Abstract
Objective
The aim of the current study is to show the clinical data of long-term (3 year) follow-up of five patients affected by Leber Congenital Amaurosis type 2 (LCA2) treated with a single unilateral injection of AAV2-hRPE65v2.
Design
clinical trial
Participants
five LCA2 patients with RPE65 gene mutations
Methods
After informed consent and confirmation of trial eligibility criteria, the eye with worse visual function was selected for subretinal delivery of Adeno-Associated Virus (AAV2-hRPE65v2). Subjects were evaluated before and after surgery at designated follow-up visits (1, 2, 3, 14, 30, 60, 90, 180, 270, 365 days, 1.5 years and 3 years) by complete ophthalmic examination. Efficacy for each subject was monitored with best corrected visual acuity, kinetic visual field, nystagmus testing and pupillary light reflex.
Main Outcome Measures
best corrected visual acuity, kinetic visual field, nystagmus testing and pupillary light reflex.
Results
The data showed a statistically significant improvement of best corrected visual acuity between baseline and 3 years after treatment in the treated eye (p<0.001). In all patients we observed an enlargement of the areas of visual field, which remained stable till 3 years post injection (average values: baseline 1058 deg2 vs 3 years post treatment: 4630 deg2) and a reduction of the nystagmus frequency compared to baseline at the 3 year time-point. Furthermore, a statistically significant difference was observed in the pupillary constriction of the treated eye (p<0.05) compared to the untreated eye in three patients at 1 and 3-year time-points. No patients suffered serious adverse events related to the vector in the 3 year post-injection period.
Conclusions
The long-term follow-up data (3 years) on the 5-patient Italian cohort involved in the LCA2 gene therapy clinical trial clearly showed a stability of improvement in visual and retinal function that had been achieved a few months after treatment. Longitudinal data analysis showed that the maximum improvement was achieved within six months after treatment, and the visual improvement was stable up to the last observed time-point.
Gene therapy has recently been explored for the treatment of previously incurable inherited retinal diseases in animal models and in human subjects. Particularly, three independent clinical trials, which initiated almost contemporaneously in 2007 (NCT00481546,1 NCT00516477,2 NCT00643747,3 clinicaltrials.gov) were performed to evaluate safety and efficacy of gene therapy for Leber Congenital Amaurosis (LCA) type 2. LCA2 is a retinal degeneration due to mutations in the RPE65 gene. Lack of functionality of this gene results in a deficiency of 11-cis-retinal, so that rod photoreceptors are unable to respond to light. Clinical assessment of LCA2 patients shows severely reduced electroretinogram (ERG) and pupillary light reflex (PLR), nystagmus and fundus abnormalities. Moreover, most patients retain visual capabilities in the first decade of life4 along with nearly normal macular thickness.
In the three initial clinical trials, the patients were treated with a single unilateral subretinal injection of adeno-associated virus 2 (AAV2) carrying the RPE65 gene in the eye with the worst vision. A safety assessment showed the presence of minimal systemic immunological response in two trials2, 5 and the absence of serious adverse events in all three trials. Moreover, the reports on short-term follow-up of the three trials showed an improvement in selected measures of vision including best corrected visual acuity (BCVA),2 kinetic visual field (VF),2 nystagmus testing,2 pupillary light reflex,2 microperimetry,3 dark-adapted perimetry3 and dark adapted full-field sensitivity testing.2, 3, 5 These short-term improvements after the unilateral injection posed questions about the longevity of gene therapy efficacy, requiring a multi-year follow-up to assess the validity of the treatment. In any case, the promising results obtained motivated a new clinical trial for the re-injection of previously treated patients in the contralateral eye (NCT01208389)6. Few long-term follow-up reports exist, among which, Jacobson et al.7 illustrating the results of three year follow-up for patients in the University of Florida/University of Pennsylvania clinical trial. Considering that the trials 2,3,5 employed multiple cohorts, each receiving different doses, different volumes, multiple surgeons, and, in several cases, different injection strategies, further data of relatively long-term follow-up are needed.
In the present report, we describe the long-term (3-year) follow-up of all five Italian subjects enrolled in the RPE65 gene therapy clinical trial performed at The Children’s Hospital of Philadelphia (CHOP) in conjunction with the Second University of Naples (SUN) (NCT00516477).2 The Italian patients are the only patients treated in the clinical trial NCT00516477 with a follow-up of at least three years after the first subretinal injection and before the treatment of the contralateral uninjected eye.
The results of the current study showed a long-term stability of visual function improvements following a single unilateral sub-retinal injection of the AAV2-hRPE65v2 vector.
Methods and materials
Study population
All details of the design, consent, and vector administration in the current phase 1 clinical trial NCT00516477 (ClinicalTrials.gov) have previously been reported.2 The study was approved by a National Ethics Committee in Italy and adhered to the tenets of the Declaration of Helsinki. Briefly, five LCA2 subjects were first evaluated at the SUN (Napoli, Italy) in 2002. The Italian patients were identified as NP01, NP02, NP03, NP04 and NP15 and the short-term follow-up data for the first three patients (till 1.5 years) have been described in Simonelli et al.,7 for NP04 (1 year) and NP15 (3 months) in Maguire et al. 8 The patients were evaluated throughout the 3 year period prior to treatment and their diagnosis was based on visual and retinal function studies and confirmed by genetic analysis.9 The main demographic characteristics of the patients are reported in Table 1. After informed consent and confirmation of trial eligibility criteria described elsewhere,2 the eye with the worse visual function was selected for delivery of AAV2-hRPE65v2. The vector was manufactured by the Center for Cellular and Molecular Therapeutics at CHOP (Philadelphia, PA) and delivered subretinally at CHOP as described elsewhere2; the injection was delivered in the macular area in all patients except NP01 who presented macular atrophy.8
Table 1.
Demographic characteristics of the patients
| Patients | NP01 | NP02 | NP03 | NP04 | NP15 |
|---|---|---|---|---|---|
| Age | 26 | 26 | 19 | 17 | 11 |
| Gender | F | M | F | M | M |
| Date of initial injection | Oct 11, 2007 | Dec 13, 2007 | Jan 24, 2008 | Apr 10, 2008 | Jun 2, 2009 |
| Months of follow-up | 36/48* | 36/48* | 36 | 36/48* | 36 |
| Injected eye | Right | Right | Right | Left | Right |
| RPE65 mutation | p.E102K/p.E102K | p.E102K/p.E102K | p.R234/p.R234 | p.R91W/p.T149N | p.D167W/p.H313R |
| Vector volume [μL] | 150 | 150 | 150 | 300 | 300 |
| Concentration [10 per μL] | 1.0 | 1.0 | 1.0 | 3.2 | 5.0 |
The last follow-up time-point available (at 48 months) is not considered in the longitudinal data analysis of the current study as these patients received an injection on the second eye
Follow-up protocol
Subjects were evaluated before and after surgery at designated follow-up visits (1, 2, 3, 14, 30, 60, 90, 180, 270, 365 days, 1.5 years and 3 years) by complete ophthalmic examination. Efficacy for each subject was monitored with objective and subjective measures of vision, as described below. Some of the procedures were unique to the SUN site, i.e. fundus autofluorescence (AF) and microperimetry.
Objective measurement of visual functionality
Objective measures included evaluation of full-field ERG, PLR, and nystagmus testing.
ERGs were recorded in Italy (at baseline and at 60, 365 days, 1.5 years and 3 years) in both eyes using LACE Elettronica Electrophysiology system (Pisa, Italy), with ERG-jet contact lens electrodes. For ERG analyses, subjects’ pupils were dilated with 1% tropicamide, and the subjects were dark adapted for 30–45 minutes following International Society for Clinical Electrophysiology of Vision standard guidelines10 as described previously.2
Pupil responses were recorded simultaneously in both eyes with a Procyon P2000 pupillometer and PupilFit4 software (Monmouthshire, UK).11 Details of the most updated protocol and related software developed ad doc were described in a technical report12 focusing on biomedical engineering issues related to pupillometry. Briefly, responses were measured in a dark room after 40 minutes of dark adaptation by sequential stimulation in each eye with white light under low, medium and high intensity conditions (0.04, 0.4 and 10 lux, respectively).11 For each intensity condition two tests were performed: Test 1 - eight consecutive cycles consisting of a light stimulus presented for 0.2 seconds followed by a 1 second dark interval; Test 2 - six consecutive cycles consisting of a light stimulus presented for 1 second followed by a 0.6 second dark interval. In both tests, the light stimulus was presented alternatively to the right and left eye and each test was repeated twice: the first time starting with the stimulation of the right eye, the second time starting with the stimulation of the left one. A series of parameters were computed for each stimulus. In our previous study12 we showed that the percentage of pupillary constriction, defined as the ratio between the minimum diameter during contraction and the baseline diameter before stimulus, is the most informative parameter and for that reason, in the current study, we will refer to this parameter.
Characteristics of nystagmus were evaluated by videotaping the eye movements at baseline and at the 3 year time-point for qualitative clinical analysis of the subjects’ oscillation and strabismus.2 Furthermore, nystagmus was characterized quantitatively by analysis of motion paths in videos taken prior to the treatment and at post-injection time-points. MATLAB software was developed ad hoc to perform the analysis in a semi-automatic manner, adopting the algorithm described for pupillometry to detect pupil position.
Subjective measurement of visual functionality
Subjective measures included standard tests of BCVA, VF, and mobility testing to assess the ability of the subjects to navigate an obstacle course. BCVA was measured by trained vision examiners using a standard protocol involving Early Treatment Diabetic Retinopathy Study (ETDRS) charts and letter counts. Letter scores were converted to the log of the Minimum Angle of Resolution (logMAR), on a scale ranging from 0.00 to 2.00, with higher values indicating poorer vision. Eyes that could detect hand motions were assigned a score that was one line worse than the largest printed line on the chart tested at a standardized distance of 4 m (<20/1600) to provide the most conservative evaluation in terms of underestimating the actual extent of visual impairment. VF was measured using Goldmann perimetry (Haag Streit Perimeter 940; Haag Streit, Mason, OH).13 The visual field isopters were obtained using the V4e test object.2 Mobility testing was carried out as previously described,2 using different mazes each time the test was performed. Scoring was based on the number of obstacles avoided or hit, the number of landmarks identified and the time necessary to complete the test.
Additional exams
Additional secondary studies included optical coherence tomography (OCT), AF measurements, multifocal ERG (mfERG) and microperimetry.
OCT scans were performed in each patient using the Cirrus HD-OCT (Carl Zeiss, Dublin, CA) and the acquisition protocol comprised both a five-line raster scan and a macular cube scan pattern (512×128 pixels) in which a 6 mm × 6 mm region of the retina was scanned within a scan time of 2.4 seconds. The retinal thickness analysis protocol provided with the instrument software was used to calculate the foveal thickness compared to age related normal-sighted controls. AF was performed in each patient both before and after the treatment and was recorded with a standard confocal scanning laser ophthalmoscope (Heidelberg Retina Angiograph; Heidelberg Engineering, Heidelberg, Germany). To amplify the AF signal, we aligned the best five images obtained using the software integrated in the instrument and calculated a mean image.
mfERG and microperimetry were performed prior to and after treatment only in the last and youngest patient (NP15, 11 years old), who had the best BCVA (0.6 logMAR) and the best fixation. Microperimetry was performed by an automatic fundus-related perimeter (MP1 Microperimeter, Nidek Technologies, Padova, Italy). For the purpose of this study, the following parameters were used: a fixation target of 2° in diameter consisting of a red ring; a white, monochromatic background with a luminance of 4 abs; and a Goldmann III–size stimulus with a projection time of 200 ms.14 The stimulus was randomly projected according to a customized radial grid of 61 points covering the central portion of the retina (108 centered onto the fovea; points aligned on the 08, 308, 608, 908, 1208, and 1508 radial axes, 18 apart), and a 4-2-1 double staircase strategy was used with an automatic eye tracker that compensated for eye movements.15 mfERG was performed by Veris Science 6.1 system (EDI, Inc., San Mateo, CA) according to ISCEV standards using DTL electrodes and a stimulus array of 103 hexagons.16
Statistics
Following the recommendation by Beck17 about BCVA analysis in case of small sample size, Wilcoxon rank sum test was adopted to compare BCVA score between baseline and last follow-up time-points included in the current study. Moreover, to explore dependency of BCVA on time or treatment, a longitudinal data analysis was performed using the method of the “quasi-least-square”18 under Markov covariance structure, as implemented by the GEEQBOX.19 Finally, correlation between visual acuity and nystagmus was explored by computing Spearman’s rank correlation coefficient. Differences in pupillometric parameters between the two eyes and the different time-points were analyzed by Wilcoxon rank sum test as previously described.12 Changes in microperimetry sensitivity between the time-points were assessed by using Kruskal-Wallis test.
Results
Results of BCVA in the five patients from baseline to 3 years after treatment are reported in Figure 1. The data showed a statistically significant improvement of BCVA between baseline and 3 years after treatment both in the treated eye (p<0.001) and in the untreated eye (p=0.041). Moreover, the longitudinal data analysis, reported in Table 2, showed a quadratic trend of BCVA along the follow-up interval. All the values of BCVA are reported in Table 3 (available at http://aaojournal.org). In particular, the maximum BCVA was observed at 6 months after treatment in NP01, NP04, NP15, and at 18 months in NP03. NP02, although displaying a macular hole 14 days after treatment, showed a sharp improvement of BCVA until it stabilized at day 90. Finally, we observed that the improved BCVA remained stable through 3 years after injection in all patients.
Figure 1. Longitudinal data analysis of Best Corrected Visual Acuity (BCVA) in all the patients.

The longitudinal data analysis showed an improvement of BCVA in treated eyes of all patients.
LogMAR: logarithm of Minimum angle of resolution
Table 2.
Results of longitudinal statistical analysis on Best Corrected Visual Acuity (BCVA) data
| Variable | Beta | Std. Error | p-value | 95% CI | |
|---|---|---|---|---|---|
| Low. Lim. | Up. Lim. | ||||
| Intercept | 1.1936 | 0.1399 | <.0001 | .9193 | 1.468 |
| Time (days) | −.0011 | 2.37e-4 | <.0001 | −.0016 | −6.59e-4 |
| Time2 (days) | 7.85e-7 | 1.83e-7 | <.0001 | 4.26e-7 | 1.14e-6 |
| Treated eye | 0.0452 | 0.2252 | .8410 | −0.396 | 0.487 |
The longitudinal data analysis showed a quadratic trend of BCVA along the follow-up interval.
Beta: Regression Coefficient
Std. Error: Standard Error of Beta
95% CI: 95% Confidence Interval of Beta
Low. Lim.: Lower Limit of 95% CI
Up. Lim.: Upper Limit of 95% CI
As shown in Figure 2, an enlargement of VF areas was observed starting from day 60, although there was variability from measurement to measurement. The largest VFs were observed at day 60 in NP02, NP03, NP04, NP15, slightly before the timing of BCVA improvement, and at day 180 in NP01, in accordance with BCVA improvement. In all patients enlargement remained relatively stable through 3 years post-injection.
Figure 2. Goldmann Visual Field (VF) at selected time-points and comparison with the injection site.
Area of retina exposed to adeno-associated virus-mediated delivery of wild-type retinal pigment epithelium (AAV2-hRPE65v2) are shown on the left; overlapped VF isopters at baseline and post-treatment time-points are shown on the right. Average values of VF areas were the following: baseline: 1058 deg2 - day 60: 6387 deg2- day 180: 6402 deg2- 1,5 years: 4565 deg2- 3 years: 4630 deg2.. Age of the patient4s at baseline are reported in brackets.
The ability of the subjects to navigate an obstacle course, quantified by the number of avoided obstacles and the time spent to complete the course, remained stable through three years post-injection in all patients (Table 4 available at http://aaojournal.org).
We observed a reduction of the nystagmus frequency compared to baseline at the 3 year time-point in all patients (Figure 3 available at http://aaojournal.org). Moreover, we observed a correlation between nystagmus reduction and improved BCVA both in the treated eye (ρ = 0.717, p<0.001) and in the untreated one (ρ = 0.684, p<0.001).
We observed a statistically significant difference between the percentage of pupillary constriction of the treated eye compared to the untreated eye in NP01, NP02 and NP03 at 1 year time-point. This asymmetry persisted through 3 years. Moreover, no significant difference in the percentage of pupillary constriction was observed between the short-term and the long-term time-points in any patients, showing a stability of PLR over the entire follow-up period (Table 5 and Table 6 available at http://aaojournal.org).
Based on OCT scan analysis (Figure 4 available at http://aaojournal.org), we observed that in the treated eyes of all patients the mean macular thickness, foveal depression and retinal lamination remained unaltered during the 3-year follow-up interval, except for NP02. The macular hole, which had developed in the treated eye of NP02 at day 14, remained stable during the whole follow-up period (hole diameter at 1.5 years: 782 μm; at 3 years: 787 μm), without development of adjacent retinal complications. The photoreceptor inner/outer segment (IS/OS) junction observed at baseline only in patients NP04 and NP15 remained preserved through 3 years post-treatment. All selected OCT parameters remained stable also in the uninjected eyes of all patients.
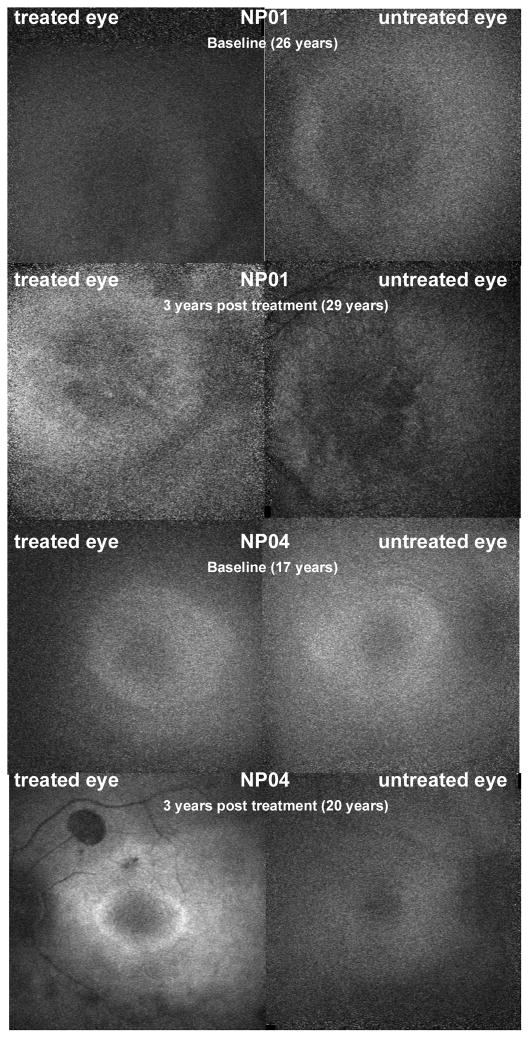
AF data for patients NP01 and NP04 are shown in Figure 5. In these patients, a marked increase of AF in the treated eye was observed as compared to baseline and remained stable till 3 years after treatment. The increase of AF in the treated eye of NP04 at the last time-point was more evident when compared to the untreated eye at the same time-point. AF was not detected in the other patients neither at baseline nor at later time-points.
Figure 5. Fundus Autofluorescence (AF) of NP01 and NP04.
An increase of AF is shown in the treated eye compared to baseline and to the untreated eye for NP01 and NP04. The macula of NP01 showed moderately decreased AF of a mottled appearance. The AF signal from the center of the macula in NP04 is surrounded by a ring of moderately increased AF more evident in the left eye after treatment.
mfERG, performed in NP15, revealed a central response of macular cone photoreceptors at day 30 and 608, although the signal was no longer recordable at the ensuing time-points.
Microperimetry results, performed in the same patient, showed an evident improvement of fixation stability both in the treated and in the untreated eye through three years after therapy (Figure 6 and Figure 7, available at http://aaojournal.org). There was a statistically significant increase in mean macular sensitivity of each post-treatment time-point compared to the baseline in both eyes (p<0.001).
Discussion
The long-term follow-up data (3 years) on the 5-patient Italian cohort involved in the CHOP LCA2 gene therapy clinical trial clearly showed that the improvements in visual and retinal function that had been achieved a few months after treatment remained stable. None of the patients underwent serious adverse events related to the vector through the 3 years after injection. Longitudinal data analysis showed that the maximum improvement was achieved within six months after treatment (eighteen months only in NP03). Concerning BCVA, the statistically significant (≥15 letters on the eye chart i.e. ≥ 0.3 logMAR) gains that we had previously reported in these subjects persisted over the 3 year timeframe. There is only one other group that has reported on improvement of BCVA after intervention,7 however, in that group, gains were substantially lower than the 0.3 logMAR limit generally considered to be clinically significant.20 Moreover, even if BCVA improvements were recorded also in the untreated eyes of four patients, they were clinically significant only in the patients who showed no nystagmus in the post-treatment time-points, consistent with the significant correlation between nystagmus and BCVA. In addition, we analyzed the trend of visual acuity over a longer follow-up by including pre-baseline data available since 2006 (Simonelli, unpublished data). We observed a clinically significant reduction of BCVA in the untreated eye only for NP04 (prebaseline 2006: 0.30 logMAR versus last follow-up timepoint 2011: 0.60 logMAR). Finally, the stability in the treated eye and the absence of degeneration of the untreated eye in four patients are consistent with results of previous studies analyzed in the review by den Hollander et al,21 who reported that in most LCA patients BCVA remained stable along multi-year follow-up. However, the evidence reported by Hollander et al21 is limited by unavailability of genetic analysis of most patients, as some studies were performed before the genome era, precluding useful genotype–phenotype correlations.
Goldmann VF analysis revealed a good correspondence between the retinal areas that displayed an increased response to the stimuli and the estimated region of sub-retinal injection. For instance, in NP01 the sub-retinal injection was performed in the superior-nasal sector, corresponding to the inferior-temporal visual field which appeared to be improved, while in NP15 the injection was performed in the superior macular sector extended to the superior and nasal retinal sectors and the improved sensitivity was on the corresponding loci, in the central, inferior and temporal visual field. The correspondence between loci was showed also in the other trials, but by using different techniques (i.e. microperimetry3 or computerized visual field7), while the technique adopted in the current study is the gold standard method for the evaluation of visual fields in retinal degeneration.
The results about pupillometric analysis showed that it is a feasible and useful technique to assess the amelioration of visual function, as described in detail elsewhere,12 particularly for patients with the worst BCVA (higher than 1.4 logMAR). A comparison with results of the other clinical gene therapy trials using pupillometry is limited by the difference in the adopted protocols.22
The changes in mean foveal thickness in both eyes before and after treatment were within published inter-visit variability for a retinal degeneration population.5, 23 In patient NP02, who developed a macular hole 14 days after treatment, OCT scans revealed no significant increase in macular hole size over time. We hypothesized that the macular hole was caused by contraction of a pre-existing retinal membrane stimulated by the surgical procedure since the hole was not detected until day 14. However, we cannot exclude that it resulted from surgical injection very close to the fovea. The latter interpretation was also suggested by Jacobson et al,7 who reported foveal thinning in two of five eyes in which retinal detachments included the fovea and also in one untreated eye and one injected eye that did not have foveal exposure. They also reported that the only eye in their study that showed a statistically significant improvement in visual acuity (surpassing the 0.3 logMAR (3-line) halving of visual angle limit) had received a subfoveal injection and that another eye in their study showed a robust improvement in visual acuity (0.29 logMAR) after receiving a subfoveal injection. However, because of the OCT data, Jacobson et al,7 concluded that the fovea should not be included in the injection site. We suggest that, while there are risks to injecting the foveal area, there are also large potential benefits. In our study, we modified the surgical procedure8 so that hydrodynamic stress and therefore the likelihood of foveal dehiscence or development of macular hole was kept at a minimum. With the exception of NP02, who developed a macular hole due to stress applied by an epiretinal membrane, measurements made with OCT before and after injection showed neither macular thinning nor loss of foveal photoreceptors, even if the retinal detachment included the fovea. The macular hole complication has not been observed since modifications to the surgical procedure were made.8 Patients NP04 and NP15 achieved a clinically significant improvement in BCVA, in nystagmus, in VF. Moreover, the longitudinal data analysis of microperimetry in NP15 showed a change of fixation from an unstable extrafoveal site to a stable foveal site. Based on all the above observations and incorporation of additional safety measures to reduce hydrodynamic stress, we conclude that foveal exposure may indeed be warranted in this population although the exact safety limitations are as yet unknown. As regards microperimetry, there was an increase in mean macular sensitivity in both eyes of NP15 after treatment that was higher than the inter-visit variability (2.17 dB) for a retinal degeneration population.24 There are a number of variables that can affect the outcome of microperimetry, including experience with the test25 (level of attentiveness and cognition, pupil size) and the instrument26 (size and duration of stimulus, test strategy, and starting stimulus luminance level). In the current study, we tried to reduce variability due to learning by providing pre-test training to allow familiarization with stimulus target size, location, and luminance as well as the correct operation of the response trigger. However, for future clinical gene therapy trials, we recommend performing more than one baseline test to exclude learning curve effects.
The AF technique was not routinely used in the clinical evaluation of RPE65 patients, as previous studies showed the lack of fundus AF in LCA2 patients.27, 28 Since we previously detected the presence of minimal AF related to preserved retinal thickness and IS/OS layer,9 we performed AF analysis in all patients. In NP01 and NP04, the AF was enhanced after the treatment, suggesting a recovery of retinoid cycle and a production of all-trans-retinal from 11-cis-retinal. Therefore, AF analysis could be useful in the follow-up of LCA2 patients undergoing AAV2-mediated RPE65 gene replacement, and also in the patients who show absence of AF at baseline, as we observed marked differences after treatment in a patient with minimal AF at baseline. However, the potential toxicity of the extra light exposure, entailed in the AF procedure, should be considered.
The current study has the following limitations: the study design did not involve the randomization of eyes, and there was an inherent imbalance between eyes because the injected eyes, by definition, had worse vision; examiners and patients were not masked to the study eye vs the control eye; only a small sample size was selected, and follow-up is described through 3 years post-treatment. However, the statistical analysis was performed following the recommendations of the guidelines on clinical trials in small populations to improve the efficiency of analysis. In particular, non-parametric methods (Wilcoxon rank sum test, Kruskal-Wallis test) were adopted instead of the most common parametric ones (t-test, ANOVA) and the Quasi Least Square method under Markov covariance structure was used to deal with the non-independence of repeated measurement of BCVA over time. Moreover, the Markov structure appeared to be useful as it assumes that the correlation between measurements depends on the temporal spacing of measurements and declines with increasing separation in time,19 which are both reasonable assumptions for data from the current study. In conclusion, the results of the current study show that the visual function improvements, achieved within a few months after a single unilateral injection, remained stable up to 3 years after the treatment in all the patients independent of age, vector dose, surgical procedure and baseline retinal status. Moreover, we observed that the surgical procedure, particularly after it was modified in order to reduce hydrodynamic stress at a minimum and performed so that the fovea was included in the treated area resulted in no clinically significant damage to macular photoreceptors up to 3 years following treatment. Moreover, clinically and statistically significant improvements in visual function were observed in the eyes treated by this modified surgical procedure. When incorporating surgical safeguards and enrolling subjects with structural evidence of photoreceptors that could benefit, we suggest that consideration should be given to including the fovea in the treatment area, particularly in those patients with low baseline visual acuity. Finally, taking into account the results of the last two patients enrolled and the updated inclusion criteria on BCVA for clinical gene therapy trials, we suggest including mfERG and microperimetry as sensitive secondary clinical tests for assessment of gene therapy efficacy for ocular disease in particular LCA patients.
Supplementary Material
Acknowledgments
Financial support: This work was supported by Italian Telethon Foundation (grant GGP10199 to Francesca Simonelli), Center for Cellular and Molecular Therapeutics at CHOP, Foundation Fighting Blindness–sponsored CHOP-PENN Pediatric Center for Retinal Degenerations, Clinical Translational Science Award NIH/National Center for Research Resources (UL1-RR-024134, 1R21EY020662, and 1R01EY019014-01A2), by Transdisciplinary Award Program in Translational Medicine and Therapeutics (TAPITMAT) from University of Pennsylvania, Research to Prevent Blindness, Howard Hughes Medical Institute, Paul and Evanina Mackall Foundation Trust at Scheie Eye Institute, and F. M. Kirby Foundation.
Footnotes
Conflict of interest: J.B. and A.M.M. are co-inventors on a patent (2797-11-US) for a method to treat or slow the development of blindness, but both waived any financial interest in this technology in 2002. K.A.H. and J.F.W. are co-inventors on a patent regarding methods of making AAV vectors for clinical studies (U.S. patent 61/299,184, 2010). J.B. and J.F.W. serve on the scientific advisory board for Avalanche Technologies. J.F.W. has consulted for Tacere Therapeutics, Genzyme, Novartis, and Genetix Inc. The other authors declare that they have no competing interests
Supplemental materials are provided at the end of the online version of this manuscript
This article contains additional online-only material.
The following should appear online-only: Figures 3, 4, 6, 7 and Table 3,4,5,6.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cideciyan AV, Hauswirth WW, Aleman TS, et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20:999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 4.Walia S, Fishman GA, Jacobson SG, et al. Visual acuity in patients with Leber’s congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology. 2010;117:1190–8. doi: 10.1016/j.ophtha.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 5.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–90. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett J, Ashtari M, Wellman J, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4:120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson SG, Cideciyan AV, Ratnakaram R, et al. Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonelli F, Ziviello C, Testa F, et al. Clinical and molecular genetics of Leber’s congenital amaurosis: a multicenter study of Italian patients. Invest Ophthalmol Vis Sci. 2007;48:4284–90. doi: 10.1167/iovs.07-0068. [DOI] [PubMed] [Google Scholar]
- 10.Holder GE, Brigell MG, Hawlina M, et al. International Society for Clinical Electrophysiology of Vision. ISCEV standard for clinical pattern electroretinography--2007 update. Doc Ophthalmol. 2007;114:111–6. doi: 10.1007/s10633-007-9053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpe NJ, Dadvand L, Kim SK, et al. Computerized binocular pupillography of the swinging flashlight test detects afferent pupillary defects. Curr Eye Res. 2009;34:606–13. doi: 10.1080/02713680902993891. [DOI] [PubMed] [Google Scholar]
- 12.Melillo P, Pecchia L, Testa F, et al. Pupillometric analysis for assessment of gene therapy in Leber congenital amaurosis patients. [Accessed November 13, 2012.];Biomed Eng Online [serial online] 2012 11:40. doi: 10.1186/1475-925X-11-40. Available at: http://www.biomedical-engineering-online.com/content/pdf/1475-925X-11-40.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross DF, Fishman GA, Gilbert LD, Anderson RJ. Variability of visual field measurements in normal subjects and patients with retinitis pigmentosa. Arch Ophthalmol. 1984;102:1004–10. doi: 10.1001/archopht.1984.01040030806021. [DOI] [PubMed] [Google Scholar]
- 14.Sohn EH, Chen FK, Rubin GS, et al. Macular function assessed by microperimetry in patients with enhanced S-cone syndrome. Ophthalmology. 2010;117:1199–206. doi: 10.1016/j.ophtha.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 15.Midena E, Vujosevic S, Convento E, et al. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91:1499–503. doi: 10.1136/bjo.2007.119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hood DC, Bach M, Brigell M, et al. ISCEV guidelines for clinical multifocal electroretinography (2007 edition) Doc Ophthalmol. 2008;116:1–11. doi: 10.1007/s10633-007-9089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck RW, Maguire MG, Bressler NM, et al. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology. 2007;114:1804–9. doi: 10.1016/j.ophtha.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 18.Chaganty NR. An alternative approach to the analysis of longitudinal data via generalized estimating equations. J Stat Plan Inference. 1997;63:39–54. [Google Scholar]
- 19.Ratcliffe SJ, Shults J. GEEQBOX: a MATLAB toolbox for generalized estimating equations and quasi-least squares. [Accessed November 13, 2012.];J Stat Softw [serial online] 2008 25(14) Available at: http://www.jstatsoft.org/v25/i14/paper. [Google Scholar]
- 20.Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29:398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2005;46:3349–54. doi: 10.1167/iovs.04-1383. [DOI] [PubMed] [Google Scholar]
- 24.Chen FK, Patel PJ, Webster AR, et al. Nidek MP1 is able to detect subtle decline in function in inherited and age-related atrophic macular disease with stable visual acuity. Retina. 2011;31:371–9. doi: 10.1097/IAE.0b013e3181e46af3. [DOI] [PubMed] [Google Scholar]
- 25.Wild JM, Searle AE, Dengler-Harles M, O’Neill EC. Long-term follow-up of baseline learning and fatigue effects in the automated perimetry of glaucoma and ocular hypertensive patients. Acta Ophthalmol (Copenh) 1991;69:210–6. doi: 10.1111/j.1755-3768.1991.tb02713.x. [DOI] [PubMed] [Google Scholar]
- 26.Artes PH, Iwase A, Ohno Y, et al. Properties of perimetric threshold estimates from Full Threshold, SITA Standard, and SITA Fast strategies. Invest Ophthalmol Vis Sci. 2002;43:2654–9. [PubMed] [Google Scholar]
- 27.Wabbels B, Demmler A, Paunescu K, et al. Fundus autofluorescence in children and teenagers with hereditary retinal diseases. Graefes Arch Clin Exp Ophthalmol. 2006;244:36–45. doi: 10.1007/s00417-005-0043-2. [DOI] [PubMed] [Google Scholar]
- 28.Lorenz B, Wabbels B, Wegscheider E, et al. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology. 2004;111:1585–94. doi: 10.1016/j.ophtha.2004.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.