The anatomic location of atrioventricular (AV) bypass tracts (or accessory pathways) is variable, but these generally traverse the tricuspid or mitral annulus and insert into the atrium and ventricle near the AV ring. There is only 1 case report of an accessory pathway ablated in the left coronary cusp (LCC),1 and this pathway had bidirectional conduction, was not decremental, and was associated with an orthodromic tachycardia. We report here the first case of an antegrade slowly conducting, decremental, accessory pathway that was also successfully ablated in the aortic LCC. This pathway generated antidromic tachycardia having a QRS morphology mimicking those seen in outflow tract ventricular tachycardia.
Case Report
A 42-year-old woman was evaluated for palpitations and pre-syncope for 2 years. These were commonly associated with shortness of breath and chest heaviness that would resolve spontaneously. Her baseline ECG was normal without ventricular preexcitation. Echocardiogram, coronary angiogram, and thyroid function were also unremarkable.
On her third presentation to a hospital, a broad complex tachycardia was captured (representative 12-lead ECG shown in Figure 1). The cycle length was 480 ms, the QRS duration was 150 ms, and a left bundle branch morphology with a right inferior axis was noted. She was referred for ablation of suspected ventricular outflow tract tachycardia. Consent was obtained for mapping and ablation of this tachycardia. During placement of catheters, a spontaneous initiation of tachycardia occurred, which was noted to have 1:1 AV association. A quadripolar catheter was placed at the right ventricular apex and His bundle, with a decapolar catheter in the coronary sinus. During ventricular pacing, retrograde conduction occurred with a long ventriculoatrial interval. Tachycardia was readily initiated with ventricular premature beats showing retrograde decremental conduction (Figure 2). An antegradely conducting accessory pathway was noted during atrial pacing maneuvers. During delivery of premature atrial stimulations, shortening of the S1S2 intervals was followed by progressive lengthening of the AV interval of preexcitation, indicating the decremental conduction nature of the accessory pathway (Figure 3).
Figure 1.
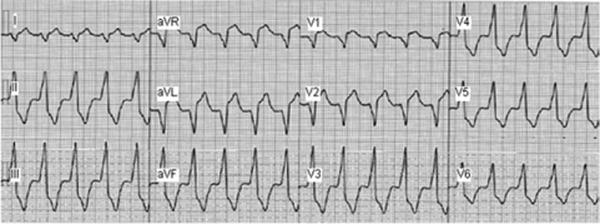
Twelve-lead ECG during the wide complex tachycardia. Based on the QRS morphology, left bundle branch block, inferior axis, and transition at V3, this tachycardia was initially suspected to be an outflow tract ventricular tachycardia.
Figure 2.
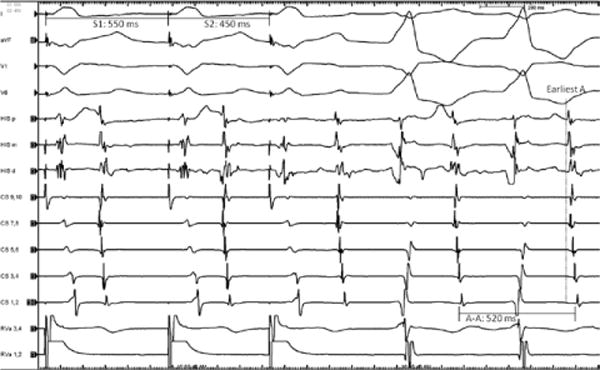
Initiation of tachycardia with ventricular premature beat. Right ventricular pacing (S1 550 ms, S2 450 ms) showed a long ventriculoatrial (VA) activation time (likely via the slow pathway), with further prolongation after the premature beat. A wide QRS echo beat having the clinical tachycardia morphology was frequently seen after A2. In this example, the echo beat is followed by tachycardia showing 1:1 VA conduction. HIS (p=proximal, m=middle, d=distal); CS indicates coronary sinus (10=proximal, 1=distal); RVa, right ventricular apex (1,2=distal, 3,4=proximal).
Figure 3.
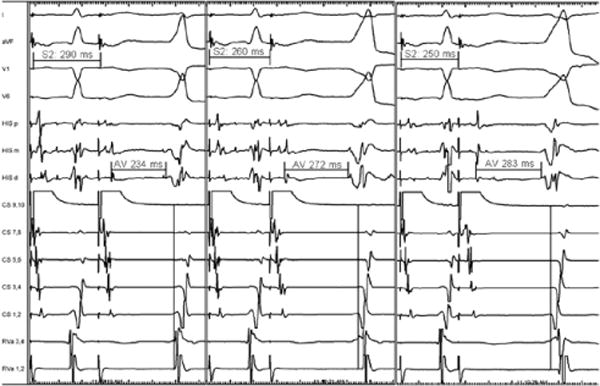
Atrial pacing from the proximal coronary sinus at a drive train of 550 ms followed by atrial extrastimuli at decreasing intervals (290, 260, and 250). The last beat of the drive train is conducted via the atrioventricular (AV) node with no evidence of preexcitation. With shortening of the extrastimulus interval, there was progressive prolongation of the AV interval. The extrastimuli show marked atrial-His increase in the first 2 panels associated with increasing preexcitation consistent with a fused QRS conducted through both the AV node and the accessory pathway. The third panel shows further prolongation of the AV interval with full preexcitation, which was followed by initiation of tachycardia. Measurements indicate the AV interval from the distal His catheter. There is also a progressive change in ventricular activation such that the right ventricular catheter precedes coronary sinus (CS) 1–2 when the QRS is minimally preexcited but reverses with increasing preexcitation, indicating earlier activation of the basal left ventricle (relative to the right ventricular apex [RVa]) when ventricular activation is via the accessory pathway. HIS (p=proximal, m=middle, d=distal); CS (10=proximal, 1=distal); RVa (1,2=distal, 3,4=proximal).
Sustained atrioventricular reentrant tachycardia was inducible with anterograde propagation via the slowly conducting accessory pathway, as well as with retrograde conduction via a slowly conducting pathway. The retrograde slowly conducting pathway used during the AV reentrant tachycardia was the AV node and not an accessory ventriculoatrial pathway (Figure 4). The tachycardia, while readily initiated with atrial or ventricular pacing, terminated multiple times spontaneously, and the termination was always associated with an AV nodal echo beat. At times, this echo beat would fuse with the antegradely conducted accessory pathway impulse. In Figure 4, despite a very late antegrade activation of the His bundle with the AV nodal echo beat showing minimal fusion, the tachycardia nevertheless terminated, demonstrating that retrograde conduction during the AV reentrant tachycardia was via the AV node. The accessory pathway was adenosine-sensitive.
Figure 4.
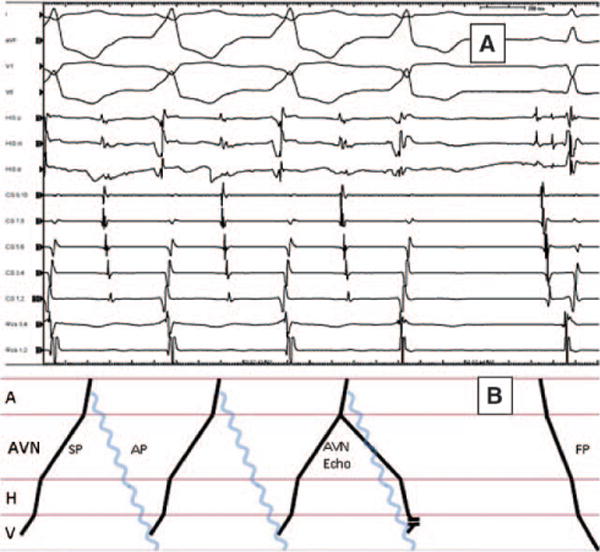
A, Tachycardia termination with antegrade His bundle activation. Multiple spontaneous terminations of tachycardia were seen during the study always associated with antegrade atrioventricular (AV) nodal activation showing varying degrees of fusion. In this panel, despite very late antegrade His bundle activation showing minimal fusion (no preexcitation of the septal V and only 20 ms preexcitation of the right ventricular apex [RVa]), the tachycardia nevertheless terminated. The retrograde conduction pathway had clearly more robust conduction during ventricular pacing. Thus, the termination of this tachycardia with retrograde block demonstrated in this figure confirmed that the retrograde pathway is via the AV node, likely the slow AV nodal pathway. Furthermore, such termination eliminated atrial tachycardia as a mechanism. There was no change in AA intervals before termination; the last A was part of the tachycardia and generated an atypical atrioventricular nodal (AVN) echo fusing with the accessory pathway generated V. Termination of the tachycardia with the anterograde AVN echo showed that the retrograde VA conduction via the AV node was part of the circuit. HIS (p=proximal, m=middle, d=distal); CS indicates coronary sinus (10=proximal, 1=distal); RVa (1,2=distal, 3,4=proximal). B, Ladder diagram corresponding to A. Blue wave line indicates the accessory pathway that, by definition, is not traversing the AVN or His. A indicates atrium; H, His; V, ventricle; SP, slow pathway; and FP, fast pathway.
During tachycardia, single late atrial premature depolarizations advanced anterograde ventricular activation with the same left bundle morphology and ventriculoatrial linking (Figure 5), demonstrating the participation of the accessory pathway in the reentrant circuit (ie, not a bystander pathway) and eliminating an atrial tachycardia, atrioventricular nodal reentrant tachycardia, and the possibility of a nodoventricular pathway generating the preexcitation.
Figure 5.
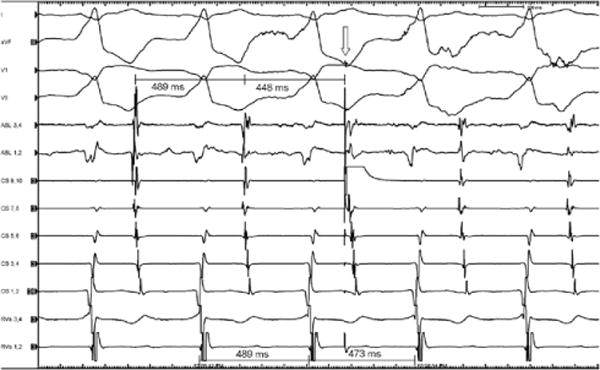
Late atrial premature beat advances the following QRS during tachycardia. This figure shows the effect of a late atrial premature beat delivered from the proximal coronary sinus (CS 9,10). Given the long ventriculoatrial (VA) conduction time during tachycardia, this late premature atrial beat would find the atrioventricular (AV) node refractory to antegrade conduction, thus demonstrating that the atrium is part of the circuit and that the accessory pathway is an AV pathway rather than a nodoventricular pathway. Advancement of anterograde ventricular activation by the atrial premature depolarization with the same left bundle morphology and VA linking with constant VA intervals also confirm the participation of the accessory pathway in the reentrant circuit and therefore excludes AV nodal reentrant tachycardia and atrial tachycardia. Upper most intervals represent AA, lower intervals VV. Arrow indicates premature atrial stimulus.
Ventricular activation was then mapped during fully preexcited QRS generated by atrial pacing. In the right ventricle, the outflow regions showed the earliest activation, but was not earlier than the onset of QRS. Given the outflow tract QRS morphology and the lack of early sites from the right ventricular outflow tract, the aortic root and left ventricle were mapped via a retrograde aortic approach. Mapping of the mitral annular and left ventricular sites, including the subvalvular aortomitral isthmus and the sinuses of Valsalva above the cusps, was performed. The earliest site of ventricular activation was in the left sinus of Valsalva at the junction of the LCC (30 ms before onset of QRS; Figure 6). An intracardiac ultrasound catheter was placed in the right atrium from a femoral venous access for aortic sinus tissue–catheter interface guidance. Fluoroscopic and intracardiac echo images, as well as activation maps of the ablation site, are shown in Figure 7. Ablation at this early site (with an irrigated catheter) at 15 W, increasing to 35 W for 60 seconds, eliminated accessory pathway conduction. Two additional lesions at the same output were also performed in close proximity. Subsequent programmed atrial and ventricular stimulation demonstrated only AV nodal conduction (supported by para-Hisian pacing, persisting dual AV nodal physiology, and V-H-A response to ventricular pacing) without induction of any tachycardia even during infusion of isoproterenol up to 5 μg/min.
Figure 6.
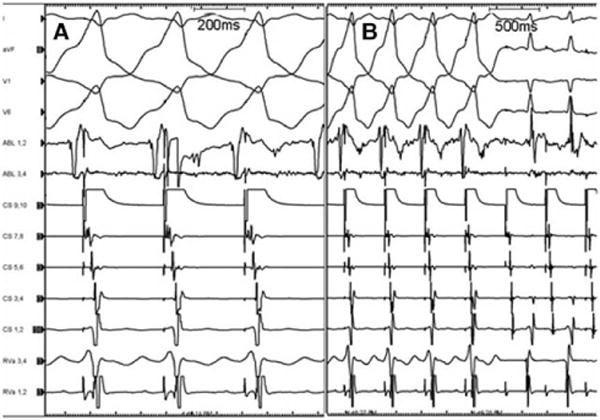
A, Earliest site of ventricular preexcitation. This electrogram is recorded from the tip of the ablation catheter located in the mid portion of the left sinus of Valsalva at the junction with the aortic cusp. Activation at this site was 30 ms before onset of QRS. Maximal preexcitation was maintained with pacing from the proximal coronary sinus. B, Ablation at this site eliminated ventricular preexcitation in 3 seconds at 15 W.
Figure 7.
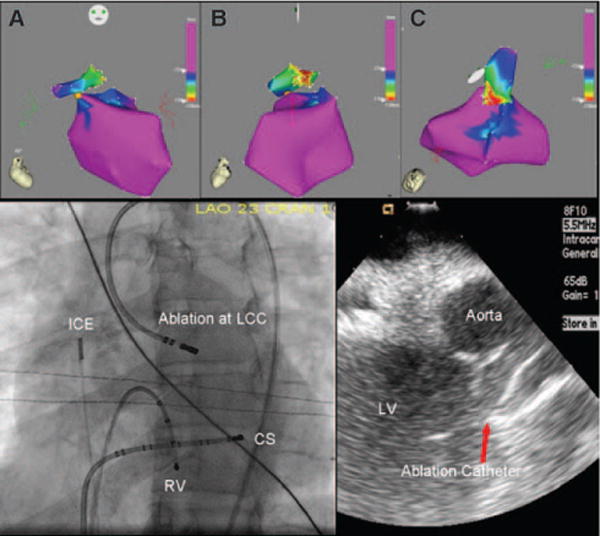
Top, Electroanatomic map (CARTO) showing the left ventricle relative to the site of earliest activation in the left coronary cusp (A, anterior-posterior view; B, left lateral view; C, Superior view). Bottom left, Fluoroscopic view of the successful ablation site. Bottom right, ICE image showing the ablation catheter at the site of successful ablation in the LCC. LV indicates left ventricle; ICE, intracardiac echocardiography catheter; RV, right ventricle; CS, coronary sinus catheter; and LCC, left coronary cusp.
Discussion
We describe an unusual, previously unreported, slowly conducting, AV accessory pathway with decremental properties, which was successfully ablated in the left sinus of Valsalva at its junction with the left aortic cusp. This pathway participated in an antidromic AV reentrant tachycardia manifesting a preexcited QRS morphology typical of those expected from an outflow ventricular tachycardia emanating from this region. The retrograde conduction was via the AV node. Although there is well-documented ablation of ventricular tachycardias and atrial tachycardias in the left sinus of Valsalva and ablation of anteroseptal typical accessory pathways from the noncoronary sinus,2 this seems to be only the second documentation of successful accessory pathway ablation in the LCC. Of note, the prior report of Godin et al1 described a typical accessory pathway with antegrade preexcitation during sinus rhythm that participated in an orthodromic reentrant tachycardia. In contrast, at no point during our procedure was retrograde conduction via the accessory pathway demonstrated. As shown in Figure 2, antidromic reentry was readily initiated with ventricular premature beats at rather long coupling intervals. Thus, the accessory pathway described in our report had poor, if any, retrograde conduction. In addition, the slow and decremental antegrade conduction of the pathway described in our report concealed its presence in sinus rhythm. Only with marked conduction delay or block in the AV node did antegrade conduction of the pathway become manifest.
Anatomically, the left sinus of Valsalva has left ventricular myocardial sleeves terminating near the insertion of the aortic cusp. This area also abuts the anterior wall of the left atrium near the mitral annulus, thus providing the proximity of atrial and ventricular myocardium for possible connection. The proximity of the right ventricular outflow tract septum to the aortic cusps (primarily the LCC and right coronary cusp) makes ECG features subtle and overlapping. The decision to map the aortic cusps was primarily based on the absence of early activation within the right ventricular outflow tract and an appreciation of the overlapping characteristics of arrhythmias originating in the right ventricular outflow tract and aortic cusps.
Slow, decrementally conducting AV accessory pathways have been commonly located along the tricuspid annulus (commonly referred to as Mahaim pathways). Rare cases have been seen along the mitral annulus. The pathophysiology of this pathway has not been directly investigated in this or the previous case report. Anatomically, the LCC lies in close proximity to the mitral annulus. Persistence of the developing conduction system in this region may have contributed to the pathway, particularly in view of the decremental nature of the pathway, and is further supported by human histological examinations that have shown crescents of ventricular musculature beyond the semilunar valves in significant number of patients.3
In summary, we present the first documented accessory pathway with slow, decremental antegrade conduction whose ventricular insertion was successfully ablated in the left sinus of Valsalva at its juncture with the coronary cusp. This pathway participated in an antidromic tachycardia. The QRS morphology of this tachycardia mimicked those seen in outflow tract ventricular tachycardia. Together with the lack of ventricular preexcitation during sinus rhythm, the tachycardia described in this report is particularly prone to be mistaken for an outflow tract ventricular tachycardia, especially because it can be ablated from the left sinus of Valsalva.
EDITOR’S PERSPECTIVE
The report by Wilsmore et al provides an opportunity to address several important teaching points relating to pathways that are decremental and in uncommon locations.
Decrement in a Pathway
How does the presence of slow and decremental conduction in an accessory pathway affect the interpretation of commonly used maneuvers to define the mechanism of tachycardia?
Delayed Excitation and Block
With orthodromic reciprocating tachycardia, the retrograde limb is readily identified as an accessory pathway by placing appropriately timed premature ventricular contractions (PVCs) during tachycardia. A PVC placed at the time of His bundle refractoriness, that preexcites the atrium without changing the atrial activation sequence and that resets the tachycardia, is diagnostic for a retrograde pathway that participates in tachycardia.
When the retrograde conducting pathway is decremental, however, it may be very difficult and at times impossible to preexcite the atrium, even with short coupled PVCs. However, a delayed excitation response, characteristic of many decremental pathways, actually makes the diagnosis easier when present. This response occurs when a PVC placed when the His bundle is refractory delays the next atrial activation without changing the activation sequence. This response proves not only the presence of a pathway but also that this pathway participates in tachycardia, even if a reset response is not present with a similar significance as when a His refractory PVC terminates tachycardia.
Antegrade or Retrograde His?
With preexcited tachycardias, a recorded His bundle electrogram may be a result of antegrade conduction via the atrioventricular (AV) node or conduction via the pathway and retrograde activation of the His. A classic technique to know whether the His is antegrade or retrograde involves placing progressively shorter coupled premature atrial beats. When the His is activated anterogradely, the A-H interval progressively lengthens as a result of decremental conduction in the AV node and then plateaus at a fixed interval once the AV node blocks and the His bundle is activated retrogradely via the antegrade conducting pathway. When the antegrade conducting pathway is decremental, however, there may be continued prolongation of the A-H interval (a retrograde A-H interval). However, on close scrutiny of the A-A versus A-H curve, an abrupt change in the slope of the curve will be noted at the time of antegrade AV block. The student of electrophysiology can gain significant insight into pacing maneuvers that help define tachycardia mechanisms by plotting and analyzing such conduction curves.
Premature Atrial Contractions to Define Antidromic Tachycardia
Wilsmore et al proved antidromic tachycardia in their case by placing premature atrial contractions (PACs) during tachycardia that preexcited the ventricle with an identical QRS morphology and reset the tachycardia without preexciting the septal atrium. This finding excludes ventricular tachycardia, their initial clinical diagnosis, and in addition confirms that the existing pathway is not a bystander in the tachycardia. The absence of septal A preexcitation with the PAC likely excludes a nodoventricular tract as the source of ventricular preexcitation as well.
Atrial pacing and PACs can be used to promote conduction via an accessory pathway rather than over the AV node. Decremental conduction over the AV node allows more ventricular activation from the pathway. When the pathway exhibits decrement, however, this finding may be difficult to demonstrate, and choosing the site where atrial pacing is done (closer to the presumed site of the pathway) is important. During tachycardia, however, because retrograde conduction is via the AV node, PACs more readily penetrate the circuit despite the decrement.
LOCATION OF THE PATHWAY
Where Do We Map?
Mahaim (atriofascicular) pathways, which are the classic examples of antegrade decremental pathways, cannot be located effectively by looking for the site of early ventricular activation, because an insulated portion of the pathway may traverse the basal ventricle and insert into the normal conduction system far from the annulus. Localization is achieved by identification of the pathway potential, typically on the tricuspid annulus. Where was this pathway?
Successful ablation and even finding early activation in the coronary cusp do not absolutely define the pathway location. This is analogous to trying to define the true site of origin for outflow tract aortic cusp ventricular tachycardia. The aortic cusp may be where energy delivery is most effective, but the true arrhythmia focus may be located within a close neighboring structure. For example, the right coronary cusp is an excellent site to ablate a focus of tachycardia originating from the posterior surface of the right ventricular outflow tract.
How Do We Know Where the Pathway Is Located?
The signature electrogram that defines pathway location is the pathway potential. In the absence of identifying a pathway potential, the operator needs to recognize that the earliest identified site may be close to, but not on the pathway. As a result, one must be prepared to increase power delivery, as was done in this case, when ablating to eliminate the pathway permanently.
Relationship to Outflow Tract Ventricular Tachycardia
The authors point out the analogy between mapping and ablation in this location and the approach to outflow tract ventricular tachycardia. The site of activation, unique electrocardiographic characteristics, and unusual local electrogram characteristics, such as finding pathway-like potentials, prepotentials, or fascicle potentials, as we see with atriofascicular Mahaim fibers, are often found in the vicinity of the cardiac fibrous trigone.
ADDITIONAL FEATURES
The tachycardia in this patient repeatedly terminated with AV nodal reentrant echoes. The authors elegantly describe the significance of this finding, particularly defining the retrograde limb of the tachycardia being the AV node. Ablation in the coronary cusps has recently been associated with aortic valve rupture and perforation (we should provide a reference). The authors show the favored positioning of the catheter in this anatomic location in Figure 7. Here, the catheter visualized with intracardiac ultrasound is not pointed directly downward the depths of the cusp but against the adjacent tissue. Real-time ultrasound imaging and ensuring that a near-field electrogram is present on the catheter tip may help prevent such complications.
Footnotes
Disclosures
None.
References
- 1.Godin B, Guiot A, Savoure A, Anselme F. The left coronary cusp as an unusual location for accessory pathway ablation. Heart Rhythm. 2011;8:1769–1772. doi: 10.1016/j.hrthm.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Tabatabaei N, Asirvatham SJ. Supravalvular arrhythmia: identifying and ablating the substrate. Circ Arrhythm Electrophysiol. 2009;2:316–326. doi: 10.1161/CIRCEP.108.847962. [DOI] [PubMed] [Google Scholar]
- 3.Hasdemir C, Aktas S, Govsa F, Aktas EO, Kocak A, Bozkaya YT, Demirbas MI, Ulucan C, Ozdogan O, Kayikcioglu M, Can LH, Payzin S. Demonstration of ventricular myocardial extensions into the pulmonary artery and aorta beyond the ventriculo-arterial junction. Pacing Clin Electrophysiol. 2007;30:534–539. doi: 10.1111/j.1540-8159.2007.00704.x. [DOI] [PubMed] [Google Scholar]


