Abstract
Due to an imbalance in oxygen supply and demand, myocardial ischemia is associated with profound tissue hypoxia. Studies of hypoxia-elicited adaptive responses during myocardial ischemia revealed a cardioprotective role for the signaling molecule adenosine. In ischemic human hearts, the A2B adenosine receptor (ADORA2B) is selectively induced. Functional studies in genetic models show that Adora2b signaling attenuates myocardial infarction by adapting metabolism towards more oxygen efficient utilization of carbohydrates. This adenosine-mediated cardio-adaptive response involves the transcription factor hypoxia-inducible factor HIF1A and the circadian rhythm protein Per2. In the present review we discuss advances in the current understanding of adenosine-elicited cardioprotection with particular emphasis on ADORA2B, its downstream targets, and their implications on novel strategies to prevent or treat myocardial ischemia.
Keywords: adenosine, A1, A2A, A2B, A3, ischemia, heart attack, hypoxia-inducible factor, HIF1, HIF2, period, PER2, CD73, ecto-nucleotidase, CD39, apyrase, prolylhydroxylases, PHD, adenosine deaminase, adenosine kinase, equilibrative nucleoside transporter
Ischemia, hypoxia, and heart disease
Myocardial ischemia is a pathological disease state characterized by an imbalance between the myocardial metabolite supply and the demand, particularly for oxygen, leading to profound myocardial hypoxia [1-3]. It is typically caused by the occlusion of a coronary artery, which limits blood flow to the myocardium, and, if prolonged, can lead to myocardial cell death and concomitant heart failure (ischemic heart disease). If reperfusion occurs, the activation of inflammatory pathways and cell death programs can cause additional injury, referred to as myocardial reperfusion injury [4].
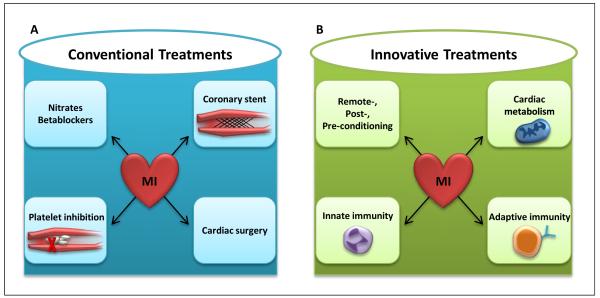
Given the numbers of patients affected every year, the search for novel therapeutic approaches for treating myocardial ischemia is highly significant for improving healthcare throughout the world. Coronary heart disease, and concomitant myocardial ischemia and infarction, caused nearly 1 of every 6 deaths in the United States in 2008 [5]. Mortality from coronary heart disease in 2008 totaled 405 309 individuals, and an estimated 785 000 Americans will have experience a new coronary attack per year. Indeed, an American will die of a heart attack approximately every minute [5]. Currently, the mainstay therapy for acute myocardial ischemia is early and persistent revascularization, in combination with anticoagulation, antiplatelet medications, and additional pharmacological strategies that reduce myocardial oxygen consumption (e.g. “beta-blockers” or nitrates). However, morbidity and mortality remain high and additional therapeutic approaches that would render the myocardium more resistant to ischemia and attenuate reperfusion injury are an intense area of investigation (see Box 1) [4, 6-8].
Box 1. Conventional and Innovative Treatments for Myocardial Ischemia.
Acute myocardial ischemia is typically caused by reduced bloodflow to the myocardium due to a mechanical obstruction of a coronary artery. In patients, myocardial ischemia causes acute coronary syndromes - a group of medical conditions that stem from the obstruction of coronary arterial blood flow, leading to myocardial ischemia. They are commonly accompanied by clinical symptoms such as chest pain radiating to the left arm, severe chest pressure, nausea, vomiting, and sweating. Indeed, myocardial ischemia is among the leading causes of death in the Western countries [5]. The mainstay therapy for myocardial ischemia (conventional treatment) is achieving coronary patency and persistent reperfusion. Frequently, this is achieved by a percutaneous coronary intervention with the placement of an intracoronary stent. Alternatively, coronary patency can be achieved surgically by coronary artery bypass grafting, a process in which a venous or an arterial graft is surgically inserted into the aorta and into the coronary artery distal to the occlusion. These mechanical means of restoring blood flow to the myocardium are typically combined with pharmacological interventions that are intended to (i) decrease myocardial oxygen consumption and work load (e.g. beta-blockers, nitrates) and (ii) prevent clot formation (e.g. by platelet inhibiting drugs such as aspirin or the ADP receptor antagonist clopidogrel, and anticoagulants such as heparin [111]).
Despite the above treatment approaches, the morbidity and mortality from myocardial ischemia and concomitant acute coronary syndromes remain extremely high [5] and the search for additional “innovative” therapies to treat or prevent myocardial ischemia and the associated tissue injury is an area of intense research. Innovative therapies include ischemic conditioning strategies [2]. For example, pre-conditioning with short ischemic time periods has provided a very potent mechanism for reducing myocardial infarct sizes [82]. However, pre-conditioning is limited by the fact that it is a “prevenitve apporach”, and most often it is unknown when an acute coronary syndrome is going to occur in an individual patient - thereby limiting its applicability as an active form of treatment. Nevertheless, ischemic pre-conditioning can be used as an experimental strategy to uncover novel therapeutic targets [34, 49, 50, 54, 55, 57]. Alternatively, treatment with additional short ischemic periods after the occurrence of myocardial ischemia has also been shown to decrease myocardial infarct sizes, and is frequently used following a coronary intervention [2]. Moreover, remote ischemic conditioning – for example, by inflating a blood pressure cuff around an extremity – has been found effective in attenuating myocardial infarct sizes in patients [6]. During myocardial ischemia and reperfusion injury, innate and adaptive immune responses are activated and many experimental studies in mice suggest that targeting post-ischemic myocardial inflammation attenuates myocardial infarct sizes and improves outcomes. An alternative approach to treating myocardial ischemia is based on the idea that myocardial metabolism could be made more oxygen efficient by, for example, switching from fatty acid metabolism towards glycolytic utilization of carbohydrates. Indeed, recent studies implicate extracellular adenosine signaling in improving myocardial metabolism during ischemia by increasing anaerobic glycolysis [8].
Although the extracellular signaling molecule adenosine has long been studied in the context of cardiovascular disease, recent experimental studies have begun to outline the intracellular signaling pathways that are responsible for transmitting signals between the plasma membrane and ischemia-responsive transcriptional pathways. As we discuss here, these studies demonstrate that adenosine signaling is enhanced during myocardial ischemia, and functions to protect the heart from the deleterious effects of ischemia and subsequent reperfusion injury. Moreover, they provide a novel link between adenosine signaling and the circadian rhythm protein Period2 (Per2), thereby connecting adenosine-elicited cardioprotection to the circadian rhythmicity of myocardial infarction [9, 10].
Adenosine signaling in the extracellular compartment
Adenosine is commonly recognized as a building block in the synthesis of ATP – the universal intracellular energy currency [11]. In contrast to its intracellular activities, adenosine in the extracellular compartment functions as a signaling molecule [12]. The signaling effects of adenosine are mediated through the activation of G protein-coupled receptors, which results in the inhibition of adenylate cyclase and concomitant changes in intracellular cyclic AMP (cAMP) levels. Four distinct adenosine receptors have been described, Adora1, -2a, -2b, and -3, which differ in their affinity for adenosine and the biological effects of their associated signaling pathways [13-16].
In a clinical setting, adenosine is used for the treatment of supraventricular tachycardia, an effect mediated by the receptor ADORA1. Patients suffering from supraventricular tachycardia are injected with a rapid bolus of adenosine, causing a transient complete heart block, which frequently results in the termination of supraventricular tachycardia [17]. Adora1 was shown to be responsible for the heart-rate slowing effects of intravenous adenosine when injection of Adora1−/− mice with adenosine failed to induce a heart block [18]. Adora2a is highly expressed on inflammatory cells and has been implicated in dampening their activation [19, 20]. Adora2b is the most adenosine-insensitive receptor and has been implicated in tissue-adaptation to hypoxic or ischemic conditions [21-25]. As we discuss below, conditions of hypoxia – such as occurs during myocardial ischemia – are associated with significant elevations in extracellular adenosine levels, thus leading to adenosine concentrations sufficient to activate this receptor [12, 26, 27]. Activation of Adora2b is coupled to the protein Gs, which subsequently stimulates the activity of adenylate cyclase and increases intracellular cAMP levels. Mice lacking Adora3 exhibit a phenotype characterized by changes in diurnal rhythm and temperature regulation [28]. As we discuss below, hypoxia-induced enhancement of extracellular adenosine generation and signaling – particularly through Adora2b – can dampen hypoxia-elicited inflammation and function to adapt ischemic tissues to conditions of limited oxygen availability [8, 26, 27, 29-35].
Enhanced adenosine production and Aora2b signaling during myocardial ischemia
Hypoxia and inflammation are interdependent [36-38], and adenosine has been implicated in attenuating hypoxia-elicited inflammation and promoting tissue-adaptation to hypoxia [39-43]. During episodes of ischemia and inflammation, extracellular adenosine is generated predominantly by the breakdown of precursor nucleotides – particularly ATP and ADP (Figure 1). Intracellular ATP concentrations are very high (5–8 mM) and its extracellular release is associated with cellular injury [1, 2] to, among other, myocytes, endothelial cells, platelets, or inflammatory cells. For example, hypoxia and inflammation are associated with neutrophil- or endothelial-dependent ATP release through connexin 43 hemichannels [24, 44, 45]. Similarly, necrotic or apoptotic cells release ATP into the extracellular compartment [46].
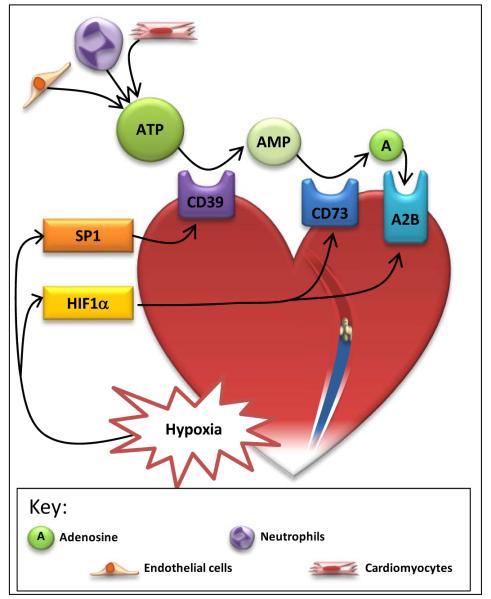
Figure 1. Hypoxia induction of adenosine signaling during myocardial ischemia.
Hypoxic cardiac tissue during myocardial ischemia stabilizes hypoxia inducible factor-1a (HIF1α) and specificity protein-1 (SP1). SP1 induces CD39, whereas HIF1α induces CD73 and the adenosine receptor -2B (A2B). Adenosine triphosphate (ATP) is released by nearby cells, such as endothelial cells, neutrophils and cardiomyocytes, during hypoxic conditions. ATP is converted to adenosine monophosphate (AMP) by CD39, and AMP is then converted to adenosine by CD73. Adenosine signals through the A2B receptor, allowing the tissue to adapt to ischemia.
Phosphohydrolysis of extracellular ATP/ADP is mainly achieved by ectonucleoside triphosphate diphosphohydrolases (E-NTPDases), a recently described family of ubiquitously expressed membrane-bound enzymes [47-49]. The initial step in the production of adenosine, the conversion of ATP/ADP to AMP, is carried out by E-NTPDase 1, also known as CD39. Previous studies demonstrated a selective induction of CD39 under conditions of hypoxia or ischemia [23-25, 50-52], and additional studies using promoter constructs and transcription factor binding assays under these conditions implicate the transcription factor Sp1 in the induction of CD39 gene expression [50, 51]. Hypoxia-dependent induction of CD39 plays a functional role in the extracellular generation of adenosine and concomitant cardioprotection, as shown by experiments demonstrating that the profound increases in extracellular adenosine levels associated with myocardial ischemia are blunted in cd39−/− mice [52]. Moreover, pharmacological inhibition or genetic deletion of cd39 is associated with increased myocardial infarct sizes due to decreased extracellular adenosine signaling [52].
The second step in extracellular adenosine generation, the conversion of AMP to adenosine, is catalyzed by the ecto-5′-nucleotidase CD73. Similar to CD39, CD73 is transcriptionally induced during inflammation, ischemia, or hypoxia [48, 53-55], with concomitant increases in extracellular adenosine generation during myocardial ischemia [56, 57]. Unlike CD39, this transcriptional pathway is controlled by hypoxia-inducible factor HIF1A. The transcription factor HIF was identified as a regulator of erythropoietin release during conditions of hypoxia [58, 59]. During normoxic conditions, HIF1A protein is rapidly degraded [60, 61] in a that process involves hydroxylation by oxygen-dependent prolylhydroxylases (PHDs) and binding of the von-Hippel-Lindau gene product, which promotes poly-ubiquitination and subsequent proteasomal degradation of HIF1A [62]. Hypoxic conditions result in the functional inhibition of PHDs and the stabilization of HIF1A [3]. Similarly, myocardial ischemia is associated with the post-translational stabilization of HIF1A, its subsequent heterodimerization with HIF1B, and a concomitant induction of HIF-dependent genes. Studies with promoter constructs, transcription factor binding assays, and HIF-loss or gain-of-function demonstrate that HIF1A controls hypoxia-associated induction of CD73 [22, 25, 63]. Studies of murine myocardial ischemia and reperfusion indicate that HIF1A controls myocardial CD73 expression during ischemia, and thus myocardial adenosine levels and concomitant cardioprotection [34]. Indeed, in vivo siRNA-mediated repression of PHDs, and subsequent HIF stabilization, results in attenuated myocardial infarct sizes, increased expression of CD73, and elevations of extracellular adenosine levels (Figure 1) [34].
Although hypoxia and/or ischemia transcriptionally enhance ATP-dependent adenosine production via CD39 [52] and CD73 [57], there are additional mechanisms of hypoxia-elicited increases in extracellular adenosine involving the termination of extracellular adenosine signaling. As such, hypoxia is also associated with the transcriptional repression of adenosine transporters – particularly the equilibrative nucleoside transporters ENT1 and ENT2 [64-67] – and the adenosine metabolizing adenosine kinase [68], thereby prolonging extracellular adenosine signaling effects. Interestingly, studies on the transcriptional control of ENTs and adenosine kinase again implicate HIF1A in transcriptional regulation. Direct binding of HIF to the ENT1, ENT2, or AK promoters results in their transcriptional repression and subsequent inhibition of adenosine transport and intracellular metabolism. As such, this pathway of hypoxia-dependent transcription factors provides another example of how limited oxygen availability – such as occurs during myocardial ischemia – enhances extracellular adenosine levels and adenosine signaling. Interestingly, the alternative pathway for adenosine degradation – adenosine deaminase (ADA)-dependent metabolism of adenosine to inosine – is transcriptionally enhanced by hypoxia. However, ADA induction occurs at a later stage, and most likely resembles a delayed off mechanism for adenosine signaling [69].
Adenosine receptor signaling during ischemic heart disease
The observation that adenosine can exert cardioprotective effects during ischemic heart disease dates back many years [70]. For example, very elegant studies in a dog model of myocardial ischemia found that inhibition of CD73 is associated with attenuated adenosine levels in the coronary sinus blood and abolished cardioprotection by ischemic preconditioning [56]. However, there has been considerable debate about which adenosine receptor signaling pathway is involved in cardioprotection. Initial studies implicated the Adora1 or Adora2a receptors, in particular [71, 72]. Studies in transgenic mice using overexpression of Adora1 showed attenuated myocardial infarct sizes in the context of slower heart rates [72]. Other studies have shown that endogenous adenosine can dampen inflammatory diseases via activation of Adora2A in in vivo models of LPS-driven inflammation [73, 74]. Indeed, studies investigating inflammatory cell signaling during ischemia and reperfusion injury implicated Adora2a signaling on inflammatory cells in the context of ischemic tissue-protection utilizing murine in vivo models, for example during renal ischemia [75, 76], hepatic ischemia [77], but also during myocardial ischemia and reperfusion injury [71]. In addition to studies on Adora1 and Adora2a signaling during ischemia and reperfusion, there has been an ongoing debate about Adora2b signaling in cardio-protection from ischemia. While a recent study indicates that Adora2b signaling could be detrimental during cardiac remodeling after acute myocardial infarction [78], several other studies implicate Adora2b agonist treatment as novel form of cardio-protection [8, 34, 57, 79, 80]. Most likely, these differences are due to the timing of treatment, or differences in the model system [81, 82]. Our laboratory observed in a head-to-head comparison in gene-targeted mice for all four individual ARs, that Adora2b−/− mice are particularly prone to myocardial ischemia [26, 27, 57, 83-86]. These studies were carried out in an in situ model of myocardial ischemia and reperfusion injury, where infarct sizes were assessed by staining techniques relative to the area that was perfused by the coronary artery that was occluded [81, 82]. Moreover, a recent paper from our group examined adenosine receptor expression in cardiac tissue samples from human hearts with ongoing myocardial ischemia (ischemic cardiomyopathy) in comparison to healthy human control heart tissues. These studies were carried out by measuring AR transcript levels by real-time RT-PCR and AR protein levels by Western blotting. This study revealed that human ischemic heart disease was associated with a selective and very robust induction of Adora2b, in comparison with healthy controls [8]. Studies on the transcriptional mechanism governing increased ADORA2B expression during ambient hypoxia or myocardial ischemia implicated HIF1A (Figure 1) [21, 34, 57]. Moreover, pharmacological studies utilizing a selective agonist for Adora2b (BAY 60-6583) show dramatic attenuation of myocardial infarct sizes in wild-type mice [57] or rats [79], but not in Adora2b−/− mice [57]. Together, these studies strongly implicate the low affinity Adora2b adenosine receptor in cardioprotection during myocardial ischemia. Indeed, in the context of other studies demonstrating dramatic elevations of extracellular adenosine during ischemic tissue injury, extracellular adenosine concentrations are likely to rise to levels that are sufficient for Adora2b activation in the context of ischemic tissue injury [8, 12, 13, 26, 83].
Adora2b signaling during ischemic pre-conditioning
Ischemic preconditioning is an experimental strategy where short time periods of nonlethal periods of myocardial ischemia are applied prior to the “actual” ischemic injury [87]. This experimental approach is associated with dramatic attenuation of myocardial infarct sizes [82]. Ischemic preconditioning has been applied in humans for example in the context of organ protection during liver transplantation surgery [88]. However, there are significant limitations in directly applying ischemic preconditioning for myocardial protection in humans. Particularly, it is extremely unlikely that a patient can receive this form of pre-treatment prior to an ischemic myocardial injury, as the timing of myocardial injury is unknown. Nevertheless, many studies have used ischemic preconditioning as an experimental strategy to identify novel therapeutic targets with the goal to find pharmacologic approaches to “imitate” cardio-protection mediated by ischemic preconditioning. Several studies had implicated extracellular adenosine production and signaling in mediating the protective effects of ischemic preconditioning. For example, mice deficient in extracellular adenosine generation (cd73−/− mice) are not protected by this experimental approach [57]. While all four adenosine receptors have been implicated in mediating cardio-protection by ischemic preconditioning, our laboratory performed a direct comparison of all four knockout mice for individual ARs [57]. This study demonstrated that the loss of cardio protection following ischemic preconditioning was particularly profound in Adora2b−/− mice. Consistent with these findings, other investigators have shown that Ador2b signaling in conjunction with signaling events through bradykinin and opioid receptors converge on protein kinase C (PKC) and that activated PKC sensitizes the low-affinity Adora2b through phosphorylation of either the receptor or its coupling proteins so that the Adora2b can be activated by endogenous adenosine released by the previously ischemic cardiomyocytes [89, 90]. The sensitized Adora2b would then be responsible for activation of the survival kinases including PI3 kinase, Akt and ERK which then act to inhibit lethal mitochondrial permeability transition pore formation which normally uncouples mitochondria and destroys many myocytes in the first minutes of reperfusion [89]. These findings are also consistent with other studies implicating this molecular pathway in cardio protection mediated by Adora2b signaling during post conditioning [91].
Several recent studies have shown that ischemic preconditioning also has strong transcriptional effects. For example, a recent study provides evidence that cardioprotective effects of ischemic preconditioning are abolished in mice with endothelial-specific deletion of Hif1a [92]. While the question if the immediate cardioprotective effects of ischemic preconditioning involves transcriptional pathways or not remains controversial, we elected to study such pathways to gain additional insight into novel pathways that could provide cardio-protection from ischemia. These studies revealed a regulatory effect of Adora2b signaling on the stabilization of circadian rhythm proteins in the heart during myocardial ischemia [8].
Circadian rhythm protein Per2 is a downstream target of ADORA2B
Although the above studies provide strong evidence for Adora2b-dependent cardioprotection during myocardial ischemia, the mechanism by which Adora2b can protect the heart from ischemia were generally unknown until recently. Microarray studies of tissues from wild-type or Adora2b−/− mice exposed to in situ myocardial ischemia revealed important information regarding potential Adora2b target genes [8]. A pathway analysis revealed that the dominant pathway with differential regulation comprised circadian rhythm genes (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19875), and of those, the most dramatic differential regulation was for circadian rhythm protein Period2 (Per2), which belongs to the family of genes expressed in the suprachiasmatic nucleus in a circadian pattern [93].
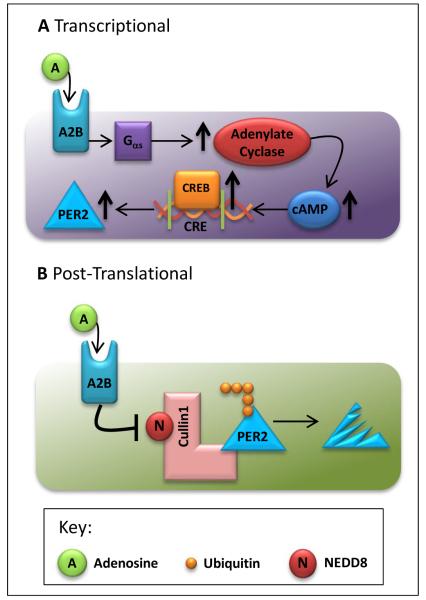
In humans, the period circadian protein homolog 2 is a protein encoded by the PER2 gene [94]. Per2 belongs to the family of Period genes that are expressed in a circadian pattern in the suprachiasmatic nucleus, the primary circadian pacemaker in the mammalian brain [93]. Genes in this family encode components of the circadian rhythms of locomotor activity, metabolism, and behavior [93-97]. Studies have confirmed increased Per2 transcript and protein levels with myocardial ischemia in wild-type mice, but not in Aorad2b−/− mice [8]. Subsequent experiments investigated the mechanism of Adora2b-dependent stabilization or induction of Per2 during myocardial ischemia and implicate a combination of transcriptional and post-translational molecular pathways [8]. The transcriptional effects of Adora2b signaling that lead to Per2 stabilization involve the G protein-mediated activation of adenylate cyclase, subsequent increases in cAMP levels, and binding of cAMP response element-binding protein (CREB) to a CREB-binding element within the Per2 promoter, thereby inducing transcription (Figure 2b). These studies are consistent with the known role of ADORA2B in elevating intracellular cAMP levels [26, 27]. In addition, Adora2b-dependent induction of Per2 involves post-translational pathways: Adora2b activity elicits inhibition of the proteasomal degradation pathway by inhibiting deneddylation of cullin-1 (Figure 2b) [8, 98]. Taken together, these pathways result in a very robust and rapid increase of Per2 transcript and protein levels upon Adora2b stimulation (Figure 2).
Figure 2. Adora2b (A2B)-elicited Period 2 (PER2) stabilization.
A) Adenosine signaling through adenosine receptor-2B (A2B) activates the Gαs protein activating adenylyl cyclase which increases the intracellular cyclic adenosine monophosphate (cAMP). Due to the increase in cAMP, higher levels of cAMP response element-binding protein (CREB) bind to the cAMP-response elements (CRE) within DNA, ultimately upregulating Period 2 (PER2). B) Adenosine signaling through A2B inhibits the neddylation of cullin-1, which inhibits the proteosomal degradation of PER2.
Functional role of Per2 during myocardial ischemia
Transgenic mice lacking Per2 are viable. However, homozygote Per2−/− mice display a shorter circadian period, followed by a loss of circadian rhythmicity in constant darkness [93]. Exposure of Per2−/− mice to myocardial ischemia revealed a very profound phenotype. Indeed, cardioprotection by ischemic preconditioning was completely abolished. Moreover, myocardial infarct sizes were dramatically increased in Per2−/− mice [8]. In wild-type animals pre-treated with the Adora2b agonist BAY 60-6583 [57], a robust decrease of myocardial infarct sizes and attenuated troponin I plasma levels – a marker of myocardial tissue injury – is observed. In contrast, Adora2b agonist treatment provides no cardioprotection in Per2−/− mice, providing evidence that Adora2b-dependent cardioprotection is abolished in Per2−/− mice [8]. Together, these studies indicate a functional role for Per2 in mediating adenosine-elicited cardioprotection.
Impaired glycolysis during myocardial ischemia in Per2−/− mice
Based on previous reports implicating Per2 in the regulation of metabolism [95, 99], we considered the possibility that adenosine-dependent increases of Per2 levels could function to adapt myocardial metabolism during ischemia towards more oxygen-efficient utilization of carbohydrates. Indeed, several levels of evidence were supporting this notion. First, we observed that the induction of transcript, protein and enzyme function for glycolytic enzymes upon exposure to myocardial ischemia was abolished in Per2−/− mice. Similarly, measurements of glycolytic flux utilizing in vivo application of labeled carbohydrates revealed dramatically attenuated glycolytic flux in Per2−/− mice [8]. Consistent with these findings we observed that increases in myocardial lactate production during ischemia were absent in Per2−/− mice. Together, these studies suggest that Adora2b-elicited stabilization of Per2 is critical for increasing the myocardial capacity to perform anaerobic glycolysis during cardiac ischemia.
Hypoxia-inducible factor 1 alpha (Hif1a) – a link between Per2 and ischemic myocardial metabolism
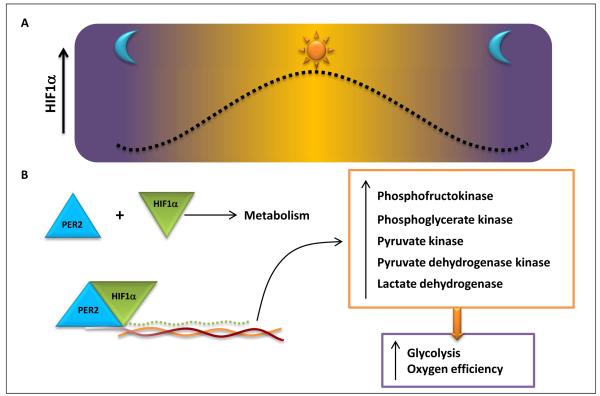
Previous studies had demonstrated a central role for HIF1 in mediating the transcriptional induction of glycolytic enzymes during conditions of limited oxygen availability [59]. Indeed, this is one of the key hypoxia-elicited adaptive responses, as the only source for cellular metabolism go generate ATP in the absence of oxygen is anaerobic glycolysis. Indeed, HIF-dependent increases in glycolytic capacity play a key role for the growth of cancer cells in an hypoxic or anoxic environment [3] or during the cell survival in the setting of ischemia and reperfusion [2, 60]. Based on the central role for HIF1 in the hypoxic induction of the glycolytic machinery, we considered the possibility that Per2 could be involved in this process via interactions with HIF1A. Utilizing a HIF reporter mouse line [100], we made the unexpected observation that HIF1A follows a circadian expression pattern with very similar kinetics as Per2 (Figure 3) [8]. Genetic deletion of Per2 in the HIF reporter mice abolished the circadian rhythmicity of HIF expression. Similarly, the hypoxic stabilization of Hif1a was abolished in isolated myocytes from Per2−/− mice, indicating a functional role for Per2 in controlling Hif1a-dependent alterations in gene expression. Moreover studies utilizing co-immunoprecipitation of Hif1a and Per2 demonstrated a direct protein-protein interaction between Hif1a and Per2 in cardiac tissues during myocardial ischemia. These studies indicate that Adora2b-dependent control of Per2 plays an important role in the HIF-elicited induction of the glycolytic machinery during myocardial ischemia [8].
Figure 3.
A) Hypoxia inducible factor 1-α (HIF1α) protein levels increase in the daytime and remain lower at night. B) Period 2 (PER2) and HIF1α bind together and upregulate glycolytic enzymes. The induction of glycolytic enzymes increases the glycoltyic rate and the generation of adenosine triphosphate (ATP), which ultimately enhances the oxygen efficiency within the cell.
Light-elicited cardioprotection mediated by cardiac Per2 stabilization (“light preconditioning”)
As final step in our studies to address the functional role of Per2 in cardioprotection from ischemia, we pursued an approach that would provide for increased cardiac Per2 expression. Indeed, previous studies had demonstrated that light exposure is associated with Per2 stabilization in different organs [101]. Therefore, we next attempted to achieve enhanced cardiac Per2 expression via exposure of mice to intense light [101]. In these studies, we exposed mice for 4h to intense daylight (13,000 lux). Indeed, this experimental approach was associated with very robust increases of Per2 protein levels in the heart. Consistent with our previous studies that Per2 functions to increase glycolytic capacity, we found that light-treated mice showed higher levels of their glycolytic enzymes in the heart. Subsequent exposure of light-treated mice to myocardial ischemia and reperfusion injury revealed that myocardial infarct sizes and plasma troponin levels were dramatically attenuated, thereby providing evidence for a myocardial preconditioning effect of light pre-treatment (“light preconditioning”). Indeed, the cardioprotection achieved by preconditioning with light was completely abolished in Per2−/− mice [8], suggesting that the light-elicited cardioprotection involves stabilization of Per2 and concomitant metabolic adaptation during myocardial ischemia [8].
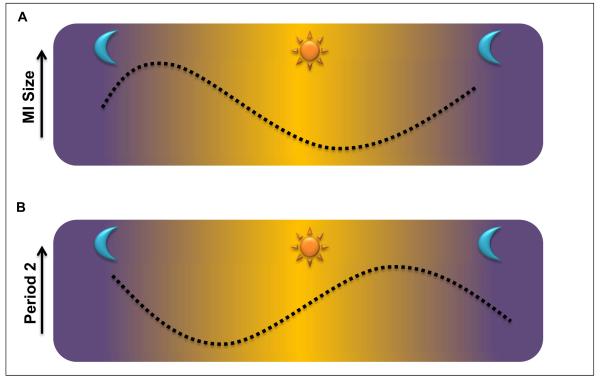
Subsequent studies examined Per2 expression over a 24h time period, and correlated Per2 protein levels with the circadian pattern of myocardial infarct sizes [8]. Utilizing a Per2 reporter mouse line, these studies revealed that Per2 protein levels were lowest in the early morning hours following a long period of total darkness. Highest Per2 protein levels were observed in the afternoon and early evening [8]. Consistent with the observation that Per2 provides cardioprotection from ischemia, myocardial infarct sizes were inversely correlated, with largest myocardial infarct sizes in the early morning hours (when Per2 levels are lowest) and smallest infarct sized in the evening (when Per2 levels are highest, Figure 4). Additional studies will be necessary to define the expression and functional role of PER2 in the human heart, as well as its role in human heart disease [8].
Figure 4.
A) Myocardial infarct sizes are highest in the morning and decrease throughout the day. B) Period 2 protein levels are at the lowest in the morning and increase throughout the day.
Conclusions and Novel Therapeutic Opportunities
Genetic and pharmacological studies during the past decade have revealed that hypoxiaelicited adaptive responses during myocardial ischemia include the HIF-dependent induction of adenosine production and signaling events – particularly through Adora2b [1]. Based on these findings, several treatment approaches could be conceived to help dampen myocardial ischemia and reperfusion injury in patients:
1. Treatment with HIF activators
Several pharmacological compounds are available that promote the stabilization of HIF under normoxic conditions [102]. These compounds typically function by inhibiting oxygen sensors (PHDs), thereby preventing hydroxylation of HIF1A and leading to HIF stabilization and subsequent induction of HIF-dependent genes. These compounds have been safely used in humans, as recently documented in a clinical trial utilizing a PHD inhibitor for the treatment of renal anemia in patients [103]. Moreover, several experimental studies indicate a protective role of these compounds during inflammatory hypoxia, such as occurs for example during intestinal inflammation [29, 63, 104-108]. In addition, experimental evidence suggests that pre-treatment with HIF activators is associated with attenuated myocardial infarct sizes in the context of increased adenosine production and signaling through Adoara2b [34]. As such, pretreatment with compounds that enhance tissue HIF levels could evolve as novel treatment approach to dampen myocardial tissue injury during myocardial ischemia. While these compounds when used locally or only during a short time period are likely to be safe for patients , there may disadvantages when using them long term, for example by causing erythrocytosis [103].
2. Treatment with compounds that promote extracellular adenosine generation
Experimental studies indicate that compounds that promote extracellular conversion of nucleotides (i.e., ATP, ADP, or AMP) to adenosine are cardioprotective during myocardial ischemia [52, 57]. Such compounds include apyrase (conversion of ATP/ADP to AMP) and nucleotidases (conversion of AMP to adenosine) [52, 57]. Nevertheless, these compounds have yet to be examined in patients and their safety profiles remain unclear. As one example of a possible unwanted side effect, treatment with nucleotidase has been reported to function as a platelet inhibitor, thereby promoting bleeding [109]. This could be an important issue in certain patient populations (e.g. patients undergoing cardiac surgery).
3. Treatment with an Adora2b agonist
Experimental studies have shown that pretreatment of myocardial ischemia with the Adora2b agonist BAY 60-6583 is effective in reducing myocardial infarct sizes in rats or in wild-type mice, but not in Adora2b−/− mice [8, 57, 79, 80]. At present the efficacy and safety of ADORA2B agonists in human remains unknown. Similarly, other ischemic diseases (e.g. the kidneys or the intestine) can be treated with Adora2b agonists [33, 63, 64, 110]. As such, experimental evidence strongly supports the use of ADORA2B agonists for attenuating myocardial infarct sizes in patients.
4. Targeting Per2 to treat myocardial ischemia
As discussed above, recent studies implicate Per2 in mediating adenosine-dependent cardioprotection from ischemia. As such, treatment approaches that would result in cardiac induction of Per2 are novel treatment approaches for myocardial ischemia. For example, exposure to intense light could be a therapeutic strategy to achieve cardiac Per2 stabilization in patients. Moreover, it would be interesting to identify delivery mechanisms, by which tissue specific targeting of PER2 could be achieved, for example via intracoronary injection of an ADORA2B agonist. However, we would like to also point out that these findings are very novel, and that additional confirmatory studies of Period-dependent cardio protection will be important for this field of research.
With some exceptions (e.g. selective induction of ADORA2B during human myocardial ischemia [8]), most of the discussed studies and experimental approaches are based on evidence from mice. As such it will be necessary to take further steps towards translating these findings from mice to man. Most of the above studies implicate hypoxia-elicited adenosine responses in preventing myocardial tissue injury during ischemia (as opposed to treating patients with ongoing myocardial ischemia or during reperfusion), and it remains unclear how adenosine signaling can contribute to myocardial injury at a later stage (e.g. during reperfusion). However, some studies suggest that Adora2b signaling may be effective in attenuating myocardial ischemia when given after the ischemic event [90, 91]. Moreover, there are large patient populations that would benefit from effective pre-treatments that would render the myocardium more resistant towards the detrimental effects of ischemia and reperfusion injury. For example, patients undergoing organ transplantation or major cardiovascular or thoracic surgery would dramatically benefit from pre-emptive ischemia-protective treatment approaches [16].
The search for novel pharmacologic or interventional therapies (e.g. light therapy) to prevent or treat myocardial ischemia and reperfusion injury remains one of the most important challenges in order to improve morbidity and mortality in patients with cardiovascular disease. As such, extracellular adenosine signaling has evolved as a direct means of providing cardioprotection (e.g. by directly activating adenosine receptors). At least similarly important, studies on the mechanisms by which adenosine provides cardioprotection has yielded several novel therapeutic ideas (for example HIF activators or activators of circadian rhythm proteins). As such, we are very optimistic that these efforts will yield novel therapeutic approaches that can be translated into novel preventive or therapeutic interventions in patients.
Figure I.
Conventional and Innovative Treatments of Myocardial Ischemia.
ACKNOWLEDGEMENT
The present research work was supported by National Heart Institute Grants R01-HL0921, R01-DK083385, R01-HL098294 to HKE, K08HL102267 to TE, and a grant by the Crohn’s and Colitis Foundation of America (CCFA) to HKE.
Glossary
- Adenosine
A purine nucleoside comprising a molecule of adenine attached to a ribose sugar via a β-N9-glycosidic bond. Functions as signaling molecule within the extracellular compartment by activating G protein-coupled receptors (adenosine receptors, see below). Available as an injectable drug for the treatment of supraventricular tachycardia [16].
- Adenosine receptors
Adenosine can signal through four distinct adenosine receptors. These are G-protein couples receptors that function through Gi to dampen adenylate cyclase activity (adenosine receptor Adora1 and Adora3) or through Gs to activate adenylate cyclase activity (adenosine receptors Adora2a and Adora2b), resulting in elevations of cyclic adenosine monophosphate [1, 112].
- Adenosine deaminase (ADA)
Enzyme that converts adenosine to inosine. Delayed induction of ADA with hypoxia indicates that it may function as delayed stop-signal for hypoxia-driven adenosine signaling [69].
- Adenosine kinase (AK)
Enzyme that converts intracellular adenosine to adenosine monophosphate (AMP). Hypoxia is associated with transcriptional repression of AK, thereby resulting in increased intracellular adenosine levels, leading to attenuated flow of adenosine across the membrane, and concomitant increases in extracellular adenosine signaling during hypoxia [68].
- Coronary patency
The most common cause of acute coronary syndromes is the occlusion of a coronary artery. If persistent, occlusion of the coronary vessel will lead to myocardial infarction. To prevent this from happening, therapeutic interventions focus on early “re-opening” of the coronary vessel, for example by placing a stent into the coronary vessel in order to achieve coronary patency.
- Ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDase) 1 (CD39)
membrane-bound enzyme with its enzymatic activity facing the extracellular space; converts extracellular ATP and/or ADP to adenosine monophosphate (AMP). Critical for the extracellular generation of adenosine, as AMP serves as the precursor molecule for adenosine generation. Conditions of hypoxia or myocardial ischemia are associated with the transcriptional induction of CD39 [24, 25, 50-52].
- Ecto-5′-nucleotidase (CD73)
membrane-bound enzyme with its enzymatic activity facing the extracellular space; converts extracellular AMP to adenosine, thereby functioning as pace-maker enzyme for the extracellular production of adenosine from precursor nucleotides. Conditions of hypoxia or myocardial ischemia are associated with the HIF-dependent induction of CD73 [22-25, 113, 114].
- Equilibrative nucleoside transporters ENT1 and ENT2
Channels located in the cell membrane that allow adenosine to cross the cell membrane following its gradient. Due to the fact that hypoxia and ischemia are associated with increases in ATP-dependent adenosine generation on the extracellular side, hypoxia-elicited repression of ENT1 and ENT2 functions as mechanism to enhance extracellular adenosine signaling [64-67].
- Heart block
A heart block is defined as a disease of the electrical conduction system of the heart. Such a heart block can involve any level of the electrical conduction system of the heart. Sometimes, pharmacological strategies to treat cardiac rhythm abnormalities include the induction of a transient “therapeutic” heart block. For example, treatment of supraventricular tachycardia (a cardiac rhythm abnormality originating at the level of the atrium) frequently includes administration of an intravenous adenosine bolus. Adenosine treatment of supraventricular tachycardia causes an iatrogenic complete block of the electrical conduction system of the heart between atrium and the ventricle. This complete heart block causes a standstill of the heart for several seconds (5 to 15 seconds) until adenosine plasma levels fall, and a normal cardiac rhythm can re-establish itself.
- Hypoxia-inducible factor (HIF)
Key transcription factor for hypoxia-adoptive responses and identified in many gene programs that are critical in adapting hypoxic or ischemic tissues to conditions of limited oxygen availability [60]. During normoxic conditions, HIF1A protein is hydroxylated, which targets it for proteasomal degradation [60, 61, 115]. During hypoxia, hydroxylation of HIF proteins are inhibited, leading to HIF stabilization, translocation to the nucleus and binding to promoter regions of hypoxia-responsive genes [3]. In most instances, this will result in the induction of hypoxia-responsive genes, while there are also some examples for HIF-dependent repression of gene expression [12, 64-68, 116].
- Ischemic conditioning
Pretreatment with repeated short time periods of myocardial ischemia results in attenuated myocardial infarct sizes (ischemic preconditioning) [82]. Alternative approaches utilizing treatment with short ischemic periods to reduce myocardial infarct sizes include ischemic postconditioning (application of short ischemic periods following myocardial ischemia) and remote conditioning (treatment with short ischemic periods to a remote place, e.g. via inflation of a blood pressure cuff) [2].
- Light preconditioning
Recent studies indicate that pre-treatment with intense light is associated with attenuated myocardial infarct sizes via light-induced stabilization of Period2 and subsequent optimization of cardiac metabolism towards more oxygen-efficient utilization of carbohydrates and concomitant attenuation of myocardial infarcts sizes [8].
- Period2 (Per2)
Per2 belongs to the family of circadian rhythm proteins and is transcriptionally induced by elevations of cAMP levels [117], including adenosine receptor Adora2b activation [8]. Recent studies implicate Per2 in cardioprotection from ischemia through re-programming myocardial metabolism towards more oxygen-efficient utilization of carbohydrates [8, 118].
- Prolylhydroxylases (PHDs)
A set of intracellular enzymes that regulate the stability of the transcription factor HIF. Under conditions of normal oxygen levels, PHDs hydroxylate the proteins HIF1A and HIF2A, thereby causing their proteasomal degradation. PHDs require oxygen as co-factor for HIF-hydroxylation. When oxygen levels fall, PHDs are functionally inhibited, and HIF is stabilized.
- Universal intracellular energy currency
In the intracellular compartment, adenosine triphosphate (ATP) is considered the universal currency of intracellular energy processes. Phosphohydrolysis of ATP is utilized as the driving energy source for intracellular reactions. In contrast, ATP functions on the extracellular side are predominantly as signaling molecules. On the one hand, ATP can activate ATP receptors (P2Y receptors or P2X receptors), or it can be enzymatically converted to adenosine, thereby providing an important source for extracellular adenosine generation [2, 11, 119].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no declared that no conflict of interest exists.
References
- 1.Eltzschig HK, et al. Mechanisms of Disease: Purinergic Signaling. N Engl J Med. 2012 in press. [Google Scholar]
- 2.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 6.Botker HE, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 7.Piot C, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 8.Eckle T, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willich SN, et al. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol. 1992;70:65–68. doi: 10.1016/0002-9149(92)91391-g. [DOI] [PubMed] [Google Scholar]
- 10.Muller JE, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 11.Khakh BS, Burnstock G. The double life of ATP. Sci Am. 2009;301:84–90. 92. doi: 10.1038/scientificamerican1209-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:153–175. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauerle JD, et al. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol. 2011;22:14–20. doi: 10.1681/ASN.2009121217. [DOI] [PubMed] [Google Scholar]
- 14.Grenz A, et al. Extracellular adenosine: a safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid Redox Signal. 2011;15:2221–2234. doi: 10.1089/ars.2010.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckle T, et al. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 16.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delacretaz E. Clinical practice. Supraventricular tachycardia. N Engl J Med. 2006;354:1039–1051. doi: 10.1056/NEJMcp051145. [DOI] [PubMed] [Google Scholar]
- 18.Koeppen M, et al. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One. 2009;4:e6784. doi: 10.1371/journal.pone.0006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronstein BN, et al. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linden J, Cekic C. Regulation of lymphocyte function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:2097–2103. doi: 10.1161/ATVBAHA.111.226837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong T, et al. HIF-dependent induction of adenosine A2B receptor in hypoxia. Faseb J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 22.Thompson LF, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eltzschig HK, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 24.Eltzschig HK, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Synnestvedt K, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koeppen M, et al. Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv Pharmacol. 2011;61:145–186. doi: 10.1016/B978-0-12-385526-8.00006-0. [DOI] [PubMed] [Google Scholar]
- 27.Aherne CM, et al. The resurgence of A2B adenosine receptor signaling. Biochim Biophys Acta. 2011;1808:1329–1339. doi: 10.1016/j.bbamem.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JN, et al. Adenosine A(3) receptors regulate heart rate, motor activity and body temperature. Acta Physiol (Oxf) 2010;199:221–230. doi: 10.1111/j.1748-1716.2010.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrentraut H, et al. Adora2b Adenosine Receptor Engagement Enhances Regulatory T Cell Abundance during Endotoxin-Induced Pulmonary Inflammation. PLoS One. 2012;7:e32416. doi: 10.1371/journal.pone.0032416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grenz A, et al. Adora2b Adenosine Receptor Signaling Protects during Acute Kidney Injury via Inhibition of Neutrophil-Dependent TNF-alpha Release. J Immunol. 2012 doi: 10.4049/jimmunol.1201651. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Zhang Y, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nat Med. 2011;17:79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckle T, et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grenz A, et al. The Reno-Vascular A2B Adenosine Receptor Protects the Kidney from Ischemia. PLoS Medicine. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckle T, et al. Hypoxia-Inducible Factor-1 Is Central to Cardioprotection: A New Paradigm for Ischemic Preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 35.Eckle T, et al. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhlicke J, et al. Hypoxia Inducible Factor (HIF)-1 Coordinates Induction of Toll-Like Receptors TLR2 and TLR6 during Hypoxia. PLoS ONE. 2007;2:e1364. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummins EP, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aherne CM, et al. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61:695–705. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grenz A, et al. Partial netrin-1 deficiency aggravates acute kidney injury. PLoS One. 2011;6:e14812. doi: 10.1371/journal.pone.0014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberger P, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 42.Schingnitz U, et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frick JS, et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faigle M, et al. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eltzschig HK, et al. Neutrophils as Sources of Extracellular Nucleotides: Functional Consequences at the Vascular Interface. Trends Cardiovasc Med. 2008;18:103–107. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chekeni FB, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reutershan J, et al. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 48.Eckle T, et al. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 49.Grenz A, et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 50.Hart ML, et al. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eltzschig HK, et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohler D, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 53.Hart ML, et al. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J. 2008;22:2784–2797. doi: 10.1096/fj.07-103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hart ML, et al. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135:1739–1750. e1733. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 55.Grenz A, et al. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 56.Kitakaze M, et al. Infarct size-limiting effect of ischemic preconditioning is blunted by inhibition of 5′-nucleotidase activity and attenuation of adenosine release. Circulation. 1994;89:1237–1246. doi: 10.1161/01.cir.89.3.1237. [DOI] [PubMed] [Google Scholar]
- 57.Eckle T, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 58.Wang G, et al. Hypoxia-Inducible Factor 1 is a Basic-Helix-Loop-Helix-PAS Heterodimer Regulated by Cellular O2 Tension. PNAS. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semenza GL, et al. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 60.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Semenza GL. Oxygen sensing, homeostasis, and disease. The New England journal of medicine. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 62.Kaelin WG. Von Hippel-Lindau disease. Annu Rev Pathol. 2007;2:145–173. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 63.Hart ML, et al. Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Grenz A, et al. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest. 2012;122:693–710. doi: 10.1172/JCI60214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Morote-Garcia JC, et al. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–618. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 66.Loffler M, et al. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 67.Eltzschig HK, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morote-Garcia JC, et al. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 69.Eltzschig HK, et al. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006;108:1602–1610. doi: 10.1182/blood-2006-02-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hori M, Kitakaze M. Adenosine, the heart, and coronary circulation. Hypertension. 1991;18:565–574. doi: 10.1161/01.hyp.18.5.565. [DOI] [PubMed] [Google Scholar]
- 71.Yang Z, et al. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–2197. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 72.Matherne GP, et al. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc Natl Acad Sci U S A. 1997;94:6541–6546. doi: 10.1073/pnas.94.12.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sitkovsky MV, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annual Review of Immunology. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 74.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 75.Li L, et al. Dendritic cells tolerized with adenosine A2AR agonist attenuate acute kidney injury. J Clin Invest. 2012;122:3931–3942. doi: 10.1172/JCI63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kinsey GR, et al. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol. 2012;23:1528–1537. doi: 10.1681/ASN.2012010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lappas CM, et al. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toldo S, et al. GS-6201, a selective blocker of the A2B adenosine receptor, attenuates cardiac remodeling after acute myocardial infarction in the mouse. J Pharmacol Exp Ther. 2012;343:587–595. doi: 10.1124/jpet.111.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maas JE, et al. Evidence that the acute phase of ischemic preconditioning does not require signaling by the A 2B adenosine receptor. J Mol Cell Cardiol. 2010;49:886–893. doi: 10.1016/j.yjmcc.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koeppen M, et al. Adora2b Signaling on Bone Marrow Derived Cells Dampens Myocardial Ischemia-Reperfusion Injury. Anesthesiology. 2012;116:1245–1257. doi: 10.1097/ALN.0b013e318255793c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eckle T, et al. Use of a hanging weight system for coronary artery occlusion in mice. J Vis Exp. 2011 doi: 10.3791/2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eckle T, et al. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–2540. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]
- 83.Grenz A, et al. Hypoxia signaling during intestinal ischemia and inflammation. Curr Opin Crit Care. 2012 doi: 10.1097/MCC.0b013e3283514bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eltzschig HK. Targeting Purinergic Signaling for Perioperative Organ Protection. Anesthesiology. 2013 doi: 10.1097/ALN.0b013e3182874686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eltzschig HK. Extracellular adenosine signaling in molecular medicine. J Mol Med (Berl) 2013;91:141–146. doi: 10.1007/s00109-013-0999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poth JM, et al. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. J Mol Med (Berl) 2013;91:183–193. doi: 10.1007/s00109-012-0988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murry CE, et al. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 88.Petrowsky H, et al. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg. 2006;244:921–928. doi: 10.1097/01.sla.0000246834.07130.5d. discussion 928-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang X, et al. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther. 2010;24:225–234. doi: 10.1007/s10557-010-6236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuno A, et al. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–271. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Philipp S, et al. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 92.Sarkar K, et al. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning. Proc Natl Acad Sci U S A. 2012;109:10504–10509. doi: 10.1073/pnas.1208314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng B, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 94.Xu Y, et al. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128:59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grimaldi B, et al. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang J, et al. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–343. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
- 97.Lee C, et al. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 98.Khoury J, et al. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J Clin Invest. 2007;117:703–711. doi: 10.1172/JCI30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lopaschuk GD, et al. Myocardial fatty acid metabolism in health and disease. Physiological reviews. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 100.Safran M, et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci U S A. 2006;103:105–110. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bendova Z, Sumova S. Photoperiodic regulation of PER1 and PER2 protein expression in rat peripheral tissues. Physiol Res. 2006;55:623–632. doi: 10.33549/physiolres.930849. [DOI] [PubMed] [Google Scholar]
- 102.Fraisl P, et al. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 2009;8:139–152. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 103.Bernhardt WM, et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21:2151–2156. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tambuwala MM, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 106.Cummins EP, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 107.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med. 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 108.Clambey ET, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hart ML, et al. Direct treatment of mouse or human blood with soluble 5′-nucleotidase inhibits platelet aggregation. Arterioscler Thromb Vasc Biol. 2008;28:1477–1483. doi: 10.1161/ATVBAHA.108.169219. [DOI] [PubMed] [Google Scholar]
- 110.Hart ML, et al. Cutting Edge: A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol. 2009;182:3965–3968. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 111.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 112.Hasko G, et al. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Colgan SP, et al. Physiological Roles of 5′-Ectonucleotidase (CD73) Purinergic Signalling. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eltzschig HK, et al. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. doi: 10.1385/1-59745-113-4:73. [DOI] [PubMed] [Google Scholar]
- 115.Taylor CT, McElwain JC. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology (Bethesda) 2010;25:272–279. doi: 10.1152/physiol.00029.2010. [DOI] [PubMed] [Google Scholar]
- 116.Hart ML, et al. Use of a hanging-weight system for liver ischemic preconditioning in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1431–1440. doi: 10.1152/ajpgi.00083.2008. [DOI] [PubMed] [Google Scholar]
- 117.O’Neill JS, et al. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lopaschuk GD, Jaswal JS. A role for period 2 in cardioprotection. Cell Metab. 2012;16:2–4. doi: 10.1016/j.cmet.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 119.Eltzschig HK, et al. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]