
| CENPC1 |
K414 |
E K P S R S K R T I K Q K |
in vivo46 |
|
| Tat (HIV) |
K51 |
I S Y G R K K R R Q R R R |
in vivo97 |
|
| FoxO3 |
K270, K271 |
G R A A K K K A A L Q T A |
in vivo98 |
|
| TAF10 |
K189 |
G S S R S K K D R K Y T L |
in vivo94 |
|
| p65 |
K37 |
G M R F R Y K C E G R S A |
in vivo51 |
|
| K314, K315 |
T F K S I M K K S P F S G |
in vivo90 |
||
| AR |
K630, K632 |
M T L G A R K L K K L G N L K |
in vivo34,36 |
|
| pRb |
K810 |
I Y I S P L K S P Y K I S |
in vivo93 |
|
| K873 |
N P P K P L K K L R F D I |
in vivo76 |
||
| STAT3 |
K140 |
A A V V T E K Q Q M L E Q |
in vivo79 |
|
| Enzymes |
ZDHC8 |
K300 |
L G R S K S K G S L D R L |
in vivo46 |
| TTK |
K708 |
R E N G K S K S K I S P K |
in vitro46 |
|
| DNMT1 |
K142 |
R T P R R S K S D G E A K |
in vivo74 |
|
| SIRT1 |
K232, K235 K236, K238 |
S E P P K R K KK R K D I N T |
in vitro82 |
|
| PCAF |
K78 |
S A R I A V K K A O L R S |
in vivo96 |
|
| K89 |
R S A P R A K K L E K L G |
|||
| K638 |
K Y V G Y I K D Y E G A T |
|||
| K671, K672 |
K Q K E I I K K L I E R K Q |
|||
| K692 |
P G L S C F K D G V R O I |
|||
| Other | AKAP6 |
K604 |
L S K H K S K K G Q A S S |
in vitro46 |
| Cullin 1 | K73 | V P P S K S K K G Q T P G | in vitro46 | |
Amino acid triplets that mark methylation motifs are underlined. Methylated lysines are highlighted bold.
Indeed, many Set7 substrates are methylated at a sequence according to this formula. For example TAF10, p53, DNMT1 and ERα display this motif particularly at sites -1 and -2. The structurally similar KSK and RSK sequences predominate the list of bona fide Set7 substrates and appear most valuable for predicting novel methylation sites. Similarly, the five novel methylation sites of H1.4 are localized to KAK motifs.55 Evidently this recognition sequence does not reflect all lysines targeted by Set7 as the sequence of amino acid motifs of substrates including p65, AR and pRb are discordant to the proposed formula. However, the ability to correctly anticipate novel in vivo substrates demonstrates its effectiveness as a predictive tool. This recognition sequence identified novel substrates by peptide array analysis, with five confirmed as Set7 methylation targets in vivo.46
A recent study identified p300/CBP-associated factor (PCAF) as a novel substrate that is methylated by Set7 at six distinct lysines including adjacent residues at K671 and K672.96 The amino acid sequences surrounding the methylation sites diverge considerably from the previously proposed consensus sequence. Subsequently, an alternative recognition motif was proposed that accounts for five of the six sites: [A/F/I/V]-K-[D/K]. Contrasting the high frequency of basic amino residues at positions downstream of the target lysine of many Set7 substrates, neutral non-polar residues precede the target lysine at five PCAF methylation sites.96 Comparison of in vitro methylation efficiency between protein targets demonstrate that binding sites that do not follow the consensus, such as the six methylation sites of PCAF and the three sites harbored by p65, are methylated by Set7 with lower efficiency than H3K4.46 Importantly, several novel substrates such as MeCP2 and MINT demonstrate stronger methylation by Set7 relative to H3 histones. Amino sequences surrounding the target lysines of these substrates differ significantly from that of the histone H3 tail, further suggesting that Set7 preferentially methylates the KSK motif. However, this sequence motif also marks the site of p53 methylation by Set7 and kinetic studies revealed Set7 to methylate histone H3 and p53 with comparable efficiency.95 Further kinetic analysis of Set7 activity on the current list of identified substrates will clarify the substrate preference of the methyltransferase.
Future Considerations
A major focus of epigenetic research is the comprehension of histone modifications and the system of enzymes and complexes that regulate them. Characterization of the Set7 lysine methyltransferase has contributed to a greater understanding of how the H3K4me1 modification contributes to an active chromatin state. Although evidence indicates Set7 activity on non-nucleosomal histones in vitro39,40 but global nucleosomal H3K4 states are unaffected by Set7 depletion,36,51,52 experimental evidence does place Set7 and H3K4 methylation at activating promoters of discrete genes. Furthermore, the potential role for Set7 in chromatin modification at enhancer elements holds intriguing possibilities for cellular differentiation, organism development and tissue-specific gene expression.58 Transcriptional regulation by Set7 could be associated with direct modification of histone tails at regulatory elements, posttranslational methylation of TFs and/or co-recruitment to activating promoters, or a combination of these mechanisms. Characterization of these events will greatly assist distinction of the subset of genes with regulatory associations to Set7.
Characterization of in vivo substrates is an avenue of investigation that deserves further attention as such studies have revealed numerous novel Set7 and target promoter associations. To date, at least 27 distinct lysines are subject to in vivo methylation by Set7. Indeed these substrates are predominantly associated with chromatin and transcription (Table 2) indicating potential for widespread transcriptional control. Intriguingly, this modification extends to components of transcriptional regulation associated with CpG methylation; DNMT1 and MeCP2.99 As CpG methylation is a central mediator of epigenetic transcriptional repression,100,101 it is tempting to speculate a role for Set7 in transcriptional enhancement through DNMT1 degradation. However, this mechanistic link remains to be established experimentally. Similarly, the in vivo consequences of MeCP2 methylation by Set7 have not been reported. The use of in vitro peptide arrays46 is an effective approach to initial substrate identification; however, it lacks in vivo context. Advances in high-throughput sequencing technologies and bioinformatics tools allow mining for TF connectivity to global gene expression changes.102 Application of this method to Set7 compromised cells or extracellular stimulation could be utilized to identify potential substrates with direct links to transcriptional changes that harbor motif substrates similar to those previously described for Set7. Several variants of the amino sequence consensus formula; KSK, RSK and KAK appear to be favored by Set7. However, other sequences are also methylated and kinetic data for substrate preference are currently incomplete.
Reports of Set7-mediated H3K4me2 enrichment of genes important for glucose metabolism in pancreatic β-cells extends to recent observations of STAT3 and MINT dimethylation by the enzyme. Although the crystal structure of Set7 in complex with H3K4 indicates that water occupies the channel necessary to accommodate the transfer of additional methyl groups,48 the product specificity of Set7 can be altered by mutation of conserved residues within the active site.48,49 Thus, it is possible that interaction with other proteins could structurally alter the active site of Set7 to allow additional methyl groups to be transferred to the target lysine of histones and non-histone proteins. Identification of the proteins that interact with Set7 could therefore reveal mechanisms of substrate specificity modulation.
Specific targeting of the enzymes implicated in chromatin structure and gene function provides potential for future epigenetic therapy for human disease. Studies suggest Set7 as a potential target for therapeutic inhibition, most notably in the context of diabetic vascular therapy and associations to glucose signaling. The therapeutic potential of Set7 inhibition may extend to other disease states, such as the important role in p53 stabilization and subsequent p21WAF/CIP induction following DNA damage.73,80 While these observations from cancer cells point toward a role for Set7 in tumor suppression, studies of Set7 knockout mouse models report conflicting results and further characterization of Set7 function in vivo is required. Set7-mediated regulation of pRb and E2F1 is also associated with proliferation, further highlighting a potential role in cell cycle regulation. Set7 was also recently found to enhance Tat-dependent viral transcription through modification and modulation of the HIV Tat protein,97 suggesting potential therapy for HIV. Several challenges to this approach are apparent in the multiplicity of pathways potentially regulated through mechanisms that encompass gene activation through H3K4me1 enrichment and regulatory TF methylation. Thus, it is important to characterize these transcriptional events in both basal and pathological contexts before Set7 inhibition can be considered for therapy.
Acknowledgments
The authors gratefully acknowledge the contribution of Dr Mark Ziemann (Epigenomics Profiling Facility, Baker IDI Heart and Diabetes Institute) for his assistance in generating the data presented as Figure 1. The authors acknowledge grant and fellowship support from the Juvenile Diabetes Research Foundation International, the Diabetes Australia Research Trust, the National Health and Medical Research Council (NHMRC) and the National Heart Foundation of Australia. S.T.K. is supported by an Australian Postgraduate Award. A.E.-O. is a senior research fellow supported by the NHMRC.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/24234
References
- 1.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–8. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 2.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 3.Turner BM. Histone acetylation and an epigenetic code. Bioessays. 2000;22:836–45. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 5.Dai Z, Dai X, Xiang Q, Feng J, Wang J, Deng Y, et al. Genome-wide analysis of interactions between ATP-dependent chromatin remodeling and histone modifications. BMC Genomics. 2009;10:304. doi: 10.1186/1471-2164-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee N, Sinha D, Lemma-Dechassa M, Tan S, Shogren-Knaak MA, Bartholomew B. Histone H3 tail acetylation modulates ATP-dependent remodeling through multiple mechanisms. Nucleic Acids Res. 2011;39:8378–91. doi: 10.1093/nar/gkr535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating ST, El-Osta A. Chromatin modifications associated with diabetes. J Cardiovasc Transl Res. 2012;5:399–412. doi: 10.1007/s12265-012-9380-9. [DOI] [PubMed] [Google Scholar]
- 8.Couture JF, Trievel RC. Histone-modifying enzymes: encrypting an enigmatic epigenetic code. Curr Opin Struct Biol. 2006;16:753–60. doi: 10.1016/j.sbi.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Wei G, Hu G, Cui K, Zhao K. Genome-wide mapping of nucleosome occupancy, histone modifications, and gene expression using next-generation sequencing technology. Methods Enzymol. 2012;513:297–313. doi: 10.1016/B978-0-12-391938-0.00013-6. [DOI] [PubMed] [Google Scholar]
- 10.Emes RD, Farrell WE. Make way for the ‘next generation’: application and prospects for genome-wide, epigenome-specific technologies in endocrine research. J Mol Endocrinol. 2012;49:R19–27. doi: 10.1530/JME-12-0045. [DOI] [PubMed] [Google Scholar]
- 11.Noma K, Allis CD, Grewal SI. Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science. 2001;293:1150–5. doi: 10.1126/science.1064150. [DOI] [PubMed] [Google Scholar]
- 12.Litt MD, Simpson M, Gaszner M, Allis CD, Felsenfeld G. Correlation between histone lysine methylation and developmental changes at the chicken beta-globin locus. Science. 2001;293:2453–5. doi: 10.1126/science.1064413. [DOI] [PubMed] [Google Scholar]
- 13.Herz HM, Mohan M, Garruss AS, Liang K, Takahashi YH, Mickey K, et al. Enhancer-associated H3K4 monomethylation by Trithorax-related, the Drosophila homolog of mammalian Mll3/Mll4. Genes Dev. 2012;26:2604–20. doi: 10.1101/gad.201327.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray K. The Occurrence of Epsilon-N-Methyl Lysine in Histones. Biochemistry. 1964;3:10–5. doi: 10.1021/bi00889a003. [DOI] [PubMed] [Google Scholar]
- 15.Jenuwein T. The epigenetic magic of histone lysine methylation. FEBS J. 2006;273:3121–35. doi: 10.1111/j.1742-4658.2006.05343.x. [DOI] [PubMed] [Google Scholar]
- 16.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–53. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Tanasa B, Tyurina OV, Zhou TY, Gassmann R, Liu WT, et al. PHF8 mediates histone H4 lysine 20 demethylation events involved in cell cycle progression. Nature. 2010;466:508–12. doi: 10.1038/nature09272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–60. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 20.Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem. 2003;278:4035–40. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- 21.Boggs BA, Cheung P, Heard E, Spector DL, Chinault AC, Allis CD. Differentially methylated forms of histone H3 show unique association patterns with inactive human X chromosomes. Nat Genet. 2002;30:73–6. doi: 10.1038/ng787. [DOI] [PubMed] [Google Scholar]
- 22.Schneider R, Bannister AJ, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat Cell Biol. 2004;6:73–7. doi: 10.1038/ncb1076. [DOI] [PubMed] [Google Scholar]
- 23.Liang G, Lin JC, Wei V, Yoo C, Cheng JC, Nguyen CT, et al. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc Natl Acad Sci U S A. 2004;101:7357–62. doi: 10.1073/pnas.0401866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–81. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Lee TI, et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–27. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Xiao T, Shibata Y, Rao B, Laribee RN, O’Rourke R, Buck MJ, et al. The RNA polymerase II kinase Ctk1 regulates positioning of a 5′ histone methylation boundary along genes. Mol Cell Biol. 2007;27:721–31. doi: 10.1128/MCB.01628-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–8. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 28.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–37. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Martens JH, Verlaan M, Kalkhoven E, Zantema A. Cascade of distinct histone modifications during collagenase gene activation. Mol Cell Biol. 2003;23:1808–16. doi: 10.1128/MCB.23.5.1808-1816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, et al. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem. 2008;283:26771–81. doi: 10.1074/jbc.M802800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–17. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, et al. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009;58:1229–36. doi: 10.2337/db08-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deering TG, Ogihara T, Trace AP, Maier B, Mirmira RG. Methyltransferase Set7/9 maintains transcription and euchromatin structure at islet-enriched genes. Diabetes. 2009;58:185–93. doi: 10.2337/db08-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko S, Ahn J, Song CS, Kim S, Knapczyk-Stwora K, Chatterjee B. Lysine methylation and functional modulation of androgen receptor by Set9 methyltransferase. Mol Endocrinol. 2011;25:433–44. doi: 10.1210/me.2010-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R. Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol. 2010;21:2069–80. doi: 10.1681/ASN.2010060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaughan L, Stockley J, Wang N, McCracken SR, Treumann A, Armstrong K, et al. Regulation of the androgen receptor by SET9-mediated methylation. Nucleic Acids Res. 2011;39:1266–79. doi: 10.1093/nar/gkq861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francis J, Chakrabarti SK, Garmey JC, Mirmira RG. Pdx-1 links histone H3-Lys-4 methylation to RNA polymerase II elongation during activation of insulin transcription. J Biol Chem. 2005;280:36244–53. doi: 10.1074/jbc.M505741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao Y, Neppl RL, Huang ZP, Chen J, Tang RH, Cao R, et al. The histone methyltransferase Set7/9 promotes myoblast differentiation and myofibril assembly. J Cell Biol. 2011;194:551–65. doi: 10.1083/jcb.201010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Cao R, Xia L, Erdjument-Bromage H, Borchers C, Tempst P, et al. Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase. Mol Cell. 2001;8:1207–17. doi: 10.1016/S1097-2765(01)00405-1. [DOI] [PubMed] [Google Scholar]
- 40.Nishioka K, Chuikov S, Sarma K, Erdjument-Bromage H, Allis CD, Tempst P, et al. Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation. Genes Dev. 2002;16:479–89. doi: 10.1101/gad.967202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson JR, Jing C, Walker PA, Martin SR, Howell SA, Blackburn GM, et al. Crystal structure and functional analysis of the histone methyltransferase SET7/9. Cell. 2002;111:105–15. doi: 10.1016/S0092-8674(02)00964-9. [DOI] [PubMed] [Google Scholar]
- 42.Veerappan CS, Avramova Z, Moriyama EN. Evolution of SET-domain protein families in the unicellular and multicellular Ascomycota fungi. BMC Evol Biol. 2008;8:190. doi: 10.1186/1471-2148-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurash JK, Lei H, Shen Q, Marston WL, Granda BW, Fan H, et al. Methylation of p53 by Set7/9 mediates p53 acetylation and activity in vivo. Mol Cell. 2008;29:392–400. doi: 10.1016/j.molcel.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 44.Mori S, Iwase K, Iwanami N, Tanaka Y, Kagechika H, Hirano T. Development of novel bisubstrate-type inhibitors of histone methyltransferase SET7/9. Bioorg Med Chem. 2010;18:8158–66. doi: 10.1016/j.bmc.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 45.Kwon T, Chang JH, Kwak E, Lee CW, Joachimiak A, Kim YC, et al. Mechanism of histone lysine methyl transfer revealed by the structure of SET7/9-AdoMet. EMBO J. 2003;22:292–303. doi: 10.1093/emboj/cdg025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhayalan A, Kudithipudi S, Rathert P, Jeltsch A. Specificity analysis-based identification of new methylation targets of the SET7/9 protein lysine methyltransferase. Chem Biol. 2011;18:111–20. doi: 10.1016/j.chembiol.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Hu P, Wang S, Zhang Y. How do SET-domain protein lysine methyltransferases achieve the methylation state specificity? Revisited by Ab initio QM/MM molecular dynamics simulations. J Am Chem Soc. 2008;130:3806–13. doi: 10.1021/ja075896n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, et al. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003;421:652–6. doi: 10.1038/nature01378. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Yang Z, Khan SI, Horton JR, Tamaru H, Selker EU, et al. Structural basis for the product specificity of histone lysine methyltransferases. Mol Cell. 2003;12:177–85. doi: 10.1016/S1097-2765(03)00224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Bruice TC. Histone lysine methyltransferase SET7/9: formation of a water channel precedes each methyl transfer. Biochemistry. 2007;46:14838–44. doi: 10.1021/bi7014579. [DOI] [PubMed] [Google Scholar]
- 51.Ea CK, Baltimore D. Regulation of NF-kappaB activity through lysine monomethylation of p65. Proc Natl Acad Sci U S A. 2009;106:18972–7. doi: 10.1073/pnas.0910439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehnertz B, Rogalski JC, Schulze FM, Yi L, Lin S, Kast J, et al. p53-dependent transcription and tumor suppression are not affected in Set7/9-deficient mice. Mol Cell. 2011;43:673–80. doi: 10.1016/j.molcel.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Okabe J, Orlowski C, Balcerczyk A, Tikellis C, Thomas MC, Cooper ME, et al. Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circ Res. 2012;110:1067–76. doi: 10.1161/CIRCRESAHA.112.266171. [DOI] [PubMed] [Google Scholar]
- 54.Wyrick JJ, Parra MA. The role of histone H2A and H2B post-translational modifications in transcription: a genomic perspective. Biochim Biophys Acta. 2009;1789:37–44. doi: 10.1016/j.bbagrm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Kassner I, Barandun M, Fey M, Rosenthal F, Hottiger MO. Crosstalk between SET7/9-dependent methylation and ARTD1-mediated ADP-ribosylation of histone H1.4. Epigenetics Chromatin. 2013;6:1. doi: 10.1186/1756-8935-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zegerman P, Canas B, Pappin D, Kouzarides T. Histone H3 lysine 4 methylation disrupts binding of nucleosome remodeling and deacetylase (NuRD) repressor complex. J Biol Chem. 2002;277:11621–4. doi: 10.1074/jbc.C200045200. [DOI] [PubMed] [Google Scholar]
- 57.Kim HJ, Seol JH, Han JW, Youn HD, Cho EJ. Histone chaperones regulate histone exchange during transcription. EMBO J. 2007;26:4467–74. doi: 10.1038/sj.emboj.7601870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blum R, Vethantham V, Bowman C, Rudnicki M, Dynlacht BD. Genome-wide identification of enhancers in skeletal muscle: the role of MyoD1. Genes Dev. 2012;26:2763–79. doi: 10.1101/gad.200113.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.UK Prospective Diabetes Study (UKPDS) UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia. 1991;34:877–90. doi: 10.1007/BF00400195. [DOI] [PubMed] [Google Scholar]
- 60.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–9. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:584–90. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 62.Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol. 2004;15(Suppl 1):S55–7. doi: 10.1097/01.ASN.0000093460.24823.5B. [DOI] [PubMed] [Google Scholar]
- 63.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Hunter T. Protein modification: phosphorylation on tyrosine residues. Curr Opin Cell Biol. 1989;1:1168–81. doi: 10.1016/S0955-0674(89)80068-7. [DOI] [PubMed] [Google Scholar]
- 65.Clarke S. Protein methylation. Curr Opin Cell Biol. 1993;5:977–83. doi: 10.1016/0955-0674(93)90080-A. [DOI] [PubMed] [Google Scholar]
- 66.Grillo MA, Colombatto S. S-adenosylmethionine and protein methylation. Amino Acids. 2005;28:357–62. doi: 10.1007/s00726-005-0197-6. [DOI] [PubMed] [Google Scholar]
- 67.Gill G. Post-translational modification by the small ubiquitin-related modifier SUMO has big effects on transcription factor activity. Curr Opin Genet Dev. 2003;13:108–13. doi: 10.1016/S0959-437X(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 68.Wilkinson KD. Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin Cell Dev Biol. 2000;11:141–8. doi: 10.1006/scdb.2000.0164. [DOI] [PubMed] [Google Scholar]
- 69.Bannister AJ, Miska EA. Regulation of gene expression by transcription factor acetylation. Cell Mol Life Sci. 2000;57:1184–92. doi: 10.1007/PL00000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berk AJ. Regulation of eukaryotic transcription factors by post-translational modification. Biochim Biophys Acta. 1989;1009:103–9. doi: 10.1016/0167-4781(89)90087-0. [DOI] [PubMed] [Google Scholar]
- 71.Seo J, Lee KJ. Post-translational modifications and their biological functions: proteomic analysis and systematic approaches. J Biochem Mol Biol. 2004;37:35–44. doi: 10.5483/BMBRep.2004.37.1.035. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X, Wen H, Shi X. Lysine methylation: beyond histones. Acta Biochim Biophys Sin (Shanghai) 2012;44:14–27. doi: 10.1093/abbs/gmr100. [DOI] [PubMed] [Google Scholar]
- 73.Chuikov S, Kurash JK, Wilson JR, Xiao B, Justin N, Ivanov GS, et al. Regulation of p53 activity through lysine methylation. Nature. 2004;432:353–60. doi: 10.1038/nature03117. [DOI] [PubMed] [Google Scholar]
- 74.Estève PO, Chin HG, Benner J, Feehery GR, Samaranayake M, Horwitz GA, et al. Regulation of DNMT1 stability through SET7-mediated lysine methylation in mammalian cells. Proc Natl Acad Sci U S A. 2009;106:5076–81. doi: 10.1073/pnas.0810362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He Y, Korboukh I, Jin J, Huang J. Targeting protein lysine methylation and demethylation in cancers. Acta Biochim Biophys Sin (Shanghai) 2012;44:70–9. doi: 10.1093/abbs/gmr109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munro S, Khaire N, Inche A, Carr S, La Thangue NB. Lysine methylation regulates the pRb tumour suppressor protein. Oncogene. 2010;29:2357–67. doi: 10.1038/onc.2009.511. [DOI] [PubMed] [Google Scholar]
- 77.Rathert P, Dhayalan A, Ma H, Jeltsch A. Specificity of protein lysine methyltransferases and methods for detection of lysine methylation of non-histone proteins. Mol Biosyst. 2008;4:1186–90. doi: 10.1039/b811673c. [DOI] [PubMed] [Google Scholar]
- 78.Del Rizzo PA, Trievel RC. Substrate and product specificities of SET domain methyltransferases. Epigenetics. 2011;6:1059–67. doi: 10.4161/epi.6.9.16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J, Huang J, Dasgupta M, Sears N, Miyagi M, Wang B, et al. Reversible methylation of promoter-bound STAT3 by histone-modifying enzymes. Proc Natl Acad Sci U S A. 2010;107:21499–504. doi: 10.1073/pnas.1016147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ivanov GS, Ivanova T, Kurash J, Ivanov A, Chuikov S, Gizatullin F, et al. Methylation-acetylation interplay activates p53 in response to DNA damage. Mol Cell Biol. 2007;27:6756–69. doi: 10.1128/MCB.00460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang J, Perez-Burgos L, Placek BJ, Sengupta R, Richter M, Dorsey JA, et al. Repression of p53 activity by Smyd2-mediated methylation. Nature. 2006;444:629–32. doi: 10.1038/nature05287. [DOI] [PubMed] [Google Scholar]
- 82.Liu X, Wang D, Zhao Y, Tu B, Zheng Z, Wang L, et al. Methyltransferase Set7/9 regulates p53 activity by interacting with Sirtuin 1 (SIRT1) Proc Natl Acad Sci U S A. 2011;108:1925–30. doi: 10.1073/pnas.1019619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campaner S, Spreafico F, Burgold T, Doni M, Rosato U, Amati B, et al. The methyltransferase Set7/9 (Setd7) is dispensable for the p53-mediated DNA damage response in vivo. Mol Cell. 2011;43:681–8. doi: 10.1016/j.molcel.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 84.Kontaki H, Talianidis I. Lysine methylation regulates E2F1-induced cell death. Mol Cell. 2010;39:152–60. doi: 10.1016/j.molcel.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Hevi S, Kurash JK, Lei H, Gay F, Bajko J, et al. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet. 2009;41:125–9. doi: 10.1038/ng.268. [DOI] [PubMed] [Google Scholar]
- 86.Subramanian K, Jia D, Kapoor-Vazirani P, Powell DR, Collins RE, Sharma D, et al. Regulation of estrogen receptor alpha by the SET7 lysine methyltransferase. Mol Cell. 2008;30:336–47. doi: 10.1016/j.molcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stark LA, Dunlop MG. Nucleolar sequestration of RelA (p65) regulates NF-kappaB-driven transcription and apoptosis. Mol Cell Biol. 2005;25:5985–6004. doi: 10.1128/MCB.25.14.5985-6004.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakajima T, Kitajima I, Shin H, Takasaki I, Shigeta K, Abeyama K, et al. Involvement of NF-kappa B activation in thrombin-induced human vascular smooth muscle cell proliferation. Biochem Biophys Res Commun. 1994;204:950–8. doi: 10.1006/bbrc.1994.2552. [DOI] [PubMed] [Google Scholar]
- 89.Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 90.Yang XD, Huang B, Li M, Lamb A, Kelleher NL, Chen LF. Negative regulation of NF-kappaB action by Set9-mediated lysine methylation of the RelA subunit. EMBO J. 2009;28:1055–66. doi: 10.1038/emboj.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stevens C, La Thangue NB. E2F and cell cycle control: a double-edged sword. Arch Biochem Biophys. 2003;412:157–69. doi: 10.1016/S0003-9861(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 92.Carr SM, Munro S, Kessler B, Oppermann U, La Thangue NB. Interplay between lysine methylation and Cdk phosphorylation in growth control by the retinoblastoma protein. EMBO J. 2011;30:317–27. doi: 10.1038/emboj.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–61. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kouskouti A, Scheer E, Staub A, Tora L, Talianidis I. Gene-specific modulation of TAF10 function by SET9-mediated methylation. Mol Cell. 2004;14:175–82. doi: 10.1016/S1097-2765(04)00182-0. [DOI] [PubMed] [Google Scholar]
- 95.Couture JF, Collazo E, Hauk G, Trievel RC. Structural basis for the methylation site specificity of SET7/9. Nat Struct Mol Biol. 2006;13:140–6. doi: 10.1038/nsmb1045. [DOI] [PubMed] [Google Scholar]
- 96.Masatsugu T, Yamamoto K. Multiple lysine methylation of PCAF by Set9 methyltransferase. Biochem Biophys Res Commun. 2009;381:22–6. doi: 10.1016/j.bbrc.2009.01.185. [DOI] [PubMed] [Google Scholar]
- 97.Pagans S, Kauder SE, Kaehlcke K, Sakane N, Schroeder S, Dormeyer W, et al. The Cellular lysine methyltransferase Set7/9-KMT7 binds HIV-1 TAR RNA, monomethylates the viral transactivator Tat, and enhances HIV transcription. Cell Host Microbe. 2010;7:234–44. doi: 10.1016/j.chom.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calnan DR, Webb AE, White JL, Stowe TR, Goswami T, Shi X, et al. Methylation by Set9 modulates FoxO3 stability and transcriptional activity. Aging (Albany NY) 2012;4:462–79. doi: 10.18632/aging.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harikrishnan KN, Chow MZ, Baker EK, Pal S, Bassal S, Brasacchio D, et al. Brahma links the SWI/SNF chromatin-remodeling complex with MeCP2-dependent transcriptional silencing. Nat Genet. 2005;37:254–64. doi: 10.1038/ng1516. [DOI] [PubMed] [Google Scholar]
- 100.Meehan R, Lewis J, Cross S, Nan X, Jeppesen P, Bird A. Transcriptional repression by methylation of CpG. J Cell Sci Suppl. 1992;16:9–14. doi: 10.1242/jcs.1992.supplement_16.2. [DOI] [PubMed] [Google Scholar]
- 101.Boyes J, Bird A. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell. 1991;64:1123–34. doi: 10.1016/0092-8674(91)90267-3. [DOI] [PubMed] [Google Scholar]
- 102.Jhamb D, Rao N, Milner DJ, Song F, Cameron JA, Stocum DL, et al. Network based transcription factor analysis of regenerating axolotl limbs. BMC Bioinformatics. 2011;12:80. doi: 10.1186/1471-2105-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]