Abstract
Loss of the tumor suppressor PTEN is observed in many human cancers that display increased chromosome instability and aneuploidy. The subcellular fractions of PTEN are associated with different functions that regulate cell growth, invasion and chromosome stability. In this study, we show a novel role for PTEN in regulating mitotic centrosomes. PTEN localization at mitotic centrosomes peaks between prophase and metaphase, paralleling the centrosomal localization of PLK-1 and γ-tubulin and coinciding with the time frame of centrosome maturation. In primary keratinocytes, knockdown of PTEN increased whole-cell levels of γ-tubulin and PLK-1 in an Akt-dependent manner and had little effect on recruitment of either protein to mitotic centrosomes. Conversely, knockdown of PTEN reduced centrosomal levels of pericentrin in an Akt-independent manner. Inhibition of Akt activation with MK2206 reduced the whole-cell and centrosome levels of PLK-1 and γ-tubulin and also prevented the recruitment of PTEN to mitotic centrosomes. This reduction in centrosome-associated proteins upon inhibition of Akt activity may contribute to the increase in defects in centrosome number and separation observed in metaphase cells. Concomitant PTEN knockdown and Akt inhibition reduced the frequency of metaphase cells with centrosome defects when compared with MK2206 treatment alone, indicating that both PTEN and pAkt are required to properly regulate centrosome composition during mitosis. The findings presented in this study demonstrate a novel role for PTEN and Akt in controlling centrosome composition and integrity during mitosis and provide insight into how PTEN functions as a multifaceted tumor suppressor.
Keywords: PTEN, centrosomes, Akt, gamma-tubulin, Polo-like kinase 1, pericentrin
Introduction
Cancers are often associated with numerous cell cycle and morphological abnormalities. Centrosome amplification (i.e., more than two centrosomes per cell) is frequently seen in both solid and hematological human cancers, while it rarely occurs in normal cells.1,2 Previous studies indicate centrosome amplification may be a causative agent in tumorigenesis.1,2 Centrosomes are commonly called the microtubule-organizing complex and are comprised of two orthogonally arranged centrioles surrounded by a dense lattice of proteins constituting the pericentrosomal matrix (PCM). The recruitment of γ-tubulin to the heart of the proximal ends of centrioles is critical for centrosome maturation and successful progression through mitosis.3,4
Organization and maturation of centrosomes is a complex and dynamic process that involves numerous proteins, many of which are regulated in a cell cycle-dependent manner. For instance, recruitment of γ-tubulin to mitotic centrosomes requires the phosphorylation of pericentrin (PCNT) by Polo-like kinase 1 (PLK-1).5,6 Gamma-tubulin then feeds back into the development of mature centrosomes, in part by enhancing the recruitment of other PCM proteins, including PLK-1.6
Multipolar spindle formation, caused either by failure of centrosomes to mature or by amplification of centrosome number, can have catastrophic effects on chromosome segregation and fidelity.7,8 Constitutive activation of Akt has been shown to cause centrosome abnormalities and amplification.9 Overexpressing the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) can reverse this amplification, at least in part by inhibiting the activation of Akt.9 PTEN is the second most commonly lost or mutated tumor suppressor in all of human cancers.10 Loss of PTEN is associated with increased growth rates, invasion, chromosome instability and aneuploidy, the latter phenotypes generally being associated with centrosome abnormalities.11,12
PTEN has many functions dependent on its location within cells. Cytoplasmic PTEN is most notably associated with Akt inhibition but is also important in suppressing invasion and migration as well as contributing to proper cell polarization through dephosphorylation of PtdIns(3,4,5)P3 (phosphatidylinositol-3,4,5-trisphosphate).13,14 Nuclear PTEN is important for inducing cell cycle arrest and maintaining chromosome stability; these processes are, at least in part, independent of Akt inhibition.15,16 Interestingly, PTEN−/− mouse embryonic fibroblasts (MEFs) escape taxol-mediated mitotic arrest more quickly than wild type cells, suggesting that PTEN is important for a mitotic spindle checkpoint.17 It is not clear if the ability of PTEN to influence mitotic checkpoints is via the regulation of centrosome number, and thus via an Akt-dependent manner, or if PTEN is capable of regulating mitotic machinery independently of Akt.
In this study we demonstrate that PTEN distribution changes through the cell cycle, and it localizes to centrosomes during mitosis. PTEN knockdown affected the whole cell and centrosome-associated levels of γ-tubulin and PLK-1 in an Akt-dependent manner while affecting centrosomal levels of PCNT in an Akt-independent manner. Akt activity is critical for recruitment of PTEN to centrosomes and maintenance of centrosome stability, as pharmacologic inhibition of Akt greatly increased the number of centrosomal defects during mitosis.
Our study identifies a novel pool of PTEN, which, in conjunction with Akt, plays an important role in regulating centrosome composition during mitosis. This study demonstrates an essential role for PTEN and Akt in regulating the mitotic machinery and provides an explanation as to how loss of PTEN in human malignancies plays a multi-faceted role in tumor progression.
Results
Localization of PTEN to mitotic centrosomes
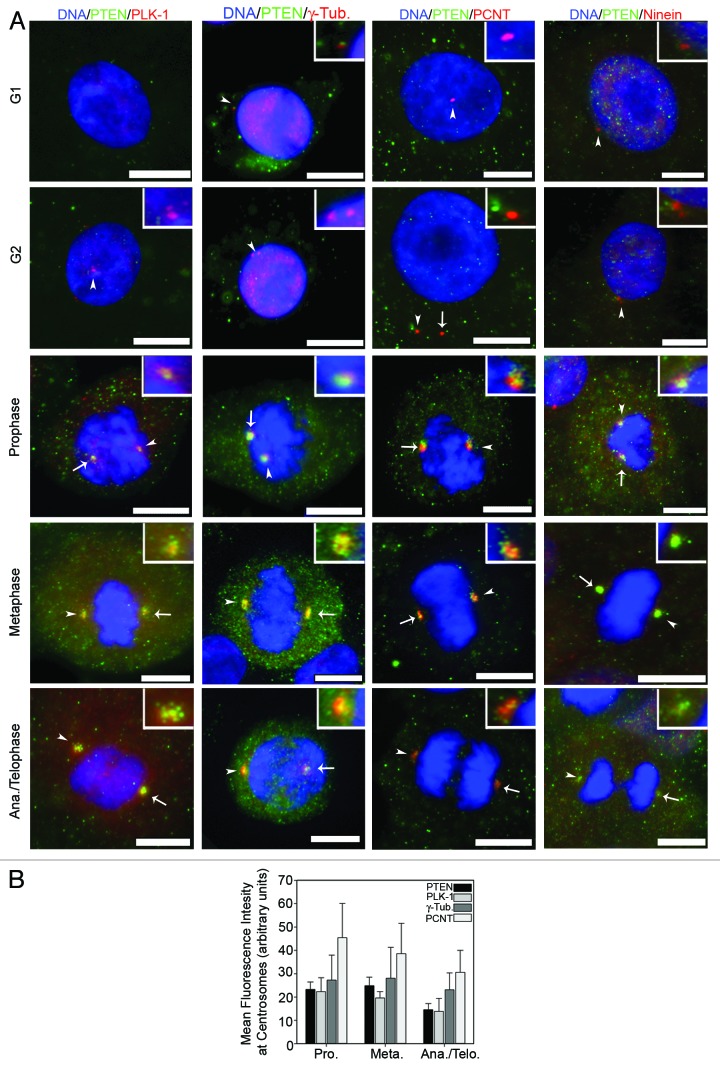
PTEN has been implicated in the mitotic checkpoint as well as in the induction of genes associated with microtubule formation and/or stabilization.17,18 To this end, we investigated a role for endogenous PTEN in centrosome function. Because of the complex interplay between centrosomes and genomic stability, we conducted all experiments, unless stated otherwise, in primary neonatal human epidermal keratinocytes (NHEK) rather than in cancer cell lines that frequently display centrosome abnormalities and genomic instability. Immunofluorescence labeling with a monoclonal PTEN-specific antibody showed that PTEN cellular distribution changes through the cell cycle (Fig. 1A). During interphase, endogenous PTEN is localized to both the cytoplasm and nucleus, with the nuclear fraction displaying a mildly stronger signal. In NHEKs, little to no centrosomal labeling of endogenous PTEN was observed during G1 or G2; however, clear labeling of PTEN at centrosomes during mitosis was observed (Fig. 1A). The intensity of PTEN at the centrosomes was most evident during prophase and metaphase (Fig. 1B), which coincides with the time frame for centrosome maturation.19 Similar patterns were also observed in the non-tumorigenic HaCaT keratinocyte cell line (Fig. S1A) and the non-small cell lung carcinoma H1299 cell line (Fig. S1B). PTEN is located in the PCM, as shown by co-localization with PLK-1, PCNT and γ-tubulin (Fig. 1A). Ninein, a protein associated with centrosomes predominantly during interphase,20 showed no co-localization with PTEN during interphase and minimal co-localization in the few mitotic cells with detectable centrosomal ninein. The relative intensity of PTEN at the PCM during mitosis was similar to PLK-1, another transiently associated PCM protein, confirming the mitosis specific centrosomal localization of PTEN (Fig. 1B).
Figure 1. PTEN localizes to mitotic centrosomes. (A) Actively growing, asynchronous, neonatal primary epidermal keratinocytes (NHEK) were fixed and stained for the indicated proteins. Representative cells from each stage of the cell cycle as determined by chromatin condensation and centrosome number are shown in each row. Arrowheads point to the centrosome enlarged within the inset box, while full arrows point to the remaining centrosome. Bars = 5 μm. (B) Mean fluorescence intensity (arbitrary units) of PTEN, PLK-1, γ-Tubulin and pericentrin (PCNT) during each stage of mitosis from NHEK. Error bars represent S.E.M. from three independent experiments. At least 25 centrosomes per experiment were measured.
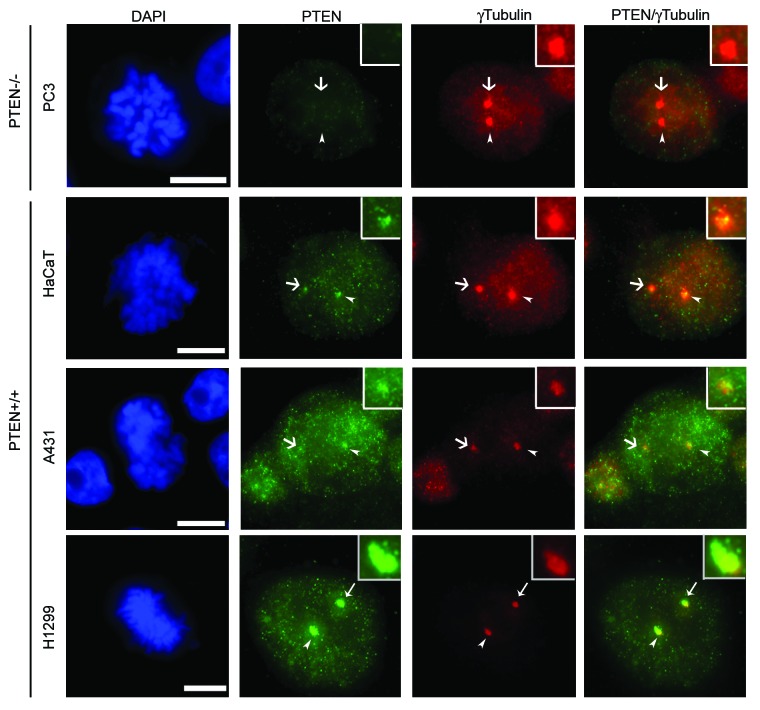
In order to further verify that detection of endogenous centrosomal PTEN was not due to cross-reaction with another protein, we performed immunofluorescence studies in PTEN-null PC3 cells. No PTEN signal was detected in asynchronously growing PC3 cells demonstrating that labeling of PTEN at mitotic centrosomes is specific (Fig. 2, top row). Co-localization of PTEN and γ-tubulin was also detected in multiple cell lines, albeit to a low extent in the atypical squamous cell line A431, further demonstrating that localization of PTEN to mitotic centrosomes is neither cell line- nor lineage-specific (Fig. 2). We confirmed the localization of PTEN to mitotic centrosomes with a second PTEN-specific antibody (Fig. S2), excluding the possibility that staining of PTEN at mitotic centrosomes is an artifact. Knockdown of ΔNp63α, previously shown by our laboratory to transcriptionally repress PTEN,21 also increased centrosomal levels of PTEN in HaCaT cells (Fig. S3A–C). This further confirms that the centrosomal labeling of PTEN is not a byproduct of the staining techniques used.
Figure 2. Centrosomal PTEN is not cell line-specific. PTEN-null PC3 cells and PTEN+/+ HaCaT, A431 and H1299 cells were stained for PTEN (green) and γ-tubulin (red). The PTEN+/+ cell lines show varying amounts of co-localization (observed as yellow in the last column) with γ-tubulin. Arrowheads indicate the centrosome enlarged within the inset box while full arrows indicate the remaining centrosome. Bars = 5 μm.
PTEN indirectly represses PLK-1 and γ-tubulin via regulation of Akt
Loss of PTEN has been associated with chromosome instability and aneuploidy,11,12 suggesting that reduced levels of PTEN at mitotic centrosomes may contribute to genetic instability. To determine if PTEN influenced genetic instability via its localization to mitotic centrosomes, we first examined the effects of silencing PTEN on PCM-associated proteins. Immunoblot analyses of whole-cell extracts after knockdown of PTEN in NHEKs resulted in modest but statistically significant increases in both PLK-1 and γ-tubulin (Fig. 3A). Previous studies in prostate cancer cells have demonstrated that PTEN catalyzes the degradation of PLK-1 independent of the phosphatase activity of PTEN, and thus independent of Akt.22 To determine if PLK-1 and γ-tubulin were similarly regulated by an Akt-independent mechanism in normal keratinocytes, we silenced PTEN and then treated cells with the Akt1/2 selective inhibitor MK2206. Although MK2206 alone had little effect on whole-cell levels of either PLK-1 or γ-tubulin (Fig. 3B), concomitant knockdown of PTEN and inhibition of Akt phosphorylation and subsequent kinase activity by MK2206 completely blocked the induction in PLK-1 and γ-tubulin caused by silencing PTEN (Fig. 3B). The panAkt inhibitor, Perifosine, displayed similar, but less robust, effects on PLK-1 and γ-tubulin expression in transformed keratinocyte cells (Fig. S4). To further confirm functionality of MK2206, the levels of ΔNp63α, which is stabilized by active Akt, were monitored.23,24 Similar to PLK-1 and γ-tubulin, increased ΔNp63α levels observed upon silencing PTEN were inhibited upon treatment with MK2206 (Fig. S3D).
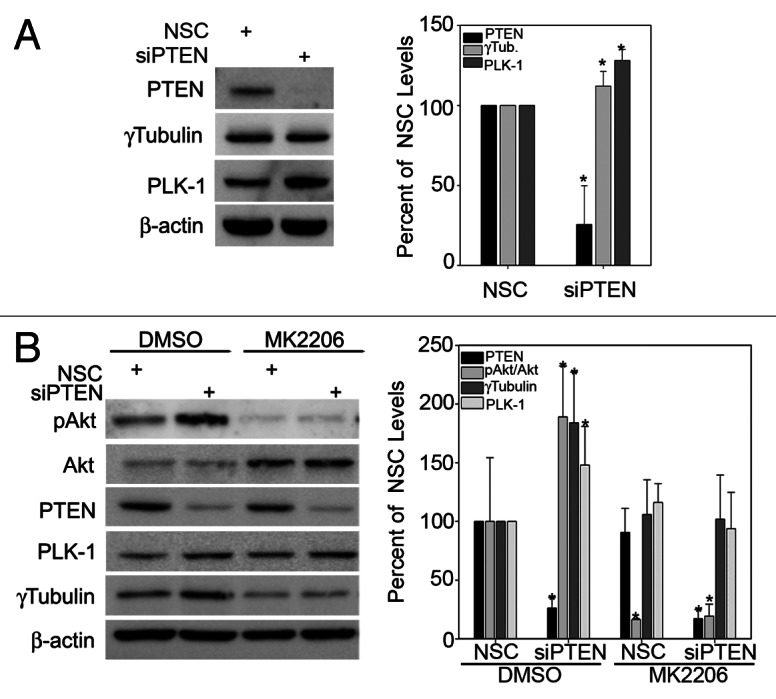
Figure 3. PTEN suppresses PLK-1 and γ-tubulin by inhibiting the activation of Akt. (A) PTEN was silenced in NHEKs followed by immunoblot analysis for the indicated proteins. Quantitation of the change in proteins levels is graphed in the right panel. Error bars represent s.d. from three separate experiments. (B) NHEKs were transfected with non-silencing control (NSC) or PTEN specific siRNA followed by treatment with 10 μM MK2206 or DMSO control for 16 h. Immunoblot analysis for the indicated proteins was performed, and the change in total protein levels is plotted in the right panel. Error bars represent s.d. from three separate experiments.
Both PTEN and Akt activity are critical to centrosome composition during mitosis
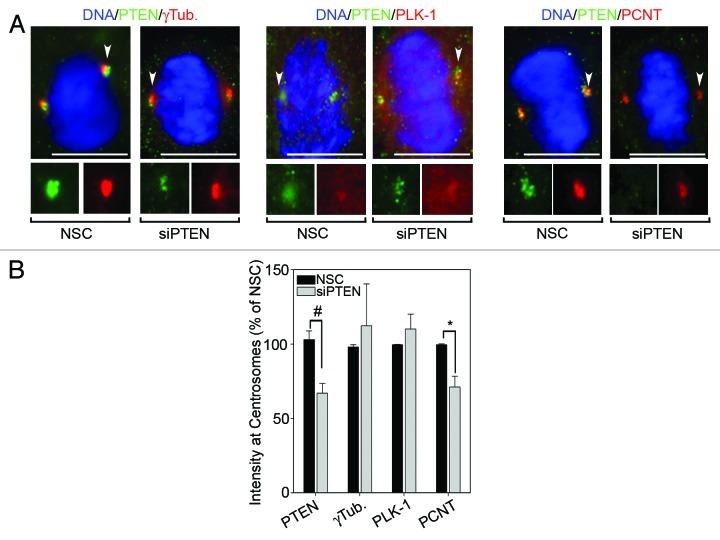
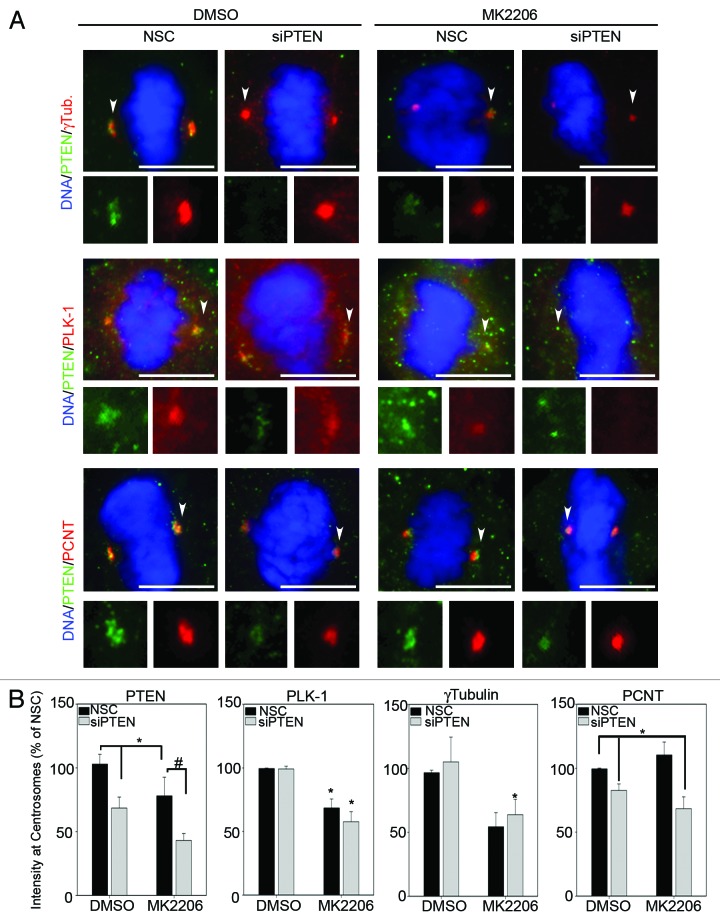
We next examined the effects of silencing PTEN on centrosome levels of PLK-1 and γ-tubulin during mitosis by immunofluorescence, since both PLK-1 and γ-tubulin have different subcellular localizations during the cell cycle.25,26 Contrary to the effects on whole-cell levels measured by immunoblot (Fig. 3), knockdown of PTEN alone did not significantly increase PLK-1 or γ-tubulin levels at mitotic centrosomes (Fig. 4A). However, re-expression of wild-type PTEN and catalytically inactive C124S PTEN, in PTEN-null PC3 cells did result in reduced expression of both PLK-1 and γ-tubulin at mitotic centrosomes (Fig. S5A and C). This further suggests that impaired organization of mitotic centrosomes is independent of inhibition of Akt by PTEN. Interestingly, a 29% reduction in centrosomal levels of the constitutive PCM protein PCNT was observed after knockdown of PTEN (Fig. 4B). A change in PCNT levels, however, was not observed in mitotic centrosomes after re-expression of wild-type or C124S PTEN, potentially because of the differences in transformed vs. primary cell lines or due to the fact that PCNT levels were already at maximum detection level in the parental PC3 cells (Fig. S5). Treatment of NHEKs with MK2206 reduced PLK-1 and γ-tubulin levels at mitotic centrosomes in both the presence and absence of PTEN-specific siRNA (Fig. 5A and B, quantitated in B). This indicates that while PTEN status is dispensable, Akt activity is required for full recruitment of PLK-1 and γ-tubulin to mitotic centrosomes. In contrast, the reduction in centrosomal levels of PCNT observed after knockdown of PTEN was shown not to be the result of downstream activation of Akt, since inhibition of Akt signaling with MK2206 had no effect on centrosomal levels of PCNT (Fig. 5A and B, quantitated in B). Unexpectedly, centrosomal levels of PTEN were also significantly reduced in cells treated with MK2206 (Fig. 5). Altogether, these studies demonstrate that Akt activity is important to the full recruitment of PTEN, PLK-1 and γ-tubulin to mitotic centrosomes. However, PTEN also contributes to centrosome composition independent of its influence on Akt activity by positively influencing the recruitment of PCNT to mitotic centrosomes.
Figure 4. Loss of PTEN reduces centrosome levels of Pericentrin. (A) PTEN was silenced in NHEKs then stained for the indicated proteins. Arrowheads point to the centrosome enlarged bottom panels. Bars = 5 μm. (B) The mean fluorescence intensities of each protein at mitotic centrosomes, relative to control treated cells, are plotted. Error bars represent S.E.M. from three separate experiments, at least 25 centrosomes measured for each protein per condition per experiment. *p values ≤ 0.05; #p values ≤ 0.009.
Figure 5. Centrosome composition is regulated by Akt. (A) Immunofluorescence analysis for the indicated proteins was performed on NHEKs transfected with NSC- or PTEN-specific siRNA followed by 10 μM MK2206 or DMSO control treatment for 16 h. Arrowheads point to the centrosome enlarged bottom panels; Bars = 5 μm. (B) The mean fluorescent intensities of each protein at mitotic centrosomes, relative to NSC/DMSO-treated cells, are plotted. Error bars represent S.E.M. from three separate experiments, at least 25 centrosomes measured for each protein per condition per experiment. *p values ≤ 0.05; #p values ≤ 0.009.
PTEN and Akt activity both contribute to centrosome integrity during mitosis
The proper recruitment of PLK-1 and γ-tubulin is critical for centrosome maturation, as inhibition of either protein leads to reduced microtubule-organizing activity, which negatively impacts the completion of mitosis. Consistent with the requirement of Akt activity to fully recruit PLK-1 and γ-tubulin to mitotic centrosomes (Fig. 5), inhibition of Akt signaling with MK2206 drastically increased the frequency of mitotic cells with centrosome defects (Fig. 6). Although MK2206 or PTEN knockdown did not dramatically alter the frequency of prophase cells with centrosome defects (Fig. 6A), MK2206 increased the frequency of metaphase cells with centrosome defects to 58.3 ± 2.37% (Fig. 6B). Although not as robust as treatment with MK2206, silencing PTEN also significantly increased the percentage of metaphase cells with centrosome abnormalities to 21.8 ± 2.37%. Concomitant knockdown of PTEN and MK2206 treatment reduced the frequency of metaphase cells with centrosome defects as compared with MK2206 treatment alone (18.7 ± 7.04% vs. 58.3 ± 2.37%), but was still significantly higher than in non-silencing control, DMSO-treated cells (Fig. 6B). This may be the result of combined PTEN knockdown and MK2206 treatment allowing cells to progress through mitosis without resolving defects, as it has been reported earlier that PTEN-null cells have a deficient mitotic checkpoint.17
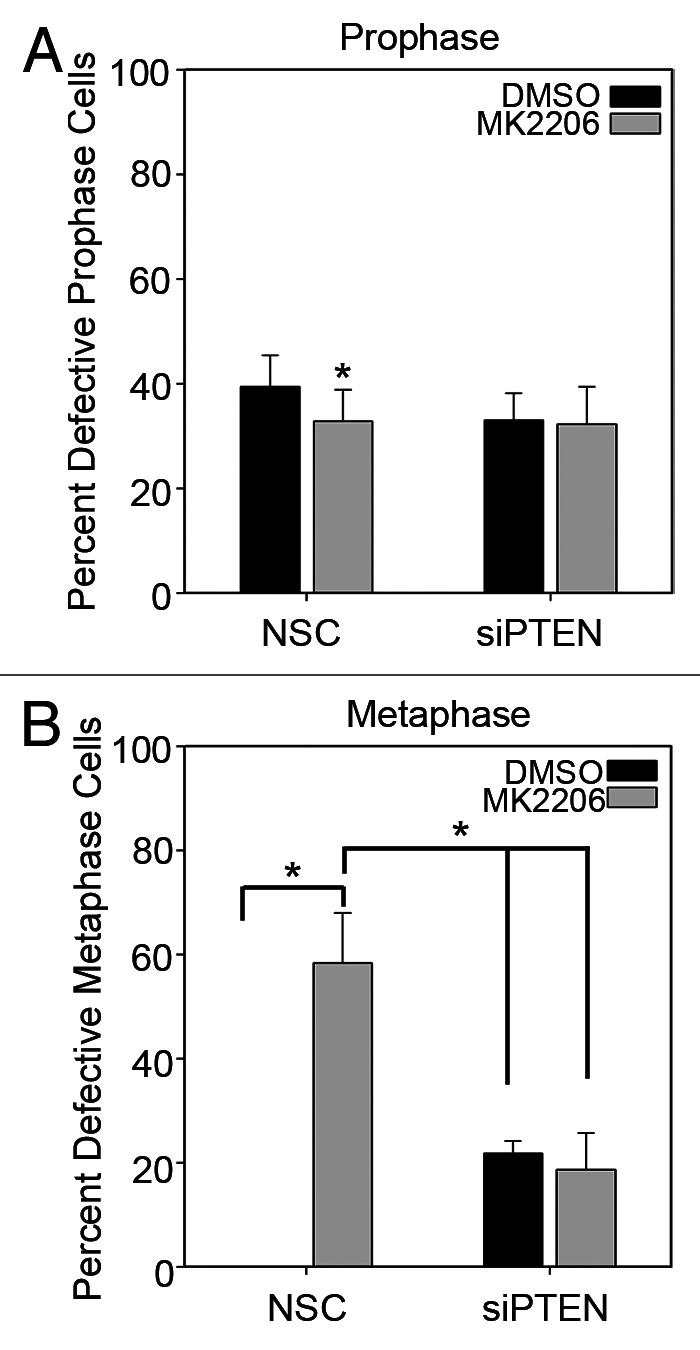
Figure 6. Loss of Akt activity and PTEN lead to centrosome defects during metaphase. Immunofluorescence analysis for the indicated proteins was performed on NHEKs transfected with NSC- or PTEN-specific siRNA followed by 10 μM MK2206 or DMSO control treatment for 16 h. (A) The number of centrosomal defects in each stage of mitosis were scored. Error bars represent s.d. from at least five separate experiments: *p values ≤ 0.05.
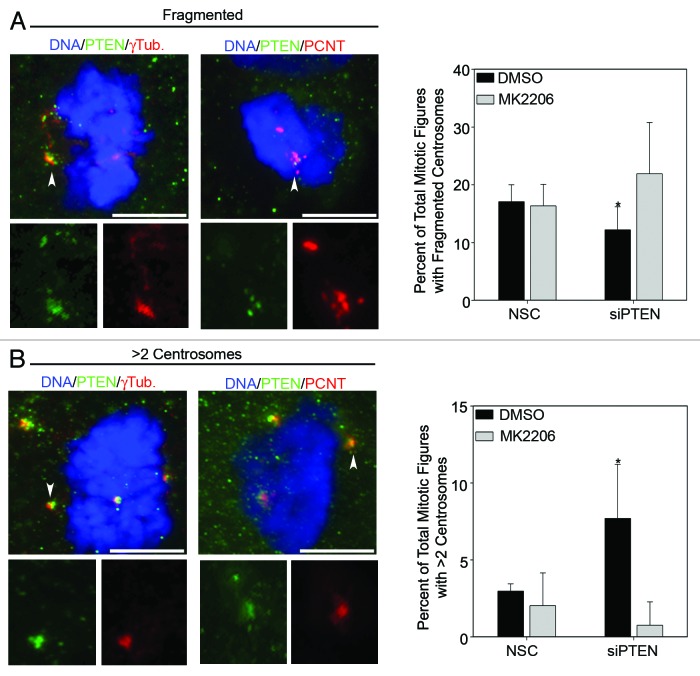
In examining mitotic cells, independently of the stage of mitosis, the most common defects observed were fragmented centrosomes (Fig. 7A) and centrosome amplification (Fig. 7B), as determined by staining for constitutive centrosome components PCNT and γ-tubulin. Interestingly, knockdown of PTEN led to a small but statistically significant decrease in the percent of mitotic cells with fragmented centrosomes as compared with DMSO-treated cells (Fig. 7A). The reduction in fragmented centrosomes may be due to increased Akt activation, since concomitant knockdown of PTEN and Akt inhibition with MK2206 ablated this reduction (Fig. 7A).
Figure 7. Loss of Akt activity and PTEN lead to increased frequencies of centrosome fragmentation and amplification. (A) Representative images of fragmented and (B) supernumerary centrosome defects. Arrowheads point to the centrosome enlarged within the inset box, while full arrows point to the remaining centrosome. Bars = 5 μm. The frequency of each type of defect is graphed in the lower panels. Error bars represent s.d. from at least five separate experiments: *p values ≤ 0.05.
Consistent with previous reports,9,27 loss of PTEN increased the frequency of centrosome amplification (Fig. 7B). Inhibition of Akt with MK2206 after knockdown of PTEN reduced the number of cells with supernumerary centrosomes, confirming that centrosome amplification is caused by increased Akt activity rather than PTEN loss (Fig. 7B, right panel). Altogether, these studies demonstrate that PTEN and Akt activity must be adequately balanced to prevent centrosome aberrations.
Discussion
Previous studies have implicated a role for PTEN in regulating the mitotic spindle, either by controlling cell polarity28 or maintaining a proper mitotic checkpoint.17 In this study we show for the first time a direct linkage between PTEN and the mitotic spindle through localization of PTEN to the pericentrisomal matrix in mitotic cells (Fig. 1). The localization of PTEN to mitotic centrosomes was found to be a conserved phenomenon in human cells, as centrosomal PTEN was neither cell line- nor lineage-specific (Fig. 2).
Microarray studies in breast29 and endometrial30 cancers have demonstrated that aberrant PTEN expression correlates with poor prognosis with concomitant upregulation of many centrosome-associated proteins. Studies that only measure global changes in proteins may not accurately represent the changes occurring at a specific cellular compartment. This appears to be the case with PTEN, as knockdown of PTEN in primary human epidermal keratinocytes differentially affected centrosome-associated proteins. Loss of PTEN increased whole-cell levels of γ-tubulin and PLK-1 in an Akt-dependent manner (Fig. 3) but did not significantly alter their levels at mitotic centrosomes (Fig. 4). Conversely, knockdown of PTEN reduced centrosomal levels of PCNT in an Akt-independent manner (Fig. 5). The recruitment of γ-tubulin to mitotic centrosomes has been shown to be mediated, in part, by PCNT31 after the phosphorylation of PCNT by PLK-1,6 and thus disruption of PCNT levels by silencing PTEN could have indirect effects on the recruitment of γ-tubulin in normal keratinocytes. There is likely a minimal threshold level of PCNT that is sufficient to recruit γ-tubulin to mitotic centrosomes and events that deplete PCNT, but failure to reach this threshold level would only have minimal effects on γ-tubulin recruitment. This notion is supported by multiple lines of evidence. First, our results in Figure 4 demonstrating that while silencing PTEN led to a modest ~29% reduction in PCNT levels, no significant change in centrosomal levels of γ-tubulin were observed. Furthermore, studies demonstrating the importance of PCNT to the recruitment of γ-tubulin have employed PCNT-specific siRNA to directly deplete PCNT levels by at least 80%.6,31,32
The full recruitment of PCNT to mitotic centrosomes appears to be dependent on PTEN (Fig. 4), and intriguingly, the centrosomal localization of PTEN in mitotic cells was found to be dependent on the kinase activity of Akt (Fig. 5). The dependence on Akt activity for centrosomal localization of PTEN is interesting given the canonical role of cytoplasmic PTEN in preventing the activation of Akt. However, the ability of Akt to control the subcellular localization of PTEN is not completely unexpected, as Akt has previously been reported to accentuate the nuclear export of PTEN by facilitating the interaction of PTEN and S6 kinase 2 (S6K2).33 One could speculate that Akt-mediated control of S6K2 could also influence recruitment of PTEN to mitotic centrosomes, since S6K2 is known to physically interact with PTEN as well as localize to centrosomes.33,34 However, further investigation would be needed to test this hypothesis. Additionally, it is possible that PTEN may influence Akt activity at mitotic centrosomes, as both PI3K and Akt have been reported to localize to centrosomes in other cell lines.35-37
The kinase activity of Akt is well known for promoting cell proliferation and also important for preventing mitotic catastrophe in cancer cells lines.38 To the best of our knowledge, however, the current study is the first to specifically demonstrate that basal Akt activity is critical for protein recruitment to mitotic centrosomes (Fig. 5) without significantly affecting steady-state levels of centrosome-associated proteins in normal, healthy cells (Fig. 3B). Inhibition of basal Akt activity with MK2206 significantly reduced centrosomal levels of PLK-1 and γ-tubulin in mitotic cells (Fig. 5). The inhibition of Akt activity seems to have a greater effect on centrosomal levels of PLK-1 and γ-tubulin in mitotic cells (Fig. 5) as compared whole-cell levels seen in Figure 3. The difference the magnitude of change in response to MK2206 treatment between immunoblot and immunofluorescence results may be due to the fact that the immunofluorescence measurements were taken of only mitotic centrosomes, while the immunoblot analyses in Figure 3B measure total protein levels (at all subcellular compartments) and at all stages of the cell cycle. This is important given that the levels of PLK-125 and γ-tubulin26 at centrosomes are dependent on the cell cycle, and changes in the mitotic centrosome levels could easily be masked in immunoblot analyses of whole-cell extracts from asynchronous cells.
Additionally, regulation of PLK-1 by Akt may have effects far beyond the recruitment of γ-tubulin to mitotic centrosomes. Phosphorylation of 4E-BP139 and AMPK40 by PLK-1 has been shown to be critical for healthy progression through mitosis. During interphase, Akt and AMPK have antagonistic effects on the mTOR pathway and subsequently on cell progression. However, the nutrient-sensing functions of AMPK are dispensable for its mitotic functions,41 and thus PLK-1 may act as a bridge to connect the AMPK and Akt pathways to ensure successful completion of mitosis. The ability of Akt and PTEN to regulate centrosome composition during mitosis likely contributes to proper spindle formation, as demonstrated by the increase in centrosome defects caused by the knockdown of PTEN and/or inhibition of Akt with MK2206 (Fig. 7). It is important to note that even though loss of PTEN and MK2206 treatment increased the number of centrosome defects, no overt chromosomal abnormalities were observed in NHEK cells during our short-term study. Because these studies were conducted in primary cells, it indicates that disruption of the PTEN/Akt pathway is sufficient to cause centrosome defects, but that additional stressors may be required before the effects on chromosome stability are detectable. Additionally, silencing of PTEN only achieved a ~50% reduction in centrosomal levels of PTEN (Fig. 4), suggesting that centrosome-associated PTEN may somehow be stabilized and continuing to assist in chromosome maintenance. Consistent with this notion are previous studies demonstrating that PTEN−/− MEFs exposed to γ-irradiation accumulate chromosomal abnormalities.17 The activity of Akt is also critically important for mitotic progression under stress conditions, as Akt removes the G2/M block caused by DNA damage by preventing the ubiquitination and degradation of PLK-1.42
Akt inhibitors in addition to MK2206 have been shown to cause spindle abnormalities during mitosis in cancer cells,43 but the studies in Figure 5 provide the first evidence that PTEN and Akt play a direct role in controlling centrosome composition during mitosis. Because of the direct effects on recruiting PTEN and PLK-1 to mitotic centrosomes in addition to the traditional growth-suppressive effects of Akt inhibition, combinatorial therapies of MK2206 with other mitotic inhibitors may prove extremely beneficial in cancer treatment. The ability of MK2206 to induce centrosome defects in the normal, primary skin cells highlight the need for more investigation into the safety of is uses as a chemotherapeutic. In fact, dose-limiting toxicities from phase I clinical trials of MK2206 were comprised mainly of skin rashes.44 More studies will be needed to determine if the rashes observed in clinical trials of MK2206 are a direct result of off-target effects in normal skin, and if so, what steps could be taken to prevent them and enhance the efficacy of MK2206. The potential therapeutic value of centrosomal PTEN and its regulation by Akt activity warrants future studies.
Materials and Methods
Cell lines and reagents
H1299, HaCaT, A431, parental PC3 (PTEN-null), PC3 cells expressing WT or mutant PTEN C142S cell lines were grown in Dulbecco’s modified Eagles medium (DMEM) supplemented with 8% fetal bovine serum and 100 U/mL of penicillin/streptomycin and maintained at 37° C in 5% CO2. Neonatal human keratinocytes (NHEK) were grown in KGM-Gold media, supplemented and maintained as per manufacturer’s instructions (Lonza). Cells were transfected with non-silencing control or PTEN specific siRNA using a previously described protocol, excepting the use of Lipofectamine RNAiMax instead of Oligofectamine (Life Technologies).21 The two Akt inhibitors, MK2206 and Perifosine, were purchased from SelleckChem.
Immunoblotting and immunofluorescence
Cells were grown, treated and either lysed and analyzed by western blot or immunofluorescence as described previously.21 For all figures, except Figure S2, PTEN was detected with mouse monoclonal anti-PTEN 6H2.1 antibody in PBS-NGS (1:50 for H1299 cells or 1:25 for all other cells from Cascade Bioscience). Confirmation of PTEN localization by immunofluorescence was shown in Figure S2 with rabbit monoclonal anti-PTEN (#9559) at a dilution of 1:25 (Cell Signaling). Gamma tubulin was detected with rabbit polyclonal anti-γ-tubulin at dilution of 1:2,000 (DQ-19, Sigma-Aldrich). Rabbit polyclonal PLK-1 (ab4448) and pericentrin (ab47867) were detected at 1 μg/mL, while ninein (ab4447) was detected at 20 μg/mL (AbCam). Analysis of relative fluorescence intensity was done as previously described.6 Briefly, the fluorescence intensity within the region of interest was defined by drawing a circle around the centrosome using ImagePro 6.2 (Media Cybernetics). The background intensity from an identically sized circle adjacent to the centrosome was subtracted from the centrosome value. At least 25 centrosomes were measured per condition for each experiment with an n = 3 independent experiments. Student’s t-tests were used to determine significant difference.
Supplementary Material
Acknowledgments
This work was supported by grant1R01CA154715 from the National Institutes of Health
Glossary
Abbreviations:
- PTEN
phosphatase and tensin homolog deleted on chromosome ten
- γ-tubulin
gamma-tubulin
- PLK-1
Polo-like kinase 1
- PCNT
pericentrin
- PCM
peri-centrosomal matrix
- PIP3
phosphatidylinositol-3,4,5-trisphosphate
- NHEK
neonatal human epidermal keratinocytes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/24516
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24516
References
- 1.Chng WJ, Ahmann GJ, Henderson K, Santana-Davila R, Greipp PR, Gertz MA, et al. Clinical implication of centrosome amplification in plasma cell neoplasm. Blood. 2006;107:3669–75. doi: 10.1182/blood-2005-09-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pihan GA, Wallace J, Zhou Y, Doxsey SJ. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 2003;63:1398–404. [PubMed] [Google Scholar]
- 3.Fuller SD, Gowen BE, Reinsch S, Sawyer A, Buendia B, Wepf R, et al. The core of the mammalian centriole contains gamma-tubulin. Curr Biol. 1995;5:1384–93. doi: 10.1016/S0960-9822(95)00276-4. [DOI] [PubMed] [Google Scholar]
- 4.O’Toole E, Greenan G, Lange KI, Srayko M, Müller-Reichert T. The role of γ-tubulin in centrosomal microtubule organization. PLoS ONE. 2012;7:e29795. doi: 10.1371/journal.pone.0029795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haren L, Stearns T, Lüders J. Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS ONE. 2009;4:e5976. doi: 10.1371/journal.pone.0005976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee K, Rhee K. PLK1 phosphorylation of pericentrin initiates centrosome maturation at the onset of mitosis. J Cell Biol. 2011;195:1093–101. doi: 10.1083/jcb.201106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khodjakov A, Rieder CL. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J Cell Biol. 2001;153:237–42. doi: 10.1083/jcb.153.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao CV, Yamada HY, Yao Y, Dai W. Enhanced genomic instabilities caused by deregulated microtubule dynamics and chromosome segregation: a perspective from genetic studies in mice. Carcinogenesis. 2009;30:1469–74. doi: 10.1093/carcin/bgp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam HJ, Chae S, Jang SH, Cho H, Lee JH. The PI3K-Akt mediates oncogenic Met-induced centrosome amplification and chromosome instability. Carcinogenesis. 2010;31:1531–40. doi: 10.1093/carcin/bgq133. [DOI] [PubMed] [Google Scholar]
- 10.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine RL, Cargile CB, Blazes MS, van Rees B, Kurman RJ, Ellenson LH. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 1998;58:3254–8. [PubMed] [Google Scholar]
- 12.Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Davidson L, Maccario H, Perera NM, Yang X, Spinelli L, Tibarewal P, et al. Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene. 2010;29:687–97. doi: 10.1038/onc.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langlois MJ, Bergeron S, Bernatchez G, Boudreau F, Saucier C, Perreault N, et al. The PTEN phosphatase controls intestinal epithelial cell polarity and barrier function: role in colorectal cancer progression. PLoS ONE. 2010;5:e15742. doi: 10.1371/journal.pone.0015742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WK. Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol. 2005;25:6211–24. doi: 10.1128/MCB.25.14.6211-6224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–70. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Yang Q, Pandita RK, Hunt CR, Xiang T, Misri S, et al. Cell cycle checkpoint defects contribute to genomic instability in PTEN deficient cells independent of DNA DSB repair. Cell Cycle. 2009;8:2198–210. doi: 10.4161/cc.8.14.8947. [DOI] [PubMed] [Google Scholar]
- 18.Tibarewal P, Zilidis G, Spinelli L, Schurch N, Maccario H, Gray A, et al. PTEN protein phosphatase activity correlates with control of gene expression and invasion, a tumor-suppressing phenotype, but not with AKT activity. Sci Signal. 2012;5:ra18. doi: 10.1126/scisignal.2002138. [DOI] [PubMed] [Google Scholar]
- 19.Palazzo RE, Vogel JM, Schnackenberg BJ, Hull DR, Wu X. Centrosome maturation. Curr Top Dev Biol. 2000;49:449–70. doi: 10.1016/S0070-2153(99)49021-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Howng SL, Cheng TS, Chou MH, Huang CY, Hong YR. Molecular characterization of human ninein protein: two distinct subdomains required for centrosomal targeting and regulating signals in cell cycle. Biochem Biophys Res Commun. 2003;308:975–83. doi: 10.1016/S0006-291X(03)01510-9. [DOI] [PubMed] [Google Scholar]
- 21.Leonard MK, Kommagani R, Payal V, Mayo LD, Shamma HN, Kadakia MP. ΔNp63α regulates keratinocyte proliferation by controlling PTEN expression and localization. Cell Death Differ. 2011;18:1924–33. doi: 10.1038/cdd.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, et al. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144:187–99. doi: 10.1016/j.cell.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segrelles C, Moral M, Lara MF, Ruiz S, Santos M, Leis H, et al. Molecular determinants of Akt-induced keratinocyte transformation. Oncogene. 2006;25:1174–85. doi: 10.1038/sj.onc.1209155. [DOI] [PubMed] [Google Scholar]
- 24.Barbieri CE, Barton CE, Pietenpol JA. Delta Np63 alpha expression is regulated by the phosphoinositide 3-kinase pathway. J Biol Chem. 2003;278:51408–14. doi: 10.1074/jbc.M309943200. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi E, Toyoshima-Morimoto F, Nishida E. Nuclear translocation of plk1 mediated by its bipartite nuclear localization signal. J Biol Chem. 2002;277:48884–8. doi: 10.1074/jbc.M206307200. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Hemmerich P, Grosse F. Centrosomal localization of DNA damage checkpoint proteins. J Cell Biochem. 2007;101:451–65. doi: 10.1002/jcb.21195. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Dutra A, Pak E, Labrie JE, 3rd, Gerstein RM, Pandolfi PP, et al. EGFRvIII expression and PTEN loss synergistically induce chromosomal instability and glial tumors. Neuro Oncol. 2009;11:9–21. doi: 10.1215/15228517-2008-081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyoshima F, Matsumura S, Morimoto H, Mitsushima M, Nishida E. PtdIns(3,4,5)P3 regulates spindle orientation in adherent cells. Dev Cell. 2007;13:796–811. doi: 10.1016/j.devcel.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA. 2007;104:7564–9. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushima-Nishiu M, Unoki M, Ono K, Tsunoda T, Minaguchi T, Kuramoto H, et al. Growth and gene expression profile analyses of endometrial cancer cells expressing exogenous PTEN. Cancer Res. 2001;61:3741–9. [PubMed] [Google Scholar]
- 31.Zimmerman WC, Sillibourne J, Rosa J, Doxsey SJ. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol Biol Cell. 2004;15:3642–57. doi: 10.1091/mbc.E03-11-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawo S, Hasegan M, Gupta GD, Pelletier L. Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nat Cell Biol. 2012;14:1148–58. doi: 10.1038/ncb2591. [DOI] [PubMed] [Google Scholar]
- 33.Liu JL, Mao Z, LaFortune TA, Alonso MM, Gallick GE, Fueyo J, et al. Cell cycle-dependent nuclear export of phosphatase and tensin homologue tumor suppressor is regulated by the phosphoinositide-3-kinase signaling cascade. Cancer Res. 2007;67:11054–63. doi: 10.1158/0008-5472.CAN-07-1263. [DOI] [PubMed] [Google Scholar]
- 34.Rossi R, Pester JM, McDowell M, Soza S, Montecucco A, Lee-Fruman KK. Identification of S6K2 as a centrosome-located kinase. FEBS Lett. 2007;581:4058–64. doi: 10.1016/j.febslet.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalous J, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J. PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biol Cell. 2006;98:111–23. doi: 10.1042/BC20050020. [DOI] [PubMed] [Google Scholar]
- 36.Kapeller R, Toker A, Cantley LC, Carpenter CL. Phosphoinositide 3-kinase binds constitutively to alpha/beta-tubulin and binds to gamma-tubulin in response to insulin. J Biol Chem. 1995;270:25985–91. doi: 10.1074/jbc.270.43.25985. [DOI] [PubMed] [Google Scholar]
- 37.Lelièvre H, Chevrier V, Tassin AM, Birnbaum D. Myeloproliferative disorder FOP-FGFR1 fusion kinase recruits phosphoinositide-3 kinase and phospholipase Cgamma at the centrosome. Mol Cancer. 2008;7:30. doi: 10.1186/1476-4598-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemström TH, Sandström M, Zhivotovsky B. Inhibitors of the PI3-kinase/Akt pathway induce mitotic catastrophe in non-small cell lung cancer cells. Int J Cancer. 2006;119:1028–38. doi: 10.1002/ijc.21927. [DOI] [PubMed] [Google Scholar]
- 39.Shang ZF, Yu L, Li B, Tu WZ, Wang Y, Liu XD, et al. 4E-BP1 participates in maintaining spindle integrity and genomic stability via interacting with PLK1. Cell Cycle. 2012;11:3463–71. doi: 10.4161/cc.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Menendez JA. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell Cycle. 2011;10:1295–302. doi: 10.4161/cc.10.8.15342. [DOI] [PubMed] [Google Scholar]
- 41.Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Menendez JA. Polo-like kinase 1 directs the AMPK-mediated activation of myosin regulatory light chain at the cytokinetic cleavage furrow independently of energy balance. Cell Cycle. 2012;11:2422–6. doi: 10.4161/cc.20438. [DOI] [PubMed] [Google Scholar]
- 42.Shtivelman E. Promotion of mitosis by activated protein kinase B after DNA damage involves Polo-like kinase 1 and checkpoint protein CHFR. Mol Cancer Res. 2003;1:959–69. [PubMed] [Google Scholar]
- 43.Liu X, Shi Y, Woods KW, Hessler P, Kroeger P, Wilsbacher J, et al. Akt inhibitor a-443654 interferes with mitotic progression by regulating aurora a kinase expression. Neoplasia. 2008;10:828–37. doi: 10.1593/neo.08408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pal SK, Reckamp K, Yu H, Figlin RA. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2010;19:1355–66. doi: 10.1517/13543784.2010.520701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.