Abstract
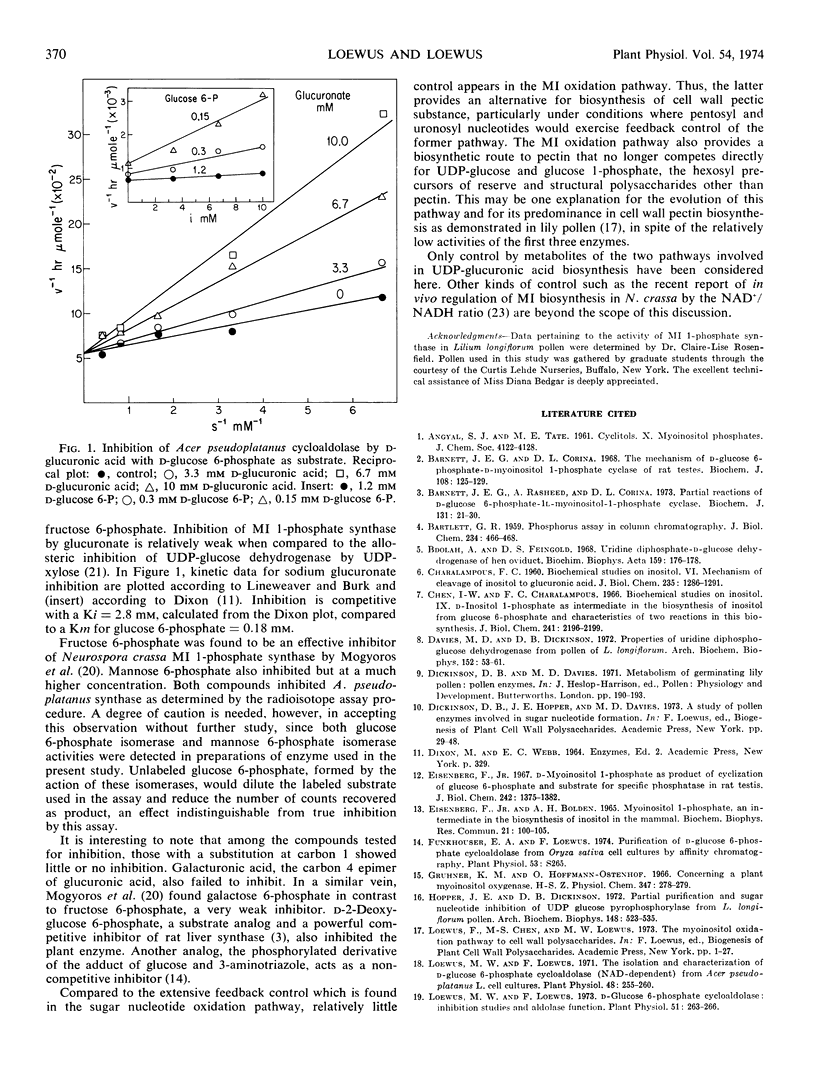
Of the eight intermediates associated with the two pathways of UDP-d-glucuronic acid biosynthesis found in plants, only d-glucuronic acid inhibited myo-inositol 1-phosphate synthase (EC 5.5.1.4), formerly referred to as d-glucose 6-phosphate cycloaldolase. Inhibition was competitive. An attempt to demonstrate over-all reversibility of the synthase indicated that it was less than 5% reversible, if at all.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Barnett J. E., Corina D. L. The mechanism of glucose 6-phosphate-D-myo-inositol 1-phosphate cyclase of rat testis. The involvement of hydrogen atoms. Biochem J. 1968 Jun;108(1):125–129. doi: 10.1042/bj1080125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J. E., Rasheed A., Corina D. L. Partial reactions of D-glucose 6-phosphate-1L-myoinositiol 1-phosphate cyclase. Biochem J. 1973 Jan;131(1):21–30. doi: 10.1042/bj1310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bdolah A., Feingold D. S. Uridine diphosphate-D-glucose dehydrogenase of hen oviduct. Biochim Biophys Acta. 1968 Apr 24;159(1):176–178. doi: 10.1016/0005-2744(68)90256-8. [DOI] [PubMed] [Google Scholar]
- Chen I. W., Charalampous C. F. Biochemical studies on inositol. IX. D-Inositol 1-phosphate as intermediate in the biosynthesis of inositol from glucose 6-phosphate, and characteristics of two reactions in this biosynthesis. J Biol Chem. 1966 May 25;241(10):2194–2199. [PubMed] [Google Scholar]
- Davies M. D., Dickinson D. B. Properties of uridine diphosphoglucose dehydrogenase from pollen of Lilium longiflorum. Arch Biochem Biophys. 1972 Sep;152(1):53–61. doi: 10.1016/0003-9861(72)90192-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg F., Jr, Bolden A. H. D-myo-inositol-1-phosphate, an intermediate in the biosynthesis of inositol in the mammal. Biochem Biophys Res Commun. 1965 Oct 26;21(2):100–105. doi: 10.1016/0006-291x(65)90093-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg F., Jr D-myoinositol 1-phosphate as product of cyclization of glucose 6-phosphate and substrate for a specific phosphatase in rat testis. J Biol Chem. 1967 Apr 10;242(7):1375–1382. [PubMed] [Google Scholar]
- Gruhner K. M., Hoffmann-Ostenhof O. Uber eine pflanzliche myo-Inosit-Oxygenase. Hoppe Seylers Z Physiol Chem. 1966;347(4):278–279. [PubMed] [Google Scholar]
- Hopper J. E., Dickinson D. B. Partial purification and sugar nucleotide inhibition of UDP-glucose pyrophosphorylase from Lilium longiflorum pollen. Arch Biochem Biophys. 1972 Feb;148(2):523–535. doi: 10.1016/0003-9861(72)90171-3. [DOI] [PubMed] [Google Scholar]
- Loewus M. W., Loewus F. The Isolation and Characterization of d-Glucose 6-Phosphate Cycloaldolase (NAD-Dependent) from Acer pseudoplatanus L. Cell Cultures: Its Occurrence in Plants. Plant Physiol. 1971 Sep;48(3):255–260. doi: 10.1104/pp.48.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus M. W., Loewus F. d-Glucose 6-Phosphate Cycloaldolase: Inhibition Studies and Aldolase Function. Plant Physiol. 1973 Feb;51(2):263–266. doi: 10.1104/pp.51.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogyoros M., Brunner A., Piña E. Behavior of cycloaldolase from Neurospora crassa towards substrate analogs and aldolase inhibitors. Biochim Biophys Acta. 1972 Dec 7;289(2):420–427. doi: 10.1016/0005-2744(72)90095-2. [DOI] [PubMed] [Google Scholar]
- NEUFELD E. F., HALL C. W. INHIBITION OF UDP-D-GLUCOSE DEHYDROGENASE BY UDP-D-XYLOSE: A POSSIBLE REGULATORY MECHANISM. Biochem Biophys Res Commun. 1965 May 3;19:456–461. doi: 10.1016/0006-291x(65)90146-4. [DOI] [PubMed] [Google Scholar]
- Northcote D. H. The synthesis and metabolic control of polysaccharides and lignin during the differentiation of plant cells. Essays Biochem. 1969;5:89–137. [PubMed] [Google Scholar]
- Pittner F., Fried W., Hoffmann-Ostenhof O. Studies on the biosynthesis of cyclitols, XXX. Purification of myo-inositol-1-phosphate synthase of rat testes to homogeneity by affinity chromatography on NAD-sepharose. Hoppe Seylers Z Physiol Chem. 1974 Feb;355(2):222–224. [PubMed] [Google Scholar]
- Piña M. Z., Brunner A., Chagoya de Sanchez V., Piña E. Role of the redox state of nicotinamide adenine dinucleotides in the in vivo regulation of inositol biosynthesis in Neurospora crassa. Biochim Biophys Acta. 1973 Aug 17;320(1):79–85. doi: 10.1016/0304-4165(73)90168-2. [DOI] [PubMed] [Google Scholar]
- Ray W. J., Jr, Mildvan A. S. Role of bivalent cations in the phosphoglucomutase system. IV. A study of the Mn2+ binding site by means of nuclear relaxation measurements on water protons. Biochemistry. 1970 Sep 29;9(20):3886–3894. doi: 10.1021/bi00822a006. [DOI] [PubMed] [Google Scholar]
- Roberts R. M. The formation of uridine diphosphate-glucuronic acid in plants. Uridine diphosphate-glucuronic acid pyrophosphorylase from barley seedlings. J Biol Chem. 1971 Aug 25;246(16):4995–5002. [PubMed] [Google Scholar]
- Rubery P. H., Northcote D. H. The effect of auxin (2,4-dichlorophenoxyacetic acid) on the synthesis of cell wall polysaccharides in cultured sycamore cells. Biochim Biophys Acta. 1970 Oct 27;222(1):95–108. doi: 10.1016/0304-4165(70)90355-7. [DOI] [PubMed] [Google Scholar]
- Seiffert U. B., Agranoff B. W. Isolation and separation of inositol phosphates from hydrolysates of rat tissues. Biochim Biophys Acta. 1965 Jun 1;98(3):574–581. doi: 10.1016/0005-2760(65)90154-2. [DOI] [PubMed] [Google Scholar]


