Abstract
Biosynthesis of glucans occurred in cell-free fractions isolated from onion stem (Allium cepa L.) enriched in either dictyosomes or plasma membranes. β-1,3- and β-1, 4-Glucans were synthesized in differing proportions and at different rates as the concentration of uridine diphosphoglucose or the proportion of dictyosomes or plasma membrane varied. At low (1.5 μm) UDP-glucose concentrations synthesis of alkali-insoluble glucan was correlated with abundance of dicytosomes; most of the substrate utilized by plasma membrane was for glycolipid synthesis. At high (1 mm) UDP-glucose concentration, the synthesis of alkali-insoluble glucans correlated with the abundance of plasma membrane. Substrate enhancement of β-1, 4-glucan synthesis in dictyosome fractions was less than proportional to increases in substrate concentration. In contrast, β-1, 4-glucan synthesis by plasma membrane was more than proportionately increased. At high substrate concentrations the synthesis of β-1, 3-glucans predominated in both dictyosome and plasma membrane fractions. The results show that the capacity to synthesize glucans resides in both Golgi apparatus and plasma membranes of onion stem, but that the plasma membrane has the greatest capacity for synthesis of alkali-insoluble glucans at high UDP-glucose concentrations.
Full text
PDF

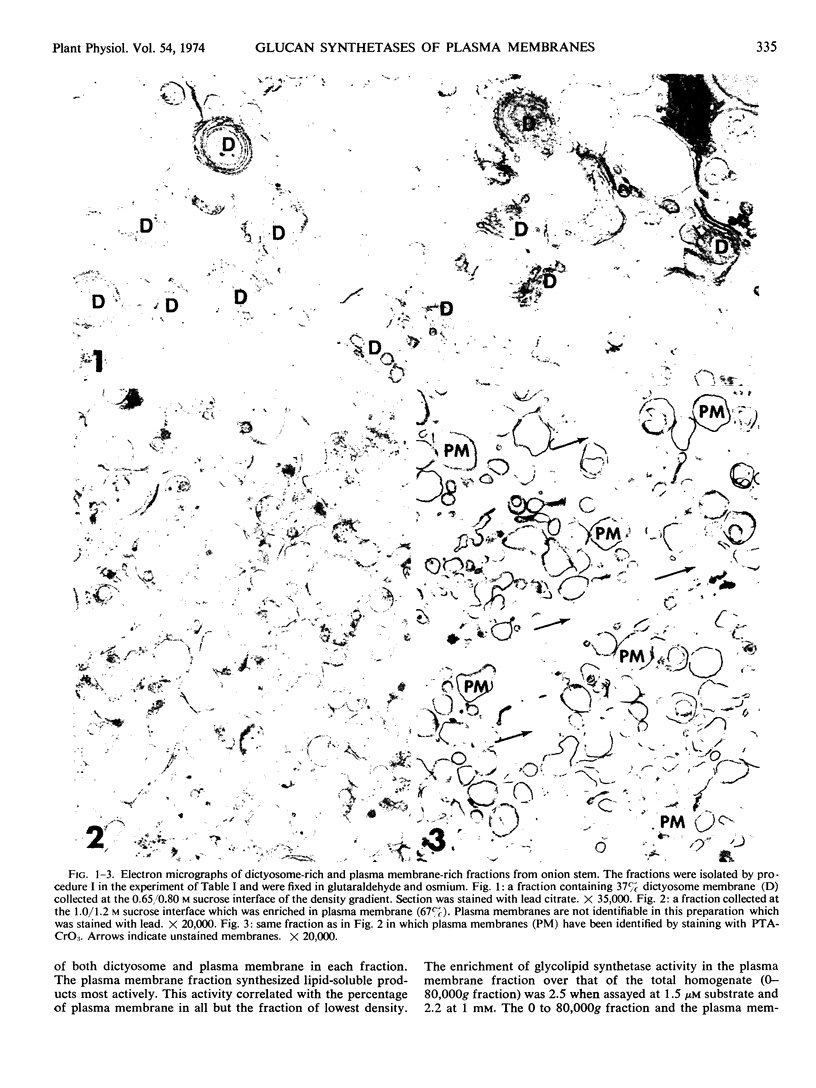
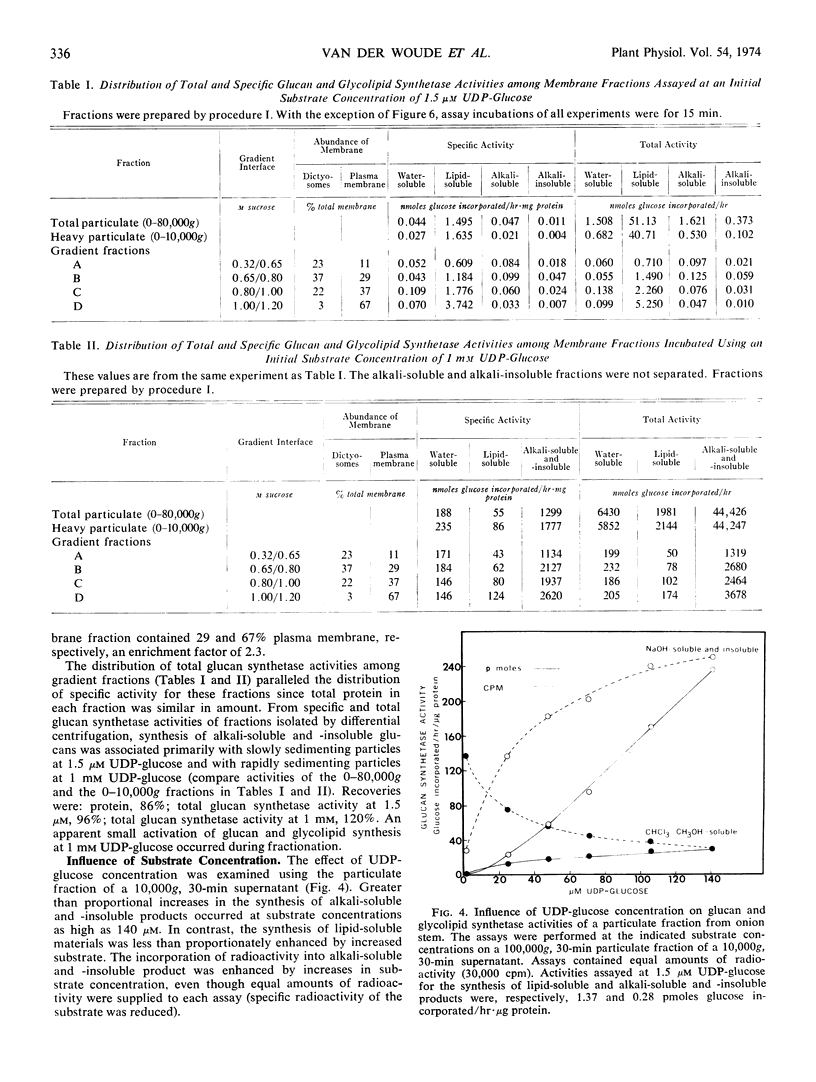
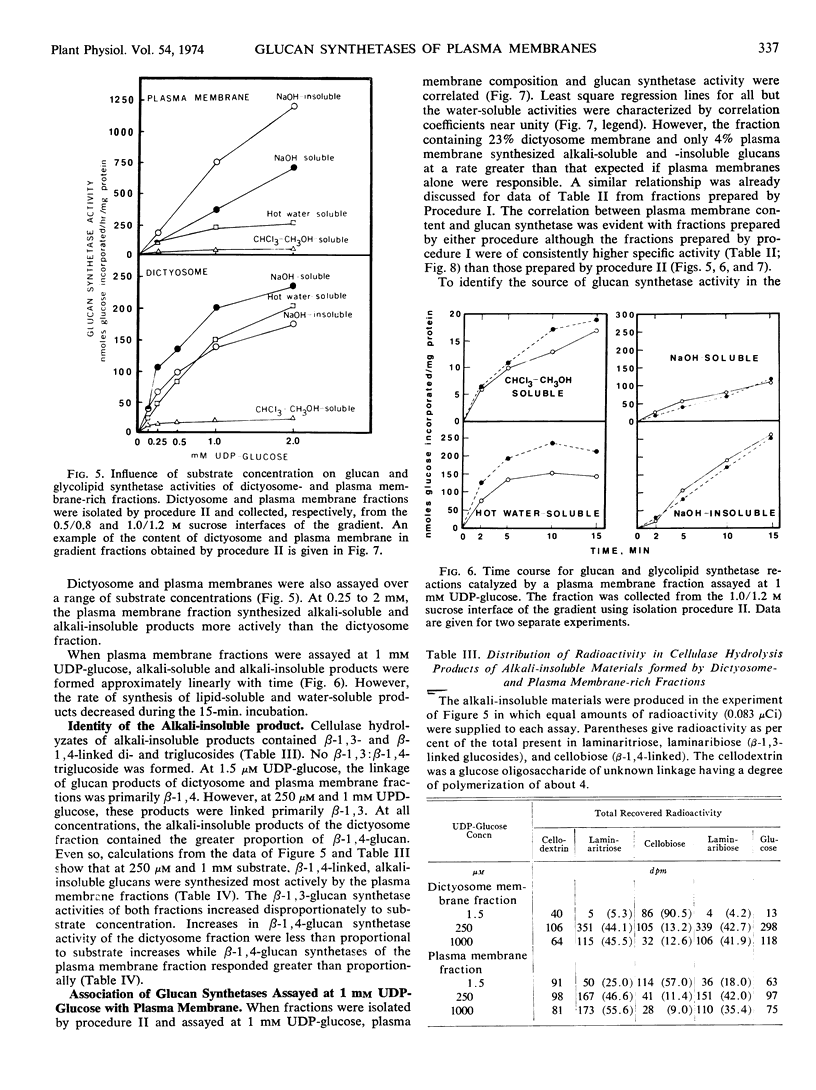


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles D. J., Northcote D. H. The sites of synthesis and transport of extracellular polysaccharides in the root tissues of maize. Biochem J. 1972 Dec;130(4):1133–1145. doi: 10.1042/bj1301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Jr, Franke W. W., Kleinig H., Falk H., Sitte P. Scale formation in chrysophycean algae. I. Cellulosic and noncellulosic wall components made by the Golgi apparatus. J Cell Biol. 1970 May;45(2):246–271. doi: 10.1083/jcb.45.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLVIN J. R. Synthesis of cellulose from ethanol-soluble precursors in green plants. Can J Biochem Physiol. 1961 Dec;39:1921–1926. doi: 10.1139/o61-212. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Morré D. J., Deumling B., Cheetham R. D., Kartenbeck J., Jarasch E. D., Zentgraf H. W. Synthesis and turnover of membrane proteins in rat liver: an examination of the membrane flow hypothesis. Z Naturforsch B. 1971 Oct;26(10):1031–1039. doi: 10.1515/znb-1971-1016. [DOI] [PubMed] [Google Scholar]
- Hardin J. W., Cherry J. H., Morré D. J., Lembi C. A. Enhancement of RNA polymerase activity by a factor released by auxin from plasma membrane. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3146–3150. doi: 10.1073/pnas.69.11.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Polysaccharide formation in plant Golgi bodies. Biochim Biophys Acta. 1971 Apr 20;237(1):56–64. doi: 10.1016/0304-4165(71)90029-8. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H. Eznymatische Glucosylierung von pflanzlichen Sterinen. Z Naturforsch B. 1968 Nov;23(11):1522–1526. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H., CHAMBERS J. E. GLUTARALDEHYDE STABILIZATION AS AN AID TO GOLGI APPARATUS ISOLATION. Exp Cell Res. 1965 Jun;38:672–675. doi: 10.1016/0014-4827(65)90392-7. [DOI] [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H. ISOLATION OF THE GOLGI APPARATUS FROM PLANT CELLS. J Cell Biol. 1964 Nov;23:295–305. doi: 10.1083/jcb.23.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J. In vivo incorporation of radioactive metabolites by Golgi apparatus and other cell fractions of onion stem. Plant Physiol. 1970 Jun;45(6):791–799. doi: 10.1104/pp.45.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcote D. H. Fine structure of cytoplasm in relation to synthesis and secretion in plant cells. Proc R Soc Lond B Biol Sci. 1969 Apr 15;173(1030):21–30. doi: 10.1098/rspb.1969.0033. [DOI] [PubMed] [Google Scholar]
- Ongun A., Mudd J. B. The biosynthesis of steryl glucosides in plants. Plant Physiol. 1970 Mar;45(3):255–262. doi: 10.1104/pp.45.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin L., Hall M. A. Cellulose synthesis in higher plants from UDP glucose. Plant Physiol. 1968 Mar;43(3):473–476. doi: 10.1104/pp.43.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péaud-Lenoël C., Axelos M. Structural features of the beta-glucans enzymatically synthesized from uridine diphosphate glucose by wheat seedlings. FEBS Lett. 1970 Jun 8;8(4):224–228. doi: 10.1016/0014-5793(70)80270-8. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Radioautographic study of cell wall deposition in growing plant cells. J Cell Biol. 1967 Dec;35(3):659–674. doi: 10.1083/jcb.35.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Regulation of beta-Glucan Synthetase Activity by Auxin in Pea Stem Tissue: I. Kinetic Aspects. Plant Physiol. 1973 Apr;51(4):601–608. doi: 10.1104/pp.51.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. G., Preston R. D. Polysaccharide synthesis in mung bean roots--an x-ray investigation. Biochim Biophys Acta. 1972 Jul 19;273(2):336–345. doi: 10.1016/0304-4165(72)90225-5. [DOI] [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Stone B. A. Beta-glucan synthesis by cell-free extracts from Lolium multiflorum endosperm. Biochim Biophys Acta. 1973 Jun 20;313(1):72–94. doi: 10.1016/0304-4165(73)90189-x. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Hassid W. Z. Solubilization and Separation of Uridine Diphospho-d-glucose: beta-(1 --> 4) Glucan and Uridine Diphospho-d-glucose:beta-(1 --> 3) Glucan Glucosyltransferases from Coleoptiles of Avena sativa. Plant Physiol. 1971 Jun;47(6):740–744. doi: 10.1104/pp.47.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Hassid W. Z. Substrate Activation of beta-(1 --> 3) Glucan Synthetase and Its Effect on the Structure of beta-Glucan Obtained from UDP-d-glucose and Particulate Enzyme of Oat Coleoptiles. Plant Physiol. 1973 Jun;51(6):998–1001. doi: 10.1104/pp.51.6.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDerWoude W. J., Lembi C. A., Morré D. J. Auxin (2,4-D) stimulation (in vivo and in vitro) of polysaccharide synthesis in plasma membrane fragments isolated from onion stems. Biochem Biophys Res Commun. 1972 Jan 14;46(1):245–253. doi: 10.1016/0006-291x(72)90656-0. [DOI] [PubMed] [Google Scholar]
- VanDerWoude W. J., Morré D. J., Bracker C. E. Isolation and characterization of secretory vesicles in germinated pollen of Lilium longiflorum. J Cell Sci. 1971 Mar;8(2):331–351. doi: 10.1242/jcs.8.2.331. [DOI] [PubMed] [Google Scholar]
- Wooding F. B. Radioautographic and chemical studies of incorporation into sycamore vascular tissue walls. J Cell Sci. 1968 Mar;3(1):71–80. doi: 10.1242/jcs.3.1.71. [DOI] [PubMed] [Google Scholar]