Abstract
Background
Delays in the release of national vital statistics hinder timely assessment of influenza severity, especially during pandemics. Inpatient mortality records could provide timelier estimates of influenza‐associated mortality.
Methods
We compiled weekly age‐specific deaths for various causes from US State Inpatient Databases (1990–2010) and national vital statistics (1990–2009). We calculated influenza‐attributable excess deaths by season based on Poisson regression models driven by indicators of respiratory virus activity, seasonality, and temporal trends.
Results
Extrapolations of excess mortality from inpatient data fell within 11% and 17% of vital statistics estimates for pandemic and seasonal influenza, respectively, with high year‐to‐year correlation (Spearman's rho = 0·87–0·90, P < 0·001, n = 19). We attribute 14 800 excess respiratory and cardiac deaths (95% CI: 10 000–19 650) to pandemic influenza activity during April 2009–April 2010, 79% of which occurred in people under 65 years.
Conclusions
Modeling inpatient mortality records provides useful estimates of influenza severity in advance of national vital statistics release, capturing both the magnitude and the age distribution of pandemic and epidemic deaths. We provide the first age‐ and cause‐specific estimates of the 2009 pandemic mortality burden using traditional ‘excess mortality’ methods, confirming the unusual burden of this virus in young populations. Our inpatient‐based approach could help monitor mortality trends in other infectious diseases.
Keywords: Age patterns, influenza, influenza‐related mortality, pandemic influenza, years of life lost
Introduction
Timely assessment of influenza mortality burden is important for monitoring the effectiveness of new intervention strategies and estimating the impact of pandemics. Laboratory‐confirmed deaths grossly underestimate influenza‐related mortality, so that burden is traditionally estimated by time series models measuring ‘excess mortality’ in broad outcomes from vital statistics.1, 2, 3 Unfortunately, US vital statistics become available 2–3 years after an influenza season has passed,4 hindering timely assessment of disease burden. More than 3 years after the emergence of the 2009 A/H1N1pdm pandemic virus, proper comparisons of pandemic burden with that of previous seasons using excess mortality approaches are lacking, although estimates by other methods exist.5, 6
Near real‐time surveillance for pneumonia and influenza (P&I) and all‐cause mortality was established in major US cities after the 1918 pandemic.7 Early estimates of the 2009–2010 pandemic mortality burden based on these data indicate low‐to‐moderate excess mortality, with disproportionate excess deaths in younger age groups compared with seasonal influenza.5 Obtaining nationally representative estimates of excess mortality from this surveillance system is difficult, however, due to a lack of well‐defined population denominators, age detail, and limited death outcomes available. More recently, an approach based on monitoring laboratory‐confirmed A/H1N1pdm hospitalizations and case fatality rates has provided additional mortality estimates, confirming a moderate pandemic impact with a young mean age of death.6 Reliance on laboratory‐confirmed diagnoses, however, underestimates the true burden of the pandemic. Furthermore, trends in laboratory testing and reporting practices limit comparisons with past seasons.
Here, we propose a novel strategy to estimate the mortality impact of influenza in the United States, using traditional excess mortality models applied to hospitalization records of patients dying in the hospital from respiratory or cardiac causes. Hospitalization data are made available 1–2 years before the release of vital statistics by the Agency for Healthcare Research and Quality and cover ~97% of the US population.8 As not all deaths occur in the hospital, we investigate the validity of this approach against traditional vital statistics data from the pre‐pandemic period and use the 2009–10 A/H1N1 pandemic season as a case study to illustrate the accuracy of our estimates. The recent release of US vital statistics for 2009 enables us to further validate our pandemic mortality estimates.
Methods
Data sources
State inpatient databases
We used the State Inpatient Databases (SID) maintained by the Agency for Healthcare Research and Quality (AHRQ), which contain all hospital discharge records from community hospitals in participating states.8 State inpatient databases data were accessed through a collaborative agreement with AHRQ. We included data from 1990–2010 to capture the 2009 pandemic and have sufficient historical seasons for comparison with US vital statistics. We compiled all records of hospitalizations resulting in death and extracted three mortality outcomes traditionally linked with influenza: pneumonia and influenza (P&I), respiratory diseases, and respiratory and cardiac diseases (R&C), listed as primary or contributing diagnoses (Table S1). Data were tabulated by week of admission and inpatient mortality rates were calculated for each disease outcome and six age categories (all ages, 0–4, 5–24, 25–44, 45–64, > = 65 years), using annual population size estimates for each state and age group from the US Census Bureau.9 We estimate that 32–37% of all US deaths occur in hospitals contributing data to the SID system; specifically, 78–86% of deaths in under 1‐year‐olds and 27–38% of deaths in older age groups. A much higher proportion of respiratory deaths is captured by inpatient records (Table S2).
Because the number of participating states has increased over time, we divided the hospitalization data into three periods: (i) 1990–2003, with data from nine states (~80‐million persons), (ii) 2003–2009, with data from 33 states (~220‐million persons, ~70% of the US population), and (iii) 2010, with data from 21 states available at the time of this study (~139‐million persons; see Supplement for list of states. This approach balanced the need for historical data for comparison with US vital statistics with the need for increased power to obtain nationally representative age‐specific time series of inpatient mortality rates in recent years.
National vital statistics
Although no gold standard exists to estimate influenza‐related mortality burden, excess mortality models applied to national vital statistics data are traditionally used to quantify influenza burden. Hence, to calibrate inpatient deaths against national vital statistics, we calculated weekly age‐specific mortality rates for P&I, respiratory, and R&C causes for 1990–2009 from national death certificates maintained by the National Center for Health Statistics4 (supplement).
Influenza and RSV laboratory surveillance data
We compiled national laboratory‐confirmed influenza and respiratory syncytial virus (RSV) surveillance data from Centers for Disease Control and Prevention (CDC).10 We used the weekly number of samples testing positive for influenza (respectively, RSV) divided by the total number of specimens tested each season as an indicator of viral activity.11
Statistical analyses
Comparison of inpatient deaths with national vital statistics
We quantified the week‐to‐week correlations between inpatient mortality and vital statistics all ages mortality time series for six outcomes (P&I, respiratory, R&C—underlying or any‐listed) to determine which inpatient outcomes were most predictive of national vital statistics.
Excess mortality models
We estimated seasonal influenza‐related excess mortality rates by age and outcome, for inpatient mortality and vital statistics. We fit quasipoisson regression models that account for over‐dispersed data and include terms for secular trends, seasonality, and viral activity, following:
where Y t is the number of age‐ and cause‐specific deaths in week t; β0 is the intercept, β1‐β2 are terms for linear and quadratic time trends, β3–β4 represent seasonal terms, RSVt and Flukt are the weekly viral activity proxies, where k indexes each season, (k = 1, 2…20; 1990/91–2009/10), βfluk are coefficients for influenza activity, and εt is an error term. The βfluk coefficients allow for different severity of influenza activity each season, linked to different subtypes and strains of circulating viruses.
To calculate influenza‐attributable excess deaths, we subtracted weekly model‐predicted values from the seasonal baseline (obtained by setting all βfluk parameters relating to influenza circulation to zero) and tabulated excess deaths by season. Confidence intervals were calculated based on the standard errors of model coefficients. Variables included in final models were determined using backwards selection F‐tests (P < 0·05). Age‐specific time series of inpatient mortality rates were smoothed using a 3‐week moving average before applying the excess mortality models described above.
Extrapolation of national excess mortality from inpatient deaths
Inpatient deaths do not capture all US deaths. Therefore, we used linear models to compare seasonal excess mortality rates derived from inpatient and vital statistics data for 19 pre‐pandemic seasons. We then used these linear models to extrapolate national pandemic excess mortality rates from our inpatient estimates for April 2009–April 2010.
Extrapolation of age‐specific excess mortality from inpatient deaths
To check the validity of age‐specific estimates from inpatient data, we compared the age distribution of excess deaths derived from inpatient and vital statistics data during the pre‐pandemic period, 2003–2009, when 21 states contributed inpatient records. Age‐specific models were based on primary‐cause mortality, as any‐listed mortality time series were unstable in children. To estimate the age‐specific burden of the 2009‐10 pandemic, we applied the age distribution of inpatient excess deaths during the pandemic season to our national extrapolation of pandemic excess mortality. The 95% confidence intervals on the age‐specific estimates were based on the standard errors of predictions from the national extrapolation approach. We note that these confidence intervals provide only a minimum estimate of uncertainty, originating from the extrapolation model used to derive national excess mortality from inpatient excess mortality.
Validation of inpatient estimates for the 2009–10 pandemic season against 2009 vital statistics
We used the newly released 2009 vital statistics data to estimate age‐ and cause‐specific excess mortality during the pandemic period April–December 2009. We compared these vital statistics estimates with those of our inpatient extrapolation approach for the truncated pandemic season, April–December 2009, and the full pandemic season, April 2009–April 2010.
Years of life lost
We integrated influenza‐related excess deaths in each age group with the life expectancy at the midpoint of each age group to estimate years of life lost.5, 12
Results
Comparison of weekly inpatient deaths and national vital statistics
Weekly mortality rates for P&I, respiratory, and R&C causes from inpatient databases, and vital statistics are depicted in Figure 1. Vital statistics mortality time series for primary‐listed diseases were most comparable to inpatient mortality time series for any‐listed diagnoses, with week‐to‐week correlations between the two systems ranging from 0·87 to 0·89 across death categories (P < 0·00001; supplement).
Figure 1.
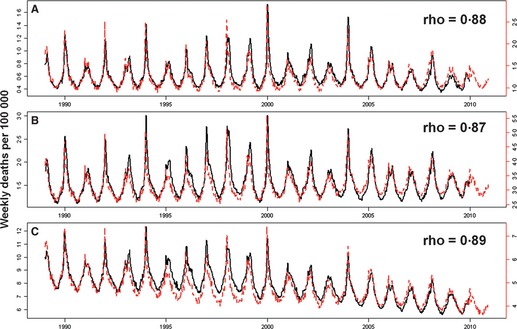
Weekly deaths per 100 000 due to (A) pneumonia and influenza, (B) respiratory causes and (C) respiratory and cardiac causes in vital statistics (NCHS, black) and inpatient mortality (SID, red) data. Legends display week‐to‐week correlations between the two datasets based on Spearman's rank correlation coefficient, rho. NCHS: National Center for Health Statistics. SID: State Inpatient Database.
All‐age excess mortality derived from inpatient deaths and national vital statistics
There was excellent agreement between pre‐pandemic excess mortality estimates derived from the two databases using quasipoisson regression models (1990/91–2008/09, R 2 values: 0·80–0·86, depending on the outcome). We used linear models linking excess mortality between the two systems to extrapolate excess mortality rates for the April 2009–April 2010 pandemic season based on inpatient data alone (Figure 2). The A/H1N1pdm pandemic excess mortality rates per 100 000 were estimated at 1·6 for P&I (95% CI: 1·28–1·91), 2·2 for respiratory diseases (95% CI: 1·5–2·8), and 4·6 for R&C diseases (95% CI: 3·0–6·2) (Table 1). The mortality burden of the 2009–10 pandemic was ~0·7 times that of a typical influenza season in the past decade and ~0·4 times that of the severe 2003–04 epidemic (Table 1). Excess mortality estimates extrapolated from inpatient deaths were within 11% of estimates based on vital statistics for seasonal influenza and 17% for the truncated April–December 2009 pandemic season.
Figure 2.
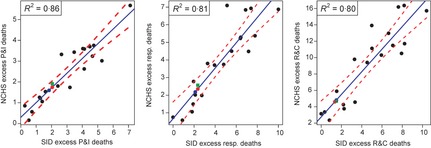
Comparison of seasonal excess mortality rates derived from vital statistics and SID inpatient mortality datasets for 19 pre‐pandemic seasons 1990/91–2008/09, by death outcome (black dots: estimated excess deaths; blue line: linear model; red dashed line: 95% confidence intervals. left panel: P&I: Pneumonia and Influenza; middle panel: Resp: Respiratory; right panel: R&C: Respiratory and Cardiac). Model R 2 values are displayed in the legends. Model predictions for the pandemic based on inpatient‐derived estimates for the April–December 2009 period are depicted as blue dots. Estimates for the April 2009–April 2010 period are depicted as red dots. For comparison, vital statistics‐derived excess mortality estimates for the April‐December 2009 period are depicted as green squares. Estimated slopes (units: NCHS excess death rate/SID excess death rate) for the simple linear models are 0·71 (P&I), 0·74 (Resp), and 1·4 (R&C). NCHS: National Center for Health Statistics. SID: State Inpatient Database.
Table 1.
Excess mortality rates due to pneumonia and influenza, respiratory diseases, and respiratory and cardiac diseases for selected seasons and the 2009–10 pandemic in the United States, based on inpatient mortality and vital statistics data
| Excess mortality rates per 100 000, all ages | |||
|---|---|---|---|
| Dataset/Outcome | Pneumonia and influenza | Respiratory diseases | Respiratory and cardiac diseases |
| A/H1N1 pandemic | |||
| Vital statistics (April–December 2009) | 1·93 (1·34–2·60) | 2·57 (1·49–3·76) | 4·68 (2·04–7·48) |
| Inpatient mortality (April–December 2009) | 1·60 (1·28–1·91) | 2·18 (1·53–2·83) | 4·61 (3·03–6·20) |
| Inpatient mortality (April 2009–April 2010) | 1·75 (1·45–2·04) | 2·35 (1·73–2·98) | 4·82 (3·27–6·4) |
| Severe A/H3N2 epidemic, 2003–04 | |||
| Vital statistics | 3·76 (3·27–4·29) | 4·49 (3·81–5·21) | 10·52 (8·89–12·20) |
| Inpatient mortality | 3·65 (3·29–4·01) | 5·82 (5·14–6·49) | 13·69 (12·16–15·21) |
| Average of pre‐pandemic seasons (2003/04–2008/09) | |||
| Vital statistics | 2·04 (0·82–3·26) | 2·97 (1·37–4·58) | 7·17 (3·94–10·39) |
| Inpatient mortality | 2·17 (1·09–3·24) | 3·60 (2·10–5·11) | 9·04 (5·90–12·18) |
Extrapolation of age‐specific excess mortality
To estimate age‐specific excess mortality, we relied on age‐specific inpatient time series for underlying causes from 2003–2010, when the number of reporting states was large (Figures 3 and S1). In inter‐pandemic seasons (2003/04–2008/09), the age distribution of excess deaths in inpatient and vital statistics data was in excellent agreement, with both systems estimating that ~85% of excess deaths occur in persons >65 years (Figure 4). Armed with this pre‐pandemic validation of the age distribution of influenza‐related deaths in inpatient data we focused on the pandemic.
Figure 3.
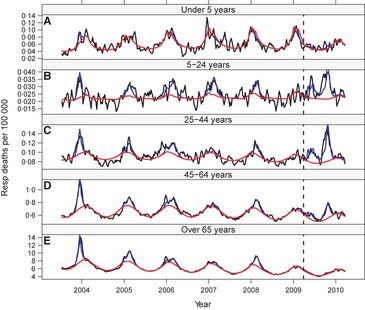
Weekly inpatient mortality rates due to respiratory causes in (A) under 5‐year‐olds, (B) 5‐ to 24‐year‐olds, (C) 25‐ to 44‐year‐olds, (D) 45‐ to 64‐year‐olds, and (E) over 65 years, 2003/04–2009/10 seasons. 3‐week moving averages of observed mortality rates are depicted in black. Model‐fitted values are depicted in blue, and seasonal baselines (adjusted for circulation of respiratory syncytial virus) are depicted in red. The vertical dashed line demarcates the onset of A/H1N1pdm circulation.
Figure 4.
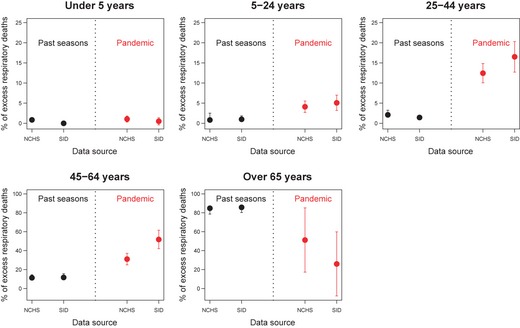
Percentage of excess respiratory deaths occurring in each, by season and database. NCHS: National Center for Health Statistics. SID: State Inpatient Database. Data labeled ‘past seasons’ represents the median, 1st and 3rd quartiles of percentages of influenza‐related deaths occurring in each age group in the pre‐pandemic period, 2003/2004 to 2008/2009. For the pandemic season, we considered the full pandemic period April 2009–April 2010 for the inpatient mortality estimates, and the truncated pandemic season April–December 2009 for vital statistics estimates. Error bars during the pandemic period depict 95% confidence intervals derived from age‐specific excess mortality models.
During the 2009–10 pandemic, the age distribution of inpatient excess deaths differed markedly from that of previous seasons, with a greater proportion of deaths occurring among persons aged 5–64 years, especially for broader outcomes (Figure 4, Table S4). We applied this pandemic age distribution to our all‐age estimate of 2009–10 pandemic mortality to obtain national age‐specific estimates (Table 2). For 5‐ to‐64‐year‐olds, pandemic mortality rates were 3–10 times those of a typical influenza season and 1–4 times those during the severe 2003/04 epidemic, depending on the outcome. In contrast, the pandemic burden in individuals over 65 years was three‐ to sevenfold lower than in a typical season.
Table 2.
Age‐ and cause‐specific excess mortality rates per 100 000 for selected seasons and the 2009–10 pandemic in the United States, based on inpatient mortality databases
| Season/Mortality Outcome | All ages | <5 years | 5–24 years | 25–44 years | 45–64 years | ≥= 65 years |
|---|---|---|---|---|---|---|
| A/H1N1 pandemic (April 2009–April 2010) | ||||||
| P&I | 1·75 (1·45–2·04) | 0·14 (0·11–0·16) | 0·26 (0·21–0·30) | 0·87 (0·72–1·01) | 2·66 (2·20–3·10) | 5·81 (4·81–6·77) |
| Respiratory | 2·35 (1·73–2·98) | 0·18 (0·13–0·22) | 0·44 (0·32–0·56) | 1·43 (1·06–1·82) | 4·71 (3·47–5·98) | 4·74 (3·49–6·01) |
| R&C | 4·82 (3·27–6·4) | – | 0·96 (0·65–1·27) | 3·03 (2·05–4·02) | 10·48 (7·11–13·91) | 8·01 (5·43–10·63) |
| Severe A/H3N2 epidemic, 2003/04 | ||||||
| P&I | 3·65 (3·29–4·01) | 0·13 (0·12–0·14) | 0·06 (0·05–0·06) | 0·20 (0·18–0·22) | 1·45 (1·31–1·59) | 24·80 (22·35 ‐27·24) |
| Respiratory | 5·82 (5·14–6·49) | 0·14 (0·13–0·16) | 0·10 (0·09–0·11) | 0·31 (0·27–0·34) | 2·84 (2·51–3·17) | 38·53 (34·03–42·96) |
| R&C | 13·69 (12·16–15·21) | 0·18 (0·16–0·20) | 0·24 (0·21–0·27) | 0·73 (0·65–0·81) | 6·20 (5·50–6·88) | 91·64 (81·40–101·82) |
| Seasonal Epidemics (2003/04–2008/09) a | ||||||
| P&I | 2·17 (1·09–3·24) | 0·06 (0·03–0·08) | 0·13 (0·07–0·20) | 0·16 (0·08–0·25) | 0·95 (0·48–1·42) | 14·27 (7·17–21·30) |
| Respiratory | 3·60 (2·10–5·11) | 0·0008 (0·0004–0·001) | 0·13 (0·08–0·19) | 0·19 (0·11‐0·27) | 1·63 (0·95–2·32) | 23·97 (13·98–34·02) |
| R&C | 9·04 (5·90–12·18) | – | 0·38 (0·25–0·51) | 0·56 (0·37–0·76) | 4·31 (2·81–5·80) | 59·52 (38·84–80·19) |
Based on the median percentage of excess deaths in each age group in pre‐pandemic seasons. P&I, pneumonia and influenza; R&C, respiratory and cardiac.
Validation of pandemic estimates against 2009 vital statistics
Although the newly released 2009 vital statistics only provide information for a truncated pandemic season, they offer a good opportunity to validate our approach. All‐age excess mortality extrapolations derived from inpatient mortality were within ~2–17% of estimates derived from vital statistics data when both models were limited to April 2009–December 2009 (Table 1). In the inpatient models, estimates for the truncated pandemic period April–December 2009 were only 4–9% lower than for the full period of A/H1N1pdm circulation, April 2009–April 2010, suggesting that most of the pandemic impact was experienced in 2009 (Table 1).
A shift in the age distribution of excess deaths was seen in vital statistics for the truncated season April–December 2009, similar to patterns in inpatient excess deaths (Figure 4). The magnitude of the age shift differed between causes of deaths and databases, though the confidence intervals of the estimates overlapped.
Years of life lost
Overall, our approach attributes 14 800 excess R&C deaths to A/H1N1pdm circulation during April 2009–April 2010 (95% CI 10 000–19 650) (Table 3, Table S5).We estimate that 474 000 years of life lost (YLL) are attributable to the pandemic (95% CI: 322–630 000) based on R&C causes, which is broadly similar to the YLL burden of a typical season, and is ~2/3 that of the severe 2003/04 epidemic season (Table 3). Years of life lost estimates based on vital statistics data for the truncated pandemic season fell within the confidence intervals of our inpatient extrapolations; estimates were stable to dataset choice and time period (data not shown).
Table 3.
Number and age distribution of excess respiratory and cardiac (R&C) deaths and years of life lost associated with the pandemic and past influenza seasons. Excess mortality estimates are based on the inpatient mortality database approach
| Excess R&C deaths | Years of life lost | |||
|---|---|---|---|---|
| No. (95% CI) | % in persons <65 years | No. in thousands (95% CI) | % in persons <65 years | |
| Pandemic April 2009‐April 2010 | 14 800 (10 000–19 650) | 79 | 474·3 (321·8–629·8) | 90 |
| Severe A/H3N2 epidemic, 2003/04 | 42 000 (37 330–46 700) | 14 | 723·5 (642·6–803·8) | 27 |
| Average seasonal epidemic, 2003/04–2008/09[Link] | 27 750 (18 110–37 390) | 15 | 490·6 (320·2–661·0) | 30 |
Based on median percentage of excess deaths in each age group.
Discussion
We have presented a robust system for estimating the mortality burden of influenza based on US inpatient mortality records, which are available 1–2 years before the release of national vital statistics. We have illustrated the utility of this system in the context of the 2009–10 A/H1N1 pandemic and validated our approach using the newly released 2009 vital statistics. We have shown that excess mortality estimates extrapolated from inpatient deaths are within 17% and 11% of estimates based on vital statistics for seasonal and pandemic influenza, respectively. Furthermore, inpatient mortality records accurately capture the age distribution of influenza‐related deaths during seasonal epidemics and detect the expected age shift associated with the 2009–10 pandemic.5, 12
Our study is the first to provide final age‐ and cause‐specific excess mortality estimates and YLL for the 2009–10 pandemic in the United States using traditional excess mortality models, allowing for fair comparisons to past pandemics and seasonal influenza. We place the overall burden of this pandemic at 14 800 deaths from R&C causes (95% CI 10 000–19 650), corresponding to 4·8 deaths per 100 000 (95% CI: 3·3–6·4). Persons aged 5–64 years experienced three‐ to tenfold elevated excess death rates relative to seasonal influenza; adults aged 20–44 years were especially affected. The YLL metric further highlights the unusually heavy burden in young populations.5, 12
Previous estimates of pandemic mortality in the United States vary substantially. Utilizing data from the 122 cities surveillance system, Viboud et al. 5 provided an early estimate at 2·4 excess P&I deaths and 14·3 excess all‐cause deaths per 100 000—albeit with large confidence intervals. A more recent study based on laboratory‐confirmed A/H1N1pdm hospitalizations suggests a rate of 4·0 deaths per 100 000 during April 2009–April 2010.6 Our new estimate of 4·8 excess R&C deaths per 100 000 (95% CI: 3·27–6·40) using traditional excess mortality approaches fits well in the range of previous estimates. Further, the signature age shift of deaths toward younger ages reported here echoes previous studies.6, 13, 14
Our estimates of pandemic mortality in the United States are higher than those reported by similar methods for Australia (−6·0/100 000), France (1·0/100 000), Hong‐Kong (1·6/100 000), the United Kingdom (2·7/100 000), and the Netherlands (3·7/100 000) but lower than those for Mexico (11/100 000).15, 16, 17, 18, 19, 20 Some of these differences could reflect estimation errors due to use of non‐specific outcomes16 or true variation in disease burden, as suggested by the unusual clinical severity of the disease reported in Mexico during the pandemic.18, 21 Considerable variation has been reported in the impact of historical pandemics (1918 and 1968) between countries,22, 23 and during this recent pandemic, between equatorial and subtropical regions of Brazil.24 Whether geographic variation in pandemic burden can be explained by differences in prior immunity, health care, or geography remains to be clarified.
In the United States, 2096 laboratory‐confirmed deaths were reported from August 30, 2009–April 6, 2010.14 Our estimate of 14 800 excess R&C deaths attributable to the pandemic suggests that 1 in ~7 excess deaths were captured by laboratory surveillance. This ratio compares favorably with diagnostic propensity in Mexico (1/7), but is not as high as in France (1/2) or the United Kingdom (1/4).16, 18, 20 Further, the age distribution of pandemic‐related deaths is in strong agreement between excess mortality models and laboratory‐confirmed deaths, with 79% (respectively, 85%) of deaths in persons under 65.14 Precise estimates of pandemic excess mortality burden are difficult to obtain for seniors, however, due to the mild nature of the 2009–10 season in this age group. This likely explains why the age distribution of influenza‐related excess mortality did not fully agree between inpatient and vital statistics databases in 2009–10, echoing inconsistencies reported in other countries.16, 18
Several limitations should be mentioned. First, our study was ecological in design and therefore causes other than influenza may contribute to our mortality estimates. However, the majority of the pandemic burden occurred in April‐December 2009, coinciding with the period of elevated influenza A/H1N1pdm activity in the United States.10 Further, adjustment for the circulation of RSV and similar age shifts reported in different mortality outcomes lend strength to our approach. Second, we have likely overestimated the absolute YLL pandemic burden due to the lack of information on co‐morbidities of deceased patients in our analysis, who may not have the average US life expectancy. However, by applying the same approach to all seasons studied, we provide a fair comparison of the relative burden of pandemic and seasonal influenza in the United States.
Another limitation of our study stems from the lack of information on coding practices in the inpatient system relative to the standardized coding algorithms of death certificates. In sensitivity analyses, we have shown that while only one‐third of total US deaths are captured by inpatient death records, the hospital system captures 90% of respiratory deaths, with an even higher fraction of deaths captured among younger age groups (Table S2). Coding differences between inpatient databases and national vital statistics certainly account for some of the observed differences in the age distribution of deaths during the pandemic between the two datasets. Additional sensitivity analyses on age‐specific excess mortality models suggest that estimates for seniors are highly accurate, while estimates for younger age groups are less well correlated between the inpatient records and vital statistics (data not shown). Acknowledging these caveats, we have shown that inpatient mortality records provide accurate all‐age excess mortality estimates for seasonal and pandemic influenza and can identify age groups experiencing unusually high pandemic burden relative to that of seasonal influenza. Based on these characteristics, inpatient mortality records could be particularly useful for monitoring the severity and mortality age patterns associated with unusual influenza seasons and help target intervention strategies to high‐risk age groups in pandemic situations.
We believe the strategy of using inpatient deaths to extrapolate the national influenza mortality burden could be generalized to other infectious and non‐infectious diseases to the benefit of public health, especially given the high coverage of the US population.8 In particular, this system could be used to monitor the impact of respiratory infections with substantial burden and year‐to‐year variability. Inpatient databases could also be helpful to assess the mortality benefits of novel or expanded vaccination programs, such as that for seasonal and pandemic influenza, pneumococcal disease, rotavirus, and in the future, RSV.25, 26
In conclusion, we have provided methodological validation for using inpatient mortality databases to monitor the severity of influenza epidemics and proposed broader applications. We hope that this work will inspire the accelerated release of hospitalization and mortality data, so that information on severe outcomes can be released in timelier manner to help guide strategies to mitigate the next emerging infectious disease outbreak.
Supporting information
Figure S1. Weekly vital statistics (NCHS) mortality time series and model predictions for pneumonia and influenza (P&I) in (A) under 5‐year‐olds, (B) 5‐ to‐24‐year‐olds, (C) 25‐ to‐44‐year‐olds, (D) 45‐ to‐64‐year‐olds, and (E) Over 65 years.
Figure S2. Same as Figure S1 but for respiratory causes.
Figure S3. Same as Figure S1 but for respiratory and cardiac (R&C) causes.
Figure S4. Weekly inpatient mortality rates due to pneumonia and influenza in (A) under 5‐year‐olds, (B) 5‐ to‐24‐year‐olds, (C) 25‐ to‐44‐year‐olds, (D) 45‐ to‐64‐year‐olds, and (E) over 65 years, 2003/04–2009/10 seasons.
Figure S5. Same as Figure S4, but for respiratory and cardiac causes (R&C).
Table S1. Diagnostic codes used to extract deaths due to pneumonia and influenza (P&I), respiratory causes, and respiratory and cardiac causes (R&C) in inpatient records and vital statistics data.
Table S2. Proportion of cause‐ and age‐specific US deaths captured by the State Inpatient Databases system for two mortality outcomes listed anywhere on the hospitalization record: Pneumonia and Influenza (P&I) and respiratory causes.
Table S3. Age‐specific excess mortality rates due to pneumonia and influenza (P&I), respiratory causes, and respiratory and cardiac causes, derived from NCHS vital statistics data.
Table S4. Age distribution of excess deaths during the pandemic and historical seasons from the NCHS and SID datasets.
Table S5. Number of influenza‐related excess deaths and years of life lost (YLL) derived from respiratory and cardiac mortality in vital statistics data (NCHS) for select historical years and the truncated pandemic period (Apr–Dec 2009).
Table S6. Spearman's and Pearson's correlation coefficients between weekly time series of vital statistics (multiple causes) and inpatient mortality (underlying and multiple causes) for three disease outcomes, 1990–2008.
Acknowledgements
We thank Richard Jordan at the Agency for Healthcare Quality and Research (AHRQ) for his help in data extraction. This research was conducted in the context of the Multinational Influenza Seasonal Mortality Study (MISMS), an ongoing international collaborative effort to understand influenza epidemiological and evolutionary patterns, led by the Fogarty International Center, National Institutes of Health (http://www.origem.info/misms/index.php). This work was supported by the in‐house Influenza Research Program of the Division of International Epidemiology and Population Studies, Fogarty International Center, National Institutes of Health, which is funded by the International Influenza Unit, Office of Global Affairs, Department of Health and Human Services. L.S. acknowledges support from the RAPIDD program of the Science and Technology Directorate, Department of Homeland Security. Part of the information contained in this manuscript was presented at the International Conference on Emerging Infectious Diseases (Atlanta, GA USA; March 2012). The authors do not have commercial or other associations that might pose a conflict of interest.
Charu et al (2013) Mortality burden of the 2009 influenza pandemic in the United States: improving the timeliness of influenza severity estimates using inpatient mortality records. Influenza and Other Respiratory Viruses 7(5), 863–871
References
- 1. Simonsen L, Viboud C. The art of modeling the mortality impact of winter‐seasonal pathogens. J Infect Dis 2012. Jun 21; 206:625–627. [DOI] [PubMed] [Google Scholar]
- 2. Thompson WW, Shay DK, Weintraub E, et al Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186. [DOI] [PubMed] [Google Scholar]
- 3. Goldstein E, Viboud C, Charu V, Lipsitch M. Improving the estimation of influenza‐related mortality over a seasonal baseline. Epidemiology 2012; 23:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Center for Health Statistics . Mortality data. Available at http://www.cdc.gov/nchs/about/major/dvs/mortdata.htm (Accessed 1 January 2012).
- 5. Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr 2010; 2:RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shrestha SS, Swerdlow DL, Borse RH, et al Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010). Clin Infect Dis 2011; 52(Suppl 1):S75–S82. [DOI] [PubMed] [Google Scholar]
- 7. Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis 2006; 194:S82–S91. [DOI] [PubMed] [Google Scholar]
- 8. Introduction to the HCUP state inpatient databases. Available at http://www.hcup-us.ahrq.gov/db/state/siddist/Introduction_to_SID.pdf (Accessed 1 September 2012).
- 9. U.S. Census Bureau . Population estimates. Available at http://www.census.gov/popest/estimates.php (Accessed 3 August 2005).
- 10. Influenza activity in the US, CDC. Available at http://www.cdc.gov/flu/. (Accessed 12 October 2012).
- 11. Zhou H, Thompson W, Viboud C et al Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 2012; 54:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller MA, Viboud C, Olson DR, Grais RF, Rabaa MA, Simonsen L. Prioritization of influenza pandemic vaccination to minimize years of life lost. J Infect Dis 2008; 198:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jhung MA, Davidson H, McIntyre A et al Preliminary results of 2009 pandemic influenza surveillance in the United States using the aggregate hospitalization and death reporting activity. Influenza Other Respi Viruses 2011; 5:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jhung MA, Swerdlow D, Olsen SJ et al Epidemiology of 2009 pandemic influenza A (H1N1) in the United States. Clin Infect Dis 2011; 52(Suppl 1):S13–S26. [DOI] [PubMed] [Google Scholar]
- 15. Muscatello DJ, Cretikos MA, MacIntyre CR. All‐cause mortality during first wave of pandemic (H1N1) 2009, New South Wales, Australia, 2009. Emerg Infect Dis 2010; 16:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lemaitre M, Carrat F, Rey G, Miller M, Simonsen L, Viboud C. Mortality burden of the 2009 A/H1N1 influenza pandemic in France: comparison to seasonal influenza and the A/H3N2 pandemic. PLoS ONE 2012; 7:e45051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang L, Chan KP, Cowling BJ et al Excess mortality associated with the 2009 pandemic of influenza A(H1N1) in Hong Kong. Epidemiol Infect 2011; 140:1542–1550. [DOI] [PubMed] [Google Scholar]
- 18. Charu V, Chowell G, Palacio Mejia LS et al Mortality burden of the A/H1N1 pandemic in Mexico: a comparison of deaths and years of life lost to seasonal influenza. Clin Infect Dis 2011; 53:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wijngaard CC, Asten L, Koopmans MP et al Comparing pandemic to seasonal influenza mortality: moderate impact overall but high mortality in young children. PLoS ONE 2012; 7:e31197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardelid P, Pebody R, Andrews N. Mortality caused by influenza and respiratory syncytial virus by age group in England and Wales 1999‐2010. Influenza Other Respi Viruses 2013; 7:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chowell G, Bertozzi SM, Colchero MA et al Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679. [DOI] [PubMed] [Google Scholar]
- 22. Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet 2006; 368:2211–2218. [DOI] [PubMed] [Google Scholar]
- 23. Viboud C, Grais RF, Lafont BA, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis 2005; 192:233–248. [DOI] [PubMed] [Google Scholar]
- 24. Schuck‐Paim C, Viboud C, Simonsen L et al Were equatorial regions less affected by the 2009 influenza pandemic? The brazilian experience. PLoS ONE 2012; 7:e41918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weinberger DM, Simonsen L, Jordan R, Steiner C, Miller M, Viboud C. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis 2012; 205:458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis 2010; 201:1617–1624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Weekly vital statistics (NCHS) mortality time series and model predictions for pneumonia and influenza (P&I) in (A) under 5‐year‐olds, (B) 5‐ to‐24‐year‐olds, (C) 25‐ to‐44‐year‐olds, (D) 45‐ to‐64‐year‐olds, and (E) Over 65 years.
Figure S2. Same as Figure S1 but for respiratory causes.
Figure S3. Same as Figure S1 but for respiratory and cardiac (R&C) causes.
Figure S4. Weekly inpatient mortality rates due to pneumonia and influenza in (A) under 5‐year‐olds, (B) 5‐ to‐24‐year‐olds, (C) 25‐ to‐44‐year‐olds, (D) 45‐ to‐64‐year‐olds, and (E) over 65 years, 2003/04–2009/10 seasons.
Figure S5. Same as Figure S4, but for respiratory and cardiac causes (R&C).
Table S1. Diagnostic codes used to extract deaths due to pneumonia and influenza (P&I), respiratory causes, and respiratory and cardiac causes (R&C) in inpatient records and vital statistics data.
Table S2. Proportion of cause‐ and age‐specific US deaths captured by the State Inpatient Databases system for two mortality outcomes listed anywhere on the hospitalization record: Pneumonia and Influenza (P&I) and respiratory causes.
Table S3. Age‐specific excess mortality rates due to pneumonia and influenza (P&I), respiratory causes, and respiratory and cardiac causes, derived from NCHS vital statistics data.
Table S4. Age distribution of excess deaths during the pandemic and historical seasons from the NCHS and SID datasets.
Table S5. Number of influenza‐related excess deaths and years of life lost (YLL) derived from respiratory and cardiac mortality in vital statistics data (NCHS) for select historical years and the truncated pandemic period (Apr–Dec 2009).
Table S6. Spearman's and Pearson's correlation coefficients between weekly time series of vital statistics (multiple causes) and inpatient mortality (underlying and multiple causes) for three disease outcomes, 1990–2008.


