Abstract
The skin has developed a hierarchy of systems that encompasses the skin immune and local steroidogenic activities in order to protect the body against the external environment and biological factors and to maintain local homeostasis. Most recently it has been established that skin cells contain the entire biochemical apparatus necessary for production of glucocorticoids, androgens and estrogens either from precursors of systemic origin or, alternatively, through the conversion of cholesterol to pregnenolone and its subsequent transformation to biologically active steroids. Examples of these products are corticosterone, cortisol, testosterone, dihydrotesterone and estradiol. Their local production can be regulated by locally produced corticotropin releasing hormone (CRH), adrenocorticotropic hormone (ACTH) or cytokines. Furthermore the production of glucocorticoids is affected by ultraviolet B radiation. The level of production and nature of the final steroid products are dependent on the cell type or cutaneous compartment, e.g., epidermis, dermis, adnexal structures or adipose tissue. Locally produced glucocorticoids, androgens and estrogens affect functions of the epidermis and adnexal structures as well as local immune activity. Malfunction of these steroidogenic activities can lead to inflammatory disorders or autoimmune diseases. The cutaneous steroidogenic system can also have systemic effects, which are emphasized by significant skin contribution to circulating androgens and/or estrogens. Furthermore, local activity of CYP11A1 can produce novel 7 -steroids and secosteroids that are biologically active. Therefore, modulation of local steroidogenic activity may serve as a new therapeutic approach for treatment of inflammatory disorders, autoimmune processes or other skin disorders. In conclusion, the skin can be defined as an independent steroidogenic organ, whose activity can affect its functions and the development of local or systemic inflammatory or autoimmune diseases.
Keywords: Glucocorticoids, androgen, estrogen, CYP11A1, skin endocrine system, skin immune system
1. Skin as an immune organ
1.1. An overview of immune barrier functions of the skin
The primary function of the skin is to protect the body against the external environment, and a hierarchy of systems has developed to fulfill this function (reviewed in [1, 2]). One of the most important is the skin immune system, which is artificially divided into “innate” and “adaptive”, based on the specificity of the offending agent and presence or absence of memory in the system [3] (Fig. 1). Nevertheless, the components of this system interact with one another, are not truly separable and also interact with the cutaneous neuro-endocrine-immune system (reviewed in [1]). Various mediators participate in interactions between these elements (reviewed in [4, 5]) (Fig. 1), and will be discussed further.
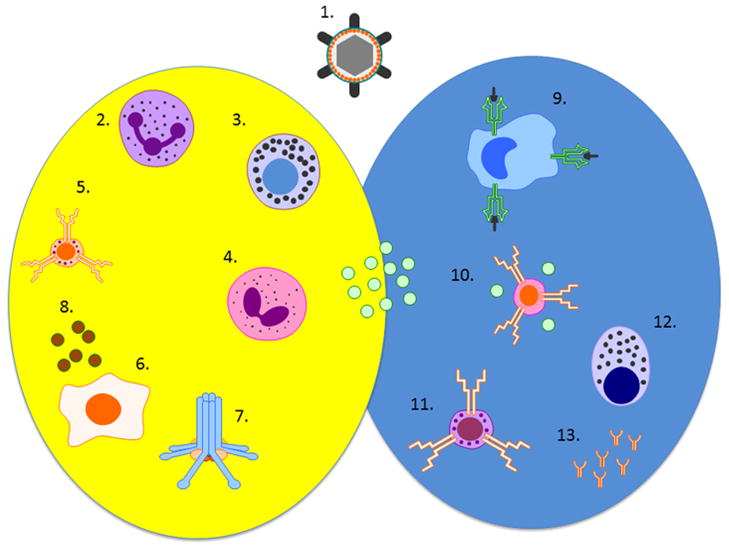
Figure 1. Elements of innate and adaptive immune system.
Yellow circle: innate part; blue circle: adaptive part; green dots: cytokines; 1. Microbe; 2. Neutrophil; 3. Mast cell; 4. Eosinophil; 5. NK cell; 6. Keratinocyte; 7. Complement; 8. Antimicrobial peptides; 9. Langerhans cell; 10. T helper 1/2/17 lymphocyte; 11. Cytotoxic lymphocyte; 12. Plasma cell; 13. Antibodies.
1.2. Skin innate immunity
The epidermis is composed of proliferating keratinocytes that differentiate to form the corneal layer. Corneocytes together with intercellular lipids isolate the body from the external environment and also protect it against dehydration [6]. A crucial role in the formation of this layer is played by the lipids and cholesterol derivatives which are synthesized locally from acetate [7, 8]. This structural barrier is complemented by components of innate immunity which are based primarily in the dermis [4]. Neutrophils and macrophages phagocytize non-self cells and organisms and kill them by oxygen-dependent or independent mechanisms. Eosinophils release major basic protein and other substances that inactivate parasites [9]. Mast cells release mediators that enhance local inflammation. Natural killer cells kill abnormal (infected or malignant) cells through antibody-dependent, MHC I-regulated and killer-activating and killer-inhibitory receptors-regulated pathways. The complement cascade kills microbes directly (through the membrane-attack complex), enhances phagocytosis (through binding to antibody-antigen complexes) and inflammation (through anaphylatoxins). Last but not least, keratinocytes and sebocytes produce beta-defensins and other antimicrobial peptides [10, 11]. Toll-like receptors are responsible for the recognition of pathogen-associated molecular patterns (PAMPs) and thus form a basis for the differentiation between self- and non-self in innate immunity [4, 12].
1.3. Skin adaptive immunity
The adaptive immune system is considered to be the more evolved part of the immune response system. Foreign antigens are presented by Langerhans/dendritic cells to T lymphocytes that in turn drive cellular or humoral responses [13]. T cell receptors and B cell/plasma cell-produced antibodies are responsible for specificity of this mode of immune response [4]. Langerhans cells are derived from the bone marrow and express CD (cluster of differentiation) 45, CD1a and CLA (cutaneous lymphocyte-associated antigen) [13]. Foreign antigens are presented in the context of MHC (major histocompatibility complex) II to CD4 positive lymphocytes and in the context of MHC I to CD8 positive lymphocytes. Variability of T cell receptors and antibodies is a function of several mechanisms that recombine and modify genes that code for variable regions [14]. The Th (T helper) 1 lymphocytes response is mediated by cytokines such as interleukin (IL)-2 and interferon (INF)γ. The humoral response is influenced by Th2 lymphocytes in part via the production of IL-4. Abnormal Th1 and Th2 responses play a role in various skin diseases [15]. Th2 lymphocytes additionally stimulate eosinophils (IL-5). CD4, CD25 and Foxp3 positive regulatory T lymphocytes suppress the immune response [3, 4]. CD4 positive Th17 lymphocytes express RORC (retinoic acid receptor -related orphan receptor C), develop in response to inflammatory cytokines, including IL-23, and release mediators such as IL-17, IL-21, IL-22, GM-CSF (granulocyte-macrophage colony stimulating factor) and CCL20 [chemokine (C-C motif) ligand 20] [16]. Several classes of antibodies are produced. Interaction between CD40 and CD40 ligand is responsible for the Ig (immunoglobulin)M to IgG isotype switch.
1.4. Physiological role of the skin immune system
The above components of the skin immune system are linked in large part by cytokines which serve as mediators connecting different components of innate and adaptive systems within those systems and between them. IL-1α and β, IL-6 and TNF (tumor necrosis factor)α are pro-inflammatory cytokines. INFα and β suppress viral infections. IL-10 inhibits cellular immunity. Transforming growth factor β (TGFβ) suppresses both Th1 and Th2 modes of immune response [17]. IL-12 facilitates Th1 cellular response. These cytokines have local and systemic effects. Moreover, an adaptive response that is initiated in the skin within Langerhans cells are fully matured in lymph nodes [13]. Lymphocytes, act in the skin in a retrograde manner but also affect other areas of the skin and the body [4]. The skin immune system is integrated into the skin neuro-endocrine system [2] through interaction with multiple pro- and anti-inflammatory neuropeptides, cytokines and hormones [1].
2. Steroidogenesis in the skin
2.1. An overview of steroidogenesis in classical steroidogenic organs
Maintenance of normal reproductive function and bodily homeostasis is dependent on steroid hormones synthesized in steroidogenic cells of the adrenal, ovary, testis, placenta, and brain. Steroid hormone biosynthesis is initiated upon mobilization of cholesterol, the substrate for steroid hormones, from cellular stores in cytoplasmic lipid granules to the mitochondrial inner membrane, the site of cytochrome P450scc (CYP11A1). This enzyme catalyses the cleavage of the side chain of cholesterol [18, 19]. In this reaction cholesterol is sequentially hydroxylated at carbons 22 and 20 producing 20R,22R-dihydroxycholesterol as a reaction intermediate [20]. CYP11A1 then catalyses the cleavage of the C20-C22 bond producing pregnenolone and isocaproic aldehyde. The concentration of cholesterol in the inner mitochondrial membrane of steroidogenic tissues is low [21], thus CYP11A1 normally works under subsaturating (limiting) cholesterol concentrations. Increased provision of cholesterol to the inner mitochondrial membrane by the action of the steroidogenic acute regulatory (StAR) protein causes a corresponding increase in the rate of pregnenolone synthesis [22, 23]. The fate of pregnenolone produced by CYP11A1 varies depending on the particular cell type or tissue, each of which contains a particular set of steroidogenic enzymes.
The biosynthesis of steroid hormones is regulated through the action of trophic hormones, ACTH (or angiotensin II for the zona glomerulosa) acting on the adrenal cortex and LH on the corpus luteum or the Leydig cells of the testis. These hormones bind to their specific receptors, which in turn activate the cAMP/PKA signaling cascade, and results in the phosphorylation of protein(s) involved in steroidogenesis [24, 25]. Hormonal regulation of steroid biosynthesis occurs within minutes (acute response) and hours (chronic response) and is mediated by cAMP/PKA signaling. The mobilization and delivery of cholesterol from the outer to the inner mitochondrial membrane initiates the acute response of steroid biosynthesis to hormonal stimulation that has an absolute requirement for de novo synthesis of the StAR protein [18, 19, 26]. On the other hand, chronic effects, associated with long-term steroid production, involve increased transcription/translation of the genes encoding steroidogenic enzymes [19].
The 30-kDa mitochondrial StAR protein was first purified from MA-10 mouse Leydig tumor cells, and its cDNA was cloned and sequenced [27]. There is now a wealth of information indicating that the StAR protein mediates the rate-limiting and regulated step in steroid biosynthesis, i.e. the delivery of cholesterol from the outer to the inner mitochondrial membrane [28–30]. Regulation of the StAR protein and thus steroid biosynthesis, is predominantly mediated by cAMP/PKA signaling in steroidogenic tissues, although several intracellular events have been demonstrated to be instrumental in this process [30–33]. Studies have demonstrated that a tight correlation exists between the synthesis of the StAR protein and the synthesis of steroids through endocrine, autocrine and paracrine regulation (reviewed in [28, 29, 33]).
Following the StAR-mediated delivery of cholesterol to CYP11A1 in the inner mitochodrial membrane and its conversion to pregnenolone, subsequent pathways are tissue-specific and involve a number of cytochrome P450 family members plus several different steroid dehydrogenases. Unlike the P450 enzymes, there are multiple isozymes for the steroid dehydrogenases catalysing the same reaction, and often varying in the preferred direction of the reaction they catalyse in vivo [23]. CYP17A1 plays a pivotal role in steroidogenesis because it is required for the synthesis of cortisol where its 17α-hydroxylase activity is essential, and androgens where both its 17α-hydroxylase and C17-C20 lyase activities are required. High lyase activity is seen when the initial substrate is pregnenolone, but with progesterone essentially only 17α-hydroxylase activity is observed, directing products to the glucocorticoid pathway [23, 34].
The major enzymes in the different classical steroidogenic tissues are listed in Table 1. Thus the glomerulosa zone of the adrenal cortex produces aldosterone, and the zona fasciculata produces cortisol. In the zona reticularis, which is deficient in 3βHSD, the pregnenolone produced by CYP11A1 undergoes complete removal of the remaining two carbons of the side chain by the 17-hydroxylase and C17-C20 lyase activities of CYP17A1, producing DHEA, much of which is sulfated [22, 35]. Cytochrome b5, which is highly expressed in the zona reticularis promotes the lyase activity of CYP17A1.
Table 1.
Expression of CYP enzymes involved in steroid biosynthesis and the major steroid products in classical steroidogenic tissues.
| Organ/tissue | CYP enzymes | Other Steroidogenic proteins | Major Steroid products |
|---|---|---|---|
| Adrenal cortex, zona glomerulosa | 11A1, 17A1, 21A2, 11B2 | StAR protein, 3βHSD2 | aldosterone |
| Adrenal cortex, zona fasciculata | 11A1, 17A1, 21A2, 11B1 | StAR protein, 3βHSD2 | cortisol |
| Adrenal cortex, zona reticularis | 11A1, 17A1, 11B1 | StAR protein, cytochrome b5, sulfotransferase | DHEA-S |
| Ovary, corpus luteum | 11A1, 17A1, 19A1 | StAR protein, 3βHSD2, | progesterone, |
| Ovary, follicle, granulosa cells | 11A1, 19A1 | StAR protein, 17βHSD1 | estradiol |
| Ovary, follicle, thecal cells | 17A1 | 3βHSD2 | androstendione (converted to estrogen in granulosa) |
| Testis, leydig cells | 11A1, 17A1, | StAR protein, 3βHSD2, 17βHSD3 | testosterone |
| Placenta, syncytiotrophoblasts | 11A1, 19A1 | MLN64, 3βHSD1, 17βHSD1, steroid sulfatase | progesterone, estradiol, estrone, estriol |
See [23] for further details of steroidogenesis in the tissues listed.
The corpus luteum produces large amounts of progesterone plus some estradiol [22]. In the ovarian follicle, pregnenolone is produced by the granulosa cells which lack CYP17A1 and cannot convert it to androgens. Conversion to androstendione occurs in the in the thecal cells which do express the required CYP17A1. The androstenedione diffuses back into the granulosa cells for conversion to estrone and estradiol by the aromatase enzyme, CYP19A1, and 17βHSD1 [22, 35]. In the Leydig cells of the testis pregnenolone is converted primarily to testosterone with only minor conversion to estrogens due to only low expression of CYP19A1 [23, 34].
The placenta displays some differences to the other steroidogenic tissues in that pregnenolone synthesis it is not limited by cholesterol availability, but rather by the level of steroidogenic electron transport protein adrenodoxin reductase [20]. The placenta does not express the StAR protein but a related START-domain protein, MLN64, appears to play a role in cholesterol transport o the inner mitochondrial membrane [20, 30]. In the placenta, all pregnenolone is converted to progesterone and cannot be converted to estrogens due to the lack of expression of the CYP17A1 necessary for its initial conversion to androgens. The large amounts of estrogens produced by the placenta are derived from the actions of 3βHSD and CYP19A1 on DHEA derived from DHEA sulfate, produced by the fetal adrenal gland [23].
2.2. Extra-adrenal and extra gonadal steroidogenesis
Several tissues besides the adrenal cortex, gonads and placenta express CYP11A1 and therefore can be considered steroidogenic, with pathways that commence form cholesterol. These tissues and their major products are listed in Table 2. The products most likely play an autocrine or paracrine regulatory role in these tissues [36]. It is noteworthy that steroidogenesis in non-classical tissues is quite modest regardless of the stimulant, usually being less than 1% of that seen with cAMP/PKA mediated StAR expression and steroid synthesis in adrenal and gonadal cells. While the magnitude of StAR-mediated response on steroidogenesis is small, it could be very important in local regulation of steroidogenesis in certain non-classical tissues. One of the first non-classical tissues identified to make steroids from cholesterol is the mammalian brain [23, 37]. Steroids produced include pregnenolone, pregnenolone sulfate, DHEA sulfate, progesterone, 3β and 5α reduced derivatives of progesterone, and corticosteroids [23, 36, 38]. Expression of CYP11A1 and its functional activity in the mouse thymus with the production of corticosterone has also been known for some time, but data for the human is lacking [36, 39]. CYP11A1 is expressed in bone but the predominant form has an N-terminal truncation that is only 30 kDa in size and has a non-mitochondrial localization, and is therefore unlikely to be catalytically active [40]. The expression of CYP11A1, 3βHSD and CYP17A1, as well as the steroidogenic capability of benign and malignant prostate and prostate carcinoma lines, as well as prostate stroma, has been demonstrated [41–47]. CYP11A1 expression has been detected at the mRNA level in both breast tumors and surrounding normal breast tissue [48]. The human gut and colon cancer cells also express glucocorticosteroidogenic activity with production of cortisol and corticosterone and expression of CYP11A1, CYP17A1 and CYP11B1 [49–51]. Other organs which display local steroidogenic pathways in humans are the heart, skin and cells of the immune system (see Table 2) including human basophils [52]. Table 3 shows the expression of steroidogenic genes in different cancers, illustrating their likely capacity to synthesize and/or metabolize steroid hormones as has already been shown with melanoma cells [53, 54].
Table 2.
Extra-adrenal and extra-adrenal steroidogenesis in humans.
| Tissue | CYP Enzymes | Other Steroidogenic proteins | Major Products from cholesterol | References |
|---|---|---|---|---|
| Brain | 11A1, 17A1, 11B1, 11B2, 2D6 (21-hydroxylase) | StAR, 3βHSD, sulfotransferase, 5α-reductase 3αHSD | pregnenolone sulfate, DHEA-S, corticosteroids | [23, 36, 38] |
| Gut | 11A1, 17A1, 21A2, 11B1 | 3βHSD | cortisol | [36, 49] |
| Heart | 11A1, 21A2, 11B1,11B2 | StAR, 3βHSD | aldosterone | [36] |
| Mammary Gland | 11A1, 19A1 | Unknown | [36, 48] | |
| Prostate (including tumors) | 11A1, 17A1 | StAR, MLN64, 3βHSD, 17βHSD, 11βHSD, 5α-reductase, cytochrome b5 | progesterone, androgens | [41–47]. |
| Skin | 11A1, 17A1, 21A2, 11B1, 19A1 | StAR, MLN64, adrenodoxin, adrenodoxin reductase, 3βHSD1, 17βHSD, 11βHSD | glucocorticoids, androgens, estrogens | [65, 69, 73, 151, 255] Also see Sections 2.3 and 2.4. |
| Thymus* (mouse) | 11A1, 11B1, 21A2 | StAR, 3βHSD | corticosterone | [36, 39] |
Steroidogenesis in thymus is for mouse species.
Table 3.
Relative expression of genes coding steroidogenic enzymes in various cancers.
| Cell Line | 7DHCR | CYP11A1 | 3βHSD | CYP21A2 | CYP11B1 | CYP17A1 | HSD11B1 |
|---|---|---|---|---|---|---|---|
| Osteosarcoma (MG63) | 14.14±0.26 | 10.07±0.01 | 21.20±0.19 | 22.38±0.01 | 20.16±0.38 | 7.16±0.26 | 10.18±0.04 |
| Glioblastoma (U87) | 9.44±0.37 | 15.57±0.05 | 16.7±0.17 | 22.1±0.02 | 22.1±0.02 | 6.57±0.03 | 16±0.04 |
| Breast carcinoma (MCF7) | 8.05±0.32 | 20.59±0.26 | 20.74±0.03 | 20.74±0.03 | 20.74±0.03 | 14.34±0.03 | 11.17±1.76 |
| Breast carcinoma (MDA-MB 231) | 13.6±0.06 | 20.59±0.26 | 18.33±0.18 | 0.47±0.48 | 18.19±0.3 | 18.57±0.4 | 16.74±0.13 |
| Breast carcinoma (MDA-MB 453) | 9.57±0.23 | 14.80±0.11 | 21.88±0.1 | 10.24±10.08 | 20.22±0.32 | 6.61±0.36 | 17.77±0.14 |
| Melanoma (WM164) | 16.36±0.08 | 13.18±0.04 | 18.34±0.07 | 9.28±0.48 | 21.6±0.18 | 19.49±0.12 | 10.51±0.06 |
| Melanoma (WM1341) | 11.44±0.26 | 19.84±0.17 | 17.25±0.08 | 8.7±0.06 | 21.9±0.34 | 7.68±0.02 | 22.81±0.49 |
| Melanoma (WM98D) | 15.93±0.19 | 13.18±0.04 | 15.93±0.19 | −19.07±0.0 | 15.93±0.19 | 15.93±0.19 | 13.84±0.22 |
| Melanoma (YUROB ) | 17.83±0.98 | 19.84±0.17 | 21.41±0.51 | −13.290.0 | 19.33±0.41 | 21.71±0.04 | 18.08±0.21 |
| Melanoma (YULAC) | 0.03±0.0 | 20.21±0.48 | 23.36±0.33 | −11.64±0.0 | 22.14±0.49 | 22.87±0.54 | 23.16±0.48 |
| Oral squamous cell carcinoma (SCC15) | 10.26±0.0 | 11.03±0.0 | 21.35±0.0 | 22.42±0.0 | 21.84±0.0 | 15.1±0.0 | 15.1±0.0 |
| Oral squamous cell carcinoma (SCC25) | 9.88±0.41 | 10.07±0.01 | 17.49±0.34 | 5.47±0.37 | 17.19±0.46 | 18±0.46 | 12.92±0.38 |
| Promyelocytic leukemia (HL-60) | 8.42±0.34 | 19.64±0.13 | 18.64±0.31 | 21.54±0.03 | 19.07±0.35 | 12.99±0.04 | 21.54±0.03 |
| Myelogenuous leukemia (K562) | 17.91±0.26 | 14.80±0.11 | 21.02±0.13 | 15.97±0.16 | 21.02±0.13 | 18.18±0.31 | 19.86±0.19 |
| Promonocytic leukemia (U937) | 14.09±0.23 | 11.03±0.0 | 19.07±0.08 | −15.93±0.0 | 17.30±0.22 | 14.07±0.09 | 18.58±0.43 |
| T-cell leukemia (Jurkat) | 13.44±0.32 | 19.64±0.13 | 19.41±0.16 | 8.18±0.29 | 20.90±0.14 | 15.03±0.15 | 20.75±0.42 |
| Embryonic kidney (HEK293) | 11.55±0.33 | 15.57±0.05 | 16.03±0.1 | 21.26±0.06 | 19.28±0.09 | 15.17±0.08 | 21.26±0.06 |
Lower the number the higher is gene expression level. Detailed methodology is in Supplementary Methods.
2.3. Glucocortico-steroidogenesis in the skin
Since the first demonstration that human skin expresses crucial genes of glucocorticosteroidogenesis including CYP11A1, CYP17, CYP21A2, CYP11B1 and MC2 [55], a series of sequential investigations have shown that the skin or skin cells can produce deoxycorticosterone (DOC), 18(OH)DOC, corticosterone and cortisol in situ [53, 56–61]. These findings have been firmly confirmed by others [62–69]. Furthermore, skin cells express functionally active CYP11A1 (there are also alternatively spliced isoforms of this enzyme in skin cells [54]), StAR and MLN64, and have the capability of starting the steroidogenic pathway de novo from cholesterol [54, 70]. They also express 3βHSD [71, 72] allowing the pathway to proceed with final production of glucocorticoids [73] and sex hormones [74]. Thus, evidence has accumulated classifying skin as an additional extra-adrenal organ with an endogenous steroidogenic capacity that is integrated into the regulatory networks of the cutaneous neuro-endocrine-immune system(s) [1]. The scheme and compartmental distribution of different elements of this steroidogenic pathway, which can start from cholesterol are presented in Figures 2 and 3. The synthesis and metabolism of cholesterol in the skin with phenotypic their phenotypic consequences are the subject of separate reviews [6, 75–77]
Figure 2. Cutaneous steroidogenic pathways.
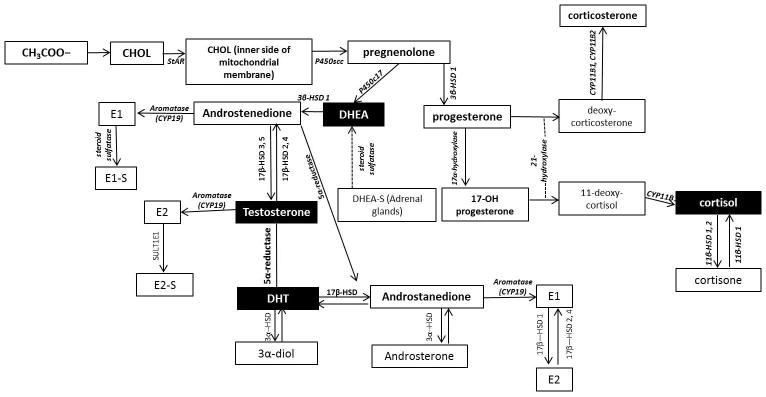
DHEA: dehydroepiandrosterone, StAR: steroidogenic acute regulatory protein, CYP11A1 or P450scc: cytochrome P450 side-chain cleavage enzyme, CYP17A1 or P450c17: cytochrome P450 17α-hydroxylase/17,20-lyase, 3β-HSD: 3β-hydroxysteroid dehydrogenase, DHEAS: dehydroepiandrosterone sulfate, DHT: dihydrotestosterone, 3α-HSD: 3α-hydroxysteroid dehydrogenase, SF-1: steroidogenic factor 1, E2: estradiol, SULT1E1: estradiol sulfotransferase, E1: Estrone, 5αR: 5α-reductase, HSD11B1 or 11-HSD1: 11-hydroxysteroiddehydrogenase type 1, HSD11B2 or 11-HSD2: 11-hydroxysteroiddehydrogenase type 2, CYP21: 21-hydroxylase, CYP11B1: 11-hydroxylase type 1, CYP11B2: 11-hydroxylase type 2
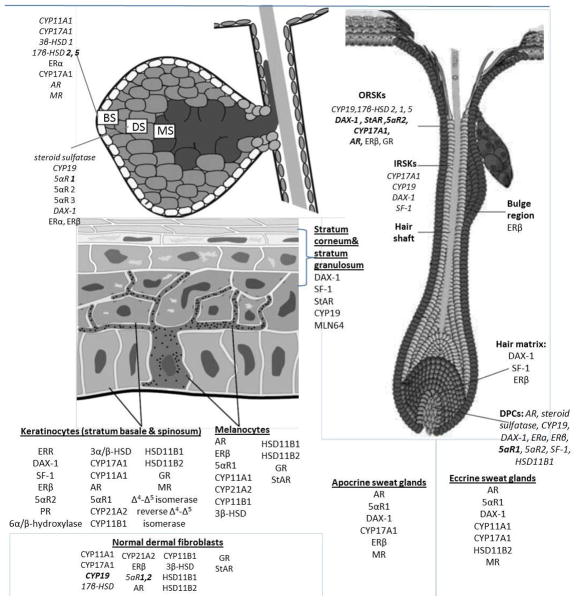
Figure 3. Compartmental expression of enzymes and co-factors involved in cutaneous steroidogenesis.
DPCs: Dermal Papilla Cells, ORSKs: outer root sheath keratinocytes, IRSKS: Inner root sheath keratinocytes, BS: Basal Sebocytes, DS: differentiating sebocytes, MS: mature sebocytes, AR: androgen receptor, HF: hair follicle, SF-1: steroidogenic factor 1, SREBP-1: sterol response binding protein-1, DAX-1:dosage-sensitive sex-reversal-adrenal hypoplasia congenital critical region on the X-chromosome, gene 1, ERR: estrogen-related receptor, ERα/β: estrogen receptor α or β, PR: progesterone receptor, GR: glucocorticoid receptor, MR: mineralocorticoid receptor,
It is noteworthy, that steroidogenesis in non-classical tissues is quite modest regardless of the stimulant, and in skin cells the rate of cholesterol conversion to pregnenolone represents 1% of that seen in the placenta [54]. This low rate of production could explain the lack of detection of cortisol formation by keratinocytes in earlier studies by Milevich et al. [78] and later by us [79]. In our studies on HaCaT keratinocytes, we have detected rapid metabolism of progesterone to DOC with further transformation to several products different from corticosterone, aldosterone and cortisol, some of which were identified by GC/MS as 3β,6α,21-trihydroxy-5α-pregnan-20-one, 3α,6α,21-trihydroxy-α-pregnan-20-one, and 3α,5α-and 3β,5α-tetrahydrodeoxycorticosterone [80]. Minor metabolites were 3α, 21-dihydroxy-5-pregnen-20-one, 3β, 21-dihydroxy-5-pregnen-20-one, 3α, 21-dihydroxy-4-pregnen-20-one, 6-hydroxy-dihydrodeoxycorticosterone, and two 5-dihydrodeoxycorticosterone species. These studies not only confirmed keratinocytic expression of 5α-reductase and 3α/βHSD but also demonstrated expression of 6α-hydroxylase, and reverse Δ4/Δ5 isomerase enzymes [80]. Other researchers using RIA or ELISA have detected cortisol in follicular [59, 68] or epidermal keratinocytes [63–65]. We have also detected cortisol as well as corticosterone production in epidermal melanocytes and dermal fibroblasts (chemical structure was confirmed by LC/MS) [57, 58, 60]. This latter finding substantiates our previous demonstration of rapid and robust transformation of progesterone and DOC into corticosterone in human malignant melanocytes [53]. So far, production of aldosterone has not been detected in epidermal and dermal cells.
Skin expresses 11βHSD1 which is primarily involved in the reduction of the 11-keto group to the alcohol, such as in the activation of cortisone to cortisol [23, 54, 66, 81]. Skin also expresses 11βHSD2 which works in the oxidative direction, converting cortisol to the inactive cortisone [23, 64–66, 81]. The type 2 enzyme plays a key role in mineralocorticoid tissues where it protects the mineralocorticoid receptor from cortisol [23]. Both 11βHSD1 and 11βHSD2 have been detected at the protein level in cultured keratinocytes [64, 81]. Keratinocytes with the type 1 isoenzyme silenced with siRNA produced less cortisol from cortisone than control cells. Conversely, the silencing of 11βHSD2 caused an elevation in cortisol levels [64]. It would appear that both the location and relative activities of these two isoenzymes determines the ability of skin to activate/inactivate both locally produced and pharmacologically administered glucocorticoids [64, 66, 81]. In the mouse skin, 11βHSD1 was found by immunohistochemical staining in keratinocytes, dermal fibroblasts and the outer root sheath of hair follicles, and appears to have a similar distribution in humans [66]. In contrast, 11βHSD2 was not detected in these sites by immunohistochemistry, so that the predominant activity was activation of cortisone to cortisol. Cortisol treatment of human dermal fibroblasts increased 11βHSD1 mRNA expression at the mRNA level, and decreased 11βHSD2 expression, providing a positive feedback loop enhancing glucocorticoid activation [66]. The mechanism for this increase in 11βHSD1 remains to be elucidated. In contrast, dexamethasone treatment of human skin was reported to increase 11βHSD2 expression at the mRNA level [65].
In the liver cortisone is mainly converted to cortisol by 11βHSD1 which is then acted on by 5α- or 5β- reductase and 3αHSD producing 5α- and 5β-tetrahydrocortisol, some of which is metabolised by 20αHSD or 20βHSD to α-cortol or β-cortol, respectively. Similar minor pathways are observed for cortisone without the initial action of 11βHSD1. The reduced products are excreted in the urine, predominantly as glucuronides [82, 83]. There is good evidence that these metabolic pathways may occur in skin. As mentioned above, 5-dihydroxycorticosterone and 3α,5α-tetrahydroxycorticosterone are produced by HaCaT keratinocytes incubated with progesterone, along with 5α-reduced pregnanes, indicating that both 3αHDS and 5α-reductase are present in these cells [80] that could potentially act on cortisol or cortisone.
The expression of StAR has been detected by RT-PCR and immunohistochemical analyses in epidermal keratinocytes, sebocytes, outer root sheath of hair follicles (HFs), vascular tissues and eccrine ducts [62, 63, 69, 84]. In addition to StAR, epidermal keratinocytes also express cholesterol transporters TSPO and MLN64 [54, 63]. Expression of positive [adrenal 4-binding protein/steroidogenic factor 1 (SF-1)] and negative (dosage-sensitive sex reversal, adrenal hypoplasia congenital, critical region on the X chromosome, gene 1) regulators of StAR has also been detected in the epidermis [63, 85–87]. DAX-1 prominent expression has been confirmed in sebaceous and sweat glands, the basal layer of the epidermis and ORSKs, while SF-1 immunoreactivity was detected across all epidermal layers except of the stratum corneum, IRSKs, matrix cells and DPCs [85, 88]. The co-factor, WT-1, modulates androgen sex steroid production by up-regulating DAX-1 and is also present in the skin [89]. It has been reported that the coiled-coil α-helical rod (CCHCR1) protein is involved in epidermal steroidogenesis and that CCHCR1 colocalizes with the StAR protein [62]. Upregulation of CCHCR1, associated with epidermal growth factor receptor expression, has been demonstrated in skin cancer [90]. We observed recently that expression of StAR mRNA was found to be aberrant in several skin diseases, including eczema, intertrigo, and seborrheic keratosis, suggesting acute steroid synthesis is disrupted in these diseased conditions (Manna et al., data not shown). In accordance with the above findings HaCaT cells treated with a cAMP analog, (Bu)2cAMP, significantly elevated StAR mRNA expression and pregnenolone synthesis over unstimulated cells, respectively (Fig. 4). However, levels of StAR mRNA and steroid synthesis in response to cAMP signaling were substantially lower in HaCaT cells when compared to responses for adrenal and gonadal cells. This suggests a slower onset of StAR localization to mitochondria and lower rates of steroid synthesis in epidermal keratinocytes. Moreover, in contrast to the rapid induction of cAMP-responsive steroidogenesis in various endocrine tissues, in HaCaT cells elevations in StAR and pregnenolone levels require a long period of time. These results may suggest differences between the classical steroidogenic organs and the cutaneous system.
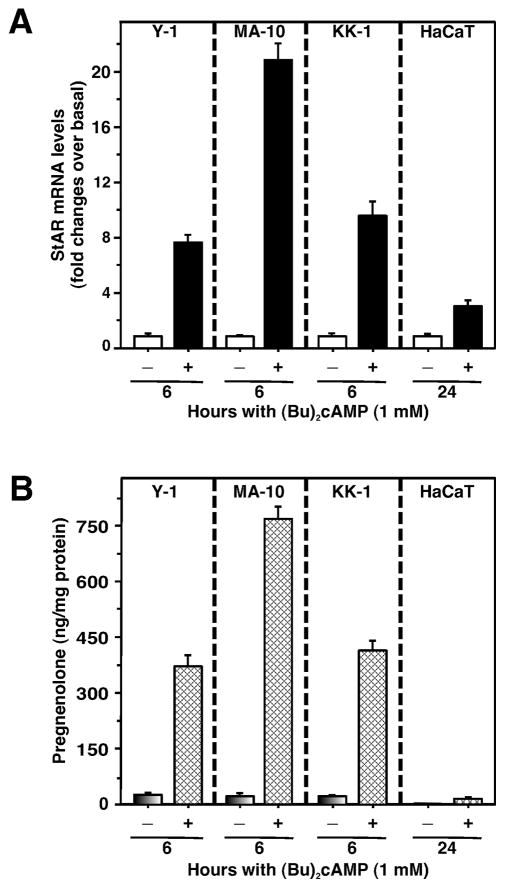
Figure 4. (Bu)2cAMP stimulates StAR gene expression and pregnenolone production.
Detailed methodology is in Supplementary Methods. Briefly, mouse adrenocortical (Y-1), Leydig turmor (MA-10), granulosa (KK-1), and human keratinocyte (HaCaT) cells were treated without or with (Bu)2cAMP (1.0 mM) as indicated, in the presence of SU-10603 (20 μM) and cyanoketone (5 μM). StAR mRNA expression (A) was measured by quantitative real-time PCR, while accumulation of pregnenolone in conditioned media (B) was determined by RIA. Gene expression is shown as fold changes in StAR mRNA levels relative to untreated cells, while levels of pregnenolone are expressed as ng/mg protein. Results represent means ± SE of four independent experiments.
2.4 Synthesis of androgens and estrogens in the skin
Plasma dehydroepiandrosterone sulfate (DHEA-S) and lesser free DHEA originate from the adrenal glands, while androstenedione is produced by the adrenal cortex and ovaries, and less by the testes [74]. Testosterone (T) is mainly secreted by the testes in males with the onset at puberty and in premenopausal females from the ovaries and the adrenal cortex. The most potent androgen, dehydrotestosterone (DHT), is mainly synthesized in peripheral tissues, including skin [74]. Since it cannot be further aromatized to estrogens, its action remains exclusively androgenic. The pilosebaceous unit has all the necessary tools to utilize sex steroid precursors for the transformation to more potent sex hormones [91, 92].
Cutaneous formation of DHEA, the main substrate for the more potent androgens T and DHT, proceeds both from endogenous cutaneous cholesterol (see section 2.3) and from DHEA-S of adrenal origin. In support of the latter, T synthesis by SZ95 sebocytes in vitro derives mainly from DHEA [93]. DHEA-S is hydrolysed in the skin to DHEA by steroid sulfatase, detected in sebaceous glands and dermal papilla cells (DPCs) of terminal HFs [94, 95]. Monocytes also exhibit steroid sulfatase activity, thus introducing the variable of “inflammation” to cutaneous androgen production [96].
Addition of pregnenolone, progesterone and 17α-hydroxyprogesterone led to a significant rise of T levels in culture media, reflecting the activity of 3βHSD1 and 17βHSD3 on SZ95 sebocytes [93]. 3βHSD converts DHEA to androstenedione, which is converted in a further step to T by the enzyme 17βHSD. Human skin expresses predominantly the 3βHSD1 isoform [97]. Interestingly, 5 isozymes of 17βHSD were identified, functioning like a “switch on-off” mechanism for the production of more potent sex steroids: Isozymes 3 and 5 catalyze the formation of T from androstenedione, in contrast to isozymes 2 and 4, which oxidize the inactivation of T to its weaker precursor [98–101]. Synthesis of T from androstenedione in skin, similar to other peripheral tissues, is catalyzed by 17βHSD type 5 [102]. 17βHSD is also detected in ORSKs of HFs, mainly type 2 and moderately type 1 in anagen ones, thus inactivating potent androgens [98, 103].
5α-reductase (5αR) type 1 is the predominant isoform detected in the skin [104, 105] and more abundantly expressed in sebaceous and sweat glands [106], keratinocytes [78, 80] and dermal fibroblasts [107]. 5αR2 is detected in genital skin fibroblasts and IRSKs [107]. The 5αR1 inhibitor MK386 blocked completely the conversion of T to DHT in SZ95 sebocytes and HaCaT keratinocytes and reduced T-induced proliferation of sebocytes [108]. The newly found 5αR3 has been detected in prostate cancer and the SZ95 sebocyte line [109]. Sebocytes normally metabolize T mainly to androstenedione, rather than DHT, since low DHT levels are required for skin homeostasis. 5aR2 mRNA levels were minimal in beard and scalp DPCs as well as normal human fibroblasts, while beard DPCs were the ones mainly expressing 5αR1 at the protein level. The formation of DHT in SZ95 sebocytes does not always require T as an intermediate, since 5α-reduction of androstenedione to 5α-androstanedione and subsequent conversion to DHT by 17βHSD provides an alternative pathway [110]. Moreover, the 3αHSD isozymes convert potent androgens to inactive compounds, which do not bind to AR [74, 111]. 3αHSD is strongly expressed in epidermal keratinocytes [80, 91, 112].
E2 derives from T and E1 from androstenedione. Local estrogen synthesis was correlated with aromatase mRNA levels and cutaneous elastic fiber content [69]. mRNA transcripts of the CYP19 gene were reported in dexamethasone-induced human fibroblasts in vitro [113], while aromatase was expressed in anagen and terminal HFs, cultured keratinocytes, melanocytes, sebaceous glands and adipose fibroblasts [114]. Interestingly, 17α-estradiol increases aromatase activity of female HFs [115]. Estrogens are inactivated through sulfation, by the enzyme estradiol sulfotransferase (SULT1E1) [116]. Its activity is higher in differentiated normal human epidermal keratinocytes (NHEKs) in comparison to proliferating ones [117], suggesting a mechanism of attenuation of estradiol-induced keratinocyte proliferation. Aromatase in skin can serve to fine-tune the relative actions of androgens and estrogens in target cells [118].
Apart from the aforementioned enzymes involved in the formation of more or less potent androgens or estrogens, tissue-specific ones are responsible for the degradation and subsequent elimination of sex steroids, via their transformation to soluble metabolites, which can be excreted in the urine, bile or feces. Enzymes which play a key role in this procedure are CYP enzymes for the hydroxylation, sulfotransferases (SULT) for the sulfation and UDP glucuronosyl transferases (UGT) for the conjugation of sex steroids with glucuronic acid respectively [119–121]. The three UGT2B enzymes, UGTB7, UGTB15, UGTB17, which are responsible for the glucuronation of DHT and its metabolites, androsterone and 3α-diol are all expressed in the skin [122, 123]. E2 is inactivated by estrogen sulfotransferase SULT1E1 in normal human keratinocytes [117]. The SULT2B1 enzyme, member of the SULT2 family, which catalyzes the sulfation of 3β-hydroxysteroids, such as DHEA and pregnenolone, was also detected in the skin [124, 125]. The isoform SULTB1b is abundantly expressed in ORSKs, sebocytes and differentiated epidermal keratinocytes [125]. The SULT1 family consists of enzymes, which primarily sulfate phenolic groups of estrogens. Its member SULT1A1 are located on the outer sheath of rat HF [126]. Expression of the aldo-keto reductases (AKR) AKRC1 and AKRC2 in keratinocytes and fibroblasts leads to inactivation of progesterone and DHT respectively, which are further metabolized through glucuronosyl transferases or hydroxylases [127]. Skin shows immunohistochemical reactivity for the enzyme CYP7B1, which catalyzes the 7α-hydroxylation of DHEA. Moreover, it turns the DHT metabolites 5-androstene-3β,17β-diol (Aene-diol) and 5-androstane-3β,17β-diol (3-Adiol) in compounds with little or no estrogenic effect [128].
2.5. Regulators of local steroidogenic activity
Steroid production in the skin is controlled by several internal and external factors, and is dependent on local enzymatic activity, substrate availability, and mobilization of signal transduction and gene expression pathways.
The hypothalamic-pituitary-adrenal (HPA) axis
Since all regulatory elements of the hypothalamus-pituitary-adrenal gland (HPA) axis, including proopiomelanocortin (POMC)-derived peptides [129], CRH and related peptides as well as the corresponding functional receptors [130, 131] are expressed in mammalian skin, the concept that skin expresses a homologue of the HPA was introduced 15 years ago [2, 73, 132]. Since then, evidence has accumulated that the cutaneous stress system follows the functional hierarchy of the central HPA with its direct local phenotypic consequences and systemic implications [1, 73, 133]. Most recently, it was proposed that the algorithm of HPA first developed in the primordial integument and then was adopted by central neuroendocrine system [134]. In the skin, induction of steroidogenesis by CRH or ACTH, or factors raising intracellular cAMP levels appears to be cell type dependent because induced corticosterone and cortisol formation has been observed in normal and malignant epidermal melanocytes [53, 58] and dermal fibroblasts [57, 60], but not epidermal keratinocytes. On the other hand HF and follicular keratinocytes were found to produce cortisol after stimulation with CRH and ACTH [59, 68]. The production of steroids in the skin after stimulation by CRH is strongly dependent on the CRH-R1 receptor [57–59]. Another important receptor with a regulatory function on steroids synthesis is the glucocorticoid receptor (GR coded by NR3C1), a ligand activated transcription factor that belongs to the nuclear hormone receptor superfamily that regulates gene expression through DNA-binding–dependent and– independent mechanisms. The wide use of glucocorticoid analogs in clinical practice relies on their great efficacy as anti-inflammatory agents, mostly due to the antagonism between ligand-activated GR and the proinflammatory NF-κB, AP-1, and signal transducer and activator of transcription (STAT) signaling pathways in the skin [135]. Though GR is alternatively spliced and species-dependent differences in aminoacid sequences occur, there are two isoforms, GRα and GRβ. Increased ratios of GRβ/GRα have been shown to correlate with resistance to GCs, and constitutes a big challenge in dermatology[136]. Modern dermatology searches for novel synthetic GR ligands that should display a better therapeutic index than the known classical ones [137, 138].
Cytokines
Cytokines serve as communicators between immunological, endocrine and nervous systems [139]. While the vast majority of both pro- and anti-inflammatory cytokines are involved in cutaneous biology, recognizable effects on steroidogenesis were caused by IL-1β and TNFα [140]. IL-1β is a critical mediator of the adaptive stress response and stress associated psycho- and neuropathology [141]. This cytokine plays a fundamental role in the pathogenesis of many inflammatory and autoagressive disorders in the skin, and its expression is up-regulated by UVB [142]. Furthermore, IL-1β can activate receptors localized on sensory nerve endings, and via a reflex switched in DRG is responsible for release of neuroinflammatory substance P (SP) [143, 144]. IL-1β stimulates components of the HPA axis (c-fos expression in CRH-producing parvocellular neurons in the PVN) to enhance cortisol production [141]. Furthermore, IL-1β can directly stimulate human adrenocortical cells [140], and similar stimulation has been also observed in epidermal keratinocytes [65]. This finding may suggest a possible feedback loop that attenuates the initial proinflammatory responses, preventing excess inflammation that can lead to further tissue damage [65]. TNFα, apart from its well-characterized proinflammatory role, has various antiinflammatory properties; an example of the latter is stimulation of the HPA axis resulting in increased steroid production [145]. Suppressor of cytokine signaling (SOCS) acts as a potent negative regulator of cytokine signaling and suppresses cytokine-induced POMC expression and ACTH release [146].
11βHSD1 and 11βHSD2
Two key enzymes that regulate the local cortisol availability for the GR are 11βHSD1 and 11βHSD2, which are expressed in many peripheral organs [147]. Recent studies have demonstrated that intracellular conversion, performed by these two enzymes, together with GR activity, represent a key mechanism of tissue-specific regulation of GC action contributing to GC deficiency, which constitutes a big challenge in dermatology [64, 66, 81, 148, 149]. The equilibrium between 11βHSD1 and 11βHSD2 expression in the skin together with sympathetic nerves density can maintain immunological homeostasis [1, 64, 150].
Ultraviolet radiation
Ultraviolet radiation (UVR) represents the electromagnetic energy of solar radiation covering wavelengths between 100–400 nm. UVC (100–280nm), when applied to the skin, stimulates cortisol production with simultaneous GR down regulation and upregulation of CRH, POMC, ACTH, β-endorphin, CYP11A1 and 11βHSD1 [81, 151]. UVB (280–320 nm), a recognized stimulator of melanin pigmentation [152], has similar stimulatory effect on CRH and POMC signaling, production of cortisol and inhibition of the GR [81, 129, 131, 151, 153, 154]. UVA (320–400 nm) had no effect on cortisol, CRH and ACTH production but stimulated β-endorphin and 11βHSD2 expression [81, 151, 155].
The CRH signaling system is highly expressed in mammalian skin (epidermis and dermis), and its expression is up-regulated by UVR [1, 131]. UVB stimulated CREB phosphorylation and the binding of phosphorylated CREB to CRE sites in the CRH promoter [156]. Next, CRH interacts with CRH-R1 stimulating cAMP production with a subsequent increase in POMC gene expression and production of ACTH [57, 60]. Pharmacological inactivation of CRH-R1 by selective inhibitors abrogated the UVB-stimulated induction of POMC production [156]. ACTH stimulates the cutaneously distributed MC2R to start steroidogenesis leading to cortisol/corticosterone production. The paracrine communication in the skin modulated by internal and external stressors can include nerve fibers, keratinocytes, melanocytes, fibroblasts and immune cells to maintain cutaneous homeostasis [1].
2. 6. Non-classical steroidogenesis
A recent review by Shackleton [157] describes in an elegant manner alternative branches in steroidogenesis that are relevant to the skin. Firstly, the 7-Δ reductase deficiency observed in Smith–Lemli–Opitz syndrome (SLOS), in an addition to severe malformations, results in development of skin photosensitivity to UVA [158, 159] and production a series of unusual steroids [79, 160, 161]. 7DHC (7-dehydrocholesterol) can be metabolized by CYP11A1 through hydroxylation at C22 followed by C20, cleavage of the site chain and subsequent metabolism of the 7DHP (7dehydropregnenolone) by existing steroidogenic enzymes [54, 162, 163] (Fig. 5). Interestingly, all of the resulting 5–7 dienes are potential source of vitamin D analogues with short side chain when subjected to UVR [54, 164], while the hypersensitivity of skin in SLOS patients has recently been explained by reactive oxigen species dependent formation of 5, 7, 9(10)-trienes. It was shown that cholesta-5,7,9(11)-trien-3β-ol (9-DDHC) derivative of 7DHC is indeed formed in the skin subjected to UVA irradiation [158]. Such a compound was capable of generating singlet oxygen in the cycle reaction stimulated by UVR [158, 165]. Interestingly, we have recently shown that another 5,7-diene, pregna-5,7-diene-3β,17α,20-triol, when subjected to UVR may also be converted to a triene, namely pregna-5,7,9(11)-triene-3β,17α,20S-triol [166]. The accumulation of unusual steroidal 5,7-dienes in skin subjected to UV irradiation may generate new classes of secosteroids with modified side-chains [166, 167]. It has to be stressed that such reactions lead not only to formation of vitamin D analogues, but also to other tachysterol- and lumisterol-like derivatives. This photoconversion of 5–7-dienes was demonstrated not only in vitro [164, 166, 167] but also ex vivo in human skin [166]. In addition, mutation of any gene encoding enzymes involved in steroidogenesis in the skin may result in local formation of unusual steroids, as extensively reviewed recently [157]. Finally, expression of CYP11A1 in the skin is also responsible for the local generation of novel secosteroids with a full-length side chain [168].
Figure 5.
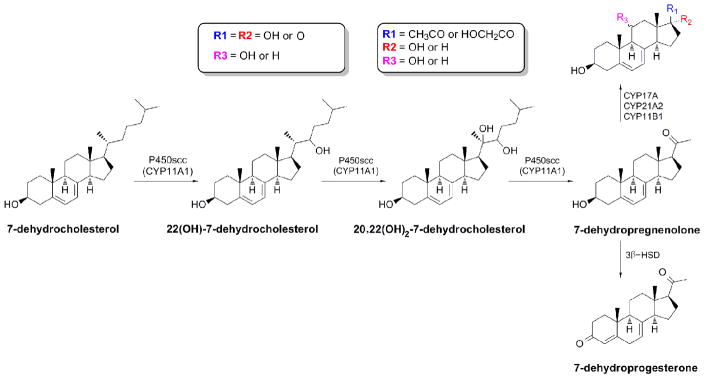
Production of 7-Δ-steroids in the skin.
3. Role of local steroidogenic pathways in inflammatory disorders
3.1. Role of local glucocosteroidogenic pathways in inflammatory disorders
Multiple components of innate and adaptive immune systems contribute to pathogenesis of different inflammatory disorders of the skin, including atopic dermatitis, psoriasis, acne vulgaris and alopecia areata. Although described mechanisms typically center on the role of various immune cells and in particular on cytokines, the role of glucocorticosteroids is also emerging. Of note, most treatments for these entities include various analogs of corticosteroids [4]. It has recently been proposed that inflammatory skin diseases may be driven by locally produced CRH that stimulates pro-inflammatory pathways that in turn are not inhibited by glucocorticosteroids [130, 169–172]. These proinflammatory activities can be counteracted by locally produced POMC derived peptides [73, 129, 173] and by locally produced glucocorticoids [1, 73]. Note, that IL-1 affects expression of steroidoigenic enzymes and production of cortisol in the skin [65].
Atopic dermatitis is characterized by extensive pruritus leading to extensive lichenification. Specifically, this is initially a Th-2 entity mediated by IL-4 and IL-5 that with time becomes driven by the cellular mode of immune response mediated by INFγ [174]. Atopic patients demonstrate a blunted response to stress (decreased production of cortisol) compared to normal controls [175]. Production of IL-4 and IL-5 is suppressed by glucocorticosteroids [176]. The absence of GRs in atopic dermatitis leads to impairment of skin permeability function [177]. Psoriatic lesions are sharply demarcated plaques with silvery scale in characteristic distribution including scalp, elbows and knees. The Th1 immune response with increased levels of IL-2 and INFγ occurs in psoriasis. IL-23, IL-17 and Th17 lymphocytes lead to increased levels of IL-22 that stimulates keratinocyte proliferation [16, 178]. Coiled-coil alpha-helical rod protein 1 (CCHCR1) promotes steroidogenesis by interacting with StAR. CCHCR1 expression in psoriatic plaques is decreased [62]. Methylprednisolone inhibits production of IL-17 by lymphocytes [179]. StAR expression has been demonstrated to be decreased or absent in psoriatic and atopic dermatitis when compared to normal skin tissues [62, 63]. Psoriatic patients have lower saliva cortisol levels [138]. Alopecia areata is a non-scarring alopecia that typically presents as well-circumscribed patches of hair loss with exclamation-mark hairs, cadaver hairs and nail pitting [180]. The HF is considered an immune privilege organ that can also be maintained by POMC-derived peptides including melanocyte stimulating hormone (MSH) [129, 180, 181] and perhaps cortisol [59, 182]. The higher elements of the HPA axis are functional within the pilosebaceous unit and provide another important element maintaining the immune privilege of this site [131, 133, 171, 183]. Down-regulation of immune privilege and local activation of the immune system is considered to be operative in alopecia areata [180]. However, in a mouse model of alopecia areata, the basal plasma levels of ACTH and cortisol are higher than in normal mice, although in response to stress the levels are lower [34]. Levels of cortisol change within the HFs, which might reflect its systemic concentration or change in local production [184]. However, it was recently documented that indeed those levels can change locally without central input [68]. In summary, effectors of the HPA and glucocorticoids in particular play significant roles in common inflammatory skin diseases, and targeted regulation of cutaneous HPA represents an exciting future approach to treat skin pathology [1, 73].
Autoimmune progesterone dermatitis is a cyclic condition of which clinical manifestations are triggered by progesterone surges during luteal phase of the cycle [185]. The symptomatology includes erythema multiforme and urticaria although disease has debatable variety of clinical and histopathological presentations [186]. Progesterone clearly causes this condition only in some women. It is possible that abnormal local cutaneous metabolism of systemically delivered progesterone is responsible for symptomatology. Different defects or levels of expression of enzymes involved in steroidogenesis and steroid metabolism might affect local processes that in turn lead to different clinical and histopathological expressions of this entity.
3.2. Role of sex hormones in inflammatory disorders
In skin, androgens mediate their effects through interaction with the androgen receptors (AR) triggering cascades of networks [91, 106, 187–189]. Higher levels of AR were detected in the balding than non-balding scalp [190], while DPCs from the occipital scalp did not express AR [191] and the expression of AR co-activator was higher in DPCs localized at the beard and frontal scalp than in the occipital scalp [192]. Very high doses of T or DHT induce apoptosis of DPCs through the bcl-2 pathway [193].
Androgens have a central role in acne, since acne onset is correlated with adrenarchal rise of blood DHEA-S levels [194], it manifests in congenital adrenal hyperplasia [195], is seen during hyperadrogenism in women [196], and it develops during anabolic steroid administration [197]. Cutaneous manifestations (SAHA syndrome [198] – seborrhea, acne, hirsutism, male-pattern alopecia) are common in cases of androgen excess. T and DHT promoted sebocyte proliferation in vitro at concentrations higher than physiological levels [70, 199], while their synergistic effect with the PPAR ligand linoleic acid resulted in increased lipogenesis [200].
Greater activity of the 17βHSD types 3 and 5 was detected in sebaceous glands of facial skin than in other, non-acne prone skin areas, suggesting the in situ more potent androgen formation in these areas [74, 101]. Strong expression of steroid sulfatase was detected in sebaceous glands of acne lesions [74]. 17βHSD2 (which can inactivate potent androgens) and was found mostly in sebaceous glands of non-acne prone areas in comparison to facial skin [99]. Although 5αR1 is the main enzyme converting T to DHT in human sebaceous glands, the use of selective inhibitors did not improve acne vulgaris lesions [201]. This might be interpreted to be due to reduced production of DHT by other 5αR isozymes to the minimal quantity required to trigger cellular responses, and/or the idea that T rather than DHT plays the main role in stimulating sebaceous lipogenesis [107, 202].
Of the two intracellular estrogen receptors, ERα and ERβ, ERβ is the predominant ER of human scalp skin [203] and is expressed in NHEKs. ERα expression of foreskin NHEKs was also reported in vitro [204, 205]. DPCs, in contrast to normal human fibroblasts, express double mRNA levels for ERα than for ERβ [111]. Both receptors have been immunohistochemically detected in human sebocytes in situ, but ERα was restricted in basal sebocytes [206]. Apocrine and eccrine glands also express ERβ, but not ERα [74, 207, 208].
Estrogens stimulate the proliferation of NHEKs [209], increase acid mucopolysaccharides, hyaluronic acid, collagen I and III synthesis [210, 211], promote wound healing [212], protect from photoageing [213] and prevent wrinkle formation and skin dryness of post-menopausal women [214]. Estrogens are believed to stimulate hair growth in men, by prolonging the anagen phase of the HF and postponing their transition to telogen phase [114]. In vitro E2 has an inhibitory effect in hair shaft elongation in female occipital scalp, while it promotes it in fronto-temporal male HFs [213, 215]. E2 stimulates HF vascular endothelial growth factor (VEGF) synthesis [216]. Estrogens suppress lipogenesis and the size of sebaceous glands in both sexes directly and indirectly, through affecting the gonadotropins released from the pituitary [106].
ERRβ and ERRγ, two orphan members of the nuclear receptor superfamily, are expressed in human skin and may also have a role in cutaneous estrogen signaling [217, 218].
Acne is the most common chronic disorder of the pilosebaceous unit, appearing usually during puberty. Its multifactorial pathogenesis includes local cutaneous sex-hormone hyperproduction or hyperresponsiveness of the epithelial cells involved, inflammatory processes and defects of adaptive immunity [219–224]. Human sebocytes express TLR-2 [225], which is activated by P. acnes, thus triggering innate immunity mechanisms. Involvement of SP, the CRH signaling system and the MC1 receptor in acne development was also suggested [226–229]. The androgen/AR complex does not only affect sebaceous gland activity, but also its inflammation, by augmenting the inflammatory responses of neutrophils and macrophages [230, 231].
Rosacea is a chronic, progressive disorder of the interfollicular skin, affecting mainly the convexities of the central face [171, 232]. The mechanisms involved in the disease can be triggered by cytokines, hormones, neuropeptides [233–235], and CRH [169, 171]. UV-radiation is considered as an initial stress factor, which triggers the cutaneous inflammatory response [236, 237]. However, UV radiation can also create a local immunosuppressive microenvironment (see 2.5).
Hidradenitis suppurativa/acne inversa (HS) is defined as “a chronic, inflammatory, recurrent, debilitating skin disease of the terminal HFs with deep-seated, painful and inflamed lesions of the apocrine gland bearing areas of the body, most commonly the axillae, inguinal and anogenital regions” [238–240]. A hypothesis of hyperandrogenism was suggested since premenstrual exacerbations, female preponderance, occurrence after menarche and improvement during pregnancy were observed [241–243]. Altered serum androgen levels [244] are rare in HS patients. Comorbidity with Crohn’s disease and other autoinflammatory Th17-induced diseases [238], subsequent bacterial colonization of the lesions [245, 246] and the response of HS patients to anti-TNFα factors strongly suggest involvement of the immune system.
3.3. Local and systemic autoimmune diseases originating in or involving the skin
Lupus erythematosus (LE) has several cutaneous variants and also a systemic form. The diagnosis is made based, on among other things, on the presence of anti-nuclear antibodies including anti-native DNA (systemic LE), anti-Sm (systemic LE), and anti-Ro (subacute cutaneous LE) [247]. Antigen-antibody complexes are deposited at the dermo-epidermal junction. What triggers production of autoantibodies is still not known, although various genetic and environmental factors are considered [4, 247]. Corticosteroids are one of the main medications used for treatment [4, 247]. Levels of androstenedione, cortisol and DHEA-S are lower in patients with systemic LE than in healthy subjects [248]. ACTH levels do not differ between LE patients and controls [248]. Glucocorticoid treatment seems not to be responsible for these changes but they are possibly due to abnormal cytokine levels or a Th17/Th1 imbalance [248, 249]. In other studies, untreated LE patients had levels of 17-hydroxypregnenolone and cortisol similar to controls, and levels of progesterone, 17-hydroxypogesterone, androstenedione, DHEA and DHEA-S lower than controls [250]. Inhibition of the C17-C20 lyase step of the of CYP17 reaction can also be responsible for decreased levels of steroids in LE patients [250].
Systemic sclerosis (SSc, scleroderma) is an autoimmune disease characterized by a poorly understood vasculopathy, autoimmunity, and extensive deposition of extracellular matrix (ECM) components in skin, lungs, gastrointestinal tract, heart and other body structures [4, 251]. Endothelial damage contributes to ongoing platelet aggregation with release of a member of fibrogenic mediators such as TGFβ1, TGFβ2, IL-4, platelet derived growth factor (PDGF), connective tissue factor, sphingosine 1-phosphate and lysophosphatidic acid [252]. Defective angiogenesis, neointimal proliferation and vascular spasm contribute to tissue hypoxia further stimulating ECM deposition [252]. There is also predominance of Th17 and Th2 cells with release of IL-17, IL-4 and IL-13 at sites of lymphocytic infiltration which contributes to ongoing fibrosis [252]. Basal levels of cortisol, androstenedione, DHEA-S and 17-hydroxyprogesterone in patients with SSc do not differ from healthy controls [253, 254]. Levels of DHEA are lower and of ACTH are higher in SSc patients [254]. They also have a diminished response to a stress test or hypoglycemia, i.e. their level of cortisol does not increase [253, 254]. Apparently, the HPA axis malfunctions in these patients.
4. Systemic implications of cutaneous steroidogenesis and conclusion
Since skin is the largest organ of the human body with powerful neuroendocrine activities (for most recent review see [1]), local production of sex steroids makes a significant contribution to circulating androgens and/or estrogens [255]. Up to half of the total circulating T is produced from skin and other peripheral organs [198]. The stromal cells of adipose tissue express CYP19A1 (aromatase) [256, 257] and provide a source of androgens for both sexes. The skin-located formation of E1 from circulating androstenedione can be the main site of estrogen biosynthesis in postmenopausal women, obese individuals and elderly men. Interestingly, CYP19A1 gene expression is higher in subcutaneous than omental adipose tissue [258–260]. The intracrine production of estrogens in peripheral tissues in women is around 75% in premenopausal and almost 100% in postmenopausal women, with a minor contribution from adrenal and ovarian T and androstenedione [97].
Although it still remains to be tested whether skin produced CRH, urocortin and ACTH can affect pituitary or adrenal functions, it is already well documented that CRH and POMC signaling systems in communication with cytokines can regulate local steroidogenic activity and skin immune activity in a context and compartment dependent manners (for most recent review see [1]). Furthermore, after exposure to UVB human skin in organ culture produces and secretes CRH, POMC derived ACTH, β-endorphin and cortisol [151]. Similarly, in vivo studies demonstrate that HF releases cortisol into the hair shaft after local pain stimuli [68]. Thus, it is likely that depending on the type and strength of the stressor(s), cutaneous elements of HPA including POMC-derived peptides and glucocorticoids will be released into the circulation. We have already obtained initial evidence that mice exposed to UVB show increased serum levels of ACTH, β-endorphin and corticosterone (Skobowiat and Slominski, in preparation). Furthermore, locally produced glucocorticoids or POMC-peptides can induce resident and circulating immune cells to express immunosuppressive phenotype with potential systemic implications as suggested previously [1]. The biological significance of novel local secosteroidogenic pathways based on action of CYP11A1 on 7DHC and vitamin D represents a new exciting challenge is skin research [163, 168].
In conclusion, skin is a steroidogenic organ in which local steroidogenic activities can regulate local and systemic immune activities, and dysregulation of cutaneous steroidogenesis may be etiologically linked to inflammatory or autoimmune skin diseases.
Supplementary Material
Highlights.
Human skin produces and metabolizes glucocorticosteroids
Human skin produces and metabolizes sex hormones
Cutaneous steroidogenesis is regulated by local factors and UVR
Skin derived steroids regulate activity of skin immune system
Dysregulation of cutaneous steroidogenesis can lead to inflammatory or autoimmune disorders
Acknowledgments
The projects described were supported by grants R01AR052190 and 1R01AR056666-01A2 from the NIH/NAIMS and IOS-0918934 from the NSF to ATS, VA Merit Award to AP and a grant from Polish Ministry of Science and Higher Education, project no. N405 623238 to MAZ.
ABBREVIATIONS LIST
- ACTH
adrenocorticotropic hormone
- cAMP/PKA
cAMP-dependent protein kinase A
- CD
cluster of differentiation
- CRH
corticotrophin releasing hormone
- CYP11A1 or P450 scc
cytochrome P450 side-chain cleavage enzyme
- CYP11B1
11-hydroxylase type 1
- CYP11B2
11-hydroxylase type 2
- CYP17A1 or P450c17
cytochrome P450 17α-hydroxylase/17,20-lyase
- CYP21
21-hydroxylase
- DAX-1
dosage-sensitive sex-reversal-adrenal hypoplasia congenital critical region on the x-chromosome, gene 1
- DHEA
dehydroepiandrosterone
- DHT
dihydrotestosterone
- DOC
deoxycorticosterone
- DPCs
Dermal Papilla Cells
- E1
Estrone
- E2
estradiol
- ECM
extracellular matrix
- ERE
estrogen response element
- ERR
estrogen-related receptor
- F-1
steroidogenic factor 1
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HPA
hypothalamus-pituitary-adrenal gland
- HF
hair follicle
- HS
Hidradenitis suppurativa/acne inversa
- 3α-HSD
3α-hydroxysteroid dehydrogenase
- 3β-HSD
3β-hydroxysteroid dehydrogenase
- HSD11B1 or 11-HSD 1
11-hydroxysteroiddehydrogenase type 1
- HSD11B2 or 11-HSD 2
11-Hydroxysteroiddehydrogenase type 2
- 17β-HSD
17β-hydroxysteroid dehydrogenase
- INF
interferon
- IRSKS
Inner root sheath keratinocytes
- LE
Lupus erythematosus
- MHC
major histocompatibility complex
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NHEKs
normal human epidermal keratinocytes
- ORSKs
outer root sheath keratinocytes
- POMC
proopiomelanocortin
- 5αR
5α-reductase
- SSc
Systemic sclerosis
- SP
substance P
- StAR
steroidogenic acute regulatory protein
- T
testosterone
- TGFβ
Transforming growth factor β
- Th
T helper
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
- UVR
ultraviolet radiation
References
- 1.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat, Embryol Cell Biol. 2012;212:1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21(5):457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 3.Minnicozzi M, Sawyer RT, Fenton MJ. Innate immunity in allergic disease. Immunol Rev. 2011;242(1):106–127. doi: 10.1111/j.1600-065X.2011.01025.x. [DOI] [PubMed] [Google Scholar]
- 4.Bolognia J, Jorizzo JL, Rapini RP. Dermatology. Elsevier; 2008. [Google Scholar]
- 5.Modlin RL. Innate immunity: ignored for decades, but not forgotten. J Invest Dermatol. 2012;132(3 Pt 2):882–886. doi: 10.1038/jid.2011.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elias PM, Williams ML, Feingold KR. Abnormal barrier function in the pathogenesis of ichthyosis: therapeutic implications for lipid metabolic disorders. Clinics in dermatology. 2012;30(3):311–322. doi: 10.1016/j.clindermatol.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proksch E, Feingold KR, Elias PM. Epidermal HMG CoA reductase activity in essential fatty acid deficiency: barrier requirements rather than eicosanoid generation regulate cholesterol synthesis. J Invest Dermatol. 1992;99(2):216–220. doi: 10.1111/1523-1747.ep12650440. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy DM, Lee CM, Laker MF, Kealey T. Lipogenesis in isolated human sebaceous glands. FEBS Lett. 1986;200(1):173–176. doi: 10.1016/0014-5793(86)80533-6. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Pathophysiology of allergic inflammation. Immunol Rev. 2011;242(1):31–50. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- 10.Arnett E, Seveau S. The multifaceted activities of mammalian defensins. Curr Pharm Des. 2011;17(38):4254–4269. doi: 10.2174/138161211798999348. [DOI] [PubMed] [Google Scholar]
- 11.Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, Seltmann H, Patrick S, Zouboulis CC, Kemeny L. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8(8):2195–2205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 12.de Koning HD, Simon A, Zeeuwen PL, Schalkwijk J. Pattern recognition receptors in immune disorders affecting the skin. J Innate Immun. 2012;4(3):225–240. doi: 10.1159/000335900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu CC, Di Meglio P, Nestle FO. Harnessing dendritic cells in inflammatory skin diseases. Semin Immunol. 2012;23(1):28–41. doi: 10.1016/j.smim.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epplen JT, Chluba J, Hardt C, Hinkkanen A, Steimle V, Stockinger H. Mammalian T-lymphocyte antigen receptor genes: genetic and nongenetic potential to generate variability. Hum Genet. 1987;75(4):300–310. doi: 10.1007/BF00284099. [DOI] [PubMed] [Google Scholar]
- 15.Sawada E, Yoshida N, Sugiura A, Imokawa G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: An implication for the disrupted barrier mechanism in atopic dermatitis. J Dermatol Sci. 2012;68(1):25–35. doi: 10.1016/j.jdermsci.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. AmJ Pathol. 2012;181(1):8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Michalak-Stoma A, Pietrzak A, Szepietowski JC, Zalewska-Janowska A, Paszkowski T, Chodorowska G. Cytokine network in psoriasis revisited. Eur Cytokine Netw. 2011;22(4):160–168. doi: 10.1684/ecn.2011.0294. [DOI] [PubMed] [Google Scholar]
- 18.Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9(3):295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 19.Waterman MR, Mason JI, Simpson ER. Regulation of steroid hydroxylase gene expression. Prog Clin Biol Res. 1988;274:543–555. [PubMed] [Google Scholar]
- 20.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26(4):273–281. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Tuckey RC, Stevenson PM. Purification and analysis of phospholipids in the inner mitochondrial membrane fraction of bovine corpus luteum, and properties of cytochrome P-450scc incorporated into vesicles prepared from these phospholipids. European journal of biochemistry/FEBS. 1985;148(2):379–384. doi: 10.1111/j.1432-1033.1985.tb08849.x. [DOI] [PubMed] [Google Scholar]
- 22.Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutrition & metabolism. 2010;7:47. doi: 10.1186/1743-7075-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine reviews. 2011;32(1):81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooke BA. Signal transduction involving cyclic AMP-dependent and cyclic AMP-independent mechanisms in the control of steroidogenesis. Mol Cell Endocrinol. 1999;151(1–2):25–35. doi: 10.1016/s0303-7207(98)00255-x. [DOI] [PubMed] [Google Scholar]
- 25.Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23(2):141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 26.Privalle CT, Crivello JF, Jefcoate CR. Regulation of intramitochondrial cholesterol transfer to side-chain cleavage cytochrome P-450 in rat adrenal gland. Proc Natl Acad Sci U S A. 1983;80(3):702–706. doi: 10.1073/pnas.80.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269(45):28314–28322. [PubMed] [Google Scholar]
- 28.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17(3):221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 29.Manna PR, Stocco DM. Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(1):93–108. doi: 10.2174/1568008053174714. [DOI] [PubMed] [Google Scholar]
- 30.Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. Journal of lipid research. 2011;52(12):2111–2135. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol. 2002;16(1):184–199. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- 32.Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19(11):2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- 33.Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod. 2009;15(6):321–333. doi: 10.1093/molehr/gap025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting humanadrenal androgen production. Trends in endocrinology and metabolism: TEM. 2002;13(6):234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 35.Patel SS, Beshay VE, Escobar JC, Carr BR. 17alpha-Hydroxylase (CYP17) expression and subsequent androstenedione production in the human ovary. Reproductive sciences. 2010;17(11):978–986. doi: 10.1177/1933719110379055. [DOI] [PubMed] [Google Scholar]
- 36.Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. American journal of physiology. Endocrinology and metabolism. 2011;301(1):E11–24. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corpechot C, Synguelakis M, Talha S, Axelson M, Sjovall J, Vihko R, Baulieu EE, Robel P. Pregnenolone and its sulfate ester in the rat brain. Brain research. 1983;270(1):119–125. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- 38.Watzka M, Bidlingmaier F, Schramm J, Klingmuller D, Stoffel-Wagner B. Sex-and age-specific differences in human brain CYP11A1 mRNA expression. Journal of neuroendocrinology. 1999;11(12):901–905. doi: 10.1046/j.1365-2826.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- 39.Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. The Journal of experimental medicine. 1994;179(6):1835–1846. doi: 10.1084/jem.179.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teplyuk NM, Zhang Y, Lou Y, Hawse JR, Hassan MQ, Teplyuk VI, Pratap J, Galindo M, Stein JL, Stein GS, Lian JB, van Wijnen AJ. The osteogenic transcription factor runx2 controls genes involved in sterol/steroid metabolism, including CYP11A1 in osteoblasts. Mol Endocrinol. 2009;23(6):849–861. doi: 10.1210/me.2008-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett NC, Hooper JD, Lambie D, Lee CS, Yang T, Vesey DA, Samaratunga H, Johnson DW, Gobe GC. Evidence for steroidogenic potential in human prostate cell lines and tissues. Am J Pathol. 2012;181(3):1078–1087. doi: 10.1016/j.ajpath.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Courtney KD, Taplin ME. The evolving paradigm of second-line hormonal therapy options for castration-resistant prostate cancer. Current opinion in oncology. 2012;24(3):272–277. doi: 10.1097/CCO.0b013e328351059d. [DOI] [PubMed] [Google Scholar]
- 43.Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PloS one. 2012;7(1):e30062. doi: 10.1371/journal.pone.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levina E, Chen M, Carkner R, Shtutman M, Buttyan R. Paracrine Hedgehog increases the steroidogenic potential of prostate stromal cells in a Gli-dependent manner. The Prostate. 2012;72(8):817–824. doi: 10.1002/pros.21500. [DOI] [PubMed] [Google Scholar]
- 45.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, Chen S, Balk SP. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer research. 2011;71(20):6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai C, Balk SP. Intratumoral androgen biosynthesis in prostate cancer pathogenesis and response to therapy. Endocrine-related cancer. 2011;18(5):R175–182. doi: 10.1530/ERC-10-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Locke JA, Fazli L, Adomat H, Smyl J, Weins K, Lubik AA, Hales DB, Nelson CC, Gleave ME, Tomlinson Guns ES. A novel communication role for CYP17A1 in the progression of castration-resistant prostate cancer. The Prostate. 2009;69(9):928–937. doi: 10.1002/pros.20940. [DOI] [PubMed] [Google Scholar]
- 48.Iscan M, Klaavuniemi T, Coban T, Kapucuoglu N, Pelkonen O, Raunio H. The expression of cytochrome P450 enzymes in human breast tumours and normal breast tissue. Breast cancer research and treatment. 2001;70(1):47–54. doi: 10.1023/a:1012526406741. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Marcos PJ, Auwerx J, Schoonjans K. Emerging actions of the nuclear receptor LRH-1 in the gut. Biochimica et biophysica acta. 2011;1812(8):947–955. doi: 10.1016/j.bbadis.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sidler D, Renzulli P, Schnoz C, Berger B, Schneider-Jakob S, Fluck C, Inderbitzin D, Corazza N, Candinas D, Brunner T. Colon cancer cells produce immunoregulatory glucocorticoids. Oncoimmunology. 2012;1(4):529–530. doi: 10.4161/onci.19459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sidler D, Renzulli P, Schnoz C, Berger B, Schneider-Jakob S, Fluck C, Inderbitzin D, Corazza N, Candinas D, Brunner T. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene. 2011;30(21):2411–2419. doi: 10.1038/onc.2010.629. [DOI] [PubMed] [Google Scholar]
- 52.Bohm M, Zmijewski MA, Wasiewicz T, Straub RH, Raap U, Luger TA, Slominski A. KU812 basophils express urocortin, CRH-R, MC1R and steroidogenic enzymes and secrete progesterone. Experimental dermatology. 2012;21(7):541–543. doi: 10.1111/j.1600-0625.2012.01513.x. [DOI] [PubMed] [Google Scholar]
- 53.Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999;455(3):364–366. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- 54.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, CTR A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. European journal of biochemistry/FEBS. 2004;271(21):4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81(7):2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 56.Slominski A, Gomez-Sanchez C, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochimica et biophysica acta. 2000;1474:1–4. doi: 10.1016/s0304-4165(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 57.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162(1–2):97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 58.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. American journal of physiology. Endocrinology and metabolism. 2005;288(4):E701–706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 59.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19(10):1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 60.Slominski A, Zbytek B, Szczesniewski A, Wortsman J. Cultured human dermal fibroblasts do produce cortisol. J Invest Dermatol. 2006;126(5):1177–1178. doi: 10.1038/sj.jid.5700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogoff D, Gomez-Sanchez CE, Foecking MF, Wortsman J, Slominski A. Steroidogenesis in the human skin: 21-hydroxylation in cultured keratinocytes. J Steroid Biochem Mol Biol. 2001;78(1):77–81. doi: 10.1016/s0960-0760(01)00076-0. [DOI] [PubMed] [Google Scholar]
- 62.Tiala I, Suomela S, Huuhtanen J, Wakkinen J, Holtta-Vuori M, Kainu K, Ranta S, Turpeinen U, Hamalainen E, Jiao H, Karvonen SL, Ikonen E, Kere J, Saarialho-Kere U, Elomaa O. The CCHCR1 (HCR) gene is relevant for skin steroidogenesis and downregulated in cultured psoriatic keratinocytes. J Mol Med (Berl) 2007;85(6):589–601. doi: 10.1007/s00109-006-0155-0. [DOI] [PubMed] [Google Scholar]
- 63.Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochemical and biophysical research communications. 2011;404(1):62–67. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 64.Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. Journal of Cellular Biochemistry. 2011;112(6):1499–1505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- 65.Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286(12):10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. Localization, age-and site-dependent expression, and regulation of 11beta-hydroxysteroid dehydrogenase type 1 in skin. Journal of Investigative Dermatology. 2011;131(1):30–36. doi: 10.1038/jid.2010.257. [DOI] [PubMed] [Google Scholar]
- 67.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski AT. Cutaneous hypothalamic pituitary adrenal (HPA) axis homologue -regulation by ultraviolet radiation. American journal of physiology. Endocrinology and metabolism. 2011 doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharpley CF, Kauter KG, McFarlane JR. An initial exploration of in vivo hair cortisol responses to a brief pain stressor: latency, localization and independence effects. Physiological Research. 2009;58(5):757–761. doi: 10.33549/physiolres.931544. [DOI] [PubMed] [Google Scholar]
- 69.Inoue T, Miki Y, Abe K, Hatori M, Hosaka M, Kariya Y, Kakuo S, Fujimura T, Hachiya A, Honma S, Aiba S, Sasano H. Sex steroid synthesis in human skin in situ: The roles of aromatase and steroidogenic acute regulatory protein in the homeostasis of human skin. Mol Cell Endocrinol. 2012;362(1–2):19–28. doi: 10.1016/j.mce.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 70.Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z, Clawson G. Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1) J Invest Dermatol. 2003;120(6):905–914. doi: 10.1046/j.1523-1747.2003.12244.x. [DOI] [PubMed] [Google Scholar]
- 71.Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocrine reviews. 2003;24(2):152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 72.Labrie F, Luu-The V, Labrie C, Pelletier G, El-Alfy M. Intracrinology and the skin. Hormone research. 2000;54(5–6):218–229. doi: 10.1159/000053264. [DOI] [PubMed] [Google Scholar]
- 73.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Hormone and metabolic research = Hormon-und Stoffwechselforschung = Hormones et metabolisme. 2007;39(2):85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 75.Elias PM, Crumrine D, Paller A, Rodriguez-Martin M, Williams ML. Pathogenesis of the cutaneous phenotype in inherited disorders of cholesterol metabolism: Therapeutic implications for topical treatment of these disorders. Dermato-endocrinology. 2011;3(2):100–106. doi: 10.4161/derm.3.2.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paller AS, van Steensel MA, Rodriguez-Martin M, Sorrell J, Heath C, Crumrine D, van Geel M, Cabrera AN, Elias PM. Pathogenesis-based therapy reverses cutaneous abnormalities in an inherited disorder of distal cholesterol metabolism. J Invest Dermatol. 2011;131(11):2242–2248. doi: 10.1038/jid.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demerjian M, Choi EH, Man MQ, Chang S, Elias PM, Feingold KR. Activators of PPARs and LXR decrease the adverse effects of exogenous glucocorticoids on the epidermis. Experimental dermatology. 2009;18(7):643–649. doi: 10.1111/j.1600-0625.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milewich L, Kaimal V, Shaw CB, Sontheimer Epidermal keratinocytes: a source of 5 alpha-dihydrotestosterone production in human skin. J Clin Endocrinol Metab. 1986;62(4):739–746. doi: 10.1210/jcem-62-4-739. [DOI] [PubMed] [Google Scholar]
- 79.Slominski A, Worstman J, Foecking MF, Shackleton CH, Gomez-Sanchez CE, Szczesniewski A. Gas chromatography/mass spectrometry characterization of corticosteroid metabolism in human immortalized keratinocytes. J Invest Dermatol. 2002;118:310–315. doi: 10.1046/j.0022-202x.2001.01648.x. [DOI] [PubMed] [Google Scholar]
- 80.Slominski A, Wortsman J, Foecking MF, Shackleton C, Gomez-Sanchez C, Szczesniewski A. Gas chromatography/mass spectrometry characterization of corticosteroid metabolism in human immortalized keratinocytes. J Invest Dermatol. 2002;118(2):310–315. doi: 10.1046/j.0022-202x.2001.01648.x. [DOI] [PubMed] [Google Scholar]
- 81.Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband dependent manner in human skin ex-vivo. British Journal of Dermatology. 2013 doi: 10.1111/bjd.12096. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palermo M, Quinkler M, Stewart PM. Apparent mineralocorticoid excess syndrome: an overview. Arquivos brasileiros de endocrinologia e metabologia. 2004;48(5):687–696. doi: 10.1590/s0004-27302004000500015. [DOI] [PubMed] [Google Scholar]
- 83.Remer T, Maser-Gluth C, Boye KR, Hartmann MF, Heinze E, Wudy SA. Exaggerated adrenarche and altered cortisol metabolism in Type 1 diabetic children. Steroids. 2006;71(7):591–598. doi: 10.1016/j.steroids.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, Tsai SJ, Liao CY, Tsai RY, Chen YJ, Pan BJ, Hung CL, Zouboulis CC. Higher levels of steroidogenic acute regulatory protein and type I 3beta-hydroxysteroid dehydrogenase in the scalp of men with androgenetic alopecia. J Invest Dermatol. 2006;126(10):2332–2335. doi: 10.1038/sj.jid.5700442. [DOI] [PubMed] [Google Scholar]
- 85.Patel MV, McKay IA, Burrin JM. Transcriptional regulators of steroidogenesis, DAX-1 and SF-1, are expressed in human skin. J Invest Dermatol. 2001;117(6):1559–1565. doi: 10.1046/j.0022-202x.2001.01587.x. [DOI] [PubMed] [Google Scholar]
- 86.Manna PR, Eubank DW, Lalli E, Sassone-Corsi P, Stocco DM. Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J Mol Endocrinol. 2003;30(3):381–397. doi: 10.1677/jme.0.0300381. [DOI] [PubMed] [Google Scholar]
- 87.Manna PR, Dyson MT, Jo Y, Stocco DM. Role of dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1 in protein kinase A-and protein kinase C-mediated regulation of the steroidogenic acute regulatory protein expression in mouse Leydig tumor cells: mechanism of action. Endocrinology. 2009;150(1):187–199. doi: 10.1210/en.2008-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206(1):57–67. doi: 10.1159/000067823. [DOI] [PubMed] [Google Scholar]
- 89.Chen W, Yang CC, Liao CY, Hung CL, Tsai SJ, Chen KF, Sheu HM, Zouboulis CC. Expression of sex-determining genes in human sebaceous glands and their possible role in the pathogenesis of acne. J Eur Acad Dermatol Venereol. 2006;20(7):846–852. doi: 10.1111/j.1468-3083.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 90.Suomela S, Elomaa O, Skoog T, Ala-aho R, Jeskanen L, Parssinen J, Latonen L, Grenman R, Kere J, Kahari VM, Saarialho-Kere U. CCHCR1 is up-regulated in skin cancer and associated with EGFR expression. PloS one. 2009;4(6):e6030. doi: 10.1371/journal.pone.0006030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zouboulis CC. Human skin: an independent peripheral endocrine organ. Horm Res. 2000;54(5–6):230–242. doi: 10.1159/000053265. [DOI] [PubMed] [Google Scholar]
- 92.Chen WC, Zouboulis CC. Hormones and the pilosebaceous unit. Dermato-endocrinology. 2009;1(2):81–86. doi: 10.4161/derm.1.2.8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen W, Tsai SJ, Sheu HM, Tsai JC, Zouboulis CC. Testosterone synthesized in cultured human SZ95 sebocytes derives mainly from dehydroepiandrosterone. Experimental dermatology. 2010;19(5):470–472. doi: 10.1111/j.1600-0625.2009.00996.x. [DOI] [PubMed] [Google Scholar]
- 94.Milewich L, Sontheimer RD, Herndon JH., Jr Steroid sulfatase activity in epidermis of acne-prone and non-acne-prone skin of patients with acne vulgaris. Arch Dermatol. 1990;126(10):1312–1314. [PubMed] [Google Scholar]
- 95.Hoffmann R, Rot A, Niiyama S, Billich A. Steroid sulfatase in the human hair follicle concentrates in the dermal papilla. J Invest Dermatol. 2001;117(6):1342–1348. doi: 10.1046/j.0022-202x.2001.01547.x. [DOI] [PubMed] [Google Scholar]
- 96.Billich A, Rot A, Lam C, Schmidt JB, Schuster I. Immunohistochemical localization of steroid sulfatase in acne lesions: Implications for the contribution of dehydroepiandrosterone sulfate to the pathogenesis of acne. Hormone and metabolic research = Hormon-und Stoffwechselforschung = Hormones et metabolisme. 2000;53:99. [Google Scholar]
- 97.Labrie F, Luu-The V, Labrie C, Pelletier G, El-Alfy M. Intracrinology and the skin. Horm Res. 2000;54(5–6):218–229. doi: 10.1159/000053264. [DOI] [PubMed] [Google Scholar]
- 98.Chen W, Thiboutot D, Zouboulis CC. Cutaneous androgen metabolism: basic research and clinical perspectives. J Invest Dermatol. 2002;119(5):992–1007. doi: 10.1046/j.1523-1747.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- 99.Thiboutot D, Martin P, Volikos L, Gilliland K. Oxidative activity of the type 2 isozyme of 17beta-hydroxysteroid dehydrogenase (17beta-HSD) predominates in human sebaceous glands. J Invest Dermatol. 1998;111(3):390–395. doi: 10.1046/j.1523-1747.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 100.Sakurai N, Miki Y, Suzuki T, Watanabe K, Narita T, Ando K, Yung TM, Aoki D, Sasano H, Handa H. Systemic distribution and tissue localizations of human 17beta-hydroxysteroid dehydrogenase type 12. J Steroid Biochem Mol Biol. 2006;99(4–5):174–181. doi: 10.1016/j.jsbmb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 101.Fritsch M, Orfanos CE, Zouboulis CC. Sebocytes are the key regulators of androgen homeostasis in human skin. J Invest Dermatol. 2001;116(5):793–800. doi: 10.1046/j.1523-1747.2001.01312.x. [DOI] [PubMed] [Google Scholar]
- 102.Dufort I, Rheault P, Huang XF, Soucy P, Luu V. The, Characteristics of a highly labile human type 5 17beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140(2):568–574. doi: 10.1210/endo.140.2.6531. [DOI] [PubMed] [Google Scholar]
- 103.Courchay G, Boyera N, Bernard BA, Mahe Y. Messenger RNA expression of steroidogenesis enzyme subtypes in the human pilosebaceous unit. Skin Pharmacol. 1996;9(3):169–176. doi: 10.1159/000211412. [DOI] [PubMed] [Google Scholar]
- 104.Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126(2):1165–1172. doi: 10.1210/endo-126-2-1165. [DOI] [PubMed] [Google Scholar]
- 105.Chen W, Zouboulis CC, Fritsch M, Blume-Peytavi U, Kodelja V, Goerdt S, Luu-The V, Orfanos CE. Evidence of heterogeneity and quantitative differences of the type 1 5alpha-reductase expression in cultured human skin cells--evidence of its presence in melanocytes. J Invest Dermatol. 1998;110(1):84–89. doi: 10.1046/j.1523-1747.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- 106.Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev. 2000;21(4):363–392. doi: 10.1210/edrv.21.4.0404. [DOI] [PubMed] [Google Scholar]
- 107.Lai JJ, Chang P, Lai KP, Chen L, Chang C. The role of androgen and androgen receptor in skin-related disorders. Arch Dermatol Res. 2012;304(7):499–510. doi: 10.1007/s00403-012-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seiffert K, Seltmann H, Fritsch M, Zouboulis CC. Inhibition of 5alpha-reductase activity in SZ95 sebocytes and HaCaT keratinocytes in vitro. Hormone and metabolic research = Hormon-und Stoffwechselforschung = Hormones et metabolisme. 2007;39(2):141–148. doi: 10.1055/s-2007-961814. [DOI] [PubMed] [Google Scholar]
- 109.Uemura M, Tamura K, Chung S, Honma S, Okuyama A, Nakamura Y, Nakagawa H. Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008;99(1):81–86. doi: 10.1111/j.1349-7006.2007.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Samson M, Labrie F, Zouboulis CC, Luu-The V. Biosynthesis of dihydrotestosterone by a pathway that does not require testosterone as an intermediate in the SZ95 sebaceous gland cell line. J Invest Dermatol. 2010;130(2):602–604. doi: 10.1038/jid.2009.225. [DOI] [PubMed] [Google Scholar]
- 111.Qin KN, New MI, Cheng KC. Molecular cloning of multiple cDNAs encoding human enzymes structurally related to 3 alpha-hydroxysteroid dehydrogenase. J Steroid Biochem Mol Biol. 1993;46(6):673–679. doi: 10.1016/0960-0760(93)90308-j. [DOI] [PubMed] [Google Scholar]
- 112.Brodie A, Inkster S, Yue W. Aromatase expression in the human male. Mol Cell Endocrinol. 2001;178(1–2):23–28. doi: 10.1016/s0303-7207(01)00444-0. [DOI] [PubMed] [Google Scholar]
- 113.Harada N. A unique aromatase (P-450AROM) mRNA formed by alternative use of tissue-specific exons 1 in human skin fibroblasts. Biochemical and biophysical research communications. 1992;189(2):1001–1007. doi: 10.1016/0006-291x(92)92303-f. [DOI] [PubMed] [Google Scholar]
- 114.Conrad F, Paus R. Estrogens and the hair follicle. J Dtsch Dermatol Ges. 2004;2(6):412–423. doi: 10.1046/j.1439-0353.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- 115.Hoffmann R, Niiyama S, Huth A, Kissling S, Happle R. 17alpha-estradiol induces aromatase activity in intact human anagen hair follicles ex vivo. Experimental dermatology. 2002;11(4):376–380. doi: 10.1034/j.1600-0625.2002.110413.x. [DOI] [PubMed] [Google Scholar]
- 116.Falany CN. Sulfation and sulfotransferases. Introduction: changing view of sulfation and the cytosolic sulfotransferases. FASEB J. 1997;11(1):1–2. doi: 10.1096/fasebj.11.1.9034159. [DOI] [PubMed] [Google Scholar]
- 117.Kushida A, Hattori K, Yamaguchi N, Kobayashi T, Date A, Tamura H. Sulfation of estradiol in human epidermal keratinocyte. Biol Pharm Bull. 2011;34(7):1147–1151. doi: 10.1248/bpb.34.1147. [DOI] [PubMed] [Google Scholar]
- 118.Ohnemus U, Uenalan M, Inzunza J, Gustafsson JA, Paus R. The hair follicle as an estrogen target and source. Endocr Rev. 2006;27(6):677–706. doi: 10.1210/er.2006-0020. [DOI] [PubMed] [Google Scholar]
- 119.Turgeon D, Carrier JS, Levesque E, Hum DW, Belanger A. Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology. 2001;142(2):778–787. doi: 10.1210/endo.142.2.7958. [DOI] [PubMed] [Google Scholar]
- 120.Tang W, Eggertsen G, Chiang JY, Norlin M. Estrogen-mediated regulation of CYP7B1: a possible role for controlling DHEA levels in human tissues. J Steroid Biochem Mol Biol. 2006;100(1–3):42–51. doi: 10.1016/j.jsbmb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 121.Zhu BT, Lee AJ. NADPH-dependent metabolism of 17beta-estradiol and estrone to polar and nonpolar metabolites by human tissues and cytochrome P450 isoforms. Steroids. 2005;70(4):225–244. doi: 10.1016/j.steroids.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 122.Chouinard S, Yueh M-F, Tukey RH, Giton F, Fiet J, Pelletier G, Barbier O, Bélanger A. Inactivation by UDP-glucuronosyltransferase enzymes: The end of androgen signaling. The Journal of Steroid Biochemistry and Molecular Biology. 2008;109(3–5):247–253. doi: 10.1016/j.jsbmb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 123.Belanger A, Hum DW, Beaulieu M, Levesque E, Guillemette C, Tchernof A, Belanger G, Turgeon D, Dubois S. Characterization and regulation of UDP-glucuronosyltransferases in steroid target tissues. J Steroid Biochem Mol Biol. 1998;65(1–6):301–310. doi: 10.1016/s0960-0760(97)00183-0. [DOI] [PubMed] [Google Scholar]
- 124.Higashi Y, Fuda H, Yanai H, Lee Y, Fukushige T, Kanzaki T, Strott CA. Expression of cholesterol sulfotransferase (SULT2B1b) in human skin and primary cultures of human epidermal keratinocytes. J Invest Dermatol. 2004;122(5):1207–1213. doi: 10.1111/j.0022-202X.2004.22416.x. [DOI] [PubMed] [Google Scholar]
- 125.Falany CN, He D, Dumas N, Frost AR, Falany JL. Human cytosolic sulfotransferase 2B1: isoform expression, tissue specificity and subcellular localization. J Steroid Biochem Mol Biol. 2006;102(1–5):214–221. doi: 10.1016/j.jsbmb.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dooley TP, Walker CJ, Hirshey SJ, Falany CN, Diani AR. Localization of minoxidil sulfotransferase in rat liver and the outer root sheath of anagen pelage and vibrissa follicles. J Invest Dermatol. 1991;96(1):65–70. doi: 10.1111/1523-1747.ep12515856. [DOI] [PubMed] [Google Scholar]
- 127.Marin YE, Seiberg M, Lin CB. Aldo-keto reductase 1C subfamily genes in skin are UV-inducible: possible role in keratinocytes survival. Experimental dermatology. 2009;18(7):611–618. doi: 10.1111/j.1600-0625.2008.00839.x. [DOI] [PubMed] [Google Scholar]
- 128.Hennebert O, Chalbot S, Alran S, Morfin R. Dehydroepiandrosterone 7alpha-hydroxylation in human tissues: possible interference with type 1 11beta-hydroxysteroid dehydrogenase-mediated processes. J Steroid Biochem Mol Biol. 2007;104(3–5):326–333. doi: 10.1016/j.jsbmb.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 129.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 130.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. Faseb J. 2001;15(10):1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 131.Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–2248. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Slominski A, Mihm MC. Potential mechanism of skin response to stress. International journal of dermatology. 1996;35(12):849–851. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 133.Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: implications for its function. Drug discovery today. 2008;5(2):137–144. doi: 10.1016/j.ddmec.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. The Journal of clinical investigation. 2007;117(11):3166–3169. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basisof biologic function. Steroids. 2010;75(1):1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perez P. Glucocorticoid receptors, epidermal homeostasis and hair follicle differentiation. Dermato-endocrinology. 2011;3(3):166–174. doi: 10.4161/derm.3.3.15332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schacke H, Zollner TM, Docke WD, Rehwinkel H, Jaroch S, Skuballa W, Neuhaus R, May E, Zugel U, Asadullah K. Characterization of ZK 245186, a novel, selective glucocorticoid receptor agonist for the topical treatment of inflammatory skin diseases. British journal of pharmacology. 2009;158(4):1088–1103. doi: 10.1111/j.1476-5381.2009.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bamberger CM, Chrousos GP. The glucocorticoid receptor and RU 486 in man. Annals of the New York Academy of Sciences. 1995;761:296–310. doi: 10.1111/j.1749-6632.1995.tb31385.x. [DOI] [PubMed] [Google Scholar]
- 139.Szelenyi J. Cytokines and the central nervous system. Brain research bulletin. 2001;54(4):329–338. doi: 10.1016/s0361-9230(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 140.Tkachenko IV, Jaaskelainen T, Jaaskelainen J, Palvimo JJ, Voutilainen R. Interleukins 1alpha and 1beta as regulators of steroidogenesis in human NCI-H295R adrenocortical cells. Steroids. 2011;76(10–11):1103–1115. doi: 10.1016/j.steroids.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 141.Gadek-Michalska A, Tadeusz J, Rachwalska P, Spyrka J, Bugajski J. Effect of prior stress on interleukin-1beta and HPA axis responses to acute stress. Pharmacological reports : PR. 2011;63(6):1393–1403. doi: 10.1016/s1734-1140(11)70703-4. [DOI] [PubMed] [Google Scholar]
- 142.Contassot E, Beer HD, French LE. Interleukin-1, inflammasomes, autoinflammation and the skin. Swiss medical weekly. 2012;142:w13590. doi: 10.4414/smw.2012.13590. [DOI] [PubMed] [Google Scholar]
- 143.Eliav E, Benoliel R, Herzberg U, Kalladka M, Tal M. The role of IL-6 and IL-1beta in painful perineural inflammatory neuritis. Brain, behavior, and immunity. 2009;23(4):474–484. doi: 10.1016/j.bbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 144.Wei T, Guo TZ, Li WW, Hou S, Kingery WS, Clark JD. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. Journal of neuroinflammation. 2012;9:181. doi: 10.1186/1742-2094-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Noti M, Corazza N, Mueller C, Berger B, Brunner T. TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis. The Journal of experimental medicine. 2010;207(5):1057–1066. doi: 10.1084/jem.20090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28(4):477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Terao M, Murota H, Kimura A, Kato A, Ishikawa A, Igawa K, Miyoshi E, Katayama I. 11beta-Hydroxysteroid dehydrogenase-1 is a novel regulator of skin homeostasis and a candidate target for promoting tissue repair. PloS one. 2011;6(9):e25039. doi: 10.1371/journal.pone.0025039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee SE, Kim JM, Jeong MK, Zouboulis CC, Lee SH. 11ss-hydroxysteroid dehydrogenase type 1 is expressed in human sebaceous glands and regulates glucocorticoid-induced lipid synthesis and toll-like receptor 2 expression in SZ95 sebocytes. The British journal of dermatology. 2012 doi: 10.1111/bjd.12009. [DOI] [PubMed] [Google Scholar]
- 149.Fitzgerald P, O’Brien SM, Scully P, Rijkers K, Scott LV, Dinan TG. Cutaneous glucocorticoid receptor sensitivity and pro-inflammatory cytokine levels in antidepressant-resistant depression. Psychological medicine. 2006;36(1):37–43. doi: 10.1017/S003329170500632X. [DOI] [PubMed] [Google Scholar]
- 150.Raza K, Hardy R, Cooper MS. The 11beta-hydroxysteroid dehydrogenase enzymes--arbiters of the effects of glucocorticoids in synovium and bone. Rheumatology (Oxford) 2010;49(11):2016–2023. doi: 10.1093/rheumatology/keq212. [DOI] [PubMed] [Google Scholar]
- 151.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. American journal of physiology. Endocrinology and metabolism. 2011;301(3):E484–493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 153.Slominski AT, Botchkarev V, Choudhry M, Fazal N, Fechner K, Furkert J, Krause E, Roloff B, Sayeed M, Wei E, Zbytek B, Zipper J, Wortsman J, Paus R. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system?”. Annals of the New York Academy of Sciences. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- 154.Slominski A, Baker J, Ermak G, Chakraborty A, Pawelek J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996;399(1–2):175–176. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
- 155.Yamate Y, Hiramoto K, Kasahara E, Jikumaru M, Sato EF, Inoue J, Inoue M. Ultraviolet-A irradiation to the eye modulates intestinal mucosal functions and properties of mast cells in the mouse. Photochemistry and photobiology. 2011;87(1):191–198. doi: 10.1111/j.1751-1097.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 156.Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone-proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20(10):2539–2547. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shackleton CH. Role of a disordered steroid metabolome in the elucidation of sterol and steroid biosynthesis. Lipids. 2012;47(1):1–12. doi: 10.1007/s11745-011-3605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chignell CF, Kukielczak BM, Sik RH, Bilski PJ, He YY. Ultraviolet A sensitivity in Smith-Lemli-Opitz syndrome: Possible involvement of cholesta-5,7,9(11)-trien-3 beta-ol. Free Radic Biol Med. 2006;41(2):339–346. doi: 10.1016/j.freeradbiomed.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 159.Gambichler T, Bader A, Vojvodic M, Bechara FG, Sauermann K, Altmeyer P, Hoffmann K. Impact of UVA exposure on psychological parameters and circulating serotonin and melatonin. BMC Drmatology. 2002;2:6. doi: 10.1186/1471-5945-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shackleton C, Roitman E, Guo LW, Wilson WK, Porter FD. Identification of 7 (8) and 8(9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosterol-delta7-reductase deficiency (Smith-Lemli-Opitz syndrome) Journal of Steroid Biochemistry and Molecular Biology. 2002;82(2–3):225–232. doi: 10.1016/s0960-0760(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 161.Shackleton CH, Roitman E, Kelley R. Neonatal urinary steroids in Smith-Lemli-Opitz syndrome associated with 7-dehydrocholesterol reductase deficiency. Steroids. 1999;64(7):481–490. doi: 10.1016/s0039-128x(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 162.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, Zjawiony JK, Tuckey RC. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PloS one. 2009;4(2):e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, Sweatman T, Janjetovic Z, Tuckey RC. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. The international journal of biochemistry & cell biology. 2012;44(11):2003–2018. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Synthesis and photo-conversion of androsta-and pregna-5,7-dienes to vitamin D3-like derivatives. Photochemical & Photobiological Sciences. 2008;7(12):1570–1576. doi: 10.1039/b809005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martin JA, Taylor C, Trehan M, Baron ED, Anstey AV. Phototesting in patients with Smith-Lemli-Opitz syndrome confirms sensitivity to UV-A. Arch Dermatol. 2006;142(5):647–648. doi: 10.1001/archderm.142.5.647. [DOI] [PubMed] [Google Scholar]
- 166.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3 beta, 17 alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74(2):218–228. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zmijewski MA, Li W, Chen J, Kim T-K, Zjawiony JK, Sweatman TW, Miller DD, Slominski AT. Synthesis and photochemical transformation of 3 beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76(1–2):193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450sccand modified by CYP27B1. FASEB J. 2012;26(9):3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Slominski A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. The British journal of dermatology. 2009;160(2):229–232. doi: 10.1111/j.1365-2133.2008.08958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Slominski A, Wortsman J, Linton E, Pisarchik A, Zbytek B. The skin as a model for the immunomodulatory effects of corticotropin-releasing hormone. In: Schaefer M, Stein C, editors. Mind over Matter-Regulation of Peripheral Inflammation by the CNS. Birkhaeuser Verlag; Basel, Boston, Berlin: 2003. pp. 149–176. [Google Scholar]
- 171.Zouboulis CC. Acne Vulgaris and Rosacea. In: Granstein R, Luger T, editors. Neuroimmunology of the Skin. Springer; Berlin Heidelberg: 2009. pp. 219–232. [Google Scholar]
- 172.Zbytek B, Slominski AT. CRH mediates inflammation induced by lipopolysaccharide in human adult epidermal keratinocytes. J Invest Dermatol. 2007;127(3):730–732. doi: 10.1038/sj.jid.5700607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zbytek B, Pfeffer LM, Slominski AT. CRH inhibits NF-kappaB signaling in human melanocytes. Peptides. 2006;27(12):3276–3283. doi: 10.1016/j.peptides.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yamanaka K, Mizutani H. The role of cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr Probl Dermatol. 2011;41:80–92. doi: 10.1159/000323299. [DOI] [PubMed] [Google Scholar]
- 175.Buske-Kirschbaum A, Ebrecht M, Hellhammer DH. Blunted HPA axis responsivenessto stress in atopic patients is associated with the acuity and severeness of allergic inflammation. Brain, behavior, and immunity. 2010;24(8):1347–1353. doi: 10.1016/j.bbi.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 176.Sewell WA, Scurr LL, Orphanides H, Kinder S, Ludowyke RI. Induction of interleukin-4 and interleukin-5 expression in mast cells is inhibited by glucocorticoids. Clin Diagn Lab Immunol. 1998;5(1):18–23. doi: 10.1128/cdli.5.1.18-23.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sevilla LM, Latorre V, Sanchis A, Perez P. Epidermal Inactivation of the Glucocorticoid Receptor Triggers Skin Barrier Defects and Cutaneous Inflammation. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.281. [DOI] [PubMed] [Google Scholar]
- 178.Tokura Y, Mori T, Hino R. Psoriasis and other Th17-mediated skin diseases. J Uoeh. 2010;32(4):317–328. doi: 10.7888/juoeh.32.317. [DOI] [PubMed] [Google Scholar]
- 179.Miljkovic Z, Momcilovic M, Miljkovic D, Mostarica-Stojkovic M. Methylprednisoloneinhibits IFN-gamma and IL-17 expression and production by cells infiltrating central nervous system in experimental autoimmune encephalomyelitis. Journal of neuroinflammation. 2009;6:37. doi: 10.1186/1742-2094-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366(16):1515–1525. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 181.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124(1):13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sharpley CF, McFarlane JR, Slominski A. Stress-linked cortisol concentrations in hair: what we know and what we need to know. Reviews in the neurosciences. 2012;23(1):111–121. doi: 10.1515/RNS.2011.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Paus R, Arck P, Tiede S. (Neuro-)endocrinology of epithelial hair follicle stem cells. Mol Cell Endocrinol. 2008;288(1–2):38–51. doi: 10.1016/j.mce.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 184.Meyer JS, Novak MA. Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012;153(9):4120–4127. doi: 10.1210/en.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nasabzadeh TJ, Stefanato CM, Doole JE, Radfar A, Bhawan J, Venna S. Recurrent erythema multiforme triggered by progesterone sensitivity. Journal of cutaneous pathology. 2010;37(11):1164–1167. doi: 10.1111/j.1600-0560.2010.01607.x. [DOI] [PubMed] [Google Scholar]
- 186.McCalmont TH. Fact or fiction? Journal of cutaneous pathology. 2010;37(11):1130–1131. doi: 10.1111/j.1600-0560.2010.01616.x. [DOI] [PubMed] [Google Scholar]
- 187.Verrijdt G, Haelens A, Claessens F. Selective DNA recognition by the androgen receptor as a mechanism for hormone-specific regulation of gene expression. Mol Genet Metab. 2003;78(3):175–185. doi: 10.1016/s1096-7192(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 188.Asada Y, Sonoda T, Ojiro M, Kurata S, Sato T, Ezaki T, Takayasu S. 5 alpha-reductase type 2 is constitutively expressed in the dermal papilla and connective tissue sheath of the hair follicle in vivo but not during culture in vitro. J Clin Endocrinol Metab. 2001;86(6):2875–2880. doi: 10.1210/jcem.86.6.7545. [DOI] [PubMed] [Google Scholar]
- 189.Inui S, Itami S. Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla. J Dermatol Sci. 2011;61(1):1–6. doi: 10.1016/j.jdermsci.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 190.Hibberts NA, Howell AE, Randall VA. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol. 1998;156(1):59–65. doi: 10.1677/joe.0.1560059. [DOI] [PubMed] [Google Scholar]
- 191.Itami S, Sonoda T, Kurata S, Takayasu S. Mechanism of action of androgen in hair follicles. J Dermatol Sci. 1994;7(Suppl):S98–103. doi: 10.1016/0923-1811(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 192.Inui S, Fukuzato Y, Nakajima T, Kurata S, Itami S. Androgen receptor co-activator Hic-5/ARA55 as a molecular regulator of androgen sensitivity in dermal papilla cells of human hair follicles. J Invest Dermatol. 2007;127(10):2302–2306. doi: 10.1038/sj.jid.5700883. [DOI] [PubMed] [Google Scholar]
- 193.Winiarska A, Mandt N, Kamp H, Hossini A, Seltmann H, Zouboulis CC, Blume-Peytavi U. Effect of 5alpha-dihydrotestosterone and testosterone on apoptosis in human dermal papilla cells. Skin Pharmacol Physiol. 2006;19(6):311–321. doi: 10.1159/000095251. [DOI] [PubMed] [Google Scholar]
- 194.Lucky AW. A review of infantile and pediatric acne. Dermatology. 1998;196(1):95–97. doi: 10.1159/000017838. [DOI] [PubMed] [Google Scholar]
- 195.New MI. An update of congenital adrenal hyperplasia. Annals of the New York Academy of Sciences. 2004;1038:14–43. doi: 10.1196/annals.1315.009. [DOI] [PubMed] [Google Scholar]
- 196.Imperato-McGinley J. 5alpha-reductase-2 deficiency and complete androgen insensitivity: lessons from nature. Adv Exp Med Biol. 2002;511:121–131. doi: 10.1007/978-1-4615-0621-8_8. discussion 131–124. [DOI] [PubMed] [Google Scholar]
- 197.Eklof AC, Thurelius AM, Garle M, Rane A, Sjoqvist F. The anti-doping hot-line, a means to capture the abuse of doping agents in the Swedish society and a new service function in clinical pharmacology. Eur J Clin Pharmacol. 2003;59(8–9):571–577. doi: 10.1007/s00228-003-0633-z. [DOI] [PubMed] [Google Scholar]
- 198.Orfanos CE, Adler YD, Zouboulis CC. The SAHA syndrome. Horm Res. 2000;54(5–6):251–258. doi: 10.1159/000053267. [DOI] [PubMed] [Google Scholar]
- 199.Zouboulis CC, Seltmann H, Neitzel H, Orfanos CE. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95) J Invest Dermatol. 1999;113(6):1011–1020. doi: 10.1046/j.1523-1747.1999.00771.x. [DOI] [PubMed] [Google Scholar]
- 200.Makrantonaki E, Zouboulis CC. Testosterone metabolism to 5alpha-dihydrotestosterone and synthesis of sebaceous lipids is regulated by the peroxisome proliferator-activated receptor ligand linoleic acid in human sebocytes. The British journal of dermatology. 2007;156(3):428–432. doi: 10.1111/j.1365-2133.2006.07671.x. [DOI] [PubMed] [Google Scholar]
- 201.Leyden J, Bergfeld W, Drake L, Dunlap F, Goldman MP, Gottlieb AB, Heffernan MP, Hickman JG, Hordinsky M, Jarrett M, Kang S, Lucky A, Peck G, Phillips T, Rapaport M, Roberts J, Savin R, Sawaya ME, Shalita A, Shavin J, Shaw JC, Stein L, Stewart D, Strauss J, Swinehart J, Swinyer L, Thiboutot D, Washenik K, Weinstein G, Whiting D, Pappas F, Sanchez M, Terranella L, Waldstreicher J. A systemic type I 5 alpha-reductase inhibitor is ineffective in the treatment of acne vulgaris. J Am Acad Dermatol. 2004;50(3):443–447. doi: 10.1016/j.jaad.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 202.Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119(Pt 4):722–732. doi: 10.1242/jcs.02786. [DOI] [PubMed] [Google Scholar]
- 203.Thornton MJ, Taylor AH, Mulligan K, Al-Azzawi F, Lyon CC, O’Driscoll J, Messenger AG. Oestrogen receptor beta is the predominant oestrogen receptor in human scalp skin. Experimental dermatology. 2003;12(2):181–190. doi: 10.1034/j.1600-0625.2003.120209.x. [DOI] [PubMed] [Google Scholar]
- 204.Verdier-Sevrain S, Yaar M, Cantatore J, Traish A, Gilchrest BA. Estradiol induces proliferation of keratinocytes via a receptor mediated mechanism. FASEB J. 2004;18(11):1252–1254. doi: 10.1096/fj.03-1088fje. [DOI] [PubMed] [Google Scholar]
- 205.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. ProcNatl Acad Sci U S A. 1986;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kariya Y, Moriya T, Suzuki T, Chiba M, Ishida K, Takeyama J, Endoh M, Watanabe M, Sasano H. Sex steroid hormone receptors in human skin appendage and its neoplasms. Endocr J. 2005;52(3):317–325. doi: 10.1507/endocrj.52.317. [DOI] [PubMed] [Google Scholar]
- 207.Beier K, Ginez I, Schaller H. Localization of steroid hormone receptors in the apocrine sweat glands of the human axilla. Histochem Cell Biol. 2005;123(1):61–65. doi: 10.1007/s00418-004-0736-3. [DOI] [PubMed] [Google Scholar]
- 208.Thornton MJ, Taylor AH, Mulligan K, Al-Azzawi F, Lyon CC, O’Driscoll J, Messenger AG. The distribution of estrogen receptor beta is distinct to that of estrogen receptor alpha and the androgen receptor in human skin and the pilosebaceous unit. J Investig Dermatol Symp Proc. 2003;8(1):100–103. doi: 10.1046/j.1523-1747.2003.12181.x. [DOI] [PubMed] [Google Scholar]
- 209.Kanda N, Watanabe S. 17beta-estradiol stimulates the growth of human keratinocytes by inducing cyclin D2 expression. J Invest Dermatol. 2004;123(2):319–328. doi: 10.1111/j.0022-202X.2004.12645.x. [DOI] [PubMed] [Google Scholar]
- 210.Raine-Fenning NJ, Brincat MP, Muscat-Baron Y. Skin aging and menopause : implications for treatment. Am J Clin Dermatol. 2003;4(6):371–378. doi: 10.2165/00128071-200304060-00001. [DOI] [PubMed] [Google Scholar]
- 211.Bentley JP, Brenner RM, Linstedt AD, West NB, Carlisle KS, Rokosova BC, MacDonald N. Increased hyaluronate and collagen biosynthesis and fibroblast estrogen receptors in macaque sex skin. J Invest Dermatol. 1986;87(5):668–673. doi: 10.1111/1523-1747.ep12456427. [DOI] [PubMed] [Google Scholar]
- 212.Kanda N, Watanabe S. 17beta-estradiol enhances the production of granulocyte-macrophage colony-stimulating factor in human keratinocytes. J Invest Dermatol. 2004;123(2):329–337. doi: 10.1111/j.0022-202X.2004.23231.x. [DOI] [PubMed] [Google Scholar]
- 213.Thornton MJ. Oestrogen functions in skin and skin appendages. Expert Opin Ther Targets. 2005;9(3):617–629. doi: 10.1517/14728222.9.3.617. [DOI] [PubMed] [Google Scholar]
- 214.Kanda N, Watanabe S. Regulatory roles of sex hormones in cutaneous biology and immunology. J Dermatol Sci. 2005;38(1):1–7. doi: 10.1016/j.jdermsci.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 215.Conrad F, Ohnemus U, Bodo E, Bettermann R, Paus A. Estrogens and human scalp hair growth-still more questions than answers. J Invest Dermatol. 2004;122(3):840–842. doi: 10.1111/j.0022-202X.2004.22344.x. [DOI] [PubMed] [Google Scholar]
- 216.Lachgar S, Charveron M, Sarraute J, Mourard M, Gall Y, Bonafe JL. In vitro main pathways of steroid action in cultured hair follicle cells: vascular approach. J Investig Dermatol Symp Proc. 1999;4(3):290–295. doi: 10.1038/sj.jidsp.5640232. [DOI] [PubMed] [Google Scholar]
- 217.Archer DF. Postmenopausal skin and estrogen. Gynecol Endocrinol. 2012:1–5. doi: 10.3109/09513590.2012.705392. [DOI] [PubMed] [Google Scholar]
- 218.Krahn-Bertil E, Bolzinger MA, Andre V, Orly I, Kanitakis J, Rousselle P, Damour O. Expression of estrogen-related receptor gamma (ERRgamma) in human skin. Eur J Dermatol. 2008;18(4):427–432. doi: 10.1684/ejd.2008.0438. [DOI] [PubMed] [Google Scholar]
- 219.Zouboulis CC, Eady A, Philpott M, Goldsmith LA, Orfanos C, Cunliffe WC, Rosenfield R. What is the pathogenesis of acne? Experimental dermatology. 2005;14(2):143–152. doi: 10.1111/j.0906-6705.2005.0285a.x. [DOI] [PubMed] [Google Scholar]
- 220.Zampeli VA, Makrantonaki E, Tzellos T, Zouboulis CC. New Pharmaceutical Concepts for Sebaceous Gland Diseases: Implementing Today’s Pre-Clinical Data into Tomorrow’s Daily Clinical Practice. Curr Pharm Biotechnol. 2012;13(10):1898–1913. doi: 10.2174/138920112802273173. [DOI] [PubMed] [Google Scholar]
- 221.Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121(1):20–27. doi: 10.1046/j.1523-1747.2003.12321.x. [DOI] [PubMed] [Google Scholar]
- 222.Anttila HS, Reitamo S, Saurat JH. Interleukin 1 immunoreactivity in sebaceous glands. The British journal of dermatology. 1992;127(6):585–588. doi: 10.1111/j.1365-2133.1992.tb14870.x. [DOI] [PubMed] [Google Scholar]
- 223.Boone DL, Lee EG, Libby S, Gibson PJ, Chien M, Chan F, Madonia M, Burkett PR, Ma A. Recent advances in understandingNF-kappaB regulation. Inflamm Bowel Dis. 2002;8(3):201–212. doi: 10.1097/00054725-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 224.Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166(6):1691–1699. doi: 10.1016/s0002-9440(10)62479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Oeff MK, Seltmann H, Hiroi N, Nastos A, Makrantonaki E, Bornstein SR, Zouboulis CC. Differential regulation of Toll-like receptor and CD14 pathways by retinoids and corticosteroids in human sebocytes. Dermatology. 2006;213(3):266. doi: 10.1159/000095056. [DOI] [PubMed] [Google Scholar]
- 226.Toyoda M, Morohashi M. New aspects in acne inflammation. Dermatology. 2003;206(1):17–23. doi: 10.1159/000067818. [DOI] [PubMed] [Google Scholar]
- 227.Thielitz A, Reinhold D, Vetter R, Bank U, Helmuth M, Hartig R, Wrenger S, Wiswedel I, Lendeckel U, Kahne T, Neubert K, Faust J, Zouboulis CC, Ansorge S, Gollnick H. Inhibitors of dipeptidyl peptidase IV and aminopeptidase N target major pathogenetic steps in acne initiation. J Invest Dermatol. 2007;127(5):1042–1051. doi: 10.1038/sj.jid.5700439. [DOI] [PubMed] [Google Scholar]
- 228.Ganceviciene R, Bohm M, Fimmel S, Zouboulis CC. The role of neuropeptides in the multifactorial pathogenesis of acne vulgaris. Dermato-endocrinology. 2009;1(3):170–176. doi: 10.4161/derm.1.3.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zouboulis CC, Seltmann H, Hiroi N, Chen W, Young M, Oeff M, Scherbaum WA, Orfanos CE, McCann SM, Bornstein SR. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci U S A. 2002;99(10):7148–7153. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. The Journal of clinical investigation. 2009;119(12):3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lee WJ, Jung HD, Chi SG, Kim BS, Lee SJ, Kim do W, Kim MK, Kim JC. Effect of dihydrotestosterone on the upregulation of inflammatory cytokines in cultured sebocytes. Arch Dermatol Res. 2010;302(6):429–433. doi: 10.1007/s00403-009-1019-6. [DOI] [PubMed] [Google Scholar]
- 232.Powell FC. Clinical practice. Rosacea. N Engl J Med. 2005;352(8):793–803. doi: 10.1056/NEJMcp042829. [DOI] [PubMed] [Google Scholar]
- 233.Kurkcuoglu N, Alaybeyi F. Substance P immunoreactivity in rosacea. J Am Acad Dermatol. 1991;25(4):725–726. doi: 10.1016/s0190-9622(08)80678-0. [DOI] [PubMed] [Google Scholar]
- 234.Powell FC, Corbally N, Powell D. Substance P and rosacea. J Am Acad Dermatol. 1993;28(1):132–133. doi: 10.1016/s0190-9622(08)80863-8. [DOI] [PubMed] [Google Scholar]
- 235.Wollina U. Rhinophyma--unusual expression of simple-type keratins and S100A in sebocytes and abundance of VIP receptor-positive dermal cells. Histol Histopathol. 1996;11(1):111–115. [PubMed] [Google Scholar]
- 236.Murphy G. Ultraviolet light and rosacea. Cutis. 2004;74(3 Suppl):13–16. 32–14. [PubMed] [Google Scholar]
- 237.Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. The British journal of dermatology. 2005;152(1):115–121. doi: 10.1111/j.1365-2133.2005.06368.x. [DOI] [PubMed] [Google Scholar]
- 238.Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa) Dermato-endocrinology. 2010;2(1):9–16. doi: 10.4161/derm.2.1.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28(4):779–793. doi: 10.1016/j.det.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 240.Nazary M, van der Zee HH, Prens EP, Folkerts G, Boer J. Pathogenesis and pharmacotherapy of Hidradenitis suppurativa. Eur J Pharmacol. 2011;672(1–3):1–8. doi: 10.1016/j.ejphar.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 241.Mortimer PS, Dawber RP, Gales MA, Moore RA. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. The British journal of dermatology. 1986;115(3):263–268. doi: 10.1111/j.1365-2133.1986.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 242.Barth JH, Layton AM, Cunliffe WJ. Endocrine factors in pre-and postmenopausal women with hidradenitis suppurativa. The British journal of dermatology. 1996;134(6):1057–1059. [PubMed] [Google Scholar]
- 243.Harrison BJ, Read GF, Hughes LE. Endocrine basis for the clinical presentation of hidradenitis suppurativa. Br J Surg. 1988;75(10):972–975. doi: 10.1002/bjs.1800751011. [DOI] [PubMed] [Google Scholar]
- 244.Harrison BJ, Kumar S, Read GF, Edwards CA, Scanlon MF, Hughes LE. Hidradenitis suppurativa: evidence for an endocrine abnormality. Br J Surg. 1985;72(12):1002–1004. doi: 10.1002/bjs.1800721223. [DOI] [PubMed] [Google Scholar]
- 245.Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2009;23(9):985–998. doi: 10.1111/j.1468-3083.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 246.Sartorius K, Killasli H, Oprica C, Sullivan A, Lapins J. Bacteriology of hidradenitis suppurativa exacerbations and deep tissue cultures obtained during carbon dioxide laser treatment. The British journal of dermatology. 2012;166(4):879–883. doi: 10.1111/j.1365-2133.2011.10747.x. [DOI] [PubMed] [Google Scholar]
- 247.Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18(6):871–882. doi: 10.1038/nm.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zietz B, Reber T, Oertel M, Gluck T, Scholmerich J, Straub RH. Altered function of the hypothalamic stress axes in patients with moderately active systemic lupus erythematosus. II. Dissociation between androstenedione, cortisol, or dehydroepiandrosterone and interleukin 6 or tumor necrosis factor. J Rheumatol. 2000;27(4):911–918. [PubMed] [Google Scholar]
- 249.Prado C, de Paz B, Gomez J, Lopez P, Rodriguez-Carrio J, Suarez A. Glucocorticoids enhance Th17/Th1 imbalance and signal transducer and activator of transcription 3 expression in systemic lupus erythematosus patients. Rheumatology (Oxford) 2011;50(10):1794–1801. doi: 10.1093/rheumatology/ker227. [DOI] [PubMed] [Google Scholar]
- 250.Vogl D, Falk W, Dorner M, Scholmerich J, Straub RH. Serum levels of pregnenolone and 17-hydroxypregnenolone in patients with rheumatoid arthritis and systemic lupus erythematosus: relation to other adrenal hormones. J Rheumatol. 2003;30(2):269–275. [PubMed] [Google Scholar]
- 251.Bhattacharyya S, Wei J, Varga J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol. 2012;8(1):42–54. doi: 10.1038/nrrheum.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pattanaik D, Brown M, Postlethwaite AE. Vascular involvement in systemic sclerosis (scleroderma) Journal of inflammation research. 2011;4:105–125. doi: 10.2147/JIR.S18145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Matsuura E, Ohta A, Suematsu R, Inoue H, Koarada S, Tada Y, Sherriff-Tadano R, Kuroki T, Ikeda D, Nagasawa K. Functional disturbance of the stress-adaptation system in patients with scleroderma. Mod Rheumatol. 2011;21(4):397–405. doi: 10.1007/s10165-010-0412-5. [DOI] [PubMed] [Google Scholar]
- 254.Imrich R, Lukac J, Rovensky J, Radikova Z, Penesova A, Kvetnansky R, Huckova M, Vigas M, Macho L, Koska J. Lower adrenocortical and adrenomedullary responsesto hypoglycemia in premenopausal women with systemic sclerosis. J Rheumatol. 2006;33(11):2235–2241. [PubMed] [Google Scholar]
- 255.Zouboulis CC. The skin as an endocrine organ. Dermato-endocrinology. 2009;1(5):250–252. doi: 10.4161/derm.1.5.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Simpson ER, Zhao Y, Agarwal VR, Michael MD, Bulun SE, Hinshelwood MM, Graham-Lorence S, Sun T, Fisher CR, Qin K, Mendelson CR. Aromatase expression in health and disease. Recent Prog Horm Res. 1997;52:185–213. discussion 213–184. [PubMed] [Google Scholar]
- 257.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15(3):342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 258.Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994;78(2):428–432. doi: 10.1210/jcem.78.2.8106632. [DOI] [PubMed] [Google Scholar]
- 259.Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing’s disease of the omentum”? Lancet. 1997;349(9060):1210–1213. doi: 10.1016/S0140-6736(96)11222-8. [DOI] [PubMed] [Google Scholar]
- 260.Kamat A, Hinshelwood MM, Murry BA, Mendelson CR. Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends in endocrinology and metabolism: TEM. 2002;13(3):122–128. doi: 10.1016/s1043-2760(02)00567-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.