Tumor-associated angiogenesis supplies the growing tumor with the necessary oxygen and nutrients and is as well critical for the progression and metastatic spread of the disease. Therefore, suppressing tumor-associated angiogenesis has often been proposed as a promising antitumor therapy. Vascular endothelial growth factor (VEGF) is one of the most recognized endothelial growth factors involved in several aspects of cancer progression, and it is not surprising that its synergy and interactions with the well-known tumor suppressor gene p53 is of keen interest.
Until recently it was accepted that wild-type p53 indirectly represses VEGF expression by interaction and inhibition of transcription factors such as SP1,1 E2F2. New evidence using primary mouse embryonic fibroblasts (MEFs) indicate that the regulation of the VEGF promoter by p53 is more complex than was originally proposed. In addition to the indirect repressive role of p53 on VEGF promoter activity, it seems that p53 is also required for VEGF induction during the initial phases of hypoxia.3 A highly conserved unconventional but functional p53-binding site adjacent to the main HIF-1α binding site was identified within the VEGF promoter and shown to be essential for this p53-mediated VEGF induction. Saturation of the cells with stabilized p53, e.g., after doxorubicin treatment, seemingly is not enough to induce VEGF promoter activation and likely requires the hypoxia-dependent HIF-1α/p53 complex.3
In all these experiments, full-length wild-type p53 was analyzed. However, we should also take into consideration that the p53 gene can theoretically produce at least 20 alternative isoforms.4 Among these potential variants, the Δ133p53 isoform is of significant interest. This isoform lacks the transactivation domain (TA) and part of the DNA-binding domain and has been suggested to play an oncogenic role.4 It was demonstrated that Δ133p53 is responsible for strong angiogenesis stimulation in glioblastomas and contributes to tumor progression.5 The expression ratio between p53 and Δ133p53 was suggested to regulate angiogenesis in human glioblastoma U87 cells by differentially modulating expression of anti/pro-angiogenic genes. However, Δ133p53 does not seem to affect VEGF expression,5 and the molecular mechanisms behind Δ133p53’s ability to modulate pro/anti-angiogenic genes remains to be elucidated.
In addition, the other two p53 family members, p63 and p73, have similar structures and mechanisms of regulation and are similarly relevant concerning their effects on angiogenesis. In cell lines that contain wild-type p53, p73 seems to increase VEGF expression, exerting a pro-angiogenic phenotype.6 It is not clear how this regulation occurs, since the VEGF promoter is only partially affected, and the half-life of the VEGF mRNA is not influenced. On the other hand, in cells with deleted or mutated p53, p73 acts as a repressor of VEGF transcription. This effect is mediated by repression at the promoter level at a 35 bp region containing SP1 binding sites,7 the same region previously described to be inhibited by p53.1 Although in the case of p73, a molecular mechanism has not been described for its ability to inhibit VEGF expression, it is likely that given its similar structure, p73 also sequesters SP1 away from its positive role on VEGF promoter transcriptional regulation.
The two major isoforms of p63, TAp63γ and dNp63α, have been shown to have opposite effects on the VEGF promoter. Similar to p53, TAp63γ represses VEGF expression by interacting with HIF-1α and mediating its proteasomal degradation. Additionally, TAp63γ binds to SP1 and prevents its association with the VEGF promoter. Conversely, dNp63α seems to have a dominant-negative effect on p53 and TAp63γ and induces VEGF expression by stabilizing HIF-1α and possibly contributing to the recruitment of p300/CBP to promote target gene expression. A 149 bp promoter region containing the HIF-1α binding site seems to be a crucial element for dNp63α to activate VEGF expression.8
It is known that TAp73 and TAp63 interact with p53 response elements and are able to induce some p53 target genes.4 Now that we have shown that there is a functional p53 binding site within the VEGF promoter,3 there is a possibility that both p73 and dNp63α are also able to bind to this region and influence VEGF expression, especially in the case of dNp63α, where not only does it interact with HIF-1α, but the promoter region containing the p53 site is crucial for its activation role (Fig. 1). p21 is also among the target genes of p73 and p63. Given the observation that VEGF repression by p53 is dependent on the p21 pathway,3 the probability that p63 and p73 can also repress VEGF via p21 pathway modulation is also high (Fig. 1).
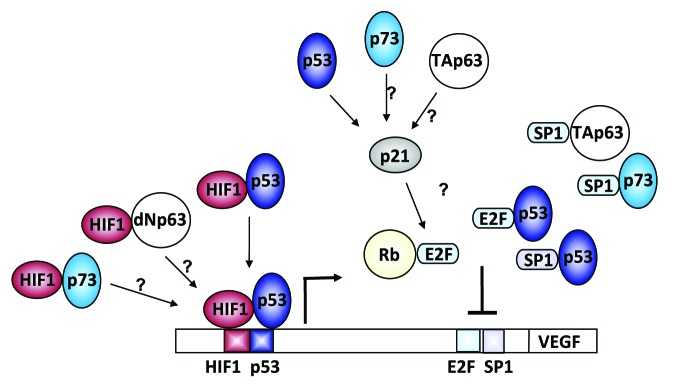
Figure 1. Schematic representation of VEGF promoter regulation by p53 family members and their isoforms. We have shown that p53 can positively regulate VEGF expression during the initial phases of hypoxia by direct binding to conserved sites in the VEGF promoter.3 It remains to be determined if p73 and dNp63 can synergistically act with HIF-1α in positively regulating VEGF expression during hypoxia by directly binding to this conserved site. We have also demonstrated that p53 can indirectly repress VEGF expression during continued hypoxic challenge that is genetically dependent on the presence of p21.3 Here it remains to be determined whether p73 and TAp63’s negative effects on VEGF expression are dependent on a functional p21-E2F pathway.
The dynamic regulation of VEGF by the p53 family members makes its regulation complex, especially given the fact that all three transcription factors are both able to induce and repress VEGF, which appears to be dependent on cellular context and stimulus. Due to their similar structure and targets, it is important to consider if the newly discovered mechanisms for p53 regulation of VEGF3 during hypoxia also apply to the two other family members. Also, the question as to whether they can substitute or compensate for each other and how this will ultimately affect the strategies used to target tumor angiogenesis needs further clarification.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24579
References
- 1.Pal S, Datta K, Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 2001;61:6952–7. [PubMed] [Google Scholar]
- 2.Qin G, Kishore R, Dolan CM, Silver M, Wecker A, Luedemann CN, et al. Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent transcriptional control of VEGF. Proc Natl Acad Sci U S A. 2006;103:11015–20. doi: 10.1073/pnas.0509533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farhang Ghahremani M, Goossens S, Nittner D, Bisteau X, Bartunkova S, Zwolinska A, et al. p53 promotes VEGF expression and angiogenesis in the absence of an intact p21-Rb pathway. Cell Death Differ. 2013 doi: 10.1038/cdd.2013.12. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei J, Zaika E, Zaika A. p53 Family: Role of Protein Isoforms in Human Cancer. J Nucleic Acids. 2012;2012:687359. doi: 10.1155/2012/687359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard H, Garmy-Susini B, Ainaoui N, Van Den Berghe L, Peurichard A, Javerzat S, et al. The p53 isoform, Δ133p53α, stimulates angiogenesis and tumour progression. Oncogene. 2012 doi: 10.1038/onc.2012.242. Epub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 6.Vikhanskaya F, Bani MR, Borsotti P, Ghilardi C, Ceruti R, Ghisleni G, et al. p73 Overexpression increases VEGF and reduces thrombospondin-1 production: implications for tumor angiogenesis. Oncogene. 2001;20:7293–300. doi: 10.1038/sj.onc.1204896. [DOI] [PubMed] [Google Scholar]
- 7.Salimath B, Marmé D, Finkenzeller G. Expression of the vascular endothelial growth factor gene is inhibited by p73. Oncogene. 2000;19:3470–6. doi: 10.1038/sj.onc.1203672. [DOI] [PubMed] [Google Scholar]
- 8.Senoo M, Matsumura Y, Habu S. TAp63gamma (p51A) and dNp63alpha (p73L), two major isoforms of the p63 gene, exert opposite effects on the vascular endothelial growth factor (VEGF) gene expression. Oncogene. 2002;21:2455–65. doi: 10.1038/sj.onc.1205330. [DOI] [PubMed] [Google Scholar]


