Abstract
Thrombosis significantly contributes to cancer morbidity and mortality. The mechanism behind thrombosis in cancer may be circulating tissue factor (TF), as levels of circulating TF are associated with thrombosis. However, circulating TF antigen level alone has failed to predict thrombosis in patients with cancer. We hypothesize that coagulation factor levels regulate the kinetics of circulating TF-induced thrombosis. Coagulation kinetics were measured as a function of individual coagulation factor levels and TF particle concentration. Clotting times increased when pooled plasma was mixed at or above a ratio of 4:6 with PBS. Clotting times increased when pooled plasma was mixed at or above a ratio of 8:2 with factor VII-depleted plasma, 7:3 with factor IX- or factor X-depleted plasmas, or 2:8 with factor II-, V- or VIII-depleted plasmas. Addition of coagulation factors VII, X, IX, V and II to depleted plasmas shortened clotting and enzyme initiation times, and increased enzyme generation rates in a concentration-dependent manner. Only additions of factors IX and X from low-normal to high-normal levels shortened clotting times and increased enzyme generation rates. Our results demonstrate that coagulation kinetics for TF particles are controlled by factor IX and X levels within the normal physiological range. We hypothesize that individual patient factor IX and X levels may be prognostic for susceptibility to circulating TF-induced thrombosis.
Keywords: cancer, coagulation factors, thrombosis, tissue factor
1. Introduction
Cancer is a hypercoagulable state. Patients with cancer are at a 4–7 fold increased risk of developing venous thrombosis.(1) The development of thrombosis confers worse outcomes for patients with cancer and contributes significantly to patient morbidity; however, the mechanisms underlying thrombosis in cancer remain ill-defined.(2, 3) The association between having cancer and developing thrombosis has been observed for centuries, yet we are unable to accurately predict if an individual patient with cancer will develop thrombosis over the course of their disease.(4) Further, only a relative minority of patients with cancer will develop thrombosis; thus, anticoagulation prophylaxis is not part of routine care for patients with cancer.
Efforts to correlate cancer-specific properties with incidence of thrombosis have found that cancer site of origin, histology and stage contribute to an increased risk for developing thrombosis.(5, 6) The observation that specific tumor characteristics influence the risk of thrombosis suggests that cancer cells are a procoagulant stimulus. Procoagulant mechanisms of cancer cells include surface expression of tissue factor (TF).(1, 7–10) TF is a 47 kDa transmembrane protein which serves as the physiological initiator of coagulation during blood vessel injury. TF is not normally exposed by cells within the blood, and therefore control of its procoagulant activity is largely due to exposure only at sites of vessel injury. Unless a bleeding diathesis is present, virtually every vessel injury results in a hemostatic response to stem blood loss. Some pathological states, such as metastatic cancer, introduce TF-expressing cells or TF-expressing cell derived microvesicles into the blood circulation.(11) Elevated levels of circulating TF are associated with thrombosis in cancer, yet presence of circulating TF by itself is not prognostic for development of thrombosis in patients with cancer.(11–14) Therefore, the impact of circulating TF on coagulation remains unclear. For instance, low levels of circulating TF have been detected in healthy subjects with no indication of coagulation initiation.(15–17) Therefore, the procoagulant activity of circulating TF appears to be inherently different than TF exposed locally at a vessel injury.(18)
Circulating TF experiences less relative blood flow than wall-bound TF exposed at the site of a vessel injury. The transmembrane nature of TF suggests that TF-dependent coagulation reactions occur on the surface of TF-expressing cells or cell-derived microvesicles. As such, coagulation factors entrained in the bloodstream must diffuse to the cell or cell-derived microvesicle surface in order for coagulation to proceed. For TF exposed at the site of an injury to a vessel wall, blood flowing past the injury provides a constant supply of coagulation factors to propagate the coagulation reaction. Conversely, for a particle of TF in the bloodstream, coagulation factors must diffuse to the TF particle, as there is very little relative flow (blood flowing past the particle) with which to supply coagulation factors. The rate at which coagulation factors diffuse to the cell or microvesicle surface can be rate-limiting for coagulation reaction kinetics.(19) Diffusion transport of coagulation factors can be quantified by a diffusive flux, which depends upon the plasma concentration of the coagulation factor (Supplemental Material). Concentrations of individual coagulation factors are known to vary by over three-fold in the normal population, implying that transport of coagulation factors vary by over three-fold in the normal population. Moreover, the rate of thrombin generation from an identical procoagulant stimulus has been shown to vary by over three-fold in healthy populations.(20, 21)
The risk to develop thrombosis in cancer is increased for patients with a prior history of thrombosis. Moreover, patients whose peak thrombin generation values (using a TF-dependent assay) rank in the upper quartile are associated with an increased incidence of thrombosis.(22, 23) Thus, patients whose blood responds more robustly to procoagulant stimuli in vitro are more susceptible to thrombosis induced by cancer. Taken together, the development of thrombosis in patients with cancer may be the culmination of the procoagulant stimulus introduced by the patient’s cancer cells and a heightened blood coagulation response to this stimulus. As such, the procoagulant stimulus of cancer itself may not be sufficient to induce thrombosis in all patients, but only in patients whose blood is more sensitive to the cancer induced procoagulant stimulus. Moreover, attempts to predict thrombosis based solely on quantification of the procoagulant stimulus (i.e. circulating TF) may lack specificity to predict thrombosis. We hypothesize that susceptibility to circulating TF-induced thrombosis lies in levels of a patient’s coagulation factors, with patients whose coagulation factor levels reside in the high-normal range to be more susceptible to cancer-derived circulating TF than patients whose coagulation factor levels reside in the low-normal range. Further, quantification of coagulation factor levels may provide added specificity for predicting thrombosis in cancer.
The goal of our study was to characterize the role of individual coagulation factor levels on coagulation kinetics for a constant burden of TF using TF and phospholipid-coated microspheres (TF particles) suspended in plasma as a model for circulating TF. This model of circulating TF allows the precise control of TF burden by controlling the amount of TF particles added (105, 104 or 103 mL−1) which presented TF levels of 3.5 pM, 350 fM and 35 fM, respectively. By varying individual coagulation factor levels below, within and above normal physiological ranges, we aimed to find the coagulation factor or factors whose physiological levels may be limiting to circulating TF-induced coagulation kinetics. The levels of coagulation factors VII, IX, X, VIII, V and II were independently controlled by mixing pooled plasma with coagulation factor-depleted plasma, by adding purified coagulation factors to coagulation factor-depleted plasma, or by diluting pooled plasma with phosphate buffered saline. Clotting times and enzyme generation assays were utilized to characterize the effect of coagulation factor levels on initiating coagulation reactions (initiation time), propagating coagulation reactions (rate), and clotting plasma (clotting time) in the presence of TF particles.
2. Materials and Methods
2.1. Materials
Anti-factor XIa antibodies were generated as described previously.(24) Thromboplastin (recombinant, lipidated tissue factor (Dade® Innovin®)) was purchased from Siemens Healthcare Diagnostics (Deerfield, IL). Immunodepleted and chemically depleted human plasmas (depleted plasmas) and purified human coagulation factors were purchased from Haematologic Technologies, Inc. (Essex Junction, VT). Polystyrene microspheres were purchased from Bangs Labs (Fishers, IN). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) or previously described sources.(25)
2.2. Generation of a model for circulating TF
Thromboplastin-coated microspheres (TF particles) were generated as previously described.(9) In brief, polymeric microspheres were immersed in a stock solution of thromboplastin for 60 minutes at room temperature. Microspheres were then washed and surface-blocked with fatty-acid free bovine serum albumin (BSA). Coated and blocked spheres were subjected to centrifugation and resuspended in Hank’s Balanced Salt Solution (HBSS), and diluted to achieve TF particle burdens from 3.3×105 to 3×103 mL−1 as determined by a hemacytometer. TF antigen levels were quantified with a TF ELISA (American Diagnostica), and found to give 3.5 *10−20 Moles/particle, which corresponds to a surface density of 33 TF molecules μm−2.
2.3. Generation of human plasmas with controlled levels of coagulation factors
Blood donors gave fully informed, written consent in accordance with the Declaration of Helsinki. Experiments were performed under approval of the Institutional Review Board of Oregon Health & Science University. All blood donations were from healthy human subjects and collected by antecubital venipuncture into 3.2% sodium citrate at a ratio of 9:1 and subjected to centrifugation at 2150 g for 10 minutes at room temperature. The supernatant was collected, pooled with supernatants prepared identically from three other donors, and subjected to centrifugation at 2150 g for 10 minutes at room temperature. The supernatant was then dispensed into 1.5 mL vials and frozen at −80°C. Globally diluted plasmas were created by mixing pooled plasma with phosphate buffered saline (PBS, pH = 7.40) in stepwise ratios from (10:0, 9:1, 4:1, 7:3, 3:2, 1:1, 2:3, 3:7, 1:4, or 1:9). In a similar manner, pooled plasma was mixed with plasma depleted of a single coagulation factor (<0.1% as reported by supplier). As the depleted plasma contains normal levels of all other coagulation factors, mixing pooled plasma with depleted plasma results in plasmas varying in levels of a single coagulation factor. Plasmas with known coagulation factor concentrations were made by adding purified coagulation factors to depleted plasmas.
2.4. Determination of clotting times
TF particle suspensions (103, 104, or 105 mL−1, final TF particle burden) were added to 50 μL of plasma and allowed to mix for 3 minutes at 37°C. The plasma-microsphere suspension was then recalcified (8.3 mM, final Ca2+ concentration) and the clotting time recorded on a KC4 coagulation analyzer (Trinity Biotech, Bray Co., Wicklow, Ireland).
2.5. Enzyme generation assays
The activation of coagulation enzymes by TF microspheres was monitored by adding the chromogenic substrate S-2366 (Chromogenix, Milan, Italy) to 50 μL each of plasma and microsphere suspension (104 mL−1, final count) in a 96-well plate. The plasma mixture was allowed to mix at 37°C for 15 minutes prior to recalcification (8.3 mM, final Ca2+ concentration) to initiate coagulation. The absorbance of 405 nm wavelength light at ambient temperature was recorded at 1 minute intervals for 120 minutes. Initiation time was recorded as the time before an increase in absorbance was detected, and rate was recorded as the maximum slope of a plot of absorbance versus time.
2.6. Statistical analysis
Data are represented as mean ± standard error (SEM). Statistical significance for clotting time increase required a 95% confidence interval (95% C.I.) of the mean increase to be greater than 0. Statistical differences between TF burdens required p<0.05 as calculated with a student’s t-test including Bonferroni’s correction for multiple comparisons.
3. Results
3.1. Clotting times for pooled plasma diluted with phosphate-buffered saline
Pooled plasma was mixed with TF particle suspensions containing 103, 104, or 105 TF particles mL−1 (TF particle burden) to determine the effect of TF particle burden on clotting time. Higher TF particle burden resulted in shorter clotting times (clotting times = 60, 110, and 210 seconds for TF burdens 105, 104, and 103, respectively). Pooled plasma was mixed with PBS to determine the effects of plasma dilution on clotting times (Fig. 1A). Clotting times from diluted plasmas were normalized to the 100% plasma value and reported as the percent increase in clotting time (Fig. 1B). Diluting plasma with PBS did not significantly increase clotting times until the dilution reached a 4:6 ratio of pooled plasma to PBS, at which point the clotting times increased by nearly 50% (clotting time increase at a TF particle burden of 105 mL−1 (95% C.I = (15.2, 68.6)). Stepwise increases in the dilution of pooled plasma resulted in stepwise increases in clotting times (dilution ratios of 3:7, 2:8 resulted in clotting time increases of 61 and 114%, respectively, at a constant TF particle burden of 105 ml−1). Clotting times were not measurable (>1200 seconds) when plasma was diluted at 2:8 with PBS for a TF burden of 103 mL−1, or when plasma was diluted at 1:9 with PBS for TF particle burdens of 104 mL−1 and 105 mL−1 (Fig. 1B).
Fig. 1.
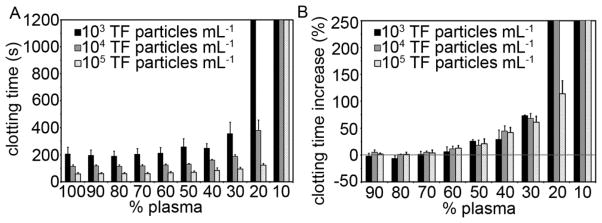
Clotting times for pooled plasma mixed with phosphate buffered saline (PBS). Citrate anticoagulated pooled human plasma was mixed with PBS and incubated with thromboplastin-coated microspheres at a count of 103, 104, or 105 mL−1 for 3 minutes at 37°C prior to recalcification (8.3 mM, final Ca2+ concentration). The time for plasma to clot was recorded on a KC4 coagulation analyzer (A). Clotting time elongation was calculated for diluted plasmas by normalizing their clotting times to clotting times obtained with non-diluted plasma (B). Experiments were performed in duplicate, with the mean clotting time reported. The bar graphs represent the mean clotting time and clotting time elongation (n=3) and the error bars represent standard error of the mean.
3.2. Clotting times for pooled plasma mixed with plasma depleted of specific coagulation factors
Pooled plasma was mixed with plasma depleted of coagulation factors VII, X, IX, VIII, V, or II at specific ratios to determine the effect of depleting individual coagulation factors on clotting time as a function of TF particle burden (Fig. 2). Plasma depleted of factors VII, X, or II did not clot (clotting time > 1200 seconds at a TF particle burden of 104 mL−1) in our assay. Mixing pooled plasma with factor VII-depleted plasma at a ratio of 8:2 significantly increased the clotting time for TF particle burdens of 104 mL−1 and 105 mL−1 (95% C.I. = (1.9, 13.7) and (7.1, 9.5), respectively). Stepwise increases in the ratio of factor VII-depleted plasma to pooled plasma resulted in stepwise increases in clotting times. Mixing pooled plasma with factor VII-depleted plasma significantly increased clotting times at a TF particle burden of 103 mL−1 by 37% (95% C.I. = (1.8, 72.6)) at a ratio of 4:6. Mixing pooled plasma with factor X-depleted plasma significantly increased clotting times at a pooled plasma to factor X-depleted plasma ratio of 7:3 (clotting time increase at TF particle burdens of 104 mL−1 and 105 mL−1 (95% C.I. = (4.3, 12.7) and (2.1, 6.5), respectively). Stepwise increases in the ratio of factor X-depleted plasma to pooled plasma resulted in stepwise increases in clotting times. Mixing pooled plasma with factor II-depleted plasma did not significantly increase clotting times until a pooled plasma to factor II-depleted plasma ratio of 1:9 was obtained (clotting time increase at TF particle burdens of 104 mL−1 and 105 mL−1 (95% C.I. = (5.3, 18.2) and (8.8, 38.5), respectively).
Fig. 2.
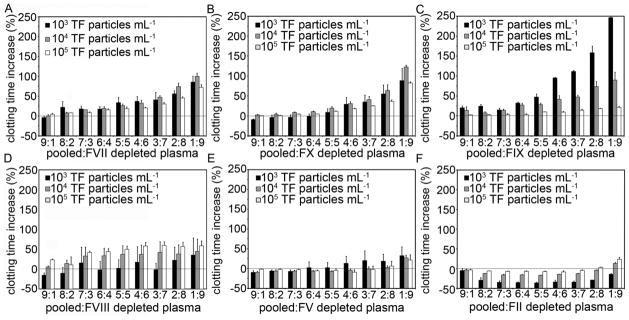
Clotting times for pooled plasma mixed with plasma immunodepleted of specific coagulation factors. Citrate anticoagulated pooled human plasma was mixed with plasma immunodepleted of either FVII (A), FX (B), FIX (C), FVIII (D), FV (E), or FII (F) and incubated with thromboplastin-coated microspheres at a count of 103, 104, or 105 mL−1 for 3 minutes at 37°C prior to recalcification (8.3 mM, final Ca2+ concentration). The time for plasma to clot was recorded on a KC4 coagulation analyzer. Clotting times elongation was calculated for mixed plasmas by normalizing their clotting times to 100% pooled plasma clotting times. Experiments were performed in duplicate, with the mean clotting time reported. The bar graphs represent the mean clotting time (n=3) and the error bars represent standard error.
Plasmas depleted of factors VIII, V and IX clotted in our assay (clotting times = 180, 890 and 500 seconds, respectively, at a constant TF burden of 104 mL−1). Mixing pooled plasma with factor VIII-depleted plasma at a ratio of 9:1 increased clotting times by 23% as compared to pooled plasma alone for a TF particle burden of 105 mL−1 (95% C.I. = (16.5, 30.3)). Increasing the ratio of factor VIII-depleted plasma to pooled plasma did not cause further increases in clotting times. Mixing pooled plasma with factor VIII-depleted plasma did not increase clotting times at any mixing ratio for TF particle burdens of 104 mL−1 or 103 mL−1 (Fig. 2D). Mixing plasma with factor V-depleted plasma significantly increased clotting times by 27% at a 1:9 ratio of pooled plasma to factor V-depleted plasma for a TF particle burden of 104 mL−1 (95% C.I. = (6.9, 47.3)). Mixing pooled plasma with factor IX-depleted plasma at a 9:1 ratio increased clotting times by 20% at a TF particle burden of 103 mL−1 (95% C.I. = (8.1,32.7)), by 14% for a 7:3 ratio of factor IX-depleted plasma:plasma for a TF particle burden of 104 mL−1 (95% C.I. = (5.4, 22.2), and by 10% for a 5:5 ratio of factor IX-depleted plasma:pooled plasma for a TF particle burden of 105 mL−1 (95% C.I. = (8.6, 11.6)).
3.3. Clotting times as function of coagulation factor concentration
Plasmas containing specific concentrations of coagulation factors were generated by adding purified coagulation factors to depleted plasma. Clotting times were determined as a function of coagulation factor concentration at a constant TF particle burden of 104 mL−1 (Fig. 3). Gray regions in the graph represent the range of coagulation factor concentrations reported for normal populations.
Fig. 3.
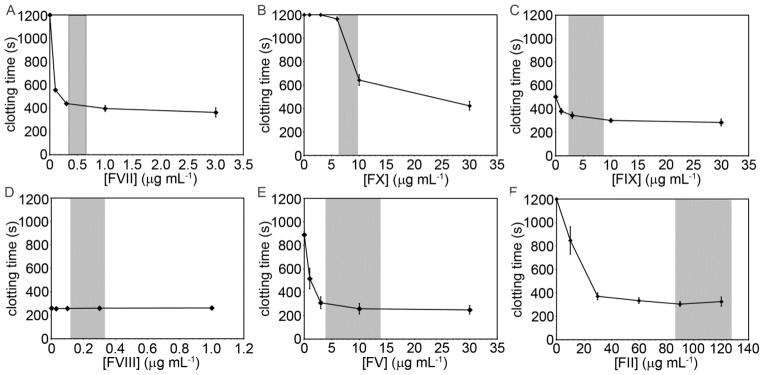
Clotting times for a single plasma as a function of coagulation factor concentration. Immunodepleted human plasma was reconstituted at indicated coagulation factor levels and incubated with thromboplastin-coated microspheres at a count of 104 mL−1 for 3 minutes at 37°C prior to recalcification (8.3 mM, final Ca2+ concentration). The time for plasma to clot was recorded on a KC4 coagulation analyzer. Experiments were performed in duplicate, with the mean clotting time reported. The data points represent the mean clotting time (n=3–4) and the error bars represent standard error. The gray regions represent the reported range of normal coagulation factor levels.
Adding purified factor VII to factor VII-depleted plasma decreased clotting times in a concentration-dependent manner (plasma factor VII concentrations of 0.1, 0.3, 1.0, and 3.0 μg mL−1 resulted in clotting times of 5600, 440, 400, and 360 seconds, respectively). Adding purified factor X to factor X-depleted plasma did not result in a measurable clotting time until a concentration of 6 μg factor X mL−1 was achieved (clotting time of 1160 seconds), after which further increasing the concentration of factor X shortened clotting times (clotting times = 640 and 430 seconds at factor X concentrations of 10 and 30 μg mL−1, respectively). Adding factor II to factor II-depleted plasma shortened clotting times in a concentration-dependent manner (clotting times = 850 and 370 seconds for factor II concentrations 10 and 30 μg mL−1, respectively). Further increasing factor II concentration above 30 μg mL−1 had no effect on clotting times. Adding factor IX to factor IX-depleted plasma shortened clotting times in a concentration-dependent manner (clotting times of 380, 350, 300, and 280 seconds for plasma factor IX concentrations of 1, 3, 10, and 50 μg mL−1, respectively). Adding factor V to factor V-depleted plasma shortened clotting times in a concentration-dependent manner, (plasma factor V concentrations of 1, 3, 10, and 30 μg mL−1 resulting in clotting times of 520, 310, 260, and 200 seconds, respectively). Adding factor VIII to factor VIII-depleted plasma at concentrations up to 1 μg mL−1 had no effect on clotting times in our study (clotting times of 260 +/− 2.5 seconds at all factor VIII concentrations tested).
3.4. Enzyme initiation times as a function of coagulation factor concentration
The time before the generation of proteolytic enzymes as a function of coagulation factor concentration was analyzed by measuring 405 nm light absorbance over time from plasmas containing the chromogenic substrate S-2366 at a constant TF particle burden of 104 mL−1 (Fig. 4).
Fig. 4.
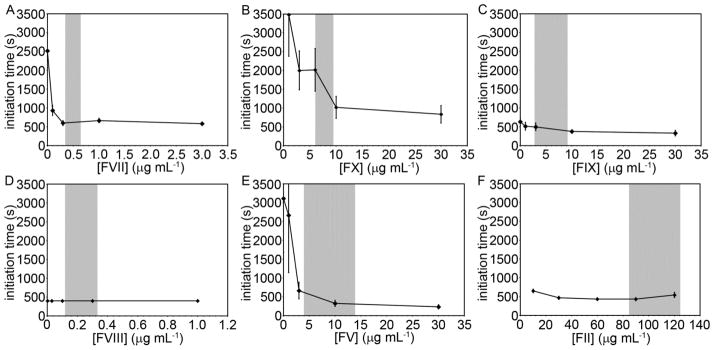
The time to initiate enzyme activity (initation times) for a single plasma containing controlled levels of specific coagulation factors. Immunodepleted human plasma was reconstituted at indicated coagulation factor levels and incubated with the chromogenic substrate S-2366 and thromboplastin-coated microspheres at a count of 104 mL−1 for 3 minutes at 37°C prior to recalcification (8.3 mM, final Ca2+ concentration). Absorbance of light (405 nm) was recorded with a spectrometer at 1 minute intervals. Samples yielded a sigmoid relationship between absorbance and time, and the initiation time was recorded as the time when absorbance increased above baseline. Experiments were performed in duplicate, with the mean clotting time reported. The data points represent the mean initiation time (n=3) and the error bars represent standard error. The gray regions represent the reported range of normal coagulation factor levels.
Adding factor VII to factor VII-depleted plasma shortened the initiation time from 2520 to 930 and 600 seconds for factor VII concentrations of <0.01, 0.1, and 0.3 μg mL−1, respectively. Increasing FVII concentration from 0.3 to 3 μg mL−1 had no effect on initiation times. Adding factor IX to factor IX-depleted plasma shortened the initiation time from 630 to 520, 500, and 380 seconds at factor IX concentrations of 1, 3, and 10 μg mL−1, respectively. Increasing factor IX concentration from 10 to 30 μg mL−1 had no effect on the initiation time. Adding factor X to factor X-depleted plasma shortened initiation times from >7200 to 3480, 2000, 2020, 1020, and 830 seconds at factor X concentrations of 1, 3, 6, 10, and 30 μg mL−1, respectively. Adding factor II to factor II-depleted plasma shortened initiation times from >7200 to 650 and 470 seconds for factor II concentrations of 10 and 30 μg mL−1, respectively. Increasing factor II concentrations from 30 to 90 μg mL−1 had no effect on the initiation time, while increasing factor II from 90 to 120 μg mL−1 increased initiation times from 430 to 540 seconds. Adding factor V to factor V-depleted plasma resulted in shortening initiation times from 3100 to 2670, 670, 330, and 240 seconds at factor V concentrations of 1, 3, 10, and 30 μg mL−1, respectively. Adding factor VIII to factor VIII-depleted plasma had no effect on initiation times for factor VIII concentrations up to 1 μg mL−1.
3.5. Enzyme generation rates as a function of coagulation factor concentration
The rate at which proteolytic enzymes were generated as a function of coagulation factor concentration was analyzed by measuring the change in 405 nm light absorbance over time from plasmas containing the chromogenic substrate S-2366 at a TF particle burden of 104 mL−1 (Fig. 5).
Fig. 5.
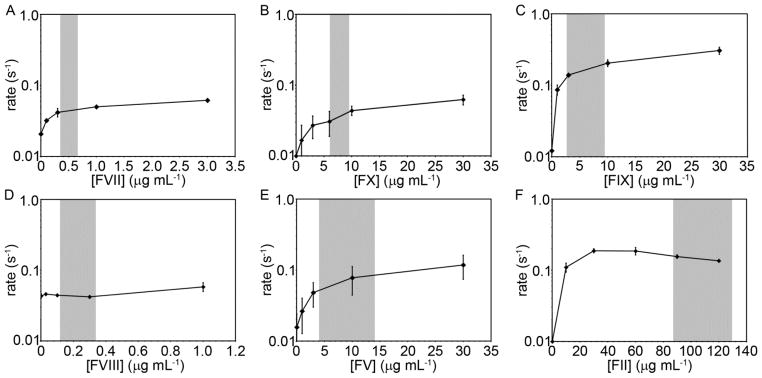
The rate of enzyme generation (rate) for a single plasma containing controlled levels of specific coagulation factors. Immunodepleted human plasma was reconstituted at indicated coagulation factor levels and incubated with the chromogenic substrate S-2366 and thromboplastin-coated microspheres at a count of 104 per mL for 3 minutes at 37°C prior to recalcification (8.3 mM, final Ca2+ concentration). Absorbance of light (405 nm) was recorded with a spectrometer at 1 minute intervals. Samples yielded a sigmoid relationship between absorbance and time, and the rate was recorded as the slope of the curve at which 50% of the total change in absorbance had occurred. Experiments were performed in duplicate, with the mean clotting time reported. The data points represent the mean initiation time (n=3) and the error bars represent standard error. The gray regions represent the reported range of normal coagulation factor levels.
Adding factor X to factor X-depleted plasma increased enzyme generation rates in a concentration dependent manner from <0.01 sec−1 for factor X-depleted plasma to 0.017, 0.027, 0.031, 0.044, and 0.063 sec−1 at factor X concentrations of 1, 3, 6, 10, and 30 μg mL−1, respectively. Adding factor II to factor II-depleted plasma increased enzyme generation rates in a concentration dependent manner from <0.01 s−1 for factor II-depleted plasma to 0.11 and 0.19 s−1 at factor II concentrations of 10 and 30 μg mL−1, respectively. Further increasing factor II concentration to 60, 90, and 120 μg mL−1 slowed enzyme generation rates in a concentration-dependent manner to 0.187, 0.156, and 0.136 s−1, respectively. Adding factor VII to factor VII-depleted plasma increased enzyme generation rates from 0.02 s−1 for factor VII-depleted plasma to 0.03, 0.04, 0.05, and 0.06 s−1 at factor VII concentrations of 0.1, 0.3, 1 and 3 μg mL−1, respectively. Adding factor IX to factor IX-depleted plasma increased enzyme generation rates in a concentration-dependent manner from 0.01 s−1 for factor IX-depleted plasma to 0.09, 0.14, 0.2 and 0.31 s−1 at factor IX concentrations of 1, 3, 10, and 30 μg mL−1, respectively. Adding factor V to factor V-depleted plasma increased enzyme generation rates in a concentration dependent manner from 0.02 s−1 for factor V-depleted plasma to 0.03 to 0.05, 0.08, and 0.12 s−1 at factor V concentrations of 1, 3, 10, and 30 μg mL−1, respectively. Adding factor VIII to factor VIII-depleted plasma had no effect on enzyme generation rates for factor VIII additions up to 0.3 μg mL−1 (rate = 0.04 s−1). Increasing factor VIII from 0.3 to 1 μg mL−1 increased the enzyme generation rate to 0.06 s−1.
4. Discussion
Thrombosis significantly contributes to morbidity and mortality in cancer. In metastatic cancer, where TF-expressing cells or cell-derived microvesicles enter the bloodstream, a minority of patients develop a thrombosis. It is unclear if the development of thrombosis in patients with cancer is due to an increased procoagulant stimulus, such as an increased level of TF-bearing microvesicles or procoagulant circulating tumor cells, or if patients that develop thrombosis are more sensitive to the procoagulant stimulus induced by cancer. A significant obstacle to implementing anticoagulant prophylaxis to patients with cancer is identifying the relative minority of patients who go on to develop a thrombosis. Moreover, risks of routine anticoagulant prophylaxis of the entire cancer patient population outweigh the benefits that would be gained by the minority that would be spared from thrombosis. A method to predict individual patient risk of developing thrombosis would allow personalized indications for anticoagulation prophylaxis.
Circulating tissue factor is elevated in the blood of patients with cancer.(11, 12, 26, 27) Concentration of circulating tissue factor is associated with thrombosis, yet this alone lacks sufficient specificity to predict thrombosis.(11–13) Patients with cancer who also have a past history of venous thrombosis, or whose peak thrombin generation values (a TF-dependent clinical assay) score in the upper quartile have an increased risk to develop thrombosis in cancer.(22, 23) We hypothesize that patients with cancer who develop thrombosis are more susceptible to the procoagulant stimulus provided by circulating TF than patients with cancer who do not develop thrombosis. Transport of coagulation factors from the blood to the TF-bearing cell or cell-derived microvesicle is a rate-limiting step in the generation of coagulation enzymes.(19, 28) The rate of generation of coagulation enzymes has been attributed to flux of zymogen from bulk solution to the surface-bound enzyme complexes.(15) The flux of substrate from bulk solution to a procoagulant surface is directly dependent upon the concentration gradient of the substrate, which itself can be directly determined by bulk concentration. The concentrations of individual coagulation factors differ by three-fold in normal populations.(29–36) Therefore, the flux of coagulation factors to and subsequent generation of procoagulant enzymes by circulating TF carriers would be expected to vary by up to three-fold for normal populations. Further, the generation of thrombin in response to an identical procoagulant stimulus has been shown to vary by over three-fold in healthy populations,(20, 21) which we hypothesize may be attributed to differences in coagulation factor levels. Along these lines, we hypothesize that patient-specific levels of coagulation factors may be predictive of susceptibility to develop thrombosis in the presence of circulating TF. Therefore, we characterized how levels of coagulation factors controlled circulating TF coagulation kinetics.
Individually diluting coagulation factors by mixing pooled plasma with coagulation factor-depleted plasma was more effective at increasing clotting times than simultaneously diluting all coagulation factors by mixing pooled plasma with PBS. For example, mixing pooled plasma with PBS at a ratio of 7:3 had no effect on clotting times as compared to pooled plasma alone, yet mixing pooled plasma with factor VII-depleted plasma at a 7:3 ratio increased clotting times by 17%. In other words, lowering factor VII levels to ~70% of normal values did not influence coagulation times if other coagulation factor levels were also lowered to 70% of normal levels. Conversely, if factor VII levels were lowered to 70% independently from other coagulation factor levels, a significant increase in clotting time was observed. Therefore, our data suggests that the ratio of coagulation factors to each other is more predictive of coagulation kinetics than overall coagulation factor levels.
In mixing studies, lowering factor VIII, V and II showed no effect on clotting times until levels reached approximately 20% of normal, well below the physiologically normal range. Therefore, normal variations in factor VIII, V and II would not influence circulating TF-induced coagulation in our assay. Conversely, lowering factor VII, IX and X levels to only 80% of normal levels, well within the physiological range of these coagulation factors, increased clotting times. Therefore levels of these coagulation factors may determine susceptibility to circulating TF-induced coagulation. Lowering factor IX levels resulted in the biggest increase in clotting times, despite factor IX not being essential to clot plasma in our assay. However, the magnitude by which lowering factor IX levels increased clotting times was diminished at higher TF burdens. Conversely, factors VII and X were essential to clotting in our assay and factor VII and X levels controlled coagulation kinetics equally for all TF burdens tested.
In studies where purified coagulation factor was added back to depleted plasma, levels of all coagulation factors except factor VIII influenced clotting times, initiation time and enzyme generation rates. For clotting time assays, the concentration at which a saturation effect was reached was different for each coagulation factor. Additions of factors VII, IX and V shortened clotting times in a concentration dependent manner up to low-normal physiological levels, after which further additions, even at supraphysiological levels did not significantly decrease clotting times. Factor X additions continued to shorten clotting times in a concentration dependent manner, even when added at supraphysiological levels. Adding factor II beyond 30 μg mL−1 (normal level ~100 μg mL−1) had no effect on clotting time. Therefore, only physiological variations of factor X levels influenced clotting times for TF particles in suspension.
Enzyme initiation times emulated the concentration-dependent shortening seen with clotting times, in that additions of factors VII and V shortened initiation times up to low-normal physiological levels, after which further increases did not significantly shorten clotting times. Converse to clotting times, additions of factor IX had minimal influence on shortening initiation times. Adding factor X to factor X-depleted plasma shortened initiation times up to a concentration of 10 μg mL−1 which is the high end of physiological levels. This suggests that physiological variations of factor X would influence initiation times in our assay.
Enzyme generation rates increased with coagulation factor levels in a concentration-dependent manner for all coagulation factors tested except factor VIII; however, the increases in enzyme generation rate levelled off as coagulation factor levels approached normal levels. Only factors IX and X showed significant variation in enzyme generation rates within the physiological ranges of these coagulation factors. However, supraphysiological additions of factors VII, IX and V showed continued increases in enzyme generation rate as coagulation factor levels crossed normal levels into supraphysiological levels. Adding factor II to factor II-depleted plasma above 30 μg mL−1 had no effect on enzyme generation rates, again suggesting that physiological variations in factor II levels would not impact enzyme generation rates. Factor IX levels had the biggest impact on enzyme generation rates.
All measures of coagulation kinetics demonstrated in this study suggest that factor II, when present at normal levels, is well in excess of levels which may influence coagulation kinetics. Factors V and VII did not significantly influence coagulation kinetics when added above low-normal physiological levels. Factor X shortened clotting and initiation times, and increased enzyme generation rates in a concentration dependent manner across the physiological range of factor X levels. Factor IX increased enzyme generation rates across the physiological range of factor IX levels. Our data suggests that physiological variations in the levels of factors X and IX, as seen in normal populations, are sufficient to influence circulating TF-induced coagulation kinetics.
Thrombosis in cancer is different from spontaneous venous thrombosis in a number of ways that suggest that the pathogenesis of cancer-associated thrombosis is unique. Along these lines, an association between circulating TF and thrombosis in cancer has been established, but circulating TF lacks specificity for identifying which patients will develop thrombosis. This suggests that patients who do develop thrombosis in the presence of circulating TF have a predisposed susceptibility to circulating TF-induced coagulation. Therefore, we provide a theoretical means by which coagulation factor levels may influence coagulation kinetics for circulating TF based on a diffusive flux analysis. Further, we demonstrate that moderate perturbations of factors X and IX levels within physiological ranges can significantly influence in vitro coagulation kinetics for a model of circulating TF. The mechanism by which coagulation factor levels are maintained in vivo are ill-defined; however, it has been shown that these levels vary widely between individuals. Similar variation has been demonstrated in individual coagulation responses to a single procoagulant stimulus.(20, 21, 37) Studies linking clinical coagulation assays and risk to develop thrombosis have shown that patients who mount the largest coagulation response to a set procoagulant stimulus are at increased risk to develop thrombosis. Whether factors X or IX levels are prognostic for thrombosis in patients with cancer remains to be seen. Moreover, therapeutic lowering of an individual coagulation factors with antisense oligonucleotides has been demonstrated in vivo to lower risk of thrombosis in a dose-dependent manner without increasing the risk of bleeding.(38) Along these lines, therapeutically lowering or pharmacologically targeting of individual levels of factors X or IX may be an attractive approach to preventing thrombosis in cancer without inducing a risk of bleeding.
5. Conclusions
The ability for TF particles in suspension to facilitate coagulation is controlled by concentrations of individual coagulation factors. Variations in individual coagulation factor levels had a bigger influence on clotting times than global coagulation factor levels. In an enzyme generation assay, adding factor IX to factor IX-depleted plasma resulted in the largest increase in enzyme generation rates, and at low TF particle burden, factor IX levels had the biggest effect on clotting times. Within normal physiological ranges, factor X levels had the biggest impact on clotting times and enzyme initiation time, while supraphysiological additions of factor X shortened clotting times as compared to normal levels. These data suggest that factor X levels within the normal physiological range control coagulation kinetics for circulating TF. Conversely, factor IX levels had the biggest effect on TF particle-induced enzyme generation. Therapeutic manipulation of factor IX or X levels may be an attractive therapeutic target for inhibiting circulating TF induced coagulation. Our data suggests that quantification of a patient’s factor IX and X levels in addition to circulating TF antigen level may improve specificity of circulating TF as a biomarker for thrombosis in cancer.
Supplementary Material
Table 1.
Calculated Coefficients of Diffusion and Diffusion Flux for TF-Dependent Coagulation Factors
| Factor | Stokes Radius (nm) | C (nM) | D20,plasma | J/JVII |
|---|---|---|---|---|
| VII | 3.53(41) | 7–13(29) | 5.07*10−7 | 1 |
| IX | 4.09(42) | 40–142(30)* | 4.38*10−7 | 3–22 |
| VIII | 8.8(43) | 0.36–1(32)* | 2.03*10−7 | ≪1 |
| X | 101–162(31) | 5.00*10−7 | 8–23 | |
| V | 9.5(44) | 12–42(33) | 1.88*10−7 | 2–10 |
| II | 4.1(45) | 1207–1727(34–36) | 4.36*10−7 | 100–266 |
calculated (FIX: 100% = 4.5 μg mL−1; FVIII: 100% = 0.2 μg mL−1)
Acknowledgments
This work was supported in part by the National Institute of Health (U54CA143906, R01HL101972, and T32-CA106195), the Oregon Clinical and Translational Research Institute (OCTRI), grant number (UL1TR000128) from the National Center for Advancing Translational Sciences (NCATS), and the Joel Drillings Award for Cardiovascular Research by the American Heart Association (12PRE11930019). AK is a Johnson Scholar. GWT is an Achievement Rewards for College Scientists scholar. OJTM is an American Heart Association Established Investigator.
7. List of Abbreviations
- 95% C.I.
95% confidence interval
- BSA
bovine serum albumin
- HBSS
Hank’s balanced salt suspension
- kDa
kiloDaltons
- PBS
phosphate buffered saline
- mM
millimolar
- mL
milliliter
- nm
nanometer
- SEM
standard error of the mean
- s
seconds
- TF
tissue factor
- μg
micrograms
- μM
micromolar
References
- 1.Iodice S, Gandini S, Lohr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008 May;6(5):781–8. doi: 10.1111/j.1538-7836.2008.02928.x. [DOI] [PubMed] [Google Scholar]
- 2.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000 Dec 21;343(25):1846–50. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 3.Tormoen GW, Haley KM, Levine RL, McCarty OJ. Do circulating tumor cells play a role in coagulation and thrombosis? Front Oncol. 2012;2:115. doi: 10.3389/fonc.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trousseau A. Clinique Medicale de l’Hotel-Dieu de Paris. Paris, France: The Syndenham Society; 1865. Phlegmasia alba dolens; pp. 654–712. [Google Scholar]
- 5.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006 Feb 27;166(4):458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 6.Chew HK, Davies AM, Wun T, Harvey D, Zhou H, White RH. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost. 2008 Apr;6(4):601–8. doi: 10.1111/j.1538-7836.2008.02908.x. [DOI] [PubMed] [Google Scholar]
- 7.Berny-Lang MA, Aslan JE, Tormoen GW, Patel IA, Bock PE, Gruber A, et al. Promotion of experimental thrombus formation by the procoagulant activity of breast cancer cells. Phys Biol. 2011 Feb;8(1):015014. doi: 10.1088/1478-3975/8/1/015014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tormoen GW, Cianchetti FA, Bock PE, McCarty OJ. Development of coagulation factor probes for the identification of procoagulant circulating tumor cells. Front Oncol. 2012;2:110. doi: 10.3389/fonc.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tormoen GW, Rugonyi S, Gruber A, McCarty OJ. The role of carrier number on the procoagulant activity of tissue factor in blood and plasma. Phys Biol. 2011 Dec;8(6):066005. doi: 10.1088/1478-3975/8/6/066005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsh J, Smith JD, Yates KR, Greenman J, Maraveyas A, Madden LA. Tissue factor expression determines tumour cell coagulation kinetics. Int J Lab Hematol. 2012 Aug;34(4):396–402. doi: 10.1111/j.1751-553X.2012.01409.x. [DOI] [PubMed] [Google Scholar]
- 11.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009 Nov 15;15(22):6830–40. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008 Nov;6(11):1983–5. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaler J, Ay C, Weinstabl H, Dunkler D, Simanek R, Vormittag R, et al. Circulating procoagulant microparticles in cancer patients. Ann Hematol. 2011 Apr;90(4):447–53. doi: 10.1007/s00277-010-1111-1. [DOI] [PubMed] [Google Scholar]
- 14.Auwerda JJ, Yuana Y, Osanto S, de Maat MP, Sonneveld P, Bertina RM, et al. Microparticle-associated tissue factor activity and venous thrombosis in multiple myeloma. Thromb Haemost. 2011 Jan;105(1):14–20. doi: 10.1160/TH10-03-0187. [DOI] [PubMed] [Google Scholar]
- 15.Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2311–5. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004 Nov 15;104(10):3190–7. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 17.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003 Jun 2;197(11):1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AM, Tormoen GW, Kanso E, McCarty OJ, Newton PK. Modeling and simulation of procoagulant circulating tumor cells in flow. Front Oncol. 2012;2:108. doi: 10.3389/fonc.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGee MP, Li LC, Xiong H. Diffusion control in blood coagulation. Activation of factor X by factors IXa/VIIIa assembled on human monocyte membranes. J Biol Chem. 1992 Dec 5;267(34):24333–9. [PubMed] [Google Scholar]
- 20.Dielis AW, Castoldi E, Spronk HM, van Oerle R, Hamulyak K, Ten Cate H, et al. Coagulation factors and the protein C system as determinants of thrombin generation in a normal population. J Thromb Haemost. 2008 Jan;6(1):125–31. doi: 10.1111/j.1538-7836.2007.02824.x. [DOI] [PubMed] [Google Scholar]
- 21.Brummel-Ziedins KE, Pouliot RL, Mann KG. Thrombin generation: phenotypic quantitation. J Thromb Haemost. 2004 Feb;2(2):281–8. doi: 10.1046/j.1538-7933.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- 22.Mandala M, Barni S, Prins M, Labianca R, Tondini C, Russo L, et al. Acquired and inherited risk factors for developing venous thromboembolism in cancer patients receiving adjuvant chemotherapy: a prospective trial. Ann Oncol. 2010 Apr;21(4):871–6. doi: 10.1093/annonc/mdp354. [DOI] [PubMed] [Google Scholar]
- 23.Ay C, Dunkler D, Simanek R, Thaler J, Koder S, Marosi C, et al. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2011 May 20;29(15):2099–103. doi: 10.1200/JCO.2010.32.8294. [DOI] [PubMed] [Google Scholar]
- 24.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, et al. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009 Jan 22;113(4):936–44. doi: 10.1182/blood-2008-06-163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berny MA, Munnix IC, Auger JM, Schols SE, Cosemans JM, Panizzi P, et al. Spatial distribution of factor Xa, thrombin, and fibrin(ogen) on thrombi at venous shear. PLoS One. 2010;5(4):e10415. doi: 10.1371/journal.pone.0010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007 Mar;5(3):520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 27.Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, et al. Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb Res. 2010 Jun;125(6):511–2. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gemmell CH, Turitto VT, Nemerson Y. Flow as a regulator of the activation of factor X by tissue factor. Blood. 1988 Oct;72(4):1404–6. [PubMed] [Google Scholar]
- 29.Fair DS. Quantitation of factor VII in the plasma of normal and warfarin-treated individuals by radioimmunoassay. Blood. 1983 Oct;62(4):784–91. [PubMed] [Google Scholar]
- 30.Yang HC. Immunologic studies of factor IX (Christmas factor). II. Immunoradiometric assay of factor IX antigen. Br J Haematol. 1978 Jun;39(2):215–24. doi: 10.1111/j.1365-2141.1978.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 31.Epstein DJ, Bergum PW, Bajaj SP, Rapaport SI. Radioimmunoassays for protein C and factor X. Plasma antigen levels in abnormal hemostatic states. Am J Clin Pathol. 1984 Nov;82(5):573–81. doi: 10.1093/ajcp/82.5.573. [DOI] [PubMed] [Google Scholar]
- 32.Hoyer L, Wyshock W, Colman R. Hemostasis and Thrombosis: basic principles and clinical practice. 3. Philadelphia: J.B. Lippincott Company; 1994. [Google Scholar]
- 33.Kamphuisen PW, Rosendaal FR, Eikenboom JC, Bos R, Bertina RM. Factor V antigen levels and venous thrombosis: risk profile, interaction with factor V leiden, and relation with factor VIII antigen levels. Arterioscler Thromb Vasc Biol. 2000 May;20(5):1382–6. doi: 10.1161/01.atv.20.5.1382. [DOI] [PubMed] [Google Scholar]
- 34.Ceelie H, Bertina RM, van Hylckama Vlieg A, Rosendaal FR, Vos HL. Polymorphisms in the prothrombin gene and their association with plasma prothrombin levels. Thromb Haemost. 2001 Jun;85(6):1066–70. [PubMed] [Google Scholar]
- 35.Legnani C, Cosmi B, Valdre L, Boggian O, Bernardi F, Coccheri S, et al. Venous thromboembolism, oral contraceptives and high prothrombin levels. J Thromb Haemost. 2003 Jan;1(1):112–7. doi: 10.1046/j.1538-7836.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 36.von Ahsen N, Lewczuk P, Schutz E, Oellerich M, Ehrenreich H. Prothrombin activity and concentration in healthy subjects with and without the prothrombin G20210A mutation. Thromb Res. 2000 Sep 15;99(6):549–56. doi: 10.1016/s0049-3848(00)00281-4. [DOI] [PubMed] [Google Scholar]
- 37.Karnicki K, Owen WG, Miller RS, McBane RD., 2nd Factors contributing to individual propensity for arterial thrombosis. Arterioscler Thromb Vasc Biol. 2002 Sep 1;22(9):1495–9. doi: 10.1161/01.atv.0000029968.34056.94. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Lowenberg EC Crosby JR, MacLeod AR, Zhao C, Gao D, Black C, Revenko AS, Meijers JC, Stroes ES, Levi M, Monia BP. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010 Nov 25;116(22):4684–92. doi: 10.1182/blood-2010-04-277798. [DOI] [PubMed] [Google Scholar]
- 39.Nemerson Y. The phospholipid requirement of tissue factor in blood coagulation. J Clin Invest. 1968 Jan;47(1):72–80. doi: 10.1172/JCI105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boltzmann L. Zur integration der diffusions gleichung bei variablen diffusion koeffizienten. Annalen der Physik and Chemie. 1894;53:959–64. [Google Scholar]
- 41.Gladhaug A, Prydz H. Purification of the coagulation factors VII and X from human serum. Some properties of factor VII. Biochim Biophys Acta. 1970 Jul 21;215(1):105–11. doi: 10.1016/0304-4165(70)90392-2. [DOI] [PubMed] [Google Scholar]
- 42.Suomela H. Human coagulation factor IX. Isolation and characterization. Eur J Biochem. 1976 Dec;71(1):145–54. doi: 10.1111/j.1432-1033.1976.tb11100.x. [DOI] [PubMed] [Google Scholar]
- 43.Hoyer LW, Trabold NC. The effect of thrombin on human factor VIII. Cleavage of the factor VIII procoagulant protein during activation. J Lab Clin Med. 1981 Jan;97(1):50–64. [PubMed] [Google Scholar]
- 44.Esmon CT. The subunit structure of thrombin-activated factor V. Isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J Biol Chem. 1979 Feb 10;254(3):964–73. [PubMed] [Google Scholar]
- 45.Stenflo J. Vitamin K and the biosynthesis of prothrombin. II. Structural comparison of normal and dicoumarol-induced bovine prothrombin. J Biol Chem. 1972 Dec 25;247(24):8167–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


