Abstract
Megakaryocytes (MKs), the platelet precursors, undergo an endomitotic cell cycle that leads to polyploidy. Lysyl oxidase propeptide (LOX-PP) is generated from lysyl oxidase (LOX) pro-enzyme after proteolytical cleavage. We recently reported that LOX, a known matrix cross-linking enzyme, contributes to MK lineage expansion. In addition, LOX expression levels are ploidy-dependent, with polyploidy MKs having minimal levels. This led us to test the effects of LOX-PP on the number and ploidy of primary MKs. LOX-PP significantly decreases mouse bone marrow MK ploidy coupled with a reduction in MK size. MK number is unchanged upon LOX-PP treatment. Analysis of LOX-PP- or vehicle-treated MKs by western blotting revealed a reduction in ERK1/2 phosphorylation and in the levels of its downstream targets, cyclin D3 and cyclin E, which are known to play a central role in MK endomitosis. Pull-down assays and immunochemistry staining indicated that LOX-PP interacts with α-tubulin and the mictotubules, which can contribute to decreased MK ploidy. Thus, our findings defined a role for LOX-PP in reducing MK ploidy. This suggests that high-level expression of LOX in aberrantly proliferating MKs could play a part in inhibiting their polyploidization via LOX-PP.
Keywords: lysyl oxidase propeptide, platelet, megakaryocyte, polyploidy, cyclin
Introduction
Lysyl oxidase (LOX) is responsible for the intermolecular cross-linking of elastin or collagen by oxidative deamination of peptidyl lysine or hydroxylysine and peptidyl lysine residues, respectively, and contributes to the accumulation of extracellular matrix by promoting intrapeptide and interpeptide chain crosslinking.1 LOX gene encodes a 50 kDa pro-enzyme (Pro-LOX), which is proteolyticaly cleaved by pro collagen C-proteinases to a 30 kDa functional LOX enzyme and an 18 kDa N-terminal LOX propeptide (LOX-PP).2 LOX-PP can then enter the cell to exert its function. A study by Guo et al.3 clearly demonstrates the intracellular localization of LOX-PP. However, the mechanism by which LOX-PP enters the cells remains elusive. It has been postulated that LOX-PP is taken up by the cells without the action of channels or membrane receptors due to its high isoelectric point.3 LOX-PP has been reported to inhibit growth and promote apoptosis of pre-existing breast cancer cells,4 and recent studies in lung cancer, oral cancer and pancreatic cancer cell lines also showed that LOX-PP has tumor-suppressor properties.5-13
Megakaryocytes (MKs), the platelet precursors, undergo endomitosis, which results in polyploid cells.14 Ploidy control is a key to understanding MK biology and platelet production.15 Platelet production and release from a single large MK is more efficient than that from several smaller ones.16 Furthermore, a subset of hematological disorders is marked with increased low-ploidy (8N) MKs.17 In primary myelofibrosis (PMF), studies demonstrated that the MKs derived from patient CD34+ cultures (or from CD45+ cultures) are of lower ploidy compared with controls.18,19 Importantly, in conditions where platelet levels are elevated, such as essential thrombocytosis (ET) or polycythemia vera (PV), the MK ploidy levels are increased.20 Major regulators of this process include upregulation of cyclin D321 and cyclin E, which has a role in endomitosis beyond its effect on mitotic cells.22,23 The process is also dependent on downregulation of non-muscle myosin heavy chain IIB.24 ERK1/2 signaling is important to MK differentiation,25 maturation and endomitosis,26 and this pathway upregulates G1 cyclins expression,27-29 which are necessary for MK ploidy.23 In this context, it has been reported that LOX-PP inhibits ERK1/2 activation in a variety of normal and cancer cell lines7,8,12,30. In addition, LOX-PP also inhibits serum and fibroblast growth factor 2 (FGF-2)-mediated ERK1/2 and Akt phosphorylation in human prostate cancer cell lines.10
The myeloproliferative neoplasms (MPN) include primary myelofibrosis (PMF) or post-ET/PV myelofibrosis, which are characterized by numerous dysplastic MKs and the presence of bone marrow fibrosis.31 Using the Gata-1low mouse model, which recapitulates the essential characteristics of myelofibrosis, our laboratory recently uncovered the role of LOX in progression of fibrosis in the bone marrow in the presence of immature MKs.32 LOX is highly expressed in diploid, but not in mature polyploid MKs, which may account for the progression of fibrosis in such MPN.32 The downregulation of LOX in polyploid MKs suggests a potentially negative effect of this protein, or of its derivative, LOX-PP, on MK polyploidization.
On the other hand, ET and frequently PV can be accompanied by severe thrombocytosis, which can lead to life threatening thrombotic events33 or, paradoxically, bleeding complications.34
Given the role of LOX-PP as a negative regulator of proliferation in a number of cancer cell lines as well as the differential pattern of LOX expression in MKs, we sought to examine the direct influence of LOX-PP on MKs. We demonstrate that MK endomitosis is inhibited in the presence of LOX-PP, along with profound changes in cyclins E and D3.
Results
LOX-PP reduces megakaryocyte ploidy, but not megakaryocyte number
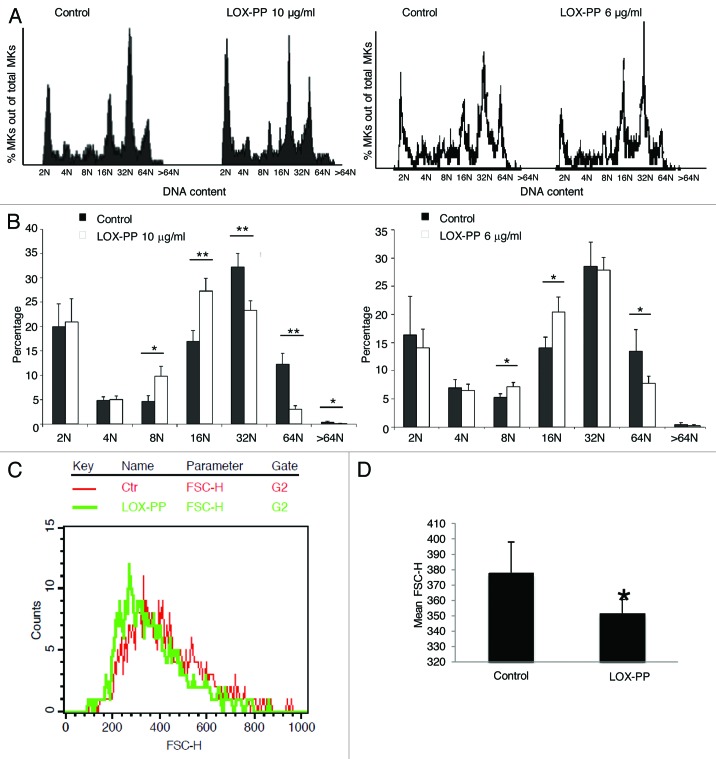
Since LOX is severely downregulated in high-ploidy MKs, and inhibition of LOX activity does not affect MK ploidy, as tested with a LOX inhibitor,32 we examined the possibility that LOX-PP, lacking enzyme activity, affects MK ploidy. Here, mouse primary MK cultures were treated with vehicle (H2O) or two different concentrations of recombinant LOX-PP. Quantitative data based on flow cytometry analysis revealed a statistically significant decrease in high ploidy level (≥ 32 N) MKs upon treatment with LOX-PP (Fig. 1A and B). MK sizes were also compared based on flow cytometry analysis. Consistent with a decrease in ploidy level, the overall MK size was smaller in LOX-PP-treated cells than that in vehicle-treated MKs (Fig. 1C and D). No evidence for apoptosis was detected in LOX-PP-treated cultures as indicated by no difference in the fractions of < 2 N cells measured by flow cytometry or in trypan blue staining (data not shown). This confirms that the effect of LOX-PP is not due to the degradation of high-ploidy MKs, but due to blocking endomitosis. We previously reported that LOX can enhance MK expansion.32 To examine whether LOX-PP affects this process in a similar manner as LOX, MK number was calculated based on CD41-positive staining detected by flow cytometry analysis. No significant difference in MK percentage or total MK cell number in bone marrow was observed between the experimental groups (Fig. 2A and B). The expression levels of the MK markers CD41 and PF4 were compared between these two groups, and no significant difference was observed (Fig. 2C).
Figure 1. Effect of LOX-PP on MK ploidy and size. (A) Bone marrow cultures were treated with 25 ng/ml TPO in the presence of 10 µg/ml or 6 µg/ml LOX-PP or vehicle (H2O) for 3 d. Cells were subjected to MK ploidy analysis, using flow cytometry. Representative histogram plots for vehicle and LOX-PP treatments are shown. (B) Quantification of ploidy status per group. Data are averages of at least four experiments ± SD. Statistical analysis was applied using the Student’s t-test, *p < 0.05, **p < 0.01. (C) Estimation of MK size based on FACS analysis. MKs were gated based on CD41 expression on a FSC-H histogram plot. A representative plot is shown (red, control; green, Lox-PP). (D) Quantification of MK size based on mean forward scatter (FSC-H) values. Statistical analysis was applied using the Student’s t-test, n = 5, *p < 0.05.
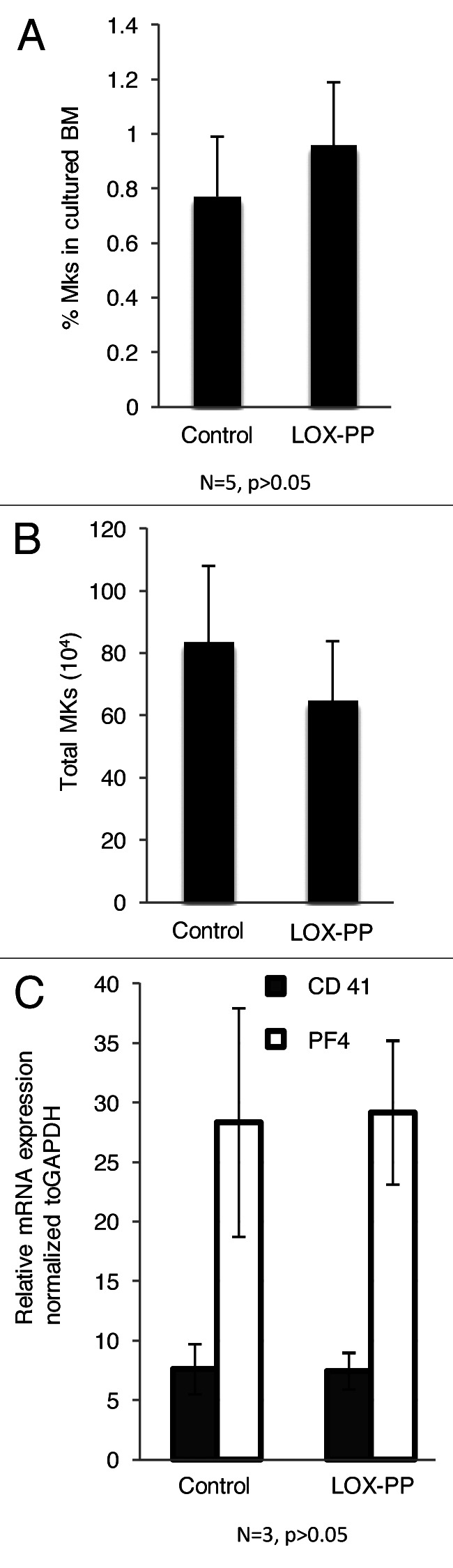
Figure 2. Effect of LOX-PP on MK number and MK maturation marker gene expression level. (A) Bone marrow cells were isolated and cultured as described in Figure 1A. Percentage of MKs was based on CD41-FITC staining with FACS analysis. (B) Total MK number was calculated based on cultured BM cell number and % MK from (A). Data are averages of ± SD of five experiments. Statistical analysis was applied using the Student’s t-test. No statistical significance was found, p > 0.05. C. qRT-PCR of isolated MKs 9, see “Materials and Methods”), pre-treated with either control or 10 µg/ml LOX-PP to evaluate CD41 and PF4 mRNA levels. Data are averages of ± SD of three experiments. Statistical analysis was applied using the Student’s t-test. No statistical significance was found, p > 0.05.
LOX-PP decreases the expression of cell cycle regulators
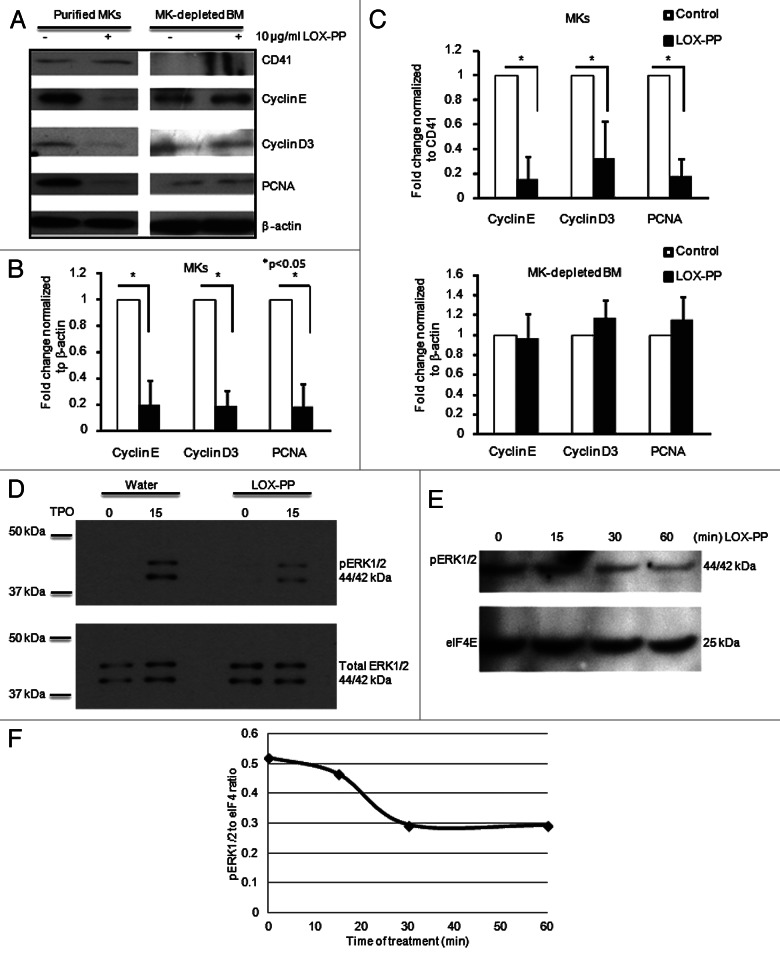
In order to further understand the mechanism by which LOX-PP is involved in controlling MK ploidy, the level of cell cycle regulators, cyclin D3 and cyclin E, which play important roles in this process,21-23 were examined by western blotting. There was a significant decrease in expression of these cyclins upon exposure to LOX-PP in TPO-treated cultures (Fig. 3). In accordance, the level of the S phase marker, proliferating cell nuclear antigen (PCNA), was also diminished in LOX-PP-treated MKs (Fig. 3A–C). In order to assert that the observed decrease was not caused by less MKs in the LOX-PP-treated samples, the expression levels of these three proteins were also normalized to CD41 (Fig. 3C). Interestingly, PCNA, cyclin D3 and cyclin E expression levels did not change in MKs-depleted bone marrow samples (Fig. 3A and B). This is not surprising, considering that the vast majority of these cells does not harbor the TPO receptor and, hence, are mildly or not proliferating. The level of cyclin E is lower in the non-MK fraction, compared with MKs (Fig. 3A). While low level cyclin E is sufficient to drive a mitotic cell cycle, high level cyclin E is needed for MK to reach higher ploidy.22,23
Figure 3. Effect of LOX-PP on ERK1/2 signaling and G1/S phase regulators. (A) BM cells were cultured in StemSpan serum-free media (SFEM) with 25 ng/ml TPO in the presence or absence of 10 µg/ml LOX-PP for 3 d and MKs were purified as described in “Materials and Methods.” The MK depleted BM cells were used for analysis as well. Western blot analysis was performed using antibodies against CD41 (MK marker), Cyclin D3, Cyclin E and PCNA. β-actin was used as a loading control. (B and C) Quantification of western blots was performed using the ImageJ software. Band densities were normalized to β-actin (B) and CD41 (C). Statistical analysis was applied using the Student’s t-test, n = 3, *p < 0.05. (D–F) BM cultures were cultured as in Figure 2A and MKs were isolated using MACS purification (see “Materials and Methods”). MK-enriched BM cultures were then re-cultured in serum-free media for 12 h and treated with vehicle (D) or 10 µg/ml LOX-PP (D and E) for the indicated times. Cell lysates were subjected to western blot analysis using anti-pERK1/2 (D and E) and anti-ERK1/2 (D). eIF4E is used as loading control (E). Shown here is one out of two representative experiments. (F) Quantification of the band density in (E) was performed using the ImageJ software.
Considering that G1 phase cyclin D1 expression is regulated by ERK1/2 signaling,35-37 and that TPO is known to signal via ERK1/2,38 we examined the possibility that LOX-PP affects ERK1/2 activation in MKs in a manner similar to its effect on ERK1/2 in other cells.4,7,8,10,12,30,39 To this end, we compared phosphorylated ERK1/2 in MKs from vehicle or LOX-PP treated cultures by western blotting. The ratio of phosphorylated ERK1/2 to total ERK1/2 was decreased within 15 min and kept decreasing until 30 min after LOX-PP was added into the medium (Fig. 3D–F).
Taken together, these data suggest that LOX-PP inhibits TPO-induced signaling to ERK1/2, the expression level of G1 cyclins and MK ploidy level. It is then not surprising that MKs downregulate LOX/LOX-PP expression as they become polyploid.32
LOX-PP interacts with microtubules in polyploid megakaryocytes
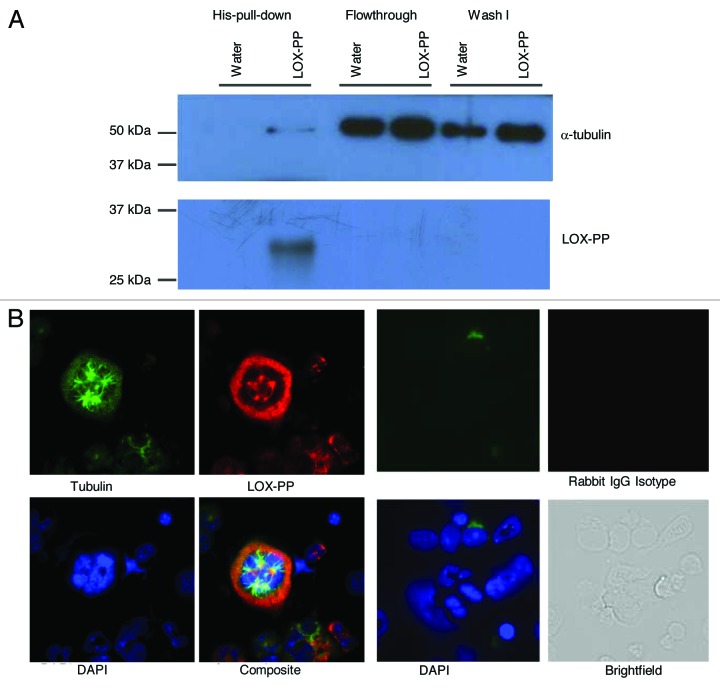
As means of further exploring its mechanism of action, we used a pull-down assay to identify proteins associated with LOX-PP in MKs. In accordance with an early report in HEK293T cells,7 direct binding of his-tagged recombinant LOX-PP with α-tubulin was observed in MKs when using pull-down assays (Fig. 4A). Immunofluorescence analysis confirmed co-localization of LOX-PP to the microtubules (Fig. 4B). The interaction of LOX-PP with the microtubules might affect ERK1/2 activation,10,30 since Raf and ERK1/2 are known to be associated with microtubules.40 A similar pull-down assay, using anti-c-Mpl showed no binding of LOX-PP to the TPO receptor (data not shown). This was not surprising since LOX-PP affects MK ploidy and minimally (or not) MK expansion, while both processes depend on c-Mpl.
Figure 4. LOX-PP interacts with tubulin in polyploidy MK. (A) Purified MKs were treated with either vehicle (H2O) or 10 µg/ml LOX-PP for 24 h (see “Materials and Methods”). The samples were lysed and incubated with Ni-NTA magnetic agrose beads for 1 h at 4°C. After wash, the elution fractions were examined by western blot analysis with the indicated antibodies. (B) Immunofluorescence analysis of LOX-PP. Mouse BM cells were cultured in the presence of 25 ng/ml TPO in IMDM media supplemented with 10% bovine calf serum BM cells were collected, fixed and stained, as described in “Materials and Methods.” LOX-PP was detected with rabbit polyclonal anti-LOX-PP and Alexa 594 anti-rabbit secondary antibody, α- tubulin was detected with mouse monoclonal anti-tubulin and Alexa 488 anti-mouse secondary antibody, and DNA was stained with DAPI. Staining with rabbit IgG was also performed to verify the specificity of staining with LOX-PP antibody (left panel).
Discussion
LOX-PP is produced extracellularly upon LOX processing, but it re-enters the cells3. Thus, cells with appreciable expression of LOX, including normally developing low-ploidy MKs generate LOX-PP. MKs downregulate LOX as they become of high ploidy.32 On the other hand, LOX is highly expressed in acute myeloid leukemia,41 and in a mouse model of abundant low-ploidy MKs.32 This raised the question whether LOX, or its product, LOX-PP has a role in controlling MK ploidy. Considering that high-ploidy MKs do not express LOX or LOX-PP, to mimic this expression in normally developing low-ploidy MKs, we needed to resort to recombinant LOX-PP. Several other studies employed recombinant LOX-PP5-7,10,12,13,39 to gauge its effect on cellular processes. For example, LOX-PP reduces the expression level of B lymphocyte-induced maturation protein 1 (Blimp1) by binding to c-Raf and inhibiting AP-1 activation, thus attenuating the migratory phenotype of lung cancer cells.5 Here, we showed that LOX-PP decreases MK ploidy level.
LOX-PP significantly downregulated cyclin D3 and cyclin E in MK cultures when induced to polyploidize by TPO. Both of these cyclins have been shown to be important for the promotion of MK endomitosis.22,23,42 Cyclin E is dispensable for mitotic proliferation but not for full polyploidization.23 Thus, it would be plausible for LOX-PP to affect high-ploidy MKs and not the low-ploidy proliferating ones. This could also explain the lack of effect of LOX-PP on the number of MKs. Analysis of S phase hallmarks, Cdc6 and Mcm2, indicates that cyclin E promotes MK progression to S phase and cell cycling by promoting the expression of the pre-replication complex components.22 Overexpression of cyclin E in MKs upregulates cyclin B1, which is associated with polyploidy in many cell types.22 The level of G1 phase cyclins is highly regulated by ERK1/2 signaling.36,37 Here, we also show that LOX-PP inhibits ERK1/2 activation in MKs.
Raf/MEK/ERK signaling is involved in many cellular functions and is required for TPO functions on hematopoietic cells.43-45 It is also important for megakaryocytic cell growth arrest.46 The involvement of ERK1/2 pathway in megakaryocytic differentiation has been shown in primary hematopoietic progenitors,47 and sustained activation of the ERK1/2 pathway is required for MK differentiation.25 Thus, a likely explanation for LOX-PP inhibition of TPO-induced ERK1/2 activity is that LOX-PP interacts with and inhibits c-Raf.7 LOX-PP has also been shown to bind to the fibroblast growth factor receptor (FGF-2).39 In our study, the decreased levels of cyclin D3 and cyclin E in MKs and unchanged levels of these cyclins in other bone marrow cells (that do not express the TPO receptor) can support a possible effect of LOX-PP on cells bearing the TPO receptor. More likely, however, these cells are not induced to cycle in our culturing conditions, and their G1 cyclin levels are low to begin with. Tetraploidy or high level ploidy has been found in certain cancers, and this cell cycle state is associated with chromosomal rearrangement and poor prognosis.48 Our results raise the hypothesis that LOX-PP might also inhibit cancer development4,8,10,12 by regulating their level of ploidy, preventing endomitotic cell cycles via downregulation of cyclin E to a level below a threshold for endoreduplication. It has been reported that in differentiating osteoblastic cells LOX-PP co-localizes with the microtubule network.3 Consistent with previous results, we also observed co-localization of LOX-PP and α-tubulin in MKs further confirmed by LOX-PP pull-down assay. Microtubules are cytoskeletal structure proteins which are important for different cellular functions, such as the determination of cell division, cell movement, intracellular transport, cell shape and polarity as well as signal transduction.49-52 It also has been shown that microtubule depolymerization early in the cell cycle is sufficient to initiate DNA synthesis53 and induce polyploidy, including in MKs.54 It is possible that LOX-PP contributes to a decrease in DNA synthesis in MKs during endomitosis by direct binding to tubulin. For instance, because ERK1/2 can phosphorylate mitogen-activated protein (MAP) kinase, it has been suggested that it contributes to instability of microtubule subsets.55,56 Decreased ERK1/2 activity in LOX-PP treated MKs may increase the stability of microtubules.
Our findings that LOX-PP affects polyploid megakaryocytes could have therapeutic implications. Namely, elucidation of the mechanism(s) that LOX-PP inhibits both polyploidization and MK size increase may lead to targeted therapies, which will reduce the amount of produced platelets. The encouraging finding that LOX-PP mechanism(s) of action were not associated with toxicity is particularly encouraging for the treatment of indolent MPN associated with thrombocytosis such as PV or ET. Currently, treatment options especially for high-risk patients are limited and are not devoid of serious side effects.57,58
Furthermore, because LOX-PP is the by-product of LOX production in low-ploidy MKs, and, as we have shown before,59 LOX expression is abundant in a mouse model recapitulating myelofibrosis, a provocative hypothesis would be that LOX-PP by inhibiting polyploidization act as a enhancer for the expansion of dysplastic MKs. If future studies support this notion then molecules that interfere with LOX-PP function may ameliorate myelofibrosis by de-repressing polyploidization. More specifically, it can be envisioned that if the amount of LOX released by MKs is reduced (due to de-repressed plolyploidization), then the positive feedback mechanism of PDGF-mediated expansion of MKs would be impaired. Seminal studies were reported exploring the potential therapeutic impact of polyploidizing agents in the context of MPN60 and acute megakaryoblastic leukemia (AMKL).61 These potential approaches merit further attention, as the clinical trials from JAK2 inhibitors point toward a palliative rather than a curative content.62-64
In summary, the results of our present study indicate that LOX-PP exerts a potent effect in MK polyploidization through mechanism(s) that include interaction with α-tubulin, modulation of ERK1/2 phosphorylation and reduction in the levels of G1 phase cyclins.
Materials and Methods
Bone marrow isolation and culture
Wild type FVB mice (6–8-wk-old) were used to isolate bone marrow from both femurs and tibias as previously described.22,65 Bone marrow cells were cultured at a density of 5 × 106 cells/ml in 1 ml plain IMDM in the presence of 10 µg/ml LOX-PP or equal volume dH20 (vehicle). Fifteen min post plating 50 ng TPO was added in all samples and cells were incubated for another 45 min. Bone marrow cultures were supplemented with 1ml IMDM with 10% BCS and penicillin/streptomycin (P/S) to obtain a final volume of 2 ml IMDM with 5% BCS, 1% 100 units/ml penicillin, 100 µg/ml streptomycin (Cellgro, cat. 30-002-CI), 25 ng/ml TPO (human PEG-rhMGDF, gift from Kirin Pharma Company). LOX-PP or vehicle were also added to achieve a final concentration of 10 μg/ml. Cells were cultured for 3 d at 37°C and 5% CO2 humidified conditions. Experiments were repeated also using a lower concentration of LOX-PP (6 µg/ml). Experiments were also conducted in serum-free conditions [StemSpan serum-free media (Stem Cell technologies, cat. 09650) supplemented with 2 mM L-Glutamine (Hyclone, cat. SH3003H.01)] and yielded similar results. All studies involving mice were approved by the Boston University Animal Care and Use Committee.
Megakaryocyte (MK) enrichment by magnetic activated cell sorting
MK enrichment from bone marrow cultures was performed as described previously32 using anti-CD41-Fluorescein isothiocyanate (FITC) (BD PharMingen, cat. 553848) at a cell concentration of 10 × 106 cells/ml and 1:150 diluted antibody to stain MKs, followed by incubation with anti-FITC labeled microbeads (10 µl per 10 × 106 cells, Miltenyi Biotech, cat. 37-048-701). Bone marrow was loaded onto an equilibrated large cell separation column (Miltenyi Biotech, cat. 130-042-202). The final eluent containing the MK-enriched fraction of the bone marrow was used for subsequent analysis. The high purity of this fraction (about 83%) was confirmed as in reference 22. This percentage of purity is an underestimation considering the larger mass of polyploid MKs as compared with diploid cells.
Analysis of polyploidy by flow cytometry
Bone marrow cultured cells were washed in Mg2+/Ca2+-free phosphate buffered saline (PBS) (Corning Cellgro, Cat. 21-031-CV) followed by fixation with 70% ethanol. Cells were washed twice with PBS and resuspended in staining solution (10% bovine calf serum). FITC-conjugated anti-CD41 (BD-PharMingen, cat. 553848) or FITC-conjugated rat-IgG1 κ isotype control (BD-PharMingen, cat. 554684) antibody was added at a dilution of 1:200 (0.625 µg/1 × 106 cells) and incubated at 4°C for 30 min. After washing twice with PBS, cells were treated with 0.1 mg/ml ribonuclease A (Sigma, cat. R51), followed by DNA staining with 0.05 mg/ml propidium iodide (Invitrogen, cat. P1304MP). Cells were incubated for 30 min at 37°C prior to analysis on a BD-FACS Calibur flow cytometer equipped with a 488 nm laser.
mRNA preparation and transcript analysis
RNA isolation was performed using the RNeasy Micro kit (Qiagen, cat. 74004) following the manufacturer's instructions. For cDNA preparation, reverse transcription was achieved using the QuantiTect Reverse Transcription kit (Qiagen, cat. 205310). The cDNA was used for quantitative reverse transcriptase PCR, performed using mouse CD41 (Mm00439741_m1) and PF4 (Mm00451315_g1) TaqMan® gene expression primers and probes (Applied Biosystems). Samples were run on an Applied Biosystems Sequence Detection System 7300. Data were normalized to GAPDH (Applied Biosystems, 4352339E) and analyzed using the ΔΔCT method.
Immunofluorescence
Bone marrow cells suspended in PBS were cytospun (3 × 105 cells/slide) to microscope slides (Fisher Scientific, cat. 12-550-15) using a Cytospin3 (Shandon) for 5 min at 120 × g. Cells were fixed in 4% fresh paraformaldehyde at room temperature for 10 min followed by permebealization in ice-cold 0.2% triton x-100 for 20 min. Slides were then blocked with 2% donkey serum or 10% bovine calf serum for 20 min at room temperature, followed by staining with the following antibodies: mouse monoclonal anti-α tubulin (1:400 dilution, Santa Cruz, cat. sc-5286), Alexa-488 anti-mouse IgG (1:800, Life technologies, cat. A1101), rabbit anti-LOX-PP (4 µg/ml, gift from Dr Philip Trackman),3 Alexa-594 anti-rabbit IgG (1:200, Life technologies, cat. A11012) and rabbit IgG (Vector Labs). All slides were observed with an Olympus 70× inverted fluorescence microscope with a 40× objective. Images were documented with a Hamamatsu CCD camera (Hamamatsu photonics) and analyzed with ImagePro software (Media Cybernetics Inc.).
Western blot analysis
Cells were collected and total protein was extracted using radio immuno precipitation assay (RIPA) buffer (25 mM Tris•HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate) supplemented with proteinase inhibitor cocktail (Roche, cat. 11697498001) and phosphatase inhibitor cocktail (Roche, 04906837001). Protein concentration was assayed using the Bradford protein assay (Biorad, cat. 500-0006). Sodium dodecyl sulfate loading buffer was added to the protein lysate that was then subjected to SDS-PAGE, followed by transfer onto a PVDF membrane. Proteins were detected by western blotting with the following antibodies as indicated: rabbit polyclonal anti-CD41 (1:1,000, Santa Cruz Biotechnology, cat. sc-15328), rabbit polyclonal anti-Cyclin E (1:1,000, Santa Cruz Biotechnology Inc., cat. sc-481), anti-Cyclin D3 (1:1,000, Santa Cruz Biotechnology Inc., cat. sc-182), PCNA (Ab-1) (1:1,000, Oncogene Research Products, cat. NA03-200 µg) rabbit monoclonal anti-phospho p44/p42 MAPK (1:200, ERK1/2, diluted 500-fold, Cell Signaling, cat. 4370), rabbit monoclonal p44/42 MAPK (Erk1/2) (1:200, Cell Signaling, cat 4695). Anti-eIF4 (1:200, Cell Signaling, cat. C46H6) and β-actin (1:10,000, Sigma, cat. A5441) antibodies were used as loading control, anti-rabbit IgG (Cell signaling, cat. 7074), goat anti-mouse IgG (Santa Cruz Biotechnology Inc., cat. sc-2060) were used as control. Finally, membranes were developed using the Immobilon Western Chemiluminescent HRP Substrate (MIllipore, cat. WBKLS0500).
Pull-down assay
Mouse bone marrow cells were cultured at a density of 1 × 107 cells/ml in IMDM medium (Gibco, Cat. 12440) with 2 mM L-Glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin, 10% bovine calf serum and 25 ng/ml TPO. MK enrichment from bone marrow cultures was performed using a BSA gradient method66 and repeated twice. MKs were resuspended in IMDM medium (10% serum and 25 ng/ml TPO) with a cell concentration of 5 × 104 cells/ml and treated with vehicle (H2O) or 10 µg/ml recombinant LOX-PP. After 24 h, MKs were collected and prepared following the manufacture’s protocol for Ni-NTA magnetic agrose beads (Qiagen, Cat. 36111). Briefly, MKs were lysed in pull down buffer [400 mM NaCl, 25 mM sucrose, 20 mM Imidazole, 0.5% Chaps and 1 mM phenylmethylsulfonyl fluoride (PMSF) in Mg2+/Ca2+ free Dulbecco’s phosphate-buffered saline] supplemented with proteinase inhibitor cocktail for 15 min. Cell lysates were passed through a 25 G needle for 5× and centrifuged for 5 min at 13,000 rpm at 4°C in a microcentrifuge to remove insoluble material. Ni-NTA magnetic agrose beads (Qiagen, Cat. 36111) were added to the supernatant and incubated for 1 h at 4°C with rotation. The beads were washed four times with pull down buffer and eluted by 450 mM Imidazole in pull down buffer. The elution fractions were collected, subjected to western blotting using anti α-tubulin antibody (1:1,000 dilution; Sigma, Cat. T6199) or anti LOX-PP antibody (1:10,000 dilution; 2.69 µg/µl, gift from Dr Philip Trackman)3 or anti-c-Mpl antibody (1:1,000 dilution) (Millipore, Cat. 06-944). In the latter case, a 10% SDS gel was used to visualize an expected band of c-Mpl of 70kDa.
Acknowledgments
K.R. is supported by NHLBI grant HL80442.
Glossary
Abbreviations:
- BM
bone marrow
- MK
megakaryocyte
- LOX-PP
lysyl oxidase propeptide
- LOX
lysyl oxidase
- ERK1/2
extracellular signal-regulated kinases ½
- FGF-2
fibroblast growth factor 2
- PCNA
proliferating cell nuclear antigen
- Blimp1
B lymphocyte-induced maturation protein 1
- MAP
mitogen-activated protein
- MPN
myeloproliferative neoplasms
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24312
References
- 1.Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–10. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- 2.Cronshaw AD, Fothergill-Gilmore LA, Hulmes DJ. The proteolytic processing site of the precursor of lysyl oxidase. Biochem J. 1995;306:279–84. doi: 10.1042/bj3060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y, Pischon N, Palamakumbura AH, Trackman PC. Intracellular distribution of the lysyl oxidase propeptide in osteoblastic cells. Am J Physiol Cell Physiol. 2007;292:C2095–102. doi: 10.1152/ajpcell.00613.2006. [DOI] [PubMed] [Google Scholar]
- 4.Bais MV, Nugent MA, Stephens DN, Sume SS, Kirsch KH, Sonenshein GE, et al. Recombinant lysyl oxidase propeptide protein inhibits growth and promotes apoptosis of pre-existing murine breast cancer xenografts. PLoS ONE. 2012;7:e31188. doi: 10.1371/journal.pone.0031188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Z, Sato S, Trackman PC, Kirsch KH, Sonenshein GE. Blimp1 activation by AP-1 in human lung cancer cells promotes a migratory phenotype and is inhibited by the lysyl oxidase propeptide. PLoS ONE. 2012;7:e33287. doi: 10.1371/journal.pone.0033287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-Morgan N, Kirsch KH, Trackman PC, Sonenshein GE. The lysyl oxidase propeptide interacts with the receptor-type protein tyrosine phosphatase kappa and inhibits β-catenin transcriptional activity in lung cancer cells. Mol Cell Biol. 2011;31:3286–97. doi: 10.1128/MCB.01426-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato S, Trackman PC, Mäki JM, Myllyharju J, Kirsch KH, Sonenshein GE. The Ras signaling inhibitor LOX-PP interacts with Hsp70 and c-Raf to reduce Erk activation and transformed phenotype of breast cancer cells. Mol Cell Biol. 2011;31:2683–95. doi: 10.1128/MCB.01148-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min C, Zhao Y, Romagnoli M, Trackman PC, Sonenshein GE, Kirsch KH. Lysyl oxidase propeptide sensitizes pancreatic and breast cancer cells to doxorubicin-induced apoptosis. J Cell Biochem. 2010;111:1160–8. doi: 10.1002/jcb.22828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min C, Yu Z, Kirsch KH, Zhao Y, Vora SR, Trackman PC, et al. A loss-of-function polymorphism in the propeptide domain of the LOX gene and breast cancer. Cancer Res. 2009;69:6685–93. doi: 10.1158/0008-5472.CAN-08-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palamakumbura AH, Vora SR, Nugent MA, Kirsch KH, Sonenshein GE, Trackman PC. Lysyl oxidase propeptide inhibits prostate cancer cell growth by mechanisms that target FGF-2-cell binding and signaling. Oncogene. 2009;28:3390–400. doi: 10.1038/onc.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Min C, Vora SR, Trackman PC, Sonenshein GE, Kirsch KH. The lysyl oxidase pro-peptide attenuates fibronectin-mediated activation of focal adhesion kinase and p130Cas in breast cancer cells. J Biol Chem. 2009;284:1385–93. doi: 10.1074/jbc.M802612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M, Min C, Wang X, Yu Z, Kirsch KH, Trackman PC, et al. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res. 2007;67:6278–85. doi: 10.1158/0008-5472.CAN-07-0776. [DOI] [PubMed] [Google Scholar]
- 13.Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, et al. The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer Res. 2007;67:1105–12. doi: 10.1158/0008-5472.CAN-06-3867. [DOI] [PubMed] [Google Scholar]
- 14.Ravid K, Lu J, Zimmet JM, Jones MR. Roads to polyploidy: the megakaryocyte example. J Cell Physiol. 2002;190:7–20. doi: 10.1002/jcp.10035. [DOI] [PubMed] [Google Scholar]
- 15.Mattia G, Vulcano F, Milazzo L, Barca A, Macioce G, Giampaolo A, et al. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99:888–97. doi: 10.1182/blood.V99.3.888. [DOI] [PubMed] [Google Scholar]
- 16.Kaushansky K. The enigmatic megakaryocyte gradually reveals its secrets. Bioessays. 1999;21:353–60. doi: 10.1002/(SICI)1521-1878(199904)21:4<353::AID-BIES12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 17.Bessman JD. The relation of megakaryocyte ploidy to platelet volume. Am J Hematol. 1984;16:161–70. doi: 10.1002/ajh.2830160208. [DOI] [PubMed] [Google Scholar]
- 18.Ciurea SO, Merchant D, Mahmud N, Ishii T, Zhao Y, Hu W, et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood. 2007;110:986–93. doi: 10.1182/blood-2006-12-064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balduini A, Badalucco S, Pugliano MT, Baev D, De Silvestri A, Cattaneo M, et al. In vitro megakaryocyte differentiation and proplatelet formation in Ph-negative classical myeloproliferative neoplasms: distinct patterns in the different clinical phenotypes. PLoS ONE. 2011;6:e21015. doi: 10.1371/journal.pone.0021015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsson S, Carneskog J, Ridell B, Wadenvik H, Swolin B, Kutti J. Flow cytometric analysis of megakaryocyte ploidy in chronic myeloproliferative disorders and reactive thrombocytosis. Eur J Haematol. 1996;56:287–92. doi: 10.1111/j.1600-0609.1996.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 21.Zimmet JM, Ladd D, Jackson CW, Stenberg PE, Ravid K. A role for cyclin D3 in the endomitotic cell cycle. Mol Cell Biol. 1997;17:7248–59. doi: 10.1128/mcb.17.12.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eliades A, Papadantonakis N, Ravid K. New roles for cyclin E in megakaryocytic polyploidization. J Biol Chem. 2010;285:18909–17. doi: 10.1074/jbc.M110.102145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431–43. doi: 10.1016/S0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 24.Lordier L, Bluteau D, Jalil A, Legrand C, Pan J, Rameau P, et al. RUNX1-induced silencing of non-muscle myosin heavy chain IIB contributes to megakaryocyte polyploidization. Nat Commun. 2012;3:717. doi: 10.1038/ncomms1704. [DOI] [PubMed] [Google Scholar]
- 25.Racke FK, Lewandowska K, Goueli S, Goldfarb AN. Sustained activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway is required for megakaryocytic differentiation of K562 cells. J Biol Chem. 1997;272:23366–70. doi: 10.1074/jbc.272.37.23366. [DOI] [PubMed] [Google Scholar]
- 26.Sungaran R, Markovic B, Chong BH. Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood. 1997;89:101–7. [PubMed] [Google Scholar]
- 27.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, et al. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–97. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 28.Rojnuckarin P, Drachman JG, Kaushansky K. Thrombopoietin-induced activation of the mitogen-activated protein kinase (MAPK) pathway in normal megakaryocytes: role in endomitosis. Blood. 1999;94:1273–82. [PubMed] [Google Scholar]
- 29.Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86:237–50. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 30.Hurtado PA, Vora S, Sume SS, Yang D, St Hilaire C, Guo Y, et al. Lysyl oxidase propeptide inhibits smooth muscle cell signaling and proliferation. Biochem Biophys Res Commun. 2008;366:156–61. doi: 10.1016/j.bbrc.2007.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadantonakis N, Matsuura S, Ravid K. Megakaryocyte pathology and bone marrow fibrosis: the lysyl oxidase connection. Blood. 2012;120:1774–81. doi: 10.1182/blood-2012-02-402594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eliades A, Papadantonakis N, Bhupatiraju A, Burridge KA, Johnston-Cox HA, Migliaccio AR, et al. Control of megakaryocyte expansion and bone marrow fibrosis by lysyl oxidase. J Biol Chem. 2011;286:27630–8. doi: 10.1074/jbc.M111.243113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson M, Gernsheimer T, Johansen K. Essential thrombocytosis: underemphasized cause of large-vessel thrombosis. J Vasc Surg. 1995;22(discussion 448-9):443–7. doi: 10.1016/S0741-5214(95)70013-7. [DOI] [PubMed] [Google Scholar]
- 34.Elliott MA, Tefferi A. Pathogenesis and management of bleeding in essential thrombocythemia and polycythemia vera. Curr Hematol Rep. 2004;3:344–51. [PubMed] [Google Scholar]
- 35.Lee CH, Yun HJ, Kang HS, Kim HD. ERK/MAPK pathway is required for changes of cyclin D1 and B1 during phorbol 12-myristate 13-acetate-induced differentiation of K562 cells. IUBMB Life. 1999;48:585–91. doi: 10.1080/713803574. [DOI] [PubMed] [Google Scholar]
- 36.Modi PK, Komaravelli N, Singh N, Sharma P. Interplay between MEK-ERK signaling, cyclin D1, and cyclin-dependent kinase 5 regulates cell cycle reentry and apoptosis of neurons. Mol Biol Cell. 2012;23:3722–30. doi: 10.1091/mbc.E12-02-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravenhall C, Guida E, Harris T, Koutsoubos V, Stewart A. The importance of ERK activity in the regulation of cyclin D1 levels and DNA synthesis in human cultured airway smooth muscle. Br J Pharmacol. 2000;131:17–28. doi: 10.1038/sj.bjp.0703454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazaki R, Ogata H, Kobayashi Y. Requirement of thrombopoietin-induced activation of ERK for megakaryocyte differentiation and of p38 for erythroid differentiation. Ann Hematol. 2001;80:284–91. doi: 10.1007/s002770000285. [DOI] [PubMed] [Google Scholar]
- 39.Vora SR, Palamakumbura AH, Mitsi M, Guo Y, Pischon N, Nugent MA, et al. Lysyl oxidase propeptide inhibits FGF-2-induced signaling and proliferation of osteoblasts. J Biol Chem. 2010;285:7384–93. doi: 10.1074/jbc.M109.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reszka AA, Seger R, Diltz CD, Krebs EG, Fischer EH. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci USA. 1995;92:8881–5. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedrich Stölzel TI. MD, Claudia Dill, Michael Kramer, Brigitte Mohr, Christian Thiede, Uwe Platzbecker, Johannes Schetelig, Christoph Röllig, Martin Bornhäuser, Gerhard Ehninger and Markus Schaich. High Plasma Lysyloxidase Concentration Is Associated with Inferior Outcome and Extramedullary Disease in Patients with Acute Myeloid Leukemia. AMERICAN SOCIETY OF HEMATOLOGY. San Diego, 2011. [Google Scholar]
- 42.Wang Z, Zhang Y, Kamen D, Lees E, Ravid K. Cyclin D3 is essential for megakaryocytopoiesis. Blood. 1995;86:3783–8. [PubMed] [Google Scholar]
- 43.Drachman JG, Rojnuckarin P, Kaushansky K. Thrombopoietin signal transduction: studies from cell lines and primary cells. Methods. 1999;17:238–49. doi: 10.1006/meth.1998.0734. [DOI] [PubMed] [Google Scholar]
- 44.Gaur M, Murphy GJ, deSauvage FJ, Leavitt AD. Characterization of Mpl mutants using primary megakaryocyte-lineage cells from mpl(-/-) mice: a new system for Mpl structure-function studies. Blood. 2001;97:1653–61. doi: 10.1182/blood.V97.6.1653. [DOI] [PubMed] [Google Scholar]
- 45.Mazharian A, Watson SP, Séverin S. Critical role for ERK1/2 in bone marrow and fetal liver-derived primary megakaryocyte differentiation, motility, and proplatelet formation. Exp Hematol. 2009;37:1238–49, e5. doi: 10.1016/j.exphem.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrera R, Hubbell S, Decker S, Petruzzelli L. A role for the MEK/MAPK pathway in PMA-induced cell cycle arrest: modulation of megakaryocytic differentiation of K562 cells. Exp Cell Res. 1998;238:407–14. doi: 10.1006/excr.1997.3847. [DOI] [PubMed] [Google Scholar]
- 47.Fichelson S, Freyssinier JM, Picard F, Fontenay-Roupie M, Guesnu M, Cherai M, et al. Megakaryocyte growth and development factor-induced proliferation and differentiation are regulated by the mitogen-activated protein kinase pathway in primitive cord blood hematopoietic progenitors. Blood. 1999;94:1601–13. [PubMed] [Google Scholar]
- 48.Nguyen HG, Ravid K. Polyploidy: mechanisms and cancer promotion in hematopoietic and other cells. Adv Exp Med Biol. 2010;676:105–22. doi: 10.1007/978-1-4419-6199-0_7. [DOI] [PubMed] [Google Scholar]
- 49.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 50.Downing KH. Structural basis for the interaction of tubulin with proteins and drugs that affect microtubule dynamics. Annu Rev Cell Dev Biol. 2000;16:89–111. doi: 10.1146/annurev.cellbio.16.1.89. [DOI] [PubMed] [Google Scholar]
- 51.Gelfand VI, Bershadsky AD. Microtubule dynamics: mechanism, regulation, and function. Annu Rev Cell Biol. 1991;7:93–116. doi: 10.1146/annurev.cb.07.110191.000521. [DOI] [PubMed] [Google Scholar]
- 52.Gundersen GG, Cook TA. Microtubules and signal transduction. Curr Opin Cell Biol. 1999;11:81–94. doi: 10.1016/S0955-0674(99)80010-6. [DOI] [PubMed] [Google Scholar]
- 53.Crossin KL, Carney DH. Evidence that microtubule depolymerization early in the cell cycle is sufficient to initiate DNA synthesis. Cell. 1981;23:61–71. doi: 10.1016/0092-8674(81)90270-1. [DOI] [PubMed] [Google Scholar]
- 54.Baatout S, Chatelain B, Staquet P, Symann M, Chatelain C. Induction and enhancement of normal human megakaryocyte polyploidization are concomitant with perturbation in the actin metabolism. Eur J Clin Invest. 1998;28:845–55. doi: 10.1046/j.1365-2362.1998.00353.x. [DOI] [PubMed] [Google Scholar]
- 55.Drewes G, Lichtenberg-Kraag B, Döring F, Mandelkow EM, Biernat J, Goris J, et al. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 1992;11:2131–8. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoshi M, Ohta K, Gotoh Y, Mori A, Murofushi H, Sakai H, et al. Mitogen-activated-protein-kinase-catalyzed phosphorylation of microtubule-associated proteins, microtubule-associated protein 2 and microtubule-associated protein 4, induces an alteration in their function. Eur J Biochem. 1992;203:43–52. doi: 10.1111/j.1432-1033.1992.tb19825.x. [DOI] [PubMed] [Google Scholar]
- 57.Barosi G, Lupo L, Rosti V. Management of myeloproliferative neoplasms: from academic guidelines to clinical practice. Curr Hematol Malig Rep. 2012;7:50–6. doi: 10.1007/s11899-011-0109-7. [DOI] [PubMed] [Google Scholar]
- 58.Finazzi G. How to manage essential thrombocythemia. Leukemia. 2012;26:875–82. doi: 10.1038/leu.2011.306. [DOI] [PubMed] [Google Scholar]
- 59.Eliades A, Papadantonakis N, Bhupatiraju A, Burridge KA, Johnston-Cox HA, Migliaccio AR, et al. Control of megakaryocyte expansion and bone marrow fibrosis by lysyl oxidase. J Biol Chem. 2011;286:27630–8. doi: 10.1074/jbc.M111.243113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen QJ, Goldenson B, Malinge S, Koppikar P, Levine RL, Tefferi A, et al. Induction of Megakaryocyte Polyploidization in Combination with JAK Inhibition As a Novel Therapeutic Strategy for Myeloproliferative Neoplasms. ASH Annual Meeting Abstracts; 118:64-. [Google Scholar]
- 61.Wen Q, Goldenson B, Silver SJ, Schenone M, Dancik V, Huang Z, et al. Identification of regulators of polyploidization presents therapeutic targets for treatment of AMKL. Cell. 2012;150:575–89. doi: 10.1016/j.cell.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 64.Yang LP, Keating GM. Ruxolitinib: in the treatment of myelofibrosis. Drugs. 2012;72:2117–27. doi: 10.2165/11209340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 65.McCrann DJ, Eliades A, Makitalo M, Matsuno K, Ravid K. Differential expression of NADPH oxidases in megakaryocytes and their role in polyploidy. Blood. 2009;114:1243–9. doi: 10.1182/blood-2008-12-195883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schulze H. Culture of murine megakaryocytes and platelets from fetal liver and bone marrow. Methods Mol Biol. 2012;788:193–203. doi: 10.1007/978-1-61779-307-3_14. [DOI] [PubMed] [Google Scholar]