Pancreatic ductal adenocarcinoma (PDAC) is remarkable for the intense fibro-inflammatory stroma that surrounds the transformed epithelial cells. Rather than being passive bystanders, the stromal cells—both immune and fibroblastic cells—are in a continuous bidirectional communication with the epithelial cells, and, though the production of inflammatory mediators, promote cancer progression and resistance to treatment.
PDAC is a quintessential example of inflammation-driven cancer. In an attempt to explore the epithelial-stromal crosstalk pathways, we studied the role of Toll-like receptors (TLRs) in pancreatitis and pancreatic carcinogenesis. Toll-like receptors are non-catalytic pattern-recognition receptors expressed on a variety of immune cells, which bind conserved microbial sequences denoted PAMPs (pathogen-associated molecular patterns) as well as endogenous by-products of sterile inflammation known as DAMPs (damage-associated molecular patterns).1 All the TLRs, with the exception of TLR3, signal via the MyD88 pathway. TLR3 and TLR4 can signal through the TRIF pathway. Regardless, TLR signaling eventually converges on NFκB and MAPK effector pathways, leading to secretion of pro-inflammatory mediators and affecting such cellular behaviors as proliferation, growth, survival and apoptosis.1,2
We found that TLR4 and TLR7 were upregulated in pancreatic cancer in both mice and humans, whereas they were undetectable in normal pancreata.3,4 These TLRs were overexpressed in both epithelial and stromal cells. We used the well-established p48Cre;LslKrasG12D pancreatic cancer mouse model to study the effects of TLR-MyD88/TRIF signaling on pancreatic carcinogenesis. p48Cre;LslKrasG12D mice treated with either TLR3, TLR4 or TLR7 agonists exhibited a dramatic acceleration of pancreatic cancer progression, characterized by more advanced pre-invasive (PanIN) lesions and a higher number of invasive foci, as well as increased fibrosis and augmented immune infiltrate.3,4 Furthermore, they had a higher fraction of proliferating epithelial cells, as evidenced by higher Ki67 staining, accompanied by a derangement of several proteins involved in cell cycle control. Specifically, TLR7 activation induced loss of p16/INK4A, the gene of which is commonly mutated in human PDAC at a relatively early stage.3 Additionally, we found higher expression of cyclin B1, the oncoprotein c-Myc and the anti-apoptotic protein Bcl-xL, all of which may contribute to the aggressiveness induced by TLR7 agonists.3 These proteins are known targets of STAT3 signaling, which was hyperactivated in mice treated with TLR7 agonists3,5.
Interestingly, we observed a decrease in cyclin D1 along with upregulation of several other tumor-suppressor proteins, such as p53, p21/WAF, pRB and p27/Kip1.3 Several of the upregulated tumor-suppressor proteins have been used as markers of oncogene-induced senescence (OIS), defined as a state of irreversible cell cycle arrest despite sufficient supply of growth factors, nutrients and oxygen.6 It is likely that TLR activation—either directly or indirectly via stromal cell activation—promotes an aggressive phenotype in the at-risk epithelial cells, which, in turn, induces OIS. However, OIS seems to be unable to restrain the at-risk cells which eventually escape and proliferate in an uncontrolled manner, culminating in cancer progression. Alternatively, the upregulation of OIS markers after TLR activation may be related to the injury of the non-transformed tissue surrounding the dysplastic areas as a result of the ongoing inflammation—a scenario consistent with a recent report demonstrating that acinar-to-ductal metaplasia (ADM) is accompanied by a significant increase in p21 and p53 levels.7 It must be noted that these effects of TLR7 activation were dependent on the presence of the KrasG12D mutation, since WT mice undergoing pancreatitis and concurrent administration of TLR7 ligands did not exhibit the above expression patterns of proto-oncogenic and tumor-suppressor proteins, despite having more severe pancreatic injury and inflammation.
Since cells at several different stages of transformation exist at a given time within the microenvironment of pancreatic cancer, it is possible that the concurrent upregulation of classical tumor-suppressor proteins and pro-carcinogenic proteins reflects the non-transformed and transformed cells, respectively, both of which are augmented by TLR stimulation. In this case, the mediators released by non-transformed cells as a result of injury and stress signal to the surrounding immune cells, which, in turn, secrete more inflammatory mediators that are beneficial for the transformed cells, thus leading to cancer progression. TLRs may participate in several phases of this process by binding DAMPs released in the tumor microenvironment from damaged cells (Fig. 1). Notably, we found elevated levels of DAMPs in pancreata of PDAC patients.4
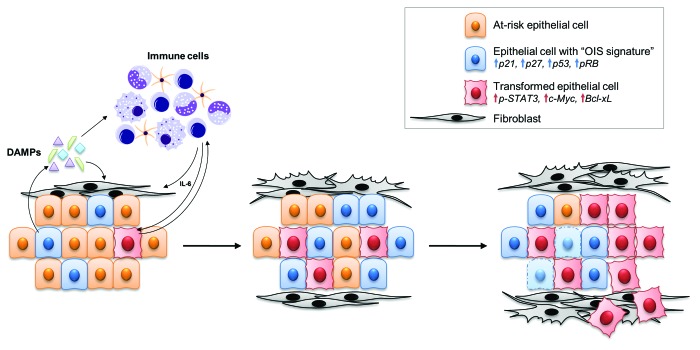
Figure 1. Central role of Toll-like receptors in the tumor-microenvironment of pancreatic cancer. Kras-mutant epithelial cells attract immune cells, which, in turn, promote inflammation and cause stress and damage to pre-malignant epithelial cells. We postulate that epithelial cells may respond to stress by undergoing the process of oncogene-induced senescence (OIS), leading to upregulation of tumor-suppressor proteins such as p21, p27, p53 and pRB. Some of these cells will bypass the barrier of OIS and transform, whereas others will die and release DAMPs. DAMPs, in turn, can bind TLRs on immune cells, leading to worsening inflammation and completing a vicious circle. At the same time, inflammatory mediators such as IL-6 can directly signal to transformed cells and activate pathways such as STAT3 that promote an aggressive cancer phenotype featuring increased expression of oncoproteins such as c-Myc and Bcl-xL. Furthermore, cancer-associated fibroblasts can be activated by released DAMPs either directly or indirectly through the immune cells, causing intense fibrosis and further perpetuating inflammation. Toll-like receptors act as the sensors of the DAMPs and therefore participate in several of the above steps.
We showed that the effects of TLR activation on pancreatic cancer progression were dependent on the stromal cells, as p48Cre;LslKrasG12D mice made chimeric with TLR4−/− or TLR7−/− bone marrow exhibited a delayed rate of pancreatic carcinogenesis.3,4 Treatment of p48Cre;LslKrasG12D mice with a TLR4 or TLR7 inhibitor protected from the accelerated carcinogenesis induced by caerulein. In addition, short treatment of the same mice with a TLR inhibitor was sufficient to reverse the changes observed in most of the aforementioned proteins, but not in pRB or p53.3 The effects of TLR ligation were mediated by the NFκB and MAPK pathways, which were activated in both the epithelial and the stromal cells.3,4 Conversely, blockade of either of these pathways with chemical inhibitors protected from TLR-accelerated pancreatic carcinogenesis.3
In conclusion, we have identified the MyD88 and TRIF downstream pathways of TLRs as mediators of intra-pancreatic cell cycle dysregulation in Kras-mutant cells. TLRs may be activated at multiple levels within the pancreatic cancer microenvironment, and continuous ligation by DAMPs, and possibly PAMPs, can accelerate pancreatic cancer. Therefore, TLR inhibition may be an attractive strategy for therapeutic intervention.
Grant Support
This work was supported by National Institutes of Health Grants CA155649, CA168611, DK085278 and DK098303 (G.M.)
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24488
References
- 1.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 3.Ochi A, Graffeo CS, Zambirinis CP, Rehman A, Hackman M, Fallon N, et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J Clin Invest. 2012;122:4118–29. doi: 10.1172/JCI63606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209:1671–87. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson LG. Oncogene- and tumor suppressor gene-mediated suppression of cellular senescence. Semin Cancer Biol. 2011;21:367–76. doi: 10.1016/j.semcancer.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell ME, DeNicola GM, Martins CP, Jacobetz MA, Maitra A, Hruban RH, et al. Cellular features of senescence during the evolution of human and murine ductal pancreatic cancer. Oncogene. 2012;31:1599–608. doi: 10.1038/onc.2011.350. [DOI] [PMC free article] [PubMed] [Google Scholar]