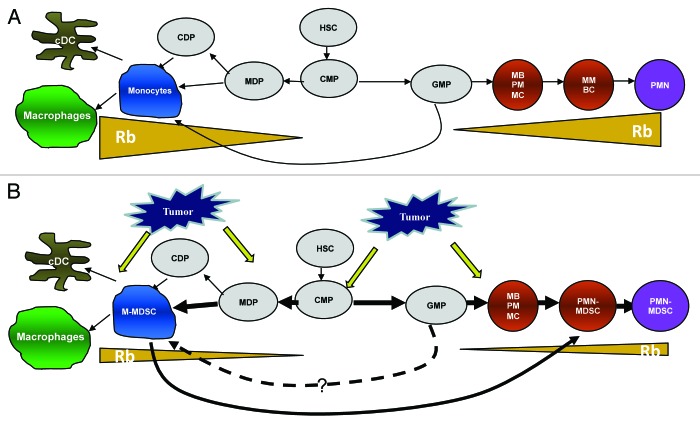
Although many different mechanisms were implicated in tumor-associated immune deficiency, myeloid-derived suppressor cells (MDSC) are now considered as one of the most prominent factors limiting the ability of immune system to control tumor progression. The accumulation of MDSCs has been documented not only in cancer, but also in many other pathological conditions associated with chronic inflammations. MDSCs inhibit the function of T cells and other cells of the immune system through a number of different mechanisms.1 Morphological and functional heterogeneity is the hallmark of MDSCs. These cells are comprised of myeloid progenitor cells and precursors of macrophages, granulocytes and dendritic cells. Recent studies demonstrated that MDSCs consist of two main subsets: polymorphonuclear (PMN-MDSCs) and monocytic MDSCs (M-MDSCs)2. In tumor-free mice, cells with the same phenotype are represented by PMN and inflammatory monocytes, respectively. It was thought that PMN-MDSCs and M-MDSCs are developed alongside separate differentiation pathways (Fig. 1). Our recent study suggested that this view needs to be revisited.3
Figure 1. Schematic of myeloid cells differentiation in cancer. (A) Physiologic conditions; (B) cancer. See explanation in the text. HSC, hematopoietic stem cell; CMP, common myeloid progenitor; MDP, macrophages and dendritic cell progenitors; CDP, common dendritic cell progenitors; GMP, granulocyte-macrophage progenitors; MB, myeloblasts; PM, promyelocytes; MC, myelocytes; MM, metamyelocytes; BC, band cells.
PMN-MDSCs comprise 60~80% of MDSCs in cancer. These cells have a relatively shorter lifespan and lower proliferation ability than M-MDSCs. Even though M-MDSCs are highly proliferative, the expansion of M-MDSCs is barely detectable in cancer. This discrepancy raised the possibility that PMN-MDSCs might be replenished from M-MDSCs. Our data demonstrated that sorted M-MDSCs from tumor-bearing mice were able to acquire polymorphonuclear morphology in the presence of tumor cell-conditioned medium in vitro, or after the adoptive transfer to a tumor-bearing recipient. PMN, generated from M-MDSCs, shared features of PMN-MDSCs, including immune-suppressive activity, a high production of reactive oxygen species and a high myeloperoxidase activity. In contrast, monocytes from tumor-free mice preferentially differentiated into dendritic cells and macrophages. Interestingly, monocytes started to differentiate into PMN cells when tumor explant supernatant was added. These results confirmed previous information that, in non-pathological conditions, monocytes differentiate into dendritic cells or macrophages. However, in a tumor environment, they preferentially differentiated into PMN-MDSCs. The dominant accumulation of PMN-MDSCs and the development of PMN-MDSCs from M-MDSCs were also observed in cancer patients.
This process was controlled by loss of the retinoblastoma protein (Rb1) in MDSCs. The Rb1 role in the cell cycle is well established; whereas, its contribution to myeloid cell differentiation is not clear. Recent study demonstrated that the inactivation of Rb1 resulted in profound myeloproliferation.4 We observed the loss of Rb1 proteins in splenocytes from tumor-bearing mice, which correlated with the expansion of MDSCs. Rb1 expression in PMN-MDSCs was barely detectable, while its high expression was found in M-MDSCs as well as mature myeloid cells from tumor-free mice. We found that bone marrow M-MDSCs and monocytes contained two populations of cells, with high and low presence of Rb1. While monocytes rapidly upregulated Rb1 in culture, in contrast, M-MDSCs failed to do so. PMN-MDSCs derived from the population of Rb1low but not Rb1high M-MDSCs. Monocytes with deleted Rb1 showed a significantly higher propensity to differentiate into PMN as compared with Rb1 wild type monocytes. The overexpression of Rb1 in M-MDSCs decreased their differentiation into PMN-MDSCs and increased their differentiation into dendritic cells and macrophages. Thus, it appears that the loss of Rb1 in M-MDSCs is directly linked with the accumulation of PMN-MDSCs in cancer.
The inhibition of Rb1 expression in MDSCs was mediated by the recruitment of histone deacetylases 2 (HDAC2) to Rb1 promoter in PMN-MDSCs. HDAC inhibitors substantially upregulated the histone acetylation of the Rb1 promoter in PMN-MDSCs. In addition, they successfully blocked the differentiation of M-MDSCs into PMN-MDSCs and redirected the M-MDSCs differentiation into macrophages and dendritic cells.
Several important questions pertinent to this study need to be addressed:
•How is PMN-MDSCs development from granulocyte-macrophage progenitors (GMP) regulated in cancer? Although PMN-MDSCs can be developed from M-MDSCs, they can also be generated from GMP (Fig. 1). The proportion of PMN-MDSCs differentiated from M-MDSCs or GMP is also not clear. Therefore, the investigation of the differentiation of PMN-MDSCs from GMP will broaden the knowledge of MDSC expansion in cancer.
•What’s the role of Rb pocket proteins in MDSCs expansion? The Rb family consists of three genes: Rb1, p107 and p13. Recent study showed that the loss of three Rb family proteins in hematopoietic stem cells resulted in myeloproliferation accompanied by increased apoptosis of lymphoid progenitor cells.5 However, their roles in myeloid cell development in cancer are not known.
•Is downregulation of Rb1, by itself, sufficient to generate immune-suppressive MDSC? If not, what factor can contribute to this process?
•Can the inhibition of HDAC in vivo improve myeloid cell differentiation and immune response in cancer?
In summary, this work provides new insights into the differentiation of MDSCs regulated by Rb1 in cancer. Future work will help to clarify the true biological role of this mechanism in MDSC accumulation in cancer.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24577
References
- 1.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14:211–20. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–95. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viatour P, Somervaille TC, Venkatasubrahmanyam S, Kogan S, McLaughlin ME, Weissman IL, et al. Hematopoietic stem cell quiescence is maintained by compound contributions of the retinoblastoma gene family. Cell Stem Cell. 2008;3:416–28. doi: 10.1016/j.stem.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]