Abstract
Meiotic divisions (meiosis I and II) are specialized cell divisions to generate haploid gametes. The first meiotic division with the separation of chromosomes is named reductional division. The second division, which takes place immediately after meiosis I without intervening S-phase, is equational, with the separation of sister chromatids, similar to mitosis. This meiotic segregation pattern requires the two-step removal of the cohesin complex holding sister chromatids together: cohesin is removed from chromosome arms that have been subjected to homologous recombination in meiosis I and from the centromere region in meiosis II. Cohesin in the centromere region is protected from removal in meiosis I, but this protection has to be removed—deprotected”—for sister chromatid segregation in meiosis II. Whereas the mechanisms of cohesin protection are quite well understood, the mechanisms of deprotection have been largely unknown until recently. In this review I summarize our current knowledge on cohesin deprotection.
Keywords: oocytes, meiosis, cyclin A, I2PP2A/Set, PP2A, separase, shugoshin, Rec8, cohesin protection, cohesin deprotection
Introduction
Haploid gametes are derived from diploid germ cells through two rounds of specialized cell divisions, meiosis I and II, without intervening S-phase. Prior to entry into meiosis I, homologous chromosomes (originating from each parent) pair and undergo meiotic recombination, thereby generating new genetic combinations in the offspring. Excellent reviews on meiotic recombination have been published and are beyond the scope of this review (for example, see refs. 1–4). After resolution of recombination products in prophase, entry into meiosis I takes place. During meiosis I, homologous chromosomes, and in meiosis II, sister chromatids are segregated into daughter cells. Whereas meiosis II can be compared with a mitotic division, meiosis I is fundamentally different due to the fact that sister chromatids are segregated to the same pole of the bipolar spindle.3
Errors in chromosome segregation during the meiotic divisions have dire consequences, because they lead to the generation of aneuploid embryos harboring the wrong number of chromosomes. In humans, female meiosis is surprisingly error-prone, and furthermore, the high error rate drastically increases with age.5-7 To get insights into what might go wrong so frequently in human oocytes, both age-related and independent of age, we need to understand the basic mechanisms and their limits in the control of chromosome and sister chromatid segregation. In this review, I will focus mainly on recent work dealing with sister chromatid segregation in meiosis II.
Meiotic Divisions
Prior to meiosis I, meiotic recombination takes place between chromosomes of maternal and paternal origin. Recombination events between non-homologous sister chromatids that have been resolved by double-Holliday junctions become visible as chiasmata upon entry into the first meiotic division, and they constitute essential structures holding the condensed chromosomes (bivalents) together.3,8 Each chromosome consists of two sister chromatids that are held together through cohesion formed by cohesin proteins that are entrapping sister chromatids through a ring-like structure, according to the widely accepted “ring model.”9,10 Cohesins are also thought to stabilize chiasmata and thereby maintain chromosomes together.5 Therefore at entry into meiosis I, bivalents are held together through chiasmata, and sister chromatids by cohesion. Crucially, both paired sister chromatids are oriented toward the same pole in a monopolar fashion.11,12 At the metaphase-to-anaphase transition, cohesin along chromosome arms, where recombination has taken place, has to be removed to allow the separation of chromosomes. Importantly, cohesion has to be maintained in the centromere region, where no recombination takes place, to prevent the precocious separation of sister chromatids. Meiosis II takes place immediately after the first meiotic division without intervening S-phase. Here, sister chromatids are oriented toward opposite spindle poles in a bipolar fashion, such as in mitosis. It is during meiosis II, and only then, that centromeric cohesion is removed, and sister chromatids are segregated.3,13
In vertebrate mitosis, cohesin is first removed from chromosome arms by the so-named prophase pathway. This requires the cohesin-associated proteins Wapl and Pds5 and phosphorylation of the cohesin subunit SA2 by Plk1 kinase.10,14-20 Remaining cohesin (mainly in the centromere region) has to be removed by the thiol-protease separase, which cleaves Scc1, the α-kleisin subunit of the cohesin complex.21-23 Separase-dependent cleavage is essential for mitotic cell cycle progression.24,25 In metaphase, when all kinetochores (the attachment sites for microtubules on chromosomes) are correctly attached to the opposite poles of the spindle, a checkpoint, the so-named spindle assembly checkpoint (SAC), is inactivated (for SAC control please refer to existing excellent reviews in refs. 26 and 27). The SAC keeps the anaphase-promoting complex/cyclosome (APC/C), an E3 ubiquitin kinase, inactive toward its substrates Cyclin B and Securin. Once the SAC has been satisified, APC/C-dependent ubiquitination targets Cyclin B1 and Securin for degradation by the 26S proteasome.28 Both proteins inhibit separase,29-32 and therefore separase can only be active and remove cohesins when the SAC has been satisfied, ensuring that chromosomes are correctly attached to the spindle. In mammalian meiosis, no prophase-dependent removal of cohesin was shown to take place, and only separase-dependent cleavage is required for chromosome segregation.33 As in mitosis, separase activation requires inactivation of the SAC, and the degradation of Cyclin B and Securin.34-38 Importantly, the mitotic cohesin subunit Scc1 is substituted with a meiotic form, named Rec8, which is a substrate of separase.33,39,40 Crucially, centromeric Rec8 is protected from separase cleavage in meiosis I, and therefore cohesion is not removed at centromeres, so that sister chromatids remain associated throughout the first division. In meiosis II, separase acquires the capacity to cleave centromeric Rec8 and therefore allows the separation of sister chromatids.3 In other words, Rec8 is deprotected.41 So how is this deprotection of centromeric Rec8 brought about in meiosis II?
Centromeric Cohesin Protection
Most of what we know about the molecular mechanisms of chromosome segregation during the meiotic divisions stems from pioneering work in yeast. Combining genetics, biochemistry and, more recently, live imaging in this simple organism provided a more complete picture of meiotic chromosome segregation than in any other model organism. I will therefore describe our current knowledge concerning cohesin protection in yeast first.
In budding and fission yeast, Rec8 has to be phosphorylated at multiple sites by two kinases, Cdc7-Dbf4 and Casein Kinase 1, for cleavage by separase.41-43 Protection of centromeric cohesin requires Shugoshin (Sgo1)-dependent recruitment of PP2A phosphatase (containing the regulatory subunit Rts1)44,45 to the centromere in meiosis I. In contrast to the unique Sgo1 in S. cerevisiae, S. pombe harbors two Shugoshin proteins (Sgo1 and Sgo2), with Sgo1 being required for centromeric cohesin protection in meiosis.46,47 In both budding and fission yeast, PP2A counteracts phophorylation of Rec8 at the centromere and thereby prevents Rec8’s cleavage by separase at the centromere, but not on chromosome arms.41-44,46-49 Accordingly, phosphomimicking mutants of Rec8 are not protected from cleavage at the centromere, whereas non-phosphorylatable mutants of Rec8 cannot be cleaved by separase.41,43 The requirement of Rec8 phosphorylation by Cdc7-Dbf4 and Casein Kinase 1 for separase-dependent cleavage is meiosis-specific and different from the situation in mitosis, where Scc1 is phosphorylated by Cdc5 (Plk1 in mammalian cells) for efficient cleavage.50,51
So how is this protective mechanism removed in meiosis II? The question is intriguing, given the fact that in budding yeast, Drosophila and mammals the respective Shugoshin proteins52 required for Rec8 protection are still found in the vicinity of centromeres in meiosis II.48,49,53-57 Is de novo phosphorylation of Rec8 indeed required for meiosis II sister chromatid segregation? And if yes, which kinases are responsible for this phosphorylation, and how do they overcome the counteracting effect of PP2A?
In mammalian meiosis the molecular mechanisms underlying cohesin protection have been less well characterized, but seem to be similar to yeast. Also, in mouse oocytes Rec8 has to be cleaved by separase.33,58-60 Sgo2 clearly is required for protection of centromeric cohesin in male and female meiosis, as has been demonstrated by analyzing meiosis in Sgo2 (Sgol2) mutant mice: Sgo2 is essential for correct chromosome segregation in male and female meiosis I, but is not required during the mitotic divisions.61 Knockdown of Sgo2 equally leads to loss of centromeric cohesin protection in oocyte meiosis I and loss of PP2A from centromeres.53 Localization of the catalytic subunit of PP2A to the centromere region in oocytes,53 and of the regulatory PP2A subunit B56 to mitotic centromeres,45 strongly suggests that, as in yeast, PP2A-B56 is required for chromosome segregation in meiosis I. Inhibiting PP2A (but not only PP2A complexes interacting with Sgo2!) with okadaic acid indeed induces precocious sister chromatid segregation in meiosis I.62,63 A caveat of using a general PP2A inhibitor as an experimental tool is the fact that presumably all PP2A complexes present in the cell are inhibited. Given the multitude of roles occupied by different PP2A complexes during cell division,64 these experiments as well as experiments using a dominant-negative form of the PP2A catalytic subunit63 are therefore difficult to interpret. Mapping Rec8 phosphorylation sites or determining whether Rec8 is phosphorylated in mammalian meiosis I and II has not been possible for technical reasons. Therefore, the formal proof for the conservation of the mechanism for centromeric cohesin protection in meiosis is still missing in mammals. Importantly though, it has been shown that all three PP2A subunits (scaffold, catalytic and regulatory B56 subunit) required for PP2A-B56 activity65 are localized to centromeres in oocyte meiosis I, but once again, also in meiosis II.57
Centromeric Cohesin Deprotection
So how is centromeric cohesin deprotection regulated in meiosis II, if PP2A is still localized to centromeres? Two not necessarily mutually exclusive models have been proposed for mammalian meiosis: in the first model, differences in kinetochore attachment between meiosis I and II (monopolar or bipolar, respectively) lead to subtle changes of Sgo2 localization that would pull associated PP2A away from centromeric cohesin and therefore allow Rec8’s phosphorylation to take place.53,55 Indeed, in fission yeast mutants that are defective in monopolar attachment due to a mutation in the SAC protein Bub1, and which attach sister chromatids in a bipolar fashion, centromeric cohesin is not protected, even when Sgo1 is correctly localized to the kinetochore.66 It seems that also in mouse oocytes, a univalent chromosome in meiosis I whose sisters are attached in a bipolar manner can separate the two sister chromatids at the first meiotic division.67-69 On the other hand in budding yeast, monopolin mutants that biorient sister chromatids in meiosis I cannot separate sisters, because centromeric cohesin is still protected in a Sgo1-dependent way.70 Furthermore in S. pombe the absence of chiasmata leads to a bipolar attachment of sister chromatids in meiosis I and, in contrast to the situation observed in Bub1 mutants,66 also to a failure in removing centromeric cohesin and separating sisters.71-73
In mammalian meiosis, subtle changes in the localization of Sgo2 in meiosis I and II are visible on squashes of spermatocytes and whole-mount immunofluorescence of oocytes.53,55 In short, in meiosis I, a colocalization of cohesin with Sgo2 is observed, whereas in meiosis II, Sgo2 is relocalized—apparently in a tension-dependent manner—toward the outside of the centromere, and colocalization with centromeric cohesin is therefore lost. According to the first model, relocalization of Sgo2 takes place in late metaphase II in oocytes that are competent to undergo metaphase II-to-anaphase II transition upon fertilization. It is intriguing that earlier in metaphase, when paired sister chromatids are already aligned at the metaphase plate, centromeric Rec8 and Sgo2 still colocalize, even though kinetochores are already attached in a bipolar manner and oriented toward the opposite poles.53 Maybe in early metaphase I, the tension applied on paired kinetochores by the newly formed metaphase II spindle is not as strong as later in metaphase II, when oocytes are competent to undergo anaphase II? Indeed, displacement of Sgo2 from Rec8 occurred in parallel with an increase in interkinetochore distance between sisters. On the other hand, PP2A has been shown to colocalize with centromeric Rec8 in late metaphase II mouse oocytes, competent to undergo the metaphase-to-anaphase II transition in a recent study.57 Differences in stainings may be due to different staining protocols used and differences in the amount of tension maintained on kinetochores throughout the staining procedure. Adding to the complexity is the fact that recently it has been shown that PP2A-B56 can be localized to the centromeres in a Sgo-independent manner in mitotic cells, insinuating that Sgo2 may not always reflect PP2A’s localization.74
The second model of how centromeric cohesin deprotection is brought about proposes that a PP2A inhibitor at the centromere is counteracting PP2A exactly where Rec8 is localized in metaphase II. Indeed, a known PP2A inhibitor named I2PP2A75,76 (also named Setβ) was recently identified as an interaction partner of Shugoshin proteins,44,57,77 and in a PP2A-associated complex after chemical crosslinking.77 In mouse oocytes, endogenous I2PP2A is colocalizing with PP2A and Rec8 in meiosis II, in agreement with a potential role in inhibiting PP2A exactly where centromeric Rec8 is found in metaphase II.57 Endogenous I2PP2A is also found in the centromere region in meiosis I but does not colocalize with Rec8. Morpholino-mediated knockdown of I2PP2A prevents sister chromatid segregation in meiosis II, even though metaphase-to-anaphase transition takes place,57 and overexpression of I2PP2A in meiosis I induces precocious sister separation.78 Importantly though, the change in I2PP2A localization at meiosis I and meiosis II centromeres does not depend on monopolar vs. bipolar tension applied on sister kinetochores.57
How can we reconcile the two models? Far from opposing each other, we can imagine that two different mechanisms are at work to ensure that no active PP2A is remaining between sister centromeres at the same place as Rec8. We propose that I2PP2A inhibition of PP2A is required to permit efficient phosphorylation of Rec8 in metaphase II, whereas physical removal of Sgo2 just before anaphase II onset is a back-up mechanism that ensures that no phosphatase activity in the vicinity of Rec8 is remaining (Fig. 1).
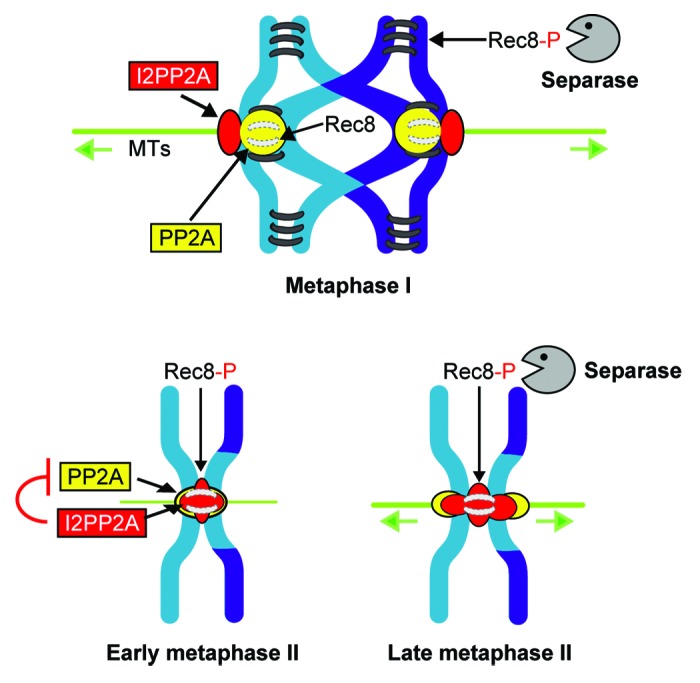
Figure 1. Scheme of how deprotection of centromeric cohesin is brought about through a combination of PP2A inhibition by I2PP2A and bipolar tension applied on sister kinetochores in meiosis II. In metaphase I, I2PP2A (in red) is localized to the centromere region, but does not colocalize with the three PP2A subunits constituting active PP2A (yellow). In metaphase II, I2PP2A is found exactly where centromeric cohesin (gray bars) remains. It is therefore localized exactly where PP2A has to be inhibited to avoid dephosphorylation of Rec8. In late metaphase II, bipolar tension applied on sister kinetochores moves potentially remaining PP2A complexes that are still free of inhibitory I2PP2A further away from Rec8. Cohesins, black bars; cohesins not visible due to PP2A or I2PP2A localization, gray bars; Rec8, unphosphorylated Rec8; Rec8-P, phosphorylated Rec8 that can be cleaved by separase; MTs, microtubules.
I2PP2A has also been identified as Set/TAF-Iβ, a component of the INHAT (inhibitor of acetyltransferases) complex, which masks histones from being acetyltransferase substrates.79 Therefore, it was also possible that I2PP2A’s role in meiosis was independent of its inhibitory activity on PP2A and, alternatively, due to its role as a component of the INHAT complex. In mitosis, sister chromatid cohesion is lost in a separase-independent manner upon knockdown of HDAC3 (histone deacetylase 3), which deacetylates centromeric histone H3K4.80 Knockdown of HDAC3 therefore induces precocious sister separation,80 and more generally, histone hyperacetylation was shown to interfere with chromosome segregation in mammalian oocyte meiosis I.81-83 If I2PP2A had a role as a component of the INHAT complex in meiosis II by preventing acetylation of histone H3K4, I2PP2A knockdown in oocytes were expected to relieve inhibition of histone acetylation and thereby induce loss of sister chromatid cohesion, such as observed upon knockdown of HDAC3. Importantly, this is not what has been observed: morpholino-oligo-mediated knockdown of I2PP2A prevents sister separation in meiosis II,57 strongly suggesting that I2PP2A functions as an inhibitor of PP2A and not as a component of the INHAT complex on meiotic centromeres.
Cyclin A2 is Required for Sister Chromatid Segregation in Meiosis II
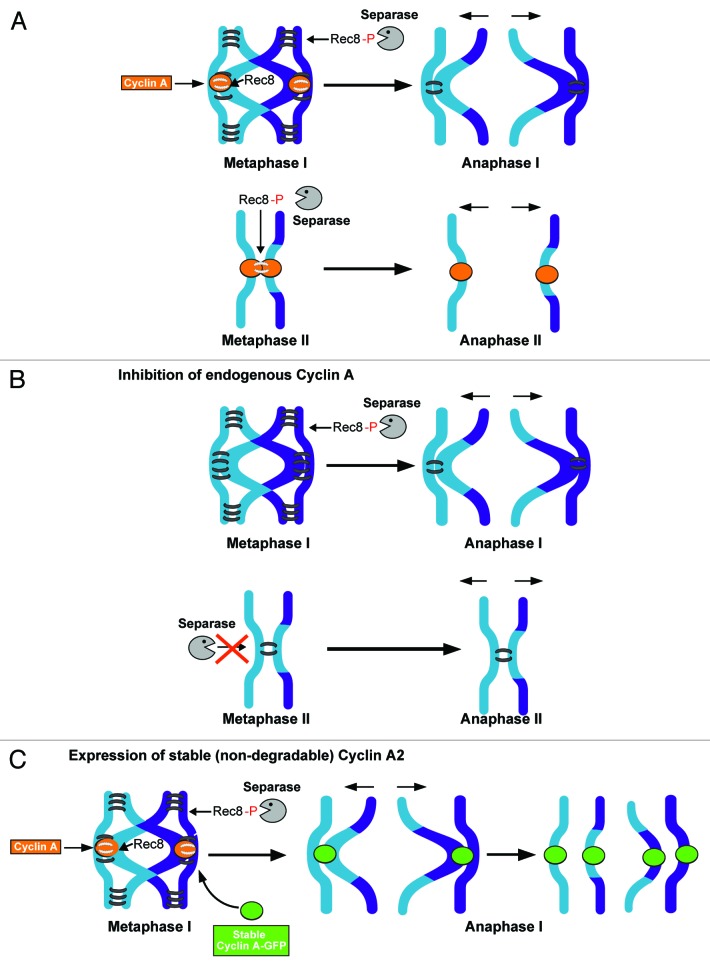
It is fascinating that in the same cytoplasm, two completely different segregation patterns take place in meiosis I and II. In budding yeast, the meiotic program with monopolar attachment, suppression of S-phase between meiosis I and II and chromosome segregation before sister chromatid segregation depends on the suppression of mitotic cell cycle regulators in meiotic prophase.84 Progression through mitosis and meiosis is regulated through rise and fall of MPF (M-phase promoting factor) activity and counterbalance by phosphatase activities.64 How do mitotic kinases support the correct pattern of chromosome and sister chromatid segregation during the meiotic divisions? Inappropriate expression during oocyte prophase arrest of Cyclin A2 in Drosophila85 and of Cyclin E in C. elegans86 perturbs the meiotic cell cycle and leads to the separation of sister chromatids. In S. cerevisiae, untimely expression of the B-type cyclin Clb3 in prophase I causes failures in monopolar attachment and therefore sister chromatid segregation.87,88 In mouse oocytes, constitutive presence of Cyclin A2-associated kinase activity leads to the loss of centromeric cohesion protection in meiosis I and sister separation, but in contrary to budding yeast with correct monopolar attachments of sisters.89 This mouse oocyte-specific phenotype of stable Cyclin A2-expressing oocytes allowed the discovery of an essential role that Cyclin A2 plays in mammalian female meiosis II, namely its requirement for the separation of sister chromatids, which cannot come appart without functional Cyclin A2. Cohesin remains protected in metaphase II when Cyclin A2 is inhibited, and constitutive presence of Cyclin A2-associated kinase activity leads to spontaneous separation of sister chromatids in a separase-dependent manner in meiosis I.89 This meiosis-specific role of Cyclin A2 is intriguing and appears specific to mammalian oocytes.
In accordance with a role in the deprotection of centromeric cohesin in meiosis II, endogenous Cyclin A2 is localized to centromeres throughout the metaphase-to-anaphase transition of meiosis II. To make matters more complicated, endogenous Cyclin A2 is also found at centromeres in meiosis I but, importantly, not during the metaphase-to-anaphase I transition89 (Fig. 2A). How is Cyclin A at centromeres protected from APC/C dependent degradation beyond prometaphase, and what are Cyclin A2’s substrates? At the metaphase-to-anaphase transition, the APC/C is active to ubiquitinate and induce degradation of the APC/C substrates Cyclin B1 and Securin upon spindle assembly checkpoint satisfaction, and even before in prometaphase to target Cyclin A for degradation in mitotic cells, so there has to be some protective mechanism prohibiting the APC/C from targeting Cyclin A2 at centromeres in meiosis.28 Concerning Cyclin A2’s substrates at the centromere, we can only speculate as to their identity: Cyclin A2 may directly or indirectly mediate phosphorylation of centromeric Rec8, and thereby render Rec8 cleavable for separase. Cyclin A2 may phosphorylate I2PP2A so that it can interact with PP2A (it has been shown previously in a different context that phosphorylation of I2PP2A is required for its interaction with the catalytic subunit of PP2A).90 PP2A itself may be a target of Cyclin A2. A recent study has identified Ppp2r1a (which corresponds to a B56 regulatory subunit of PP2A) as an interaction partner of Cyclin A2 in G2 cells by immunoprecipitation and mass spectrometry.91 It is therefore possible that Cyclin A2 influences PP2A’s phosphatase activity (either directly, or indirectly) at the centromere in meiosis II. Sgo2 and PP2A localization were not affected in stable Cyclin A2-expressing oocytes that were maintained in a metaphase I arrest, excluding that Cyclin A2 removes PP2A from the centromere.89 Moreover, Cyclin A2 inhibition cannot prevent sister separation induced by PP2A inhibition by okadaic acid. With the reserve that inhibition of total PP2A activity has other pleiotropic effects, this result may indicate that Cyclin A2 functions upstream of PP2A and requires PP2A to induce sister separation.89
Figure 2. Cyclin A2 is required for sister chromatid segregation in meiosis II in mouse oocytes. (A) Endogenous Cyclin A (orange) is localized in the centromere region in metaphase I and II. In meiosis II, Cyclin A remains associated with centromeres throughout the metaphase-to-anaphase transition, whereas in meiosis I Cyclin A is lost from centromeres at anaphase onset. (B) Inhibition of endogenous Cyclin A-associated kinase activity does not interfere with chromosome segregation in meiosis I, but prevents sister chromatid segregation in meiosis II, even though metaphase-to-anaphase transition takes place. (C) Expression of stable Cyclin A2 in meiosis I induces sister chromatid segregation in oocytes that remain blocked in an anaphase I-like state due to high Cdk1 kinase activity. Stable Cyclin A2 (in green) is localized to centromeres at the metaphase-to-anaphase transition in meiosis I. Cohesins, black bars; cohesins not visible due to Cyclin A colocalization, gray bars; Rec8, unphosphorylated Rec8; Rec8-P, phosphorylated Rec8 that can be cleaved by separase.
So how does Cyclin A2 induce sister chromatid segregation only in meiosis II, if it is localized to centromeres in meiosis I as well? As mentioned above, Cyclin A2-associated kinase activity is required for its function in meiosis II. One obvious question concerns the identity of the Cdk that Cyclin A2 is associated with. It has been shown that only Cdk1 and not Cdk2 is required for meiosis in mouse oocytes92 and therefore Cyclin A2 is expected to be associated with Cdk1 for sister chromatid separation in meiosis II. Now it will be important to address whether Cyclin A2 is found in association with Cdk1 only on metaphase II and not metaphase I centromeres, and therefore capable of phosphorylating its targets only in metaphase II. Alternatively, association of Cdk1 with Cyclin A2 may not be stable at metaphase I centromeres due to missing T-loop phosphorylation.93 We can also imagine that removal of Cyclin A2 shortly before anaphase onset in meiosis I is sufficient for maintaining protection of centromeric Cohesin, whereas in meiosis II, the presence of Cyclin A2 at centromeres throughout the metaphase-to-anaphase transition induces removal of centromeric Cohesin. Accordingly, inhihibition of Cyclin A affects only the second meiotic division and has no effect on meiosis I (Fig. 2B).89 We can speculate that exogenously expressed stable Cyclin A2 associates with Cdk1 before being recruited to the meiosis I centromere. This and stable Cyclin A’s centromere localization throughout the metaphase-to-anaphase transition in meiosis I are probably required to induce precocious sister separation in meiosis I (Fig. 2C).89 Likely, a combination of Cyclin A’s localization and local regulation of associated kinase activity is responsible for inducing loss of Cohesin protection only in meiosis II.
Generating Oocytes of the Correct Ploidy
It is estimated that a staggering 20–40% of all human oocytes are aneuploid.6,94 Errors in chromosome segregation during the meiotic divisions lead to either spontaneous abortions or the development of trisomies, with trisomy 21 being the most frequent.6,94 Furthermore, the incidence of missegregations in oocytes augments with the age of the mother, which has led to an increase of clinically recognized trisomy 21 pregnancies by more than 70% in the UK in the past 20 y, due to the fact that today women get their first child on average later in life than 20 y ago.95 The reasons for this high error rate are still unknown and are probably due to multiple factors, such as weakening of the SAC, failures to maintain a functional cohesin complex throughout the several-decades-long prophase arrest and environmental factors.5,6 Excellent reviews have been published on this topic,5-7 and I will therefore only briefly outline how loss of cohesins with age is thought to affect oocyte ploidy.
Cohesion holding sister chromatids together is generated in the immature oocytes of the female embryo, and has to be maintained until entry into the first meiotic division upon hormonal stimulation and fertilization in the adult. Prior to entry into meiosis I, oocytes undergo a lengthy growth phase that takes up to nearly 3 mo in human oocytes. In the mouse, it has been shown that no Rec8 cohesin turnover takes place during the growing phase of the oocyte.60 Cohesin complexes are therefore extremely stable and long-lived, but probably for this reason they also constitute the weak point of mammalian female meiosis. Indeed, diminished levels of cohesin proteins have been described in oocytes of aged mice.68,96,97 Not only are cohesins required for keeping sister chromatids together, but also for the maintenance of chiasmata.33 Therefore, less cohesin is expected to have severe consequences, leading both to failures to maintain sisters and homologous chromosomes together. Indeed, this loss of cohesin leads to the destabilization of chiasmata and, therefore, the presence of unpaired chromosomes in meiosis I5. Diminished levels of cohesin proteins in the centromere region may also be responsible for the age-dependent loss of Sgo2 that has been observed in mouse oocytes.96 We can hypothesize that this loss of Sgo2 further increases precocious sister chromatid separation in oocytes from older mice.
So named “cohesin fatigue” has been shown to affect mitotic cells kept in metaphase for prolonged periods of time.98 The force applied by the bipolar spindle leads to a gradual loss and rupture of the cohesive forces holding sister chromatids together, and thereby contributes to the generation of aneuploid daughter cells. Mammalian oocytes are arrested in metaphase II (cytostatic factor or CSF-arrest) for a prolonged period of time to await fertilization.99,100 It is only upon fertilization that metaphase-to-anaphase transition and exit from meiosis II takes place. During this CSF-arrest paired sister chromatids are attached to the bipolar spindle and under tension. In combination with the age-dependent loss of cohesin in oocytes, cohesin fatigue may additional make matters worse and contribute to the high aneuploidy rate of human oocytes.
Concluding Remarks
Protection of centromeric cohesin has been studied in a wide variety of model organisms, and it seems that the protective mechanisms are conserved from yeast to man. But as important as it is to understand how protection takes place, it is as important to know the mechanisms of deprotection. Not being able to get rid of centromeric cohesin protection in meiosis II will have the same fatal consequences as not being able to protect centromeric cohesin in the first place, with the generation of aneuploid gametes that will give rise to embryos with the wrong number of chromosomes. It is striking that proteins involved in protection and deprotection (Sgo2, PP2A, I2PP2A, Cyclin A) are localized to the centromere region in both meiosis I and II, with only subtle changes. Therefore it is reasonable to expect that posttranslational modifications further regulate activities and specificities of these proteins to confer protection in meiosis I and deprotection in meiosis II. I believe that future work will help us better understand chromosome segregation in meiosis by identifying the signaling pathways involved in regulating these proteins during meiotic progression. This will also help us understand why oocytes missegregate their chromosomes so frequently.
Acknowledgments
I thank C. Carron, V. Galy and S. Touati (all UMR7622, Paris) for comments on the manuscript, and S. Gournet (UMR7622, Paris) for help with figures. Work in my group is supported by the CNRS, UPMC and an ANR grant (ANR-12-BSV2-0005-01).
Glossary
- Abreviations: APC/C
anaphase promoting complex/cyclosome
- Bub1
Budding uninhibited by Benomyl 1
- Cdk
cyclin-dependent kinase
- CSF
cytostatic factor
- I2PP2A
inhibitor 2 of PP2A
- MPF
M-phase promoting factor
- PP2A
protein phosphatase 2A
- SAC
spindle assembly checkpoint
- Sgo
shugoshin/MEI-S332
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24600
References
- 1.Lichten M, de Massy B. The impressionistic landscape of meiotic recombination. Cell. 2011;147:267–70. doi: 10.1016/j.cell.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roig I, Keeney S. Probing meiotic recombination decisions. Dev Cell. 2008;15:331–2. doi: 10.1016/j.devcel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Petronczki M, Siomos MF, Nasmyth K. Un ménage à quatre: the molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–40. doi: 10.1016/S0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 4.Zickler D, Kleckner N. Meiotic chromosomes: integrating structure and function. Annu Rev Genet. 1999;33:603–754. doi: 10.1146/annurev.genet.33.1.603. [DOI] [PubMed] [Google Scholar]
- 5.Jessberger R. Age-related aneuploidy through cohesion exhaustion. EMBO Rep. 2012;13:539–46. doi: 10.1038/embor.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21:703–8. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page SL, Hawley RS. Chromosome choreography: the meiotic ballet. Science. 2003;301:785–9. doi: 10.1126/science.1086605. [DOI] [PubMed] [Google Scholar]
- 9.Losada A. Cohesin regulation: fashionable ways to wear a ring. Chromosoma. 2007;116:321–9. doi: 10.1007/s00412-007-0104-x. [DOI] [PubMed] [Google Scholar]
- 10.Mehta GD, Rizvi SM, Ghosh SK. Cohesin: a guardian of genome integrity. Biochim Biophys Acta. 2012;1823:1324–42. doi: 10.1016/j.bbamcr.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Hauf S, Watanabe Y. Kinetochore orientation in mitosis and meiosis. Cell. 2004;119:317–27. doi: 10.1016/j.cell.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Brar GA, Amon A. Emerging roles for centromeres in meiosis I chromosome segregation. Nat Rev Genet. 2008;9:899–910. doi: 10.1038/nrg2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marston AL, Amon A. Meiosis: cell-cycle controls shuffle and deal. Nat Rev Mol Cell Biol. 2004;5:983–97. doi: 10.1038/nrm1526. [DOI] [PubMed] [Google Scholar]
- 14.Shintomi K, Hirano T. Sister chromatid resolution: a cohesin releasing network and beyond. Chromosoma. 2010;119:459–67. doi: 10.1007/s00412-010-0271-z. [DOI] [PubMed] [Google Scholar]
- 15.Hauf S, Roitinger E, Koch B, Dittrich CM, Mechtler K, Peters JM. Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol. 2005;3:e69. doi: 10.1371/journal.pbio.0030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, Nigg EA, et al. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell. 2002;9:515–25. doi: 10.1016/S1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- 17.Shintomi K, Hirano T. Releasing cohesin from chromosome arms in early mitosis: opposing actions of Wapl-Pds5 and Sgo1. Genes Dev. 2009;23:2224–36. doi: 10.1101/gad.1844309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giménez-Abián JF, Sumara I, Hirota T, Hauf S, Gerlich D, de la Torre C, et al. Regulation of sister chromatid cohesion between chromosome arms. Curr Biol. 2004;14:1187–93. doi: 10.1016/j.cub.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi R, Gillespie PJ, Hirano T. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr Biol. 2006;16:2406–17. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, et al. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127:955–67. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima M, Kumada K, Hatakeyama K, Noda T, Peters JM, Hirota T. The complete removal of cohesin from chromosome arms depends on separase. J Cell Sci. 2007;120:4188–96. doi: 10.1242/jcs.011528. [DOI] [PubMed] [Google Scholar]
- 22.Waizenegger IC, Hauf S, Meinke A, Peters JM. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 2000;103:399–410. doi: 10.1016/S0092-8674(00)00132-X. [DOI] [PubMed] [Google Scholar]
- 23.Hauf S, Waizenegger IC, Peters JM. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science. 2001;293:1320–3. doi: 10.1126/science.1061376. [DOI] [PubMed] [Google Scholar]
- 24.Wirth KG, Wutz G, Kudo NR, Desdouets C, Zetterberg A, Taghybeeglu S, et al. Separase: a universal trigger for sister chromatid disjunction but not chromosome cycle progression. J Cell Biol. 2006;172:847–60. doi: 10.1083/jcb.200506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumada K, Yao R, Kawaguchi T, Karasawa M, Hoshikawa Y, Ichikawa K, et al. The selective continued linkage of centromeres from mitosis to interphase in the absence of mammalian separase. J Cell Biol. 2006;172:835–46. doi: 10.1083/jcb.200511126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol. 2012;22:R966–80. doi: 10.1016/j.cub.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–93. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 28.Acquaviva C, Pines J. The anaphase-promoting complex/cyclosome: APC/C. J Cell Sci. 2006;119:2401–4. doi: 10.1242/jcs.02937. [DOI] [PubMed] [Google Scholar]
- 29.Gorr IH, Boos D, Stemmann O. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol Cell. 2005;19:135–41. doi: 10.1016/j.molcel.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Holland AJ, Taylor SS. Cyclin-B1-mediated inhibition of excess separase is required for timely chromosome disjunction. J Cell Sci. 2006;119:3325–36. doi: 10.1242/jcs.03083. [DOI] [PubMed] [Google Scholar]
- 31.Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–22. doi: 10.1126/science.285.5426.418. [see comments] [DOI] [PubMed] [Google Scholar]
- 32.Hagting A, Den Elzen N, Vodermaier HC, Waizenegger IC, Peters JM, Pines J. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J Cell Biol. 2002;157:1125–37. doi: 10.1083/jcb.200111001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudo NR, Wassmann K, Anger M, Schuh M, Wirth KG, Xu H, et al. Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell. 2006;126:135–46. doi: 10.1016/j.cell.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 34.Wassmann K, Niault T, Maro B, Metaphase I. Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Curr Biol. 2003;13:1596–608. doi: 10.1016/j.cub.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 35.Herbert M, Levasseur M, Homer H, Yallop K, Murdoch A, McDougall A. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nat Cell Biol. 2003;5:1023–5. doi: 10.1038/ncb1062. [DOI] [PubMed] [Google Scholar]
- 36.Homer HA, McDougall A, Levasseur M, Murdoch AP, Herbert M. Mad2 is required for inhibiting securin and cyclin B degradation following spindle depolymerisation in meiosis I mouse oocytes. Reproduction. 2005;130:829–43. doi: 10.1530/rep.1.00856. [DOI] [PubMed] [Google Scholar]
- 37.Homer HA, McDougall A, Levasseur M, Yallop K, Murdoch AP, Herbert M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 2005;19:202–7. doi: 10.1101/gad.328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGuinness BE, Anger M, Kouznetsova A, Gil-Bernabé AM, Helmhart W, Kudo NR, et al. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr Biol. 2009;19:369–80. doi: 10.1016/j.cub.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 39.Parisi S, McKay MJ, Molnar M, Thompson MA, van der Spek PJ, van Drunen-Schoenmaker E, et al. Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family conserved from fission yeast to humans. Mol Cell Biol. 1999;19:3515–28. doi: 10.1128/mcb.19.5.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell. 2005;8:949–61. doi: 10.1016/j.devcel.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Katis VL, Lipp JJ, Imre R, Bogdanova A, Okaz E, Habermann B, et al. Rec8 phosphorylation by casein kinase 1 and Cdc7-Dbf4 kinase regulates cohesin cleavage by separase during meiosis. Dev Cell. 2010;18:397–409. doi: 10.1016/j.devcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rumpf C, Cipak L, Dudas A, Benko Z, Pozgajova M, Riedel CG, et al. Casein kinase 1 is required for efficient removal of Rec8 during meiosis I. Cell Cycle. 2010;9:2657–62. doi: 10.4161/cc.9.13.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishiguro T, Tanaka K, Sakuno T, Watanabe Y. Shugoshin-PP2A counteracts casein-kinase-1-dependent cleavage of Rec8 by separase. Nat Cell Biol. 2010;12:500–6. doi: 10.1038/ncb2052. [DOI] [PubMed] [Google Scholar]
- 44.Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, et al. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441:46–52. doi: 10.1038/nature04663. [DOI] [PubMed] [Google Scholar]
- 45.Riedel CG, Katis VL, Katou Y, Mori S, Itoh T, Helmhart W, et al. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature. 2006;441:53–61. doi: 10.1038/nature04664. [DOI] [PubMed] [Google Scholar]
- 46.Kitajima TS, Kawashima SA, Watanabe Y. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature. 2004;427:510–7. doi: 10.1038/nature02312. [DOI] [PubMed] [Google Scholar]
- 47.Rabitsch KP, Gregan J, Schleiffer A, Javerzat JP, Eisenhaber F, Nasmyth K. Two fission yeast homologs of Drosophila Mei-S332 are required for chromosome segregation during meiosis I and II. Curr Biol. 2004;14:287–301. doi: 10.1016/j.cub.2004.01.051. [DOI] [PubMed] [Google Scholar]
- 48.Katis VL, Galova M, Rabitsch KP, Gregan J, Nasmyth K. Maintenance of cohesin at centromeres after meiosis I in budding yeast requires a kinetochore-associated protein related to MEI-S332. Curr Biol. 2004;14:560–72. doi: 10.1016/j.cub.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Marston AL, Tham WH, Shah H, Amon A. A genome-wide screen identifies genes required for centromeric cohesion. Science. 2004;303:1367–70. doi: 10.1126/science.1094220. [DOI] [PubMed] [Google Scholar]
- 50.Alexandru G, Uhlmann F, Mechtler K, Poupart MA, Nasmyth K. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell. 2001;105:459–72. doi: 10.1016/S0092-8674(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 51.Hornig NC, Uhlmann F. Preferential cleavage of chromatin-bound cohesin after targeted phosphorylation by Polo-like kinase. EMBO J. 2004;23:3144–53. doi: 10.1038/sj.emboj.7600303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao Y, Dai W. Shugoshins function as a guardian for chromosomal stability in nuclear division. Cell Cycle. 2012;11:2631–42. doi: 10.4161/cc.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, et al. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol. 2008;10:42–52. doi: 10.1038/ncb1667. [DOI] [PubMed] [Google Scholar]
- 54.Kerrebrock AW, Moore DP, Wu JS, Orr-Weaver TL. Mei-S332, a Drosophila protein required for sister-chromatid cohesion, can localize to meiotic centromere regions. Cell. 1995;83:247–56. doi: 10.1016/0092-8674(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 55.Gómez R, Valdeolmillos A, Parra MT, Viera A, Carreiro C, Roncal F, et al. Mammalian SGO2 appears at the inner centromere domain and redistributes depending on tension across centromeres during meiosis II and mitosis. EMBO Rep. 2007;8:173–80. doi: 10.1038/sj.embor.7400877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gregan J, Rumpf C, Li Z, Cipak L. What makes centromeric cohesion resistant to separase cleavage during meiosis I but not during meiosis II? Cell Cycle. 2008;7:151–3. doi: 10.4161/cc.7.2.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chambon JP, Touati SA, Berneau S, Cladière D, Hebras C, Groeme R, et al. The PP2A Inhibitor I2PP2A Is Essential for Sister Chromatid Segregation in Oocyte Meiosis II. Curr Biol. 2013;23:485–90. doi: 10.1016/j.cub.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Lee J, Iwai T, Yokota T, Yamashita M. Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J Cell Sci. 2003;116:2781–90. doi: 10.1242/jcs.00495. [DOI] [PubMed] [Google Scholar]
- 59.Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C. Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1beta and SMC3. J Cell Biol. 2003;160:657–70. doi: 10.1083/jcb.200212080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, et al. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 2010;24:2505–16. doi: 10.1101/gad.605910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Llano E, Gómez R, Gutiérrez-Caballero C, Herrán Y, Sánchez-Martín M, Vázquez-Quiñones L, et al. Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev. 2008;22:2400–13. doi: 10.1101/gad.475308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mailhes JB, Hilliard C, Fuseler JW, London SN. Okadaic acid, an inhibitor of protein phosphatase 1 and 2A, induces premature separation of sister chromatids during meiosis I and aneuploidy in mouse oocytes in vitro. Chromosome Res. 2003;11:619–31. doi: 10.1023/A:1024909119593. [DOI] [PubMed] [Google Scholar]
- 63.Chang HY, Jennings PC, Stewart J, Verrills NM, Jones KT. Essential role of protein phosphatase 2A in metaphase II arrest and activation of mouse eggs shown by okadaic acid, dominant negative protein phosphatase 2A, and FTY720. J Biol Chem. 2011;286:14705–12. doi: 10.1074/jbc.M110.193227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Domingo-Sananes MR, Kapuy O, Hunt T, Novak B. Switches and latches: a biochemical tug-of-war between the kinases and phosphatases that control mitosis. Philos Trans R Soc Lond B Biol Sci. 2011;366:3584–94. doi: 10.1098/rstb.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barr FA, Elliott PR, Gruneberg U. Protein phosphatases and the regulation of mitosis. J Cell Sci. 2011;124:2323–34. doi: 10.1242/jcs.087106. [DOI] [PubMed] [Google Scholar]
- 66.Vaur S, Cubizolles F, Plane G, Genier S, Rabitsch PK, Gregan J, et al. Control of Shugoshin function during fission-yeast meiosis. Curr Biol. 2005;15:2263–70. doi: 10.1016/j.cub.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 67.Kouznetsova A, Lister L, Nordenskjöld M, Herbert M, Höög C. Bi-orientation of achiasmatic chromosomes in meiosis I oocytes contributes to aneuploidy in mice. Nat Genet. 2007;39:966–8. doi: 10.1038/ng2065. [DOI] [PubMed] [Google Scholar]
- 68.Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20:1522–8. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LeMaire-Adkins R, Hunt PA. Nonrandom segregation of the mouse univalent X chromosome: evidence of spindle-mediated meiotic drive. Genetics. 2000;156:775–83. doi: 10.1093/genetics/156.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petronczki M, Matos J, Mori S, Gregan J, Bogdanova A, Schwickart M, et al. Monopolar attachment of sister kinetochores at meiosis I requires casein kinase 1. Cell. 2006;126:1049–64. doi: 10.1016/j.cell.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 71.Dudas A, Ahmad S, Gregan J. Sgo1 is required for co-segregation of sister chromatids during achiasmate meiosis I. Cell Cycle. 2011;10:951–5. doi: 10.4161/cc.10.6.15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirose Y, Suzuki R, Ohba T, Hinohara Y, Matsuhara H, Yoshida M, et al. Chiasmata promote monopolar attachment of sister chromatids and their co-segregation toward the proper pole during meiosis I. PLoS Genet. 2011;7:e1001329. doi: 10.1371/journal.pgen.1001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakuno T, Tanaka K, Hauf S, Watanabe Y. Repositioning of aurora B promoted by chiasmata ensures sister chromatid mono-orientation in meiosis I. Dev Cell. 2011;21:534–45. doi: 10.1016/j.devcel.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 74.Ricke RM, Jeganathan KB, Malureanu L, Harrison AM, van Deursen JM. Bub1 kinase activity drives error correction and mitotic checkpoint control but not tumor suppression. J Cell Biol. 2012;199:931–49. doi: 10.1083/jcb.201205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M, Guo H, Damuni Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry. 1995;34:1988–96. doi: 10.1021/bi00006a020. [DOI] [PubMed] [Google Scholar]
- 76.Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–62. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- 77.Herzog F, Kahraman A, Boehringer D, Mak R, Bracher A, Walzthoeni T, et al. Structural probing of a protein phosphatase 2A network by chemical cross-linking and mass spectrometry. Science. 2012;337:1348–52. doi: 10.1126/science.1221483. [DOI] [PubMed] [Google Scholar]
- 78.Qi ST, Wang ZB, Ouyang YC, Zhang QH, Hu MW, Huang X, et al. Overexpression of SETβ, a protein localizing to centromeres, causes precocious separation of chromatids during the first meiosis of mouse oocyte. J Cell Sci. 2013 doi: 10.1242/jcs.116541. [DOI] [PubMed] [Google Scholar]
- 79.Seo SB, McNamara P, Heo S, Turner A, Lane WS, Chakravarti D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104:119–30. doi: 10.1016/S0092-8674(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 80.Eot-Houllier G, Fulcrand G, Watanabe Y, Magnaghi-Jaulin L, Jaulin C. Histone deacetylase 3 is required for centromeric H3K4 deacetylation and sister chromatid cohesion. Genes Dev. 2008;22:2639–44. doi: 10.1101/gad.484108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang F, Baumann C, Viveiros MM, De La Fuente R. Histone hyperacetylation during meiosis interferes with large-scale chromatin remodeling, axial chromatid condensation and sister chromatid separation in the mammalian oocyte. Int J Dev Biol. 2012;56:889–99. doi: 10.1387/ijdb.120246rd. [DOI] [PubMed] [Google Scholar]
- 82.van den Berg IM, Eleveld C, van der Hoeven M, Birnie E, Steegers EA, Galjaard RJ, et al. Defective deacetylation of histone 4 K12 in human oocytes is associated with advanced maternal age and chromosome misalignment. Hum Reprod. 2011;26:1181–90. doi: 10.1093/humrep/der030. [DOI] [PubMed] [Google Scholar]
- 83.Akiyama T, Nagata M, Aoki F. Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. Proc Natl Acad Sci USA. 2006;103:7339–44. doi: 10.1073/pnas.0510946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okaz E, Argüello-Miranda O, Bogdanova A, Vinod PK, Lipp JJ, Markova Z, et al. Meiotic prophase requires proteolysis of M phase regulators mediated by the meiosis-specific APC/CAma1. Cell. 2012;151:603–18. doi: 10.1016/j.cell.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 85.Sugimura I, Lilly MA. Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev Cell. 2006;10:127–35. doi: 10.1016/j.devcel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 86.Biedermann B, Wright J, Senften M, Kalchhauser I, Sarathy G, Lee MH, et al. Translational repression of cyclin E prevents precocious mitosis and embryonic gene activation during C. elegans meiosis. Dev Cell. 2009;17:355–64. doi: 10.1016/j.devcel.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Miller MP, Unal E, Brar GA, Amon A. Meiosis I chromosome segregation is established through regulation of microtubule-kinetochore interactions. Elife. 2012;1:e00117. doi: 10.7554/eLife.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carlile TM, Amon A. Meiosis I is established through division-specific translational control of a cyclin. Cell. 2008;133:280–91. doi: 10.1016/j.cell.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Touati SA, Cladière D, Lister LM, Leontiou I, Chambon JP, Rattani A, et al. Cyclin A2 is required for sister chromatid segregation, but not separase control, in mouse oocyte meiosis. Cell Rep. 2012;2:1077–87. doi: 10.1016/j.celrep.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Vasudevan NT, Mohan ML, Gupta MK, Hussain AK, Naga Prasad SV. Inhibition of protein phosphatase 2A activity by PI3Kγ regulates β-adrenergic receptor function. Mol Cell. 2011;41:636–48. doi: 10.1016/j.molcel.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pagliuca FW, Collins MO, Lichawska A, Zegerman P, Choudhary JS, Pines J. Quantitative proteomics reveals the basis for the biochemical specificity of the cell-cycle machinery. Mol Cell. 2011;43:406–17. doi: 10.1016/j.molcel.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adhikari D, Zheng W, Shen Y, Gorre N, Ning Y, Halet G, et al. Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Hum Mol Genet. 2012;21:2476–84. doi: 10.1093/hmg/dds061. [DOI] [PubMed] [Google Scholar]
- 93.Merrick KA, Larochelle S, Zhang C, Allen JJ, Shokat KM, Fisher RP. Distinct activation pathways confer cyclin-binding specificity on Cdk1 and Cdk2 in human cells. Mol Cell. 2008;32:662–72. doi: 10.1016/j.molcel.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 95.De Souza E, Alberman E, Morris JK. Down syndrome and paternal age, a new analysis of case-control data collected in the 1960s. Am J Med Genet A. 2009;149A:1205–8. doi: 10.1002/ajmg.a.32850. [DOI] [PubMed] [Google Scholar]
- 96.Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–21. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 97.Liu L, Keefe DL. Defective cohesin is associated with age-dependent misaligned chromosomes in oocytes. Reprod Biomed Online. 2008;16:103–12. doi: 10.1016/S1472-6483(10)60562-7. [DOI] [PubMed] [Google Scholar]
- 98.Daum JR, Potapova TA, Sivakumar S, Daniel JJ, Flynn JN, Rankin S, et al. Cohesion fatigue induces chromatid separation in cells delayed at metaphase. Curr Biol. 2011;21:1018–24. doi: 10.1016/j.cub.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu JQ, Kornbluth S. Across the meiotic divide - CSF activity in the post-Emi2/XErp1 era. J Cell Sci. 2008;121:3509–14. doi: 10.1242/jcs.036855. [DOI] [PubMed] [Google Scholar]
- 100.Schmidt A, Rauh NR, Nigg EA, Mayer TU. Cytostatic factor: an activity that puts the cell cycle on hold. J Cell Sci. 2006;119:1213–8. doi: 10.1242/jcs.02919. [DOI] [PubMed] [Google Scholar]