Abstract
Background
Caring for an ill or disabled family member imposes a well-documented burden on the caregiver. The benefits of a health intervention may be underestimated if “spillover” effects on family members are not captured, resulting in inaccurate conclusions of economic evaluations.
Objective
To provide an estimate of, and to summarize measurement approaches for, the spillover disutility of illness on family members, relatives, and caregivers, through a systematic review of the literature.
Methods
The medical (PubMED), psychology (PsycINFO) and economics (EconLit) literatures were searched from inception through February, 2012 for published studies measuring spillover disutility of illness on family members and caregivers. Inclusion criteria were (1) studies using preference-based measures of health-related quality of life, and (2) studies reporting spillover disutility, or (3) studies reporting data from which a spillover disutility could be inferred.
Results
Fifteen studies were included in this review: 7 reported estimates of spillover disutility and 8 reporting data from which disutility could be inferred. Three studies found no disutility associated with spillover while 12 found measurable effects as large as −0.718 (and two found evidence of positive spillover in subsets of their samples). Generic (indirect) utility instruments were primarily used to measure spillover, including the EQ-5D, QWB and HUI (n=13), though two studies used modified versions of the time trade-off technique. Illnesses studied included childhood disorders (e.g., spina bifida, congenital malformations), diseases of the elderly (e.g., Alzheimer’s disease and dementia), physically disabling conditions (e.g., arthritis, multiple sclerosis), and medical conditions such as cancer and stroke. The persons affected by spillover included parents, grandparents, spouses/partners, other family caregivers, and household members.
Conclusions
There is a limited literature on the spillover disutility of illness on family members and caregivers, providing some specific estimates of a generally small, negative effect for particular conditions and individuals. Measurement methods vary across studies and a consensus approach has not yet been reached. Evidence suggests that the inclusion of spillover effects in economic evaluations would increase the relative effectiveness of interventions that address conditions with spillover compared to those without, though such differential benefits may be limited to such specific circumstances.
Keywords: caregivers, spillover, utility, externalities, family effects
1. Introduction
Illness affects more than just the solitary individual who suffers from an acute or chronic condition. Social network research has shown a “contagion effect” in which individuals’ obesity and smoking extend to their family members and others.[1–3] Other research has shown that taking care of an ill or disabled individual imposes a well-documented burden on the caregiver, both in health effects and quality of life.[4–8] An ill individual also exerts an emotional toll on family members who care about the patient, distinct from the toll of caregiving.[9,10] Though less-commonly noted, care taking also confers a positive benefit to some, through feelings of altruism and fulfillment of familial obligations.[11] Overall, research has established that health effects extend beyond one individual to include those surrounding him or her, including those physically present and those emotionally connected. The landscape of health decision making is altered by the conceptualization of health as a family affair. This paper reviews the literature quantifying the effect of an individual’s health on surrounding persons in terms useful for economic evaluation, specifically as health utility.
The definition of spillover effects in health is broad, encompassing a range of what the spillover effect is and whom it affects. The person affected may be providing care to the ill individual or may be related to the individual who is ill, or both. [4,6,7,12] Persons affected by spillover can include caregivers (both formal (paid) and informal (unpaid)), and family members whether caregivers or not[10], including parents[13–15], spouses/partners[16,17], children (young children and adult children)[18], siblings[19,20], more distant familial relatives, and sometimes friends acting as caregivers.[21–24] Spillover effects can include somatic and psychological health[25,26], emotional health[27], quality of life[15,28–32], and well-being (including happiness, life satisfaction, etc.[10,33]). Other effects extending beyond health include finances[34], relationship stability (e.g., marriage)[35], and work (e.g., ability to maintain employment).[36] Spillover effects can be a direct result of caregiving responsibilities, such as physical stress caused by lifting and dressing a disabled spouse or fatigue from the hours of caregiving tasks[26], or an indirect result of knowing a relative is ill[10], such as anxiety or depression due to caring for a parent with dementia[21] or a child with cancer.[13] Caregivers can feel guilt at what they provide or do not provide, and worry about finances and the future.[36] The burden imposed by caregiving can also cycle back to the ill person in terms of guilt at needing assistance, and worry about the demands placed on the caregiver.[36]
The range and scope of spillover effects in terms of people and areas of life affected confirm that spillover is an important component of the total effect of individuals’ illnesses, which implies that it is a piece that should be “counted” to fully assess both the burden of disease and the benefit accrued by treatment. The original guidance proffered for economic evaluations in health and medicine recommended casting a wide net when measuring costs and benefits of interventions, including these spillover effects on friends and family members.[37] However, in contrast to the direct costs and time costs of caregiving which are typically included in economic evaluations, spillover quality of life effects are generally not included in evaluations of the effectiveness or comparative effectiveness of health interventions, which can lead to an understatement of the benefit of reducing disease.[31,38,39] Diseases that have substantial spillover onto others may therefore be undervalued in comparative analyses of treatments where benefits accrue to both the “patient” and to surrounding family members, such as childhood conditions where a parent caregiver is substantially affected by the illness.
The importance of including these spillover effects has been noted by many[8,40,41], as has the lack of established methods to do so.[31,42,43] While there is a large literature on the existence of spillover effects, a smaller literature exists on the measurement of such effects, and an even smaller one on the quantification of spillover in metrics that are comparable across conditions and persons affected. These latter measures are generally in terms of health utility[44], and are the most useful for understanding the scope and range of spillover because they allow for the relative assessment of effects across people and conditions, such as the comparison of the spillover effect on an adult child of a parent’s Alzheimer’s disease[21] with the spillover effect on a parent of a child’s spina bifida.[45] Capturing spillover disutility allows for such comparisons and therefore has the potential to enable the inclusion of family spillover effects into economic evaluations assessing the usefulness of interventions. This paper summarizes the evidence to date from studies that assess spillover disutility of illness on family members and caregivers using health utility. We present both the methodologies used to estimate spillover effects and quantitative estimates of spillover disutility. Our goals are to inform the discussion of the scope and magnitude of spillover effects in health, the measurement of these effects, and their inclusion effects into cost effectiveness and comparative effectiveness analyses.
2. Methods of review
A systematic review of the medical, economics and psychology literatures was conducted to identify articles that measured the spillover disutility of illness on caregivers and family members. The PubMed, PsycINFO, and EconLit databases were searched from inception (1947 for MEDLINE, 1967 for PsycINFO, 1969 for EconLit) through February 2012 for articles published in English, using combinations of the following categories of search terms to cast the widest possible net for identifying utility studies of spillover: “spillover”, “family effect”, “externalities”, “external effects”, “caregiver” or “carer”; and “Health-related quality of life”, “utility”, “preferences”, “QALY”, “HYE”, “SF-6D”, “EQ-5D”, “EuroQol”, “HUI”, “QWB”, “Standard gamble”, or “Time tradeoff” (see Technical appendix for example of search strategy). Supplementary sources of articles for inclusion in the review were articles known to the investigators by researchers working in this field, and articles from Tables of Contents of journals that commonly publish studies in this field (including Quality of Life Research, Medical Decision Making, PharmacoEconomics and Value in Health). A first round of screening was conducted using the titles and abstracts of all identified articles and applying three exclusion criteria: (1) the study was a duplicate, (2) no spillover utility/disutility was reported, and (3) no comparison group identified and accompanying utility reported (for calculation of spillover utility). For the purposes of this review, utility/disutility was defined as a preference-based measure of health-related quality of life elicited using the time trade-off (TTO), standard gamble (SG) or visual analog scale (VAS, to which a utility transformation was or could be applied), or an indirect utility elicitation method based on these techniques.[46] Of the articles remaining after this screening, the full texts were reviewed to assess eligibility for inclusion using the same criteria. And finally, the bibliographies of the articles remaining after all screening were reviewed for possible additions, which were subjected to the same screening steps described for the database and other source search results.
Articles included in the review were abstracted by a trained research assistant and reviewed by the authors. Elements extracted included the primary person’s illness, the person affected by spillover (their relationship to the ill person and caregiving role, if any), the comparison group or method used to calculate spillover, the valuation technique used to measure utility/disutility, and the reported spillover disutility or caregiver/family member utility. Calculations were performed on reported data by the authors of this review to further describe spillover disutility estimates in a consistent manner across studies: utilities were converted into disutilities by subtracting from 1.0; and for studies reporting comparison group utilities only, an estimate of spillover disutility was calculated as the difference between mean family member/caregivers’ utility and mean comparison group utility, with EQ-VAS scores transformed into utilities using an established algorithm (1-(1-EQ-VAS score)^1.95[46]) prior to calculation.
3. Results
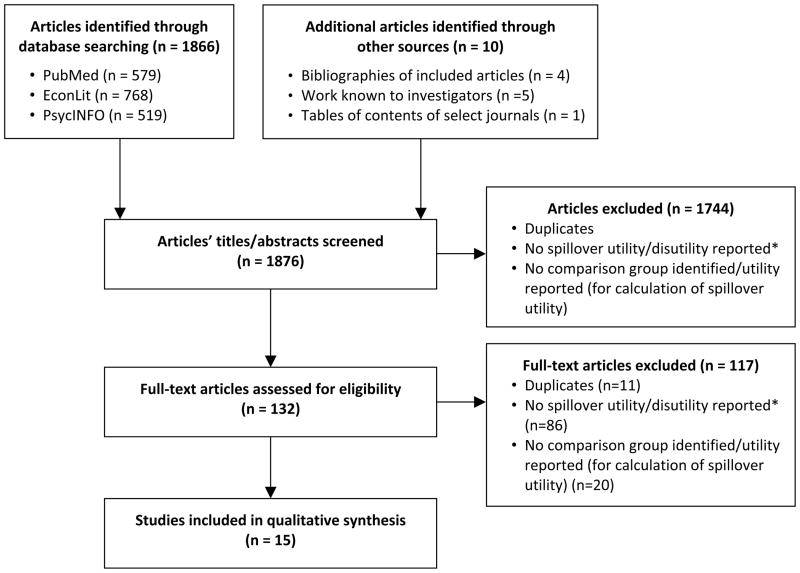
The initial database search resulted in 1,866 articles, which were supplemented with 10 articles from other sources (references lists of screened articles (n=4), articles by researchers known to the authors (n=5), tables of contents of relevant journals (n=1)), for a total of 1,876 included in screening. Of these, 1,861 were excluded using established criteria, resulting in 15 studies for inclusion in this review (Figure 1). Of these 15, 7 articles reported spillover disutility and the other 8 reported data from which spillover disutility could be inferred/calculated by the authors of this review (Table I). In some cases these data were not the primary outcome measure or focus of the study, but their inclusion nevertheless provided sufficient means to infer spillover disutility from the study results.
Figure 1.
Literature search procedure
*Utility/disutility defined as preference-based measure of health-related quality of life measured using time trade-off, standard gamble, or visual analog scale to which a utility transformation is applied, or an indirect utility method based on these techniques (such as the EQ-5D or HUI)
Table I.
Literature reporting spillover disutility of illness on surrounding individuals
| Studies reporting directly elicited spillover utility of family members of ill individuals
| ||||||
|---|---|---|---|---|---|---|
| Study | Primary person’s illness | Person(s) affected by spillover (n) | Comparison group (n) | Valuation technique | Reported utility (mean; SD or CI) | Review-calculated spillover disutility (1- utility) |
| Basu et al., 2010[16] | Prostate cancer | Partners (n=26) | None: partners’ spillover utility directly assessed | Modified TTO | By patient’s state: incontinent: 0.675 (0.344); impotent: 0.664 (0.356); under watchful waiting: 0.705 (0.327); metastasis: 0.497 (0.409); death: 0.282 (0.340) | By patient’s state: incontinent: −0.325; impotent: −0.336; under watchful waiting: −0.295; metastasis: −0.503; death: −0.718 |
|
| ||||||
|
Studies reporting spillover disutility by comparison group method (population norms or control/comparison group utilities)
| ||||||
| Study | Primary person’s illness | Person(s) affected by spillover (n) | Comparison group (n) | Valuation technique | Reported disutility (mean; SD or CI) | |
|
| ||||||
| Brouwer et al., 2004[51] | Rheumatoid arthritis | Informal caregivers (n=145) (partner or other) | Population norms | EQ-5D | Non-significant: 0.0173 (95% CI: −0.02, 0.05) | |
| Davidson et al., 2008[22] | Dementia, stroke, general weakness, musculoskeletal and cardiovascular diseases in elderly persons | “Family” caregivers (n=894) (spouse, child, other) | (a) Population-norms, (b) comparison group: hypothetical healthy relative (n=329) | “R-QALY” (using EQ-5D) | (a) −0.015a
(b) −0.062a |
|
| Neumann et al., 1999[49]; Bell et al., 2001[21] | Alzheimer’s disease | Primary family caregivers or friends (n=679) (spouse, child, other) | Population norms | HUI2 | Noneb | |
| Poley et al., 2012[29] | Congenital anomalies in children (anorectal malformations and diaphragmatic hernia) | Parents (n=306) | (a) Population norms, (b) comparison group: hypothetically absolved of caregiving responsibilities | EQ-5D | (a) −0.10 (95% CI: −0.16, −0.04) for mothers age 25–34 of children with anorectal malformations; all other parent/age groups non-significant | |
| Wittenberg et al., 2012[28] | All chronic health conditions | Household members (n=11,207) | Comparison group: Individuals living in households with healthy household members or alone. (n=12,981) | EQ-5D | By adult household member’s condition: existing mental or musculoskeletal conditions: −0.01 (95% CI:−0.02, −0.00); new mental conditions: −0.02 (95% CI:−0.04, −0.00); new musculoskeletal conditions: −0.03 (95% CI: −0.05, −0.01)c | |
|
| ||||||
|
Studies reporting family member/caregiver and comparison group utilities only
| ||||||
| Study | Primary person’s illness | Person(s) affected by spillover (n) | Comparison group (n) | Valuation technique | Reported utilities (mean; SD or CI) | Implied spillover disutility |
|
| ||||||
| Argyriou et al., 2011[30] | Multiple Sclerosis | Caregivers (n=35) (spouse, parent, or other family member living in same household) | Comparison group: age-, gender-, & education-matched healthy controls (n=35) | EQ-VAS | Caregivers: 61.9 (13.8); controls: 90.3 (7.1) (raw VAS scores) | −0.142d |
| Brisson et al., 2010[15] | Rotavirus-associated gastroenteritis in children | Parents (during episode) (n=186) | Comparison group: parents post-episode (n=186) | EQ-5D | During-episode: 0.875 (95% CI: 0.844, 0.907); week 2 (after episode): 0.967 (95% CI: 0.951, 0.983) | −0.092 |
| Kuhlthau et al., 2008[48] | Activity limitations in children | Parents (n=2,412) | Comparison group: parents of children without activity limitations (n=13,560) | EQ-5D | With activity limitations: 0.82; without activity limitations: 0.9e | −0.08 |
| Mohide et al., 1988[50] | Chronic degenerative disorders in elderly persons | Family caregivers (n=23) (spouse, child, or other relatives) | Comparison group: relatives of well-elderly (n=10) | CQLI/TTO | Caregivers of physically impaired: 0.795 (95% CI: 0.621, 0.969); caregivers of cognitively impaired: 0.412 (95% CI: 0.204, 0.619); Comparison group: 0.990 (95% CI: 0.977, 1.0) | −0.195 physically Impaired; −0.578 cognitively impaired |
| Serrano-Aguilar et al., 2006[23] | Alzheimer’s disease | Informal caregivers (n=237) (partner, child, or other) | Population norms | EQ-VAS | Caregivers: 61.4 (16.6); population norms: 65.9 (18.3) (raw VAS scores) | −0.034d |
| Tilford et al., 2005[45]; Grosse et al., 2009[47] | Spina bifida in children | Caregivers (n=98) (adult family member) | Comparison group: caregivers of unaffected children (n=49) | QWB | Caregivers: 0.76 (0.1); comparison group: 0.8 (0.1) | −0.04f |
| Van Exel et al., 2005[24] | Stroke | Informal caregivers (n=145) (spouse or other) | Population norms | EQ-5D | Moderately burdened caregivers: 0.89; substantially burdened caregivers: 0.67; population norms: 0.81g | −0.14 substantially burdened caregivers; (moderately burdened caregivers +0.08 utility gain) |
TTO= time trade-off; EQ-5D= EuroQol 5 dimensions; EQ-VAS= EuroQol visual analog scale; HUI2= Health Utilities Index version 2; QWB= Quality of Well-Being scale; CQLI= Caregiver Quality of Life Index; SD= standard deviation; CI=confidence interval.
No confidence interval reported, p-value=(a)0.04, (b)<0.001;
Reported that caregiver scores were similar to population norms, no actual scores reported;
Disutility for subset of adults who reported EQ-5D scores<1.0;
VAS scores transformed to utilities before spillover disutility calculated;
p-value for difference <0.0001, no SD or CI reported;
Adjusted disutility= −0.072;
No standard deviation, CI or p-value reported.
3.1 Illnesses/conditions and spillover populations studied
Most articles on spillover disutility reported on the effect on family members and caregivers of specific conditions among specific patient populations (Table I). Conditions included childhood disorders (n=5), diseases of the elderly (n=5), physically disabling conditions (multiple sclerosis and arthritis, n=2), medical conditions such as cancer and stroke (n=2), and the general category of all chronic health conditions (n=1). Children’s conditions included spina bifida (n=2) [45,47], activity limitations[48], congenital anomalies[29], and gastroenteritis.[15] Conditions among the elderly included Alzheimer’s disease (n=3)[21,23,49] and general conditions affecting this age group (including dementia; n=2).[22,50] Four studies focused on illness in adults, including stroke[24], multiple sclerosis[30], cancer[16], and rheumatoid arthritis.[51] The study focusing on all chronic health conditions included those among children and adults.[28]
Of the three studies that were not disease or condition-specific, two were designed to develop measures of caregiver utility. Mohide et al.[50] developed the “Caregiver Quality of Life Instrument” to measure caregiver well-being, not specifically spillover, using a sample of family caregivers of elderly individuals with chronic degenerative disorders. Davidson et al.[22] developed the “R-QALY” to measure spillover in a sample of relatives caring for or supporting older persons with any condition. The third study by Wittenberg et al.[28] estimated spillover disutility on household members of all chronic health conditions reported by adults and children in the US population.[28]
Most of the studies included in this review focused on spillover specifically for caregivers of ill persons as opposed to non-caregiving family members/relatives, and in all cases these were informal family caregivers (with a broad definition of “family” to include extended relatives and close friends). Parents, and often mothers, were studied as the primary caregivers of children and the person experiencing spillover from the child’s condition.[15,29,45,47,48] Spouses and adult children were generally the caregivers/spillover individual for ill elderly persons[21–23,49,50], while for ill younger adults the caregiver was a spouse or a parent.[12,16,24,30] One study focused on household members of ill individuals without specifying the type of relationship, if any.[28]
3.2 Methods used to measure spillover
Methods used to assess spillover included three types of approaches: (i) directly measuring caregivers’/family members’ spillover utility or disutility using the standard gamble, time trade-off or visual analog techniques; and (ii) comparison methods, including assessing caregiver/family member utility and comparing it to (a) utility from a comparison population (population norms or a non-spillover affected control group), or (b) utility assessed using a hypothetical scenario in which the family member was healthy or caregiving was not required. While comparison methods could in principle use any utility elicitation technique to estimate utility scores for the caregiver/family member and comparison group(s), in practice most used generic (indirect) utility measures (some studies included in this review used multiple comparison methods). The direct spillover measurement approach requires the adaptation of existing direct utility assessment methods to capture spillover, while the comparison approaches use existing utility assessment techniques for family members/caregivers and either population valuation sets for the same technique or data from an appropriate control/comparison group (also using same technique).
3.2.1 Direct spillover utility/disutility assessment
Only one study directly assessed spillover utility using the SG, TTO or VAS techniques (Table I): Basu and colleagues[16] developed a modified version of the TTO to isolate and measure the spillover effect of prostate cancer on a patient’s spouse/partner. The authors used a chained TTO in which the anchor state was represented by the spouse/partner’s and prostate cancer patient’s current health, including any accompanying “worries” for the spouse/partner related to the patient’s cancer. The TTO asked spouse/partners to trade off a shorter time in perfect health for themselves, without change in the patient’s health state, against longer time in their own current health “with added burdens and concerns stemming from the patient experiencing an adverse health event.”[16] The modification of the standard TTO focuses respondents on the specific aspects of a relative’s illness that produce spillover: the effect of a patient’s adverse health event on the spouse/partner, to measure the isolated spillover effect of another’s illness.
3.2.2 Spillover utility/disutility assessed by comparison method (between caregiver utility and comparison/control group)
All other studies estimated spillover disutility by comparing utilities of family members/caregivers of ill individuals with utilities from a comparison/control group. The studies reporting spillover calculated unadjusted between-group differences in means or adjusted differences through regression analysis or matched samples, while the others reported means for each group but did not calculate spillover disutility. Types of comparison groups used included country-specific, age and gender-adjusted population norms (n=7), empirically selected control samples (n=6), and within-subject scores, either across time points (n=1) or hypothetical scenario evaluations (n=2) (note: two studies used two comparator groups).
Most of the seven studies using population norms as comparators utilized the EQ-5D. Brouwer and colleagues[51] compared EQ-5D scores from Dutch caregivers of patients with rheumatoid arthritis and UK EQ-5D age and gender-adjusted population norms. Van Exel et al.[24] compared EQ-5D scores from Dutch informal caregivers of stroke patients with age and gender-adjusted UK norms as well. Poley et al.[29] collected EQ-5D data from Dutch parents of children with major congenital anomalies, including both the EQ-5D index score and the EQ-5D VAS (EQ-VAS), and compared the former with UK general population norms and the latter with EQ-VAS scores for a hypothetical scenario in which parents’ caregiving responsibilities were absolved. Davidson and colleagues[22] compared Swedish EQ-5D scores for relatives of elderly individuals with Swedish population values for the EQ-5D, and with relatives’ EQ-5D scores for a hypothetical scenario in which the elderly individual was completely healthy. The EQ-VAS was used by Serrano-Aguilar et al.[23] in a design comparing caregivers of persons with Alzheimer’s disease in the Canary Islands to age and gender-adjusted population norms for the Canary Islands. And finally, Neumann et al.[49] and Bell et al.[21] compared Health Utilities Index scores of US family caregivers of patients with Alzheimer’s Disease with population norms for this instrument.
The six studies that estimated spillover disutility by comparing data from specified control/comparison groups rather than using population norms used a variety of instruments/techniques. Kuhlthau and colleagues[48] analyzed EQ-5D data from the Medical Expenditures Panel Survey (MEPS) for parents with and without children with activity limitations to assess the spillover effect of having a child with this type of condition. Wittenberg et al.[28] compared EQ-5D scores from MEPS of individuals living in households with a child or adult with a chronic medical condition with scores of individuals living alone or with healthy household members. Argyriou et al.[30] compared EQ-VAS data collected from caregivers of patients with multiple sclerosis with EQ-VAS data collected from age, gender and education-matched “healthy controls”. Mohide and colleagues[50] compared TTO scores of caregivers of elderly individuals with chronic degenerative disorders with TTO scores from individuals with healthy elderly relatives. And Grosse et al.[47] and Tilford et al.[45] compared Quality of Well-Being scale data from parents of children with spina bifida with a control sample of parents of non-disabled children.
The studies that used within-subject comparisons included Brisson et al.[15], who assessed parents’ EQ-5D scores at the onset of a child’s acute condition (gastroenteritis) and again after the event concluded. Poley et al.[29] compared EQ-VAS scores from parents of children with congenital anomalies for their current health and for a hypothetical health state in which they assumed that “someone would take on their caregiving activities completely and free of charge”. And Davidson and colleagues[22] compared current health EQ-5D scores for family caregivers’ of elderly persons with these caregivers’ EQ-5D scores described by considering a hypothetical health state in which their relative was healthy.
3.3 Spillover disutility estimates
Acknowledging the heterogeneity in measurement methods and populations in the spillover literature, reported disutility ranged from −0.015 for family caregivers of the elderly[22] to −0.718 for the spouse of a prostate cancer patient who dies[16] (Table I). Several studies reported no significant effect, or a disutility of zero.[21,29,49,51] One study compared spillover across conditions within one population sample to assess relative effect, finding (adjusted) effect sizes from none to −0.03.[28] Studies reporting data that allowed for our calculation of spillover fell similarly within this range. [23,50] A couple of outliers in the literature reported positive spillover, meaning utility associated with having an ill family member, including a small positive effect of 0.08 for moderately burdened caregivers of stroke patients[24] and 0.01 for household members of adults with cancer.[28] Most of the spillover literature reported small negative effects, however, with infrequent reports of very large disutility or small positive utility. Variability seems to depend on the ill person’s condition and the method used to measure the spillover effect. Results of typical magnitude included −0.04 for parents of children with spina bifida[45,47], −0.08 for parents of children with activity limitations[48], −0.09 for parents of children with gastroenteritis[15], and −0.14 for caregivers of persons with multiple sclerosis[30] and substantially burdened caregivers of stroke patients.[24]
4. Discussion
Though the burden of illness on caregivers has been noted for some time, empiric estimates of spillover disutility are sparse and measurement methodologies unproven, creating challenges for the incorporation of this element into economic evaluation. The available studies reporting spillover estimates vary widely in the illnesses studied and the measurement approach used. The general conclusion of the literature is that spillover disutility is small but measurable, and likely correlated with condition, population affected, as well as measurement technique. This evidence suggests that there is a quantifiable component of disease burden that is not currently captured by traditional utility measurement and therefore not included in calculating quality-adjusted life years or other health economic outcome measures. Further work is required to establish valid methods for measuring spillover utility and for integrating this aspect of ill health into economic evaluations and decision analyses.
To the extent that spillover disutility exists, it is an important component of the entirety of benefits associated with an intervention or treatment. That is, if family members’ health or well-being is improved by the alleviation of a patient’s health condition, their benefit should be factored into the equation of how much “good” is received by the remission of that condition.[31] Similarly, if treatments have negative effects on utility, such as increased caregiving or emotional burden to family members, these effects should also be considered in the total utility associated with an intervention. This broader scope of outcomes more accurately captures the entirety of effects that interventions accrue, both positive and negative, to the patient and family members/caregivers, and thereby improves the validity of economic evaluations and comparative effectiveness analyses based on outcome measures. This full scope of costs and benefits has been promulgated since the initial recommendations for economic evaluation of health interventions[37], and frameworks have been presented to justify its inclusion in cost effectiveness analyses.[38,52] The barrier to fulfilling this mandate has been the methodology to measure spillover disutility and to incorporate it into analyses.
The methods of incorporating family/caregiver effects into analyses have in part framed the development of spillover measurement methods. Starting from the premise that illness affects more than just the affected individual (i.e., the patient), analyses can be framed on a multiple-individual basis, including the “patient” plus an other, and both individuals’ costs and benefits. Such a framework seems reasonable for parent/child pairs or organ transplant pairs, for example, but pushes the question of where to draw the line beyond one “other.” Drug abuse treatment, for example, may accrue benefit to co-workers, neighbors and the broader public, but including all these QALYs seems less reasonable than the parent/child pair. A paired or multiple individual approach suggests inclusion of the pair’s QALYs in analyses, including each individual’s utility and therein spillover as well. Measurement methods of these utilities could include assessment of each individual’s current health state which would be combined for analysis, or assessment of a dyad health state (by one or the other) for use as a single value in analysis, as would be more appropriate for a parent/child or caregiver/very ill individual pair. Alternately, an individual-based analysis would require the measurement of spillover alone, as an isolated quantity, to be combined with the patient’s utility as an additional component of total QALYs. This approach would dictate measurement of “individual” spillover, meaning that affecting the “other” separate of the patient and separate of the other’s baseline utility/current health. The literature includes both measurement approaches as the field has not yet decided upon an incorporation method. Basu[16] and Wittenberg[28], for example, measure spillover disutility alone, while Mohide[50] and current efforts by Al-Janabi and others[53] measure caregiver utility, which could also include the caregiver’s baseline health. Little attention has been paid to the distinction between patient-perspective and community-perspective spillover, as it is as yet unknown whether this difference exists nor which perspective is appropriate for use in analyses. This discussion will continue on dual fronts as the field advances.
Attempts to measure spillover disutility alone have been stymied by the small scale and the external nature of the effect, both of which are not well-handled by existing utility assessment methods. The measurement of spillover utility is as much a focus of the existing literature as are the estimates of this value. Only one article included in this review used a direct utility assessment method; most used indirect methods and varied the comparison groups for the purpose of estimating spillover. Direct utility elicitation methods are defined by individuals making trade-offs while thinking about themselves and their own health. Adapting direct techniques to measure spillover of illness from another person (usually a relative) becomes tricky and framing is critical: the effect of the relative’s illness on the respondent must be isolated and measured, and the trade-off is now an improvement in just the aspect of the individual’s own health that is a result of the relative being ill, separate of his or her own health or the relative’s health. This degree of compartmentalization of effects is conceptually difficult for respondents to imagine and to construct in responses, which may lead to double-counting of effects if the separation is impossible. Basu and colleagues’ chained time trade-off for the spillover effects of prostate cancer on a partner/spouse attempted to isolate this effect and arrived at substantially larger spillover disutilities compared with studies using other methodologies.[16] It is also worth noting that this method effectively assessed patient-assessed hypothetical states, which are variants of patient perspective utilities, and it is as yet unknown how patient-perspective spillover compares to community-perspective spillover (and whether this distinction matters for CEA/CUA). Direct utility elicitation methods are in principle applicable to community-perspective spillover elicitation, though would require considerable cognitive effort on the part of respondents to imagine a hypothetically ill relative as well as the spillover effect on themselves.[36]
Other investigators experimented with combined measurement designs, incorporating elements of direct elicitation and comparison group methods, and patient and community perspectives. Mohide and colleagues used direct utility elicitation (TTO) to assess patient-perspective utilities for family caregivers of elderly persons with cognitive or physical disability, and relatives of well-elderly as a control group, as well as TTO scores for hypothetical states describing the health of caregivers in relevant domains (such as worry, time to socialize, undisturbed sleep and relationship with ill relative).[50] While the comparison between utilities for caregivers and relatives of the healthy elderly shed light on spillover disutility, Mohide et al.’s method was primarily designed to measure caregiver utility rather than spillover, which would be useful for analyses using a combined patient/caregiver perspective. Their results also implied spillover disutilities on the high end of the scale found in the literature. Davidson and colleagues defined the “R-QALY” (relative’s QALY) as the difference between caregivers’ current EQ-5D scores and their hypothetical EQ-5D scores imagining that the patient for whom they were caring was healthy(in addition to comparing caregivers’ scores with population norms).[22] This approach estimates spillover isolated from the caregiver’s current health, through the use of the hypothetical scenario versus current health comparison, though runs the risk of double-counting the patient’s improved health benefit with the respondent’s spillover benefit if the respondent cannot effectively separate the two. Their spillover estimates were, however, similar to much of those reported in the literature. In a similar vein, Poley et al. elicited current EQ-VAS scores for parents’ of children with congenital anomalies and their imagined EQ-VAS scores for a hypothetical state in which all caregiving responsibilities were provided free of charge by someone else, with the comparison in scores representing the value of the caregiving element along of parents’ utility.[29] An advantage of the use of hypothetical scenarios to measure spillover is that it allows for the incorporation of both positive and negative aspects of caregiving, such as the altruism in addition to the burden, which together would be lost if health is restored (or caregiving is “outsourced”). These dual effects may be a realistic portrayal of spillover utility and may be present in other caregiving circumstances as well, so their inclusion is appropriate.[9,10] Hypothetical scenarios also control for the caregiver characteristics that affect utility separate of spillover, to isolate the spillover effect, though generally elicit patient-perspective values rather than presenting these scenarios to community members as is more common with the hypothetical scenario approach. They are similar to other comparison designs to measure spillover except for the nature of the comparison group.
Most of the comparison group designs used population norms as a comparator, though some used empiric values from a matched control group. Anecdotal evidence suggests that caregivers are often individuals who otherwise can accommodate the time demands of the role, and may therefore be unemployed, disabled, or retired, all situations that may be correlated with lower quality of life than the average person.[36] Caregivers may therefore be a select group of individuals who have the time or inclination to take on such responsibility. This self-selection into caregiving suggests that caregivers may have lower health related quality of life than non-caregivers or the general population regardless of spillover effects, which may confound or obscure spillover estimates based on comparison designs. The use of select comparison groups rather than population norms may control for some caregiver characteristics and therefore more accurately capture spillover disutility. Examples of these designs include Kuhlthau and colleagues, who compared EQ-5D scores of parents of children with and without activity limitations, which effectively controlled for the utility of being a parent.[48] And Tilford,[45] Grosse[47] and Mohide[50], who matched caregivers of a certain type of patient with individuals with the same type of family member who was not ill, controlling for all the attributes associated with being a family member of a certain type of person, minus the caregiving role. These comparison groups do not, however, control for the attributes of choosing or ending up being a caregiver, which may in and of itself confer utility decrements (or increments) independent of the spillover effect of illness. Wittenberg et al.[28] tried to overcome this bias by using regression analysis to compare EQ-5D scores of all individuals with and without ill household members to adjust for underlying differences in these types of individuals that might explain apparent spillover disutility. They found a small, persistent effect within some conditions after controlling for individuals’ own health and other known predictors of utility.
Since most spillover studies used generic (indirect) utility instruments, it is worthwhile to comment on the limitations of these measures in this context, namely the relevance of the domains included in these instruments to accurately capture spillover effects.[54] The combination of positive and negative effects that is inherent to spillover of illness underscores the need for the measurement instrument used to include the correct definition of domains in order to capture this effect. It is likely that mental and emotional health are the domains most affected by spillover, though physical health can be compromised and comorbid conditions exacerbated.[36] Generic utility instruments that include only one or two dimensions related to mental/emotional health may be inadequate to completely capture spillover effects. Supporting this hypothesis, a number of spillover studies using generic utility instruments have found no effect, including the HUI for caregivers of Alzheimer’s disease (Bell et al.[21], and Neumann et al.[49]), and the EQ-5D for caregivers of patients with rheumatoid arthritis (Brouwer et al.[51]), household members of adults and children with many chronic conditions (Wittenberg et al.[28]), and the majority of parents studied by Poley et al.[29] It may be that for certain conditions and/or caregiver relationships, there is in fact so little spillover that it is undetectable (and unimportant for comparative effectiveness analyses), or that certain instruments are less able to capture these effects. Some have gone further to suggest that the dimensions of health included in patient-focused health related quality of life measures may be fundamentally inadequate to capture caregiver effects, explaining the apparent lack of detectable spillover in some studies.[31] A direct approach such as that used by Basu et al.[16] allows for the inclusion of all aspects of health related quality of life of significance to caregiver and is not limited by the dimensions/domains included in a generic health utility instrument, possibly explaining the larger values estimated by these authors compared with others. On the flip side, however, direct measurement is far more resource-intensive than the use of generic utility instruments, limiting its applicability in practice.
In summary, the studies included in this review used mostly established utility methods and had quite reasonable sample sizes, and provide evidence of the existence of a small spillover effect of illness. This effect is often smaller than the minimally important clinical difference for utility (approximately 0.03)[55], but may be important when aggregated across a population. The literature suggests patterns of variation in spillover effects, with higher spillover associated with higher severity of conditions, and variation by the attributes of the condition. In the studies included in this review, conditions among children consistently produced small estimates of spillover disutility compared with conditions of adults that were highly variable in their spillover, possibly because of the relatively lesser-severity of children’s conditions in general or the nature of the parent/child relationship. Conditions among elderly relatives (which were often dementia or Alzheimer’s disease) showed high variability in spillover disutility across studies, from none to a very high level. The one study that focused on spillover from cancer (that also used a different measurement technique than the other studies included in the review), showed very high spillover disutility. Some additional evidence reported in the studies in this review suggests that spillover varies by the age and educational status of the caregiver. One area that has been relatively unexplored is the spillover effect of parental illness on young children, likely because of the dearth of utility instruments for children. The persistence of spillover effects across conditions and relationships points to the need to explore the extent of these effects and to identify areas where they do and do not exist which can only be known with larger studies that control for condition, caregiver characteristics, caregiver relationship to the patient, and measurement method. Validation of findings will be supported by more and larger studies, since no gold standard exists for comparison of spillover values, as is true with all utility studies.
5. Conclusion
The literature presents a strong case that spillover of illness creates disutility for caregivers and family members. What remains unclear is the magnitude of this effect and the situations in which it does and does not occur. There is a need for validation of existing estimates of spillover and for measurement methods. Beyond establishing the existence of spillover, the question of how to integrate these effects into economic evaluation remains. Research is emerging on the methods of including family member’s benefits and costs into analyses, including a recent review on approaches.[39] Accurate and valid measurement of spillover and caregiving utility is a necessary prerequisite to inclusion, as current methods may contain biases of overestimation or double-counting. A valid estimate of spillover utility could reasonably be added to the QALYs accrued to an intervention, provided that spillover was in fact accurately isolated from other effects. Consideration of family-level or dyad QALYs could be an alternative approach if isolation of spillover is impossible, with subsequent integration into analyses. Spillover of illness is clearly an important part of the outcomes picture but many questions remain about how to capture it. This review provides a baseline view of our knowledge to date to guide and inform future efforts.
Key points for decision makers.
Illness has collateral effects on family members’ and caregivers’ health and well-being that, if considered, may influence clinical decision making and resource allocation decisions.
The literature describes these “spillover” effects as varying by the type and severity of the patient’s health condition and the family member’s/caregiver’s relationship to the patient.
The methodology for quantifying spillover and for formally integrating it into analyses is as yet unproven, and further work is needed before estimates can be relied upon with confidence.
Acknowledgments
The authors thank Kara Lamarand, MPH, Lisa Lee, MS, and Gail Strickler, PhD for helpful research assistance in the review, Adrianna Saada, MPH for assistance in preparing the manuscript, and Tara Lavelle, PhD, 2 anonymous reviewers, and the Editor of this journal for helpful comments on previous drafts. EW and LAP conceived of and received funding for the study; EW directed the study and wrote the final manuscript, and takes responsibility for the integrity and accuracy of the analysis; both authors reviewed and contributed to the final manuscript. This work was supported by awards numbers 7R01NR011880 from the National Institute of Nursing Research and 7K02HS014010 from the Agency for Health Care Research and Quality, both to EW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Health Care Research and Quality, the National Institute of Nursing Research, or the National Institutes of Health.
Footnotes
Neither author has conflicts to report.
References
- 1.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. The New England journal of medicine. 2007;357:370–9. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 2.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. The New England journal of medicine. 2008;358:2249–58. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christakis NA. Social networks and collateral health effects. BMJ. 2004;329:184–5. doi: 10.1136/bmj.329.7459.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigby H, Gubitz G, Phillips S. A systematic review of caregiver burden following stroke. Int J Stroke. 2009;4:285–92. doi: 10.1111/j.1747-4949.2009.00289.x. [DOI] [PubMed] [Google Scholar]
- 5.Vellone E, Piras G, Talucci C, Cohen M. Quality of life of caregivers of people with Alzheimer’s disease. J Adv Nurs. 2008;61:222–31. doi: 10.1111/j.1365-2648.2007.04494.x. [DOI] [PubMed] [Google Scholar]
- 6.Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: a review of the literature. J Am Acad Nurse Pract. 2008;20:423–8. doi: 10.1111/j.1745-7599.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- 7.Awad AG, Voruganti LN. The burden of schizophrenia on caregivers: a review. Pharmacoeconomics. 2008;26:149–62. doi: 10.2165/00019053-200826020-00005. [DOI] [PubMed] [Google Scholar]
- 8.Brouwer WBF. Too important to ignore: informal caregivers and other significant others. Pharmacoeconomics. 2006;24:39–41. doi: 10.2165/00019053-200624010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Al-Janabi H, Coast J, Flynn TN. What do people value when they provide unpaid care for an older person? A meta-ethnography with interview follow-up. Soc Sci Med. 2008;67:111–21. doi: 10.1016/j.socscimed.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Bobinac A, van Exel NJ, Rutten FF, Brouwer WB. Caring for and caring about: disentangling the caregiver effect and the family effect. J Health Econ. 2010;29:549–56. doi: 10.1016/j.jhealeco.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer WB, van Exel NJ, van den Berg B, van den Bos GA, Koopmanschap MA. Process utility from providing informal care: the benefit of caring. Health Policy. 2005;74:85–99. doi: 10.1016/j.healthpol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer WBF, van Exel NJa, van Gorp B, Redekop WK. The CarerQol instrument: a new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual Life Res. 2006;15:1005–21. doi: 10.1007/s11136-005-5994-6. [DOI] [PubMed] [Google Scholar]
- 13.Klassen A, Raina P, Reineking S, Dix D, Pritchard S, O’Donnell M. Developing a literature base to understand the caregiving experience of parents of children with cancer: a systematic review of factors related to parental health and well-being. Support Care Cancer. 2007;15:807–18. doi: 10.1007/s00520-007-0243-x. [DOI] [PubMed] [Google Scholar]
- 14.Klassen AF, Gulati S, Granek L, et al. Understanding the health impact of caregiving: a qualitative study of immigrant parents and single parents of children with cancer. Qual Life Res. 2011 doi: 10.1007/s11136-011-0072-8. [DOI] [PubMed] [Google Scholar]
- 15.Brisson M, Senecal M, Drolet M, Mansi JA. Health-related quality of life lost to rotavirus-associated gastroenteritis in children and their parents: a Canadian prospective study. Pediatr Infect Dis J. 2010;29:73–5. doi: 10.1097/INF.0b013e3181b41506. [DOI] [PubMed] [Google Scholar]
- 16.Basu A, Dale W, Elstein A, Meltzer D. A time tradeoff method for eliciting partner’s quality of life due to patient’s health states in prostate cancer. Med Decis Making. 2010;30:355–65. doi: 10.1177/0272989X09349959. [DOI] [PubMed] [Google Scholar]
- 17.Couper J, Bloch S, Love A, Macvean M, Duchesne G, Kissane D. Psychosocial adjustment of female partners of men with prostate cancer: a review of the literature. Psycho-oncology. 2006;15:937–53. doi: 10.1002/pon.1031. [DOI] [PubMed] [Google Scholar]
- 18.Osborn T. The psychosocial impact of parental cancer on children and adolescents: a systematic review. Psycho-oncology. 2007;16:101–26. doi: 10.1002/pon.1113. [DOI] [PubMed] [Google Scholar]
- 19.Koehly LM, Peters Ja, Kuhn N, et al. Sisters in hereditary breast and ovarian cancer families: communal coping, social integration, and psychological well-being. Psycho-oncology. 2008;17:812–21. doi: 10.1002/pon.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packman W, Greenhalgh J, Chesterman B, et al. Siblings of pediatric cancer patients: the quantitative and qualitative nature of quality of life. J Psychosoc Oncol. 2005;23:87–108. doi: 10.1300/J077v23n01_06. [DOI] [PubMed] [Google Scholar]
- 21.Bell C, Araki S, Neumann P. The association between caregiver burden and caregiver health-related quality of life in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2001;15:129–36. doi: 10.1097/00002093-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Davidson T, Krevers B, Levin LA. In pursuit of QALY weights for relatives: empirical estimates in relatives caring for older people. Eur J Health Econ. 2008;9:285–92. doi: 10.1007/s10198-007-0076-z. [DOI] [PubMed] [Google Scholar]
- 23.Serrano-Aquilar PG, Lopez-Bastida J, Yanes-Lopez V. Impact on health-related quality of life and perceived burden of informal caregivers of individuals with Alzeheimer’s disease. Neuroepidemiology. 2006;27:136–42. doi: 10.1159/000095760. [DOI] [PubMed] [Google Scholar]
- 24.van Exel N, Koopmanshap M, van de Berg B, Brouwer W, van den Bos G. Burden of informal caregiving for stroke patients: Identification of caregivers at risk of adverse health effects. Cerebrovascular Diseases. 2005;19:11–7. doi: 10.1159/000081906. [DOI] [PubMed] [Google Scholar]
- 25.Pinquart M, Sorensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18:250–67. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- 26.Pinquart M, Sorensen S. Correlates of physical health of informal caregivers: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2007;62:P126–37. doi: 10.1093/geronb/62.2.p126. [DOI] [PubMed] [Google Scholar]
- 27.Kuster PA, Merkle CJ. Caregiving stress, immune function, and health: implications for research with parents of medically fragile children. Issues Compr Pediatr Nurs. 2004;27:257–76. doi: 10.1080/01460860490884165. [DOI] [PubMed] [Google Scholar]
- 28.Wittenberg E, Ritter GA, Prosser LA. Evidence of spillover of illness among household members: EQ-5D Scores from a US sample. Med Decis Making. doi: 10.1177/0272989X12464434. epub 10/27/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poley MJ, Brouwer WB, van Exel NJ, Tibboel D. Assessing health-related quality-of-life changes in informal caregivers: an evaluation in parents of children with major congenital anomalies. Qual Life Res. 2012;21:849–61. doi: 10.1007/s11136-011-9991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Argyriou AA, Karanasios P, Ifanti AA, et al. Quality of life and emotional burden of primary caregivers: a case-control study of multiple sclerosis patients in Greece. Qual Life Res. 2011;20:1663–8. doi: 10.1007/s11136-011-9899-2. [DOI] [PubMed] [Google Scholar]
- 31.Al-Janabi H, Flynn TN, Coast J. QALYs and carers. Pharmacoeconomics. 2011;29:1015–23. doi: 10.2165/11593940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Gusi N, Prieto J, Madruga M, Garcia J, Gonzalez-Guerrero J. Health-Related Quality of Life and Fitness of the Caregivers of Patient with Dementia. Med Sci Sports Exerc. 2009;41:1182–7. doi: 10.1249/MSS.0b013e3181951314. [DOI] [PubMed] [Google Scholar]
- 33.Northouse L, Mood D, Montie J, et al. Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25:4171–7. doi: 10.1200/JCO.2006.09.6503. [DOI] [PubMed] [Google Scholar]
- 34.Northouse L, Williams AL, Given B, McCorkle R. Psychosocial care for family caregivers of patients with cancer. J Clin Oncol. 2012;30:1227–34. doi: 10.1200/JCO.2011.39.5798. [DOI] [PubMed] [Google Scholar]
- 35.Carmack Taylor CL, Badr H, Lee JH, et al. Lung cancer patients and their spouses: psychological and relationship functioning within 1 month of treatment initiation. Ann Behav Med. 2008;36:129–40. doi: 10.1007/s12160-008-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wittenberg E, Saada A, Prosser LA. How illness affects family members: domains of well-being affected by "spillover": abstract. Med Decis Making. 2012 [Google Scholar]
- 37.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 38.Basu A, Meltzer D. Implications of spillover effects within the family for medical cost-effectiveness analysis. J Health Econ. 2005;24:751–73. doi: 10.1016/j.jhealeco.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Goodrich K, Kaambwa B, Al-Janabi H. The inclusion of informal care in applied economic evaluation: a review. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012;15:975–81. doi: 10.1016/j.jval.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Bonomi AE, Boudreau DM, Fishman PA, Meenan RT, Revicki DA. Is a family equal to the sum of its parts? Estimating family-level well-being for cost-effectiveness analysis. Qual Life Res. 2005;14:1127–33. doi: 10.1007/s11136-004-2578-9. [DOI] [PubMed] [Google Scholar]
- 41.van Exel J, Bobinac A, Koopmanschap M, Brouwer W. The invisible hands made visible: recognizing the value of informal care in healthcare decision-making. Expert Rev Pharmacoecon Outcomes Res. 2008;8:557–61. doi: 10.1586/14737167.8.6.557. [DOI] [PubMed] [Google Scholar]
- 42.Davidson T, Levin LA. Is the societal approach wide enough to include relatives? Incorporating relatives’ costs and effects in a cost-effectiveness analysis Applied health economics and health policy. 2010;8:25–35. doi: 10.1007/BF03256163. [DOI] [PubMed] [Google Scholar]
- 43.Koopmanschap MA, van Exel JN, van den Berg B, Brouwer WB. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics. 2008;26:269–80. doi: 10.2165/00019053-200826040-00001. [DOI] [PubMed] [Google Scholar]
- 44.Weeks J. Valuing Outcomes. In: Hunink M, Glasziou P, editors. Decision making in health and medicine Integrating evidence and values. New York, NY: Cambridge University Press; 2001. pp. 88–127. [Google Scholar]
- 45.Tilford JM, Grosse SD, Robbins JM, Pyne JM, Cleves MA, Hobbs CA. Health state preference scores of children with spina bifida and their caregivers. Qual Life Res. 2005;14:1087–98. doi: 10.1007/s11136-004-3305-2. [DOI] [PubMed] [Google Scholar]
- 46.Hunink M, Glasziou P. Integrating Evidence and Values. Cambridge: Cambridge University Press; 2001. Decision Making in Health and Medicine. [Google Scholar]
- 47.Grosse SD, Flores A, Ouyang L, Robbins JM, Tilford JM. Impact of Spina Bifida on parental caregivers: findings from a survey of Arkansas families. J Child Fam Stud. 2009;18:574–81. [Google Scholar]
- 48.Kuhlthau K, Kahn R, Hill KS, Gnanasekaran S, Ettner SL. The well-being of parental caregivers of children with activity limitations. Matern Child Health J. 2008;14:155–63. doi: 10.1007/s10995-008-0434-1. [DOI] [PubMed] [Google Scholar]
- 49.Neumann PJ, Kuntz KM, Leon J, et al. Health utilities in Alzheimer’s disease: a cross-sectional study of patients and caregivers. Med Care. 1999;37:27–32. doi: 10.1097/00005650-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Mohide EA, Torrance GW, Streiner DL, Pringle DM, Gilbert R. Measuring the wellbeing of family caregivers using the time trade-off technique. J Clin Epidemiol. 1988;41:475–82. doi: 10.1016/0895-4356(88)90049-2. [DOI] [PubMed] [Google Scholar]
- 51.Brouwer WB, van Exel NJ, van de Berg B, Dinant HJ, Koopmanschap MA, van den Bos GA. Burden of caregiving: evidence of objective burden, subjective burden, and quality of life impacts on informal caregivers of patients with rheumatoid arthritis. Arthritis Rheum. 2004;51:570–7. doi: 10.1002/art.20528. [DOI] [PubMed] [Google Scholar]
- 52.Davidson T, Levin L. Is the societal approach wide enough to include relatives? Incorporating relatives’ costs and effects in a cost-effectiveness analysis. Applied health economics and health policy. 2010;8:25–35. doi: 10.1007/BF03256163. [DOI] [PubMed] [Google Scholar]
- 53.Al-Janabi H, Flynn TN, Coast J. Estimation of a preference-based carer experience scale. Med Decis Making. 2011;31:458–68. doi: 10.1177/0272989X10381280. [DOI] [PubMed] [Google Scholar]
- 54.Prosser LA, Grosse SD, Wittenberg E. Health utility elicitation: is there still a role for direct methods? Pharmacoeconomics. 2012;30:83–6. doi: 10.2165/11597720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care. 2010;48:365–71. doi: 10.1097/mlr.0b013e3181c162a2. [DOI] [PubMed] [Google Scholar]