Abstract
The anti-inflammatory actions of Vitamin D have long been recognized and its importance in modulating colon cancer and colitis development is becoming apparent. The Vitamin D receptor (VDR) is downregulated in human Ulcerative Colitis (UC) and colitis-associated cancer (CAC); however, its status in murine models of colitis has yet to be explored. Snail and Snail2, zinc-finger transcription factors regulated by inflammatory pathways and able to transcriptionally silence VDR, are upregulated in human UC and are associated with localized VDR silencing. To signal, VDR must heterodimerize with Retinoid X Receptor alpha (RXRα). If either VDR or RXRα are compromised, Vitamin D cannot regulate inflammatory pathways. RXRα is downregulated in human colorectal cancer (CRC), yet its expression in human and murine colitis has yet to be investigated. To explore the importance of Vitamin D and VDR in murine colitis, we utilized acute and chronic Azoxymethane/Dextran Sulfate Sodium (AOM/DSS) models of murine colitis. VDR was downregulated early in the onset of colitis whereas RXRα downregulation only occurred as colitis became chronic and developed into CAC. Receptor downregulation was associated with an early increase in the expression of the inflammatory markers, Snail and Snail2. The acute colitis model induced in combination with a Vitamin D deficient diet resulted in increased morbidity, receptor downregulation, inflammatory marker expression and Snail and Snail2 upregulation. These experiments demonstrate the importance of Vitamin D and VDR in modulating murine colitis development.
Keywords: Vitamin D, VDR, Colitis, RXRα, Cancer Prevention
Introduction
Vitamin D and its receptor are intimately involved in the regulation of inflammation [1–5] and have been implicated in the progression of inflammatory diseases such as diabetes, cardiovascular disease and cancer [6]. This has been supported by in vitro data where Vitamin D provided anti-tumor activity in numerous cell lines [7–9] and by epidemiological evidence showing that reduced Vitamin D intake or production increases cancer prevalence [10–15].
The role of Vitamin D in the development of colitis, a disease state that can develop into colitis-associated cancer (CAC), has yet to be elucidated. Low circulating 25(OH) Vitamin D has been associated with an increased risk for colorectal cancer (CRC) [16–18], and as Vitamin D supplementation has been proposed as a potential chemopreventive tool for colorectal cancer (CRC) [19], it follows that Vitamin D may play a role in the development and prevention of colitis. The association between Vitamin D and colitis is based on the observation that colitis incidence is proportional to distance from the equator and thus, sunlight exposure and dermal Vitamin D production [20]. Vitamin D deficiency is common in both Ulcerative Colitis (UC) and Crohn’s Disease patients [21], but it has yet to be determined if this deficiency is a cause or effect of colitis.
The importance of Vitamin D in colitis development is illustrated by the observation that Vitamin D receptor (VDR)−/− mice, when challenged with a chemically induced colitis, exhibit increased mortality as compared to wild-type mice [22]. It has been shown that VDR expression is downregulated in human UC [23], yet the mechanism behind its downregulation has yet to be elucidated. VDR can be silenced at the transcript level via the zinc-finger transcription factors Snail and Snail2 [24–26] which are upregulated or stabilized by inflammatory mediators [27–31].
Snail and Snail2 expression are increased in ulcerated tissue of UC patients and in CRC with expression corresponding to a localized downregulation in VDR [25, 32–33] [34] It has yet to be investigated if the upregulation of Snail and Snail2 and subsequent, localized decrease in VDR expression is a factor in human and murine colitis and CAC.
To study colitis and CAC, the Dextran Sulfate Sodium (DSS) model, a well accepted proxy for human UC can be used in combination with azoxymethane (AOM), a carcinogen [35]. Our study demonstrates the importance of VDR and Vitamin D in modulating colitis development and severity. By examining localization of VDR downregulation and Snail and Snail2 expression, a potential mechanism for VDR downregulation is suggested. Also, the role that dietary Vitamin D has on colitis is exemplified by the severe colitis we observed in Vitamin D deficient mice.
Material and Methods
Mice
Female, 6-week-old C57BL/6J mice weighing approximately 20g were used in all experiments (Jackson Labs). Mice were cared for within Institutional Animal Care Committee (IACUC) guidelines and all procedures were approved by the Medical University of South Carolina (MUSC) IACUC. Mice were housed in groups of five at 22–24°C using a 12 hour light-12 hour dark cycle with lights on at 06:00. Animals were fed normal chow (Harlan Teklad Diet 2918) except for experiments utilizing a deficient Vitamin D diet. For animals receiving DSS, automatic water was removed from cages at six weeks of age or three weeks of age for the mice on the Vitamin D deficient diet and replaced with bottled water.
Induction of colitis
To induce colitis, DSS (MW 36,000–50,000D, MP Biomedical, Santa Ana, CA) and AOM (Sigma-Aldrich, St. Louis, MO) were utilized. 30 mice were divided into control and treatment groups for four different treatment modalities. For the acute AOM/DSS model, mice were allowed to acclimate for one week and were then injected intraperitoneally with 10mg/kg AOM or saline (control) on day eight. The mice recovered for one week with water, and on day fifteen, the mice injected with AOM were given 2%DSS in water for seven days and the mice injected with saline remained on normal water. The mice were sacrificed on the seventh day of the DSS/water treatment. For the CAC model, the same procedure was performed with two additional DSS/water cycles. For the CAC model, 12mg/kg of AOM was used with 4% DSS and this protocol is associated with a high degree of colon tumor development [36].
Study design
Fifteen animals per treatment group were utilized. The colon from ten mice were used for RNA and protein extraction and the remaining five mice had their colons Swiss-rolled as described [37] for colitis scoring and immunohistochemistry.
Sacrifice and tissue harvesting
Mice were sacrificed via CO2 inhalation followed by cervical dislocation. Blood was removed via cardiac puncture, allowed to clot at room temperature for one hour, centrifuged for 15 min at 1000g and plasma was removed. The colon of each mouse was removed, measured, flushed with ice-cold PBS, flayed and the mucosa was scraped and separated into two fractions. One fraction was flash frozen in liquid nitrogen and the other fraction was placed in RNAlater (Ambion, Grand Island, NY) and then flash frozen in liquid nitrogen.
Vitamin D quantification
Plasma was transported to the laboratory of Dr. Bruce Hollis (Medical University of South Carolina) for quantification of systemic 25(OH)-Vitamin D via a 25(OH)-Vitamin D ELISA as described [38].
Colitis scoring
The colons from mice reserved for colitis scoring and immunohistochemistry were removed, flushed with ice-cold PBS, flayed and Swiss-rolled. Colons were fixed overnight in 70% ethanol and paraffin embedded. Five-micrometer sections were cut and either stained for H&E or left unstained for immunohistochemistry. H&E stained slides were scored blind by a MUSC pathologist on a scale from 0 to 4 as described [39]. Briefly, Grade 0 was normal colon tissue, Grade 1 was mild focal ulceration, Grade 2 was moderate multifocal ulceration, Grade 3 was moderate to severe multifocal ulceration and Grade 4 was widespread ulceration.
Immunohistochemistry (IHC)
Paraffin sections were rehydrated through xylenes and graded ethanols. Cells were permeabilized with 0.5% Triton X-100 (USB Products, Cleveland, OH) for five minutes at room temperature, rinsed and then incubated with 3% hydrogen peroxide for twenty minutes. Slides were washed with PBS, boiled for twenty minutes in 10mM sodium citrate buffer (Vector Labs, Burlingame, CA), rinsed with water to cool and then placed in iced methanol for ten minutes and rinsed with PBS. Slides were serum blocked with the appropriate species Vectastain ABC system (Vectastain, Burlingame, CA) for one hour at room temperature. Slides were then incubated with the primary antibody (anti-VDR, -RXRα, -PCNA, (Santa Cruz Biotechnology, Santa Cruz, CA) -Snail, -Snail2 (Abcam, Cambridge, MA), -COX-2 (Cayman Chemical, Ann Arbor, MI), -iNOS (Calbiochem, Darmstadt, Germany)) diluted in water 1:50–1:2000. The appropriate species secondary antibody and ABC reagent (Vectastain, Burlingame, CA) were added for 30 minutes each at room temperature with a PBS wash between solution incubation. DAB solution (Vectastain, Burlingame, CA) was added for three minutes, slides were rinsed with PBS and then counterstained with hematoxylin (Thermo Scientific, Pittsburgh, PA) for 30 seconds or until adequate staining had occurred. Slides were dehydrated through graded ethanols and xylene and coverslipped using Cytosol 60 (Thermo Scientific, Pittsburgh, PA). Specimens were visualized by a Zeiss Axiophot Microscope (Carl Zeiss AG, Oberkochen, Germany) and pictures were taken with an Insight digital camera (Spot Imaging, Sterling Heights, Michigan, USA) at 10–20x magnification.
Protein extraction and Immunobloting
The colonic mucosa fraction not placed in RNAlater was homogenized in 500uL of T-Per tissue protein extraction (Thermo Scientific, Rockport, IL) and .05% protease inhibitor cocktail via sonification. The homogenate was centrifuged at 10,000g for 5′ and supernatant was collected. Protein purity and concentration was quantified with a GE Nanovue. Protein samples (standardized to 50 μg of nuclear protein) were mixed in loading buffer containing 2% sodium dodecyl sulfate and 10% β-mercaptoethanol. Protein was denatured at 95°C for 5′ and then run in a 10% polyacrylamide gel with a Precision Plus Protein Standard (BioRad, Hercules, CA).
Proteins were transferred to a nitrocellulose membrane at 65 mA for 4hrs. The blot was saturated in PBS and 0.1% Tween 20 (PBS-T buffer) containing 10% nonfat dry milk at 4° C for a minimum of 1hr and incubated o/n at 4°C with primary antibody. Antibodies were anti-VDR, -RXRα (Millipore, Billerica, MA) and -GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA) as a loading control 1:1000–1:5000 in 5% nonfat dry milk. Blots were washed three times in PBS-T for 10′ at room temperature before incubating with horseradish peroxidase secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:10,000–1:20,000 in 5% nonfat dry milk for 2hr at room temperature. Blots were washed three times in PBS-T for 10′ each at room temperature and detection of protein was performed using West Pico and Femto blot detection reagents (Thermo Scientific, Pittsburgh, PA). Films were scanned and bands were quantified using Image J software. Proteins of interest were normalized to GAPDH.
RNA extraction and real-time PCR
RNA was isolated with a miRNAeasy kit (Qiagen, Valencia, CA) according to manufacturer’s instructions and each sample was resuspended in 40uL RNAse-free water. RNA purity and concentration was quantified with a GE Nanovue. Reactions were set up in duplicate for each sample. PCR was performed in a 25uL reaction containing 12.5uL of the 2X SYBR PCR reaction mix, 300nM of each primer, .5uL of iScript reverse transcriptase and 25–150ng of RNA. Reaction protocol was as follows: initial incubations at 50°C for 10 minutes to allow for CDNA synthesis, reverse transcriptase inactivation at 95°C for 5 minutes and then 40 cycles of PCR cycling and detection with 95°C for 10 seconds, 55°C for 30 seconds and 95°C for one minute. The melt curve was done at 55°C for one minute and 80 cycles of 0.5°C increments from 55°C to 95°C. Primers sequences were: RXRα 5′-CCATGAACCCTGTGAGCAG-3′ (sense) and 5′-CCTCTTGAAGAAGCCCTTGC-3′ (anti-sense), VDR 5′-ATGGCGGCCAGCACTTCCCTGCCTGAC-3′ (sense) and 5′-CTCCTCCTTCCGCTTCAGGATCATCTC-3′ (anti-sense), GAPDH 5′-CCCAGCAAGGACACTGAGCAAG-3′ (sense) and 5′-AGGCCCCTCCTGTTATTATGGGG-3′ (anti-sense), Amphiregulin 5′-GCTGCTCCGTGGTTCCGCTG-3′ (sense) and 5′-GCTCAAGTCCACCGGCACTGT-3′ (anti-sense), COX-2 5′-CCAGGGCCCTTCCTCCCGTAG-3′ (sense) and 5′-TGAGCCTTGGGGGTCAGGGA-3′ (anti-sense), Snail 5′-TGTACCCGCCCAGAGCCTCC-3′ (sense) and 5′-CCCCTGAGCGGGGTTCAAGC-3′ (anti-sense), Snail2 5′-GCCGGGTGACTTCAGAGGCG-3′ (sense) and 5′-GATAACGGTCCAGGCGGCGG-3′ (anti-sense), TNFα 5′-TGTCCCTTTCACTCACTGGC-3′ (sense) and 5′-CATCTTTTGGGGGAGTGCCT-3′ (anti-sense).
Vitamin D Deficiency Studies
For the Vitamin D intervention experiment, female, C57BL/6J mice were fed a diet deficient in Vitamin D (TD.89123) (Harlan, Indianapolis, IN) beginning at three weeks of age. Thirty mice were placed on each diet, half of which would be induced to have colitis and the other half serving as diet controls. The diets were maintained for the duration of the experiment.
Statistical Analysis
For the statistical analysis of immunoblotting and real time PCR data, expression levels from the animals were averaged in the control or treated group. Treatment comparisons are as follows. For the acute and CAC models, the averages of treated and control mice were compared via a Student’s t-test. For the Vitamin D intervention trials, the averages of the treated and control mice on the same diet were compared Student’s t-test. To perform comparisons between the acute and CAC trials and the Vitamin D deficient diet and acute normal diet, a two-way ANOVA with interactions was used to evaluate differences in treatment effects.
Results
Challenge with AOM/DSS results in acute colitis with receptor downregulation and expression of inflammatory markers
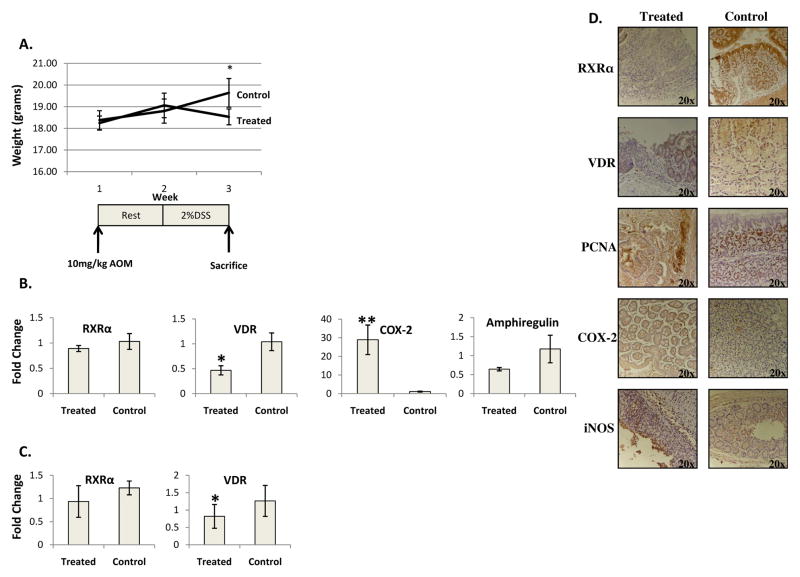
To study murine colitis, the AOM/DSS model was utilized. Mice were injected intraperitoneally with 10mg/kg of AOM and after a week of recovery, were challenged with 2%DSS for seven days. This resulted in a decrease in body weight that became significant at the third week (p=.02)(Figure 1A) with gross blood loss, an indicator of disease severity, apparent two days prior to sacrifice. Challenged mice exhibited a shortened colon of 57.6 +/− 9.4mm as compared to 67.8 +/− 6.1mm in control mice (p<.05). Challenged mice had a decreased systemic 25(OH)-Vitamin D of 20.1 +/− 5.1ng/mL as compared to control mice with 36.5 +/− 4.7ng/mL (p<.005).
Figure 1. Acute AOM/DSS colitis results in a down regulation of VDR, but not RXRα, and increased expression of inflammatory markers.
A) Weight loss in acute model occurs simultaneously with DSS challenge. B) Average expression levels of real time PCR of RXRα, VDR, COX-2 and Amphiregulin for treated and control mice, n=4 treated and 4 controls. C) \Average expression levels of quantified immunoblotting for RXRα and VDR for treated an control mice, n=8 treated and 8 control. D) RXRα and VDR are downregulated in ulcerated tissue of challenged mice while normal adjacent tissue and control mice have normal nuclear expression. COX-2 and iNOS are expressed in ulcerated tissue of challenged mice but not in normal adjacent tissue or in control mice. PCNA is expressed in both ulcerated tissue and control mice *=p<.05, **=p<.005
To analyze receptor expression, PCR results or immunoblots were quantified and the average expression levels were calculated for the treated and control groups. VDR mRNA (Figure 1B) and protein (Figure 1C) were downregulated in challenged mice, but RXRα mRNA and protein were not significantly decreased (Figure 1B and C). COX-2 mRNA was upregulated in challenged mice (Figure 1B). The expression of Amphiregulin (Figure 1B), a downstream Vitamin D target, was not significantly downregulated in challenged mice.
Upon microscopic analysis, challenged mice demonstrated a loss of crypts and ulcerations most prevalent in the distal colon. The average colitis score of challenged mice was 3.5. Immunohistochemistry indicated that the downregulation of VDR was confined to ulcerated tissue with normal adjacent tissue exhibiting nuclear VDR expression. RXRα protein was downregulated in ulcerated tissue. Localized with the receptor downregulation was expression of iNOS and COX-2 which were not evident in normal tissue or in the proximal colons of treated mice. PCNA, a marker of cell proliferation, was present in ulcerated tissue and normal adjacent crypts, indicating cell turnover due to tissue damage (Figure 1D).
Colitis associated cancer results in further receptor downregulation and expression of inflammatory markers
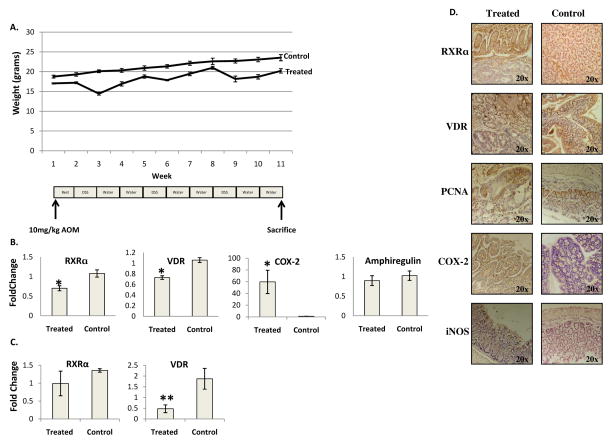
To model CAC, an extended study of thirteen weeks was conducted with an initial 12mg/kg AOM injection and three cycles of 4% DSS and water. Challenged mice demonstrated fluctuations in weight with decreases corresponding to DSS challenges (Figure 2A). This model had a mortality rate between 10 and 40%. Challenged mice had a shortened colon of 52.5 +/−3.5mm as compared to control mice with a colon length of 74.6 +/− 6.8mm (p<.005). Challenged mice had a decreased systemic 25(OH)-Vitamin D of 14.7 +/− 6.1ng/mL as compared to control mice with 30.8 +/− 9.6ng/mL (p<.005), but this was not significantly decreased as compared to acute treated mice.
Figure 2. CAC results in a downregulation of VDR and RXRα and increased expression of inflammatory markers.
A) Weight loss in CAC occurs simultaneously with each DSS challenge with periods of remission and weight gain observed when DSS is replaced with water. B) Average expression levels of real time PCR of RXRα, VDR, Amphiregulin and COX-2 for treated and control mice n=4 treated and 4 controls. C) Average expression levels of quantified immunoblotting for RXRα and VDR for treated and control mice. RXRα n=4 treated and 4 control, VDR n=6 treated and 5 control. D) RXRα and VDR are downregulated in dysplastic and ulcerated tissue of challenged mice while normal adjacent tissue and control mice have normal nuclear expression. COX-2 and iNOS are expressed in dysplastic and ulcerated tissue of challenged mice but not in normal adjacent tissue or in control mice. PCNA is expressed in dysplastic, ulcerated tissue and control mice *=p<.05, **=p<.005
Real time PCR demonstrated that challenged mice had a downregulation of VDR mRNA (Figure 2B) and protein (Figure 2C), but in this model, RXRα mRNA (Figure 2B) and protein (Figure 2C) were also downregulated. VDR protein was downregulated to a greater extent in the CAC model as compared to the acute model (p=.03). There was no significant change in the downregulation of VDR mRNA between the two models. COX-2 mRNA was upregulated in CAC challenged mice (Figure 2B) and it was upregulated more significantly than in the acute model (p=.02). There was no difference in Amphiregulin (Figure 2B) expression between CAC challenged and control mice.
In CAC challenged mice, microscopic analysis demonstrated phases of damage and regeneration with ulcerated areas and loss of crypts along with areas of abnormal appearing, branched crypts consistent with regeneration. Three out of five mice developed intramucosal adenocarcinomas. Immunohistochemistry demonstrated a downregulation of VDR and RXRα in ulcerated and dysplastic tissue. Normal adjacent tissue in CAC challenged mice and control mice exhibited nuclear VDR and RXRα. Dysplastic tissue and nearby normal tissue exhibited a high degree of iNOS and COX-2 expression with no staining evident in the proximal colon. PCNA was present in ulcerated tissue, dysplastic tissue, normal adjacent crypts and in control mice (Figure 2D).
Snail and Snail2 expression in acute colitis and CAC
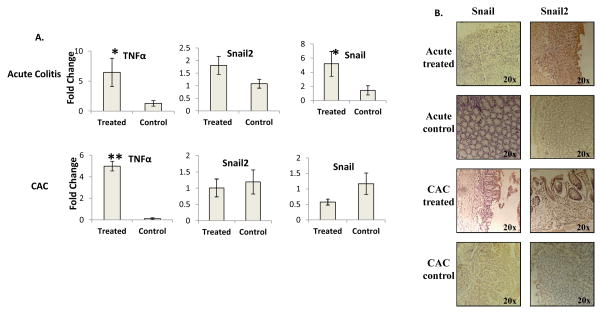
To investigate a possible mechanism through which VDR was being silenced, the expression of SNAIL and SNAIL2 and their upstream regulator, TNFα were investigated. TNFα and Snail mRNA were significantly upregulated in acute colitis with Snail2 just short of significance. In the CAC model, the upregulation of TNFα was still present, but Snail2 and Snail were no longer upregulated (Figure 3A). Snail and Snail2 were evident in ulcerated areas or dysplastic tissue in the acute and CAC models, thus, in the same locations where RXRα and VDR were downregulated. No Snail or Snail2 expression was in normal adjacent tissue or in control mice (Figure 3B).
Figure 3. Real time PCR analysis of Snail, Snail2 and their upstream modulator.
A) TNFα is upregulated in treated mice in the acute model and CAC. Snail is upregulated in acute colitis with Snail2 being upregulated just short of significance (p=.1). The upregulation of Snail and Snail2 is not observed in the CAC model N=4 treated and 4 controls. B) Snail and Snail2 upregulation is confined to ulcerated or dysplastic tissue. *=p<.05, **=p<.005
The effect of Vitamin D Deficiency on colitis development and nuclear receptor expression
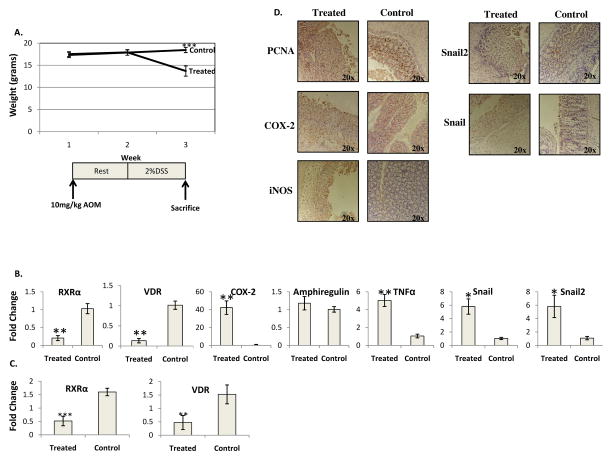
To study the effects of Vitamin D deficiency on the development and progression of acute AOM/DSS colitis, mice were started on a Vitamin D deficient diet at three weeks of age and the diet was continued for the duration of the experiment. Acute AOM/DSS colitis was introduced after three weeks on the diet at six weeks of age. Treated mice exhibited a decreased body weight that became significant at the third week (p<.005) (Figure 4A) and gross blood loss four days prior to sacrifice. Mice on a Vitamin D deficient diet had between a 7 and 10% mortality rate. A shortened colon of 44.1 +/− 4.6mm in challenged mice was found as compared to 63.3 +/− 3.8 mm in control mice (p<.005). This decrease in colon length was greater than the decrease observed in acute treated mice on normal chow (p=.02). AOM/DSS treated mice had a systemic 25(OH)-Vitamin D of 4.9 +/− 4.1ng/mL as compared to saline/water control mice on the Vitamin D deficient diet that evidenced an average systemic Vitamin D level of 2.6+/− 2.1ng/mL, a difference that was not statistically significant. This can be contrasted to mice fed normal chow where AOM/DSS treated mice had a decreased systemic 25(OH)-Vitamin D of 20.1 +/−5.1ng/mL as compared to saline/water control mice with 36.5 +/− 4.7ng/mL (p<.005). Thus, the Vitamin D deficient diet reduced systemic Vitamin D levels in both AOM/DSS treated and saline/water control mice; DSS treatment also reduced circulating vitamin D levels, likely due to induction of colitis. VDR mRNA was significantly downregulated (Figure 4B), but not significantly more so than the downregulation observed in the normal chow colitis. RXRα mRNA was significantly downregulated in this model (Figure 4B and C). COX-2 mRNA was upregulated in treated mice on a Vitamin D deficient diet but not significantly more so than the upregulation observed in acute treated mice on a normal chow. No difference in the expression of Amphiregulin was found between treated and control mice (Figure 4B). VDR protein was reduced, but not significantly more so than observed downregulation found in acute treated mice on a normal chow. (Figure 4C). In this Vitamin D deficient acute model, TNFα, Snail and Snail2 mRNA were all significantly upregulated (Figure 4B).
Figure 4. The effects of Vitamin D deficiency on acute murine colitis.
A) Vitamin D deficient mice challenged with AOM/DSS mice lose weight during treatment with weight loss parallel to DSS challenge. B) Average expression levels of real time PCR of RXRα, VDR, COX-2, Amphiregulin, TNFα, Snail and Snail2 for treated compared to control mice. n=4 control and 4 treated, C) Average expression levels of quantified immunoblotting for RXRα and VDR for treated an control mice, n=6 treated and 7 control. D) COX-2, iNOS, Snail and Snail2are expressed throughout the entire colon of treated mice. PCNA is expressed in ulcerated tissue and control mice *=p<.05, **=p<.005
The average colitis score was 3.8 to 4 out of 4. This was greater than the 3.5 observed in acute treated mice but not significantly so. Ulceration was observed not only in the distal colon, but throughout the entire colon. Very few normal appearing mucosal areas remained with almost the entire colon being ulcerated. COX-2 and iNOS, as well as Snail and Snail2, had widespread expression in the colon, both distally and proximally, in the ulcerated tissue and stroma (Figure 4D).
Discussion
Vitamin D has been implicated in the pathogenesis of numerous inflammatory diseases [6] and its anti-inflammatory effect [1–5] is being explored as a potential interventional agent. It has been shown in vitro that Vitamin D can prevent the growth of cancer cells [7–9] and epidemiological data suggests reduced Vitamin D intake or production can increase rates of various cancers [10–15]. Because of the epidemiological relationship between Vitamin D status and UC, we probed the influence of RXRα and VDR in acute and chronic animals models of colitis.
To study an acute murine colitis, the AOM/DSS model was utilized as this model is accepted as a proxy for human UC. We found a loss of crypts and the development of colonic ulcerations in treated mice that, along with the decreased systemic 25-(OH) Vitamin D, resembles findings in UC patients [40].
We have shown that the downregulation of VDR occurs early in acute murine colitis at both the protein and the mRNA level which agrees with data from UC patients [23]. This downregulation was confined to ulcerated tissue and corresponded to a localized increase of COX-2 and iNOS. RXRα was not downregulated in acute colitis at the protein or mRNA level, suggesting that VDR and RXRα undergo different mechanisms of silencing. Upon IHC analysis, it was clear that RXRα was downregulated in areas of ulceration in the acute colitis. The apparent disagreement in expression of RXRα between IHC compared to real time PCR and immunoblotting is likely due to the fact that for IHC, the entire colon can be visualized and it was noted that the RXRα downregulation was confined to distal tissue. However, for mRNA and protein extraction, the entire colon was scraped as it was not feasible to separate out ulcerated and normal tissue. The introduction of unaffected, proximal tissue with normal RXRα expression likely masked any receptor downregulation that was occurring distally.
As colitis develops into CAC, mice exhibited a 10 to 40% mortality rate with 60% of surviving mice developing adenomas. It has been proposed that the development of AOM/DSS CAC is due to TNFα production that encourages inflammatory cell invasion and the subsequent production of COX-2, a major player in CAC development [41]. In fact, in has been shown that dietary calcium and active Vitamin D in tandem were able to reduce colitis in Vitamin D deficient IL-10−/− mice due to a suppression of the TNFα pathway [42]. Our finding of increased COX-2 protein and mRNA in challenged mice in ulcerated and cancerous tissue supports this notion and it is likely that other pro-inflammatory mediators are involved.
To investigate a potential mechanism for VDR downregulation Snail and Snail2, two transcription factors that have shown to inhibit VDR transcription [24–26], were accessed. We found an early upregulation of Snail mRNA in the acute model, but Snail2 mRNA upregulation was short of significance. Snail and Snail2 expression correlated with an increase in mRNA of their upstream modulator, TNFα. The upregulation of Snail and Snail2 dissipated as CAC developed, despite a continued upregulation of TNFα mRNA. This suggests a temporal relationship of Snail and Snail2 expression. The observed upregulation of Snail in the acute model was likely due to the disease time course where mice were sacrificed without any recovery post DSS challenge. This can be contrasted to the CAC treated mice that had a two week recovery period after DSS challenge which might have allowed for Snail expression to normalize. It was surprising that Snail and Snail2 were not upregulated in the CAC model as these transcription factors have been linked to cancer progression. However, previous studies were mostly conducted in cell lines and, to our knowledge, expression patterns have not been reported in murine colitis or CAC models [43]. Immunohistochemistry demonstrated the upregulation of Snail and Snail2 to be confined to ulcerated tissue, similar to findings in UC patients [29]. The fact that Snail and Snail2 were found to be upregulated in CAC on IHC, but not via real time PCR, was once again, likely due to the method of colon scraping and subsequent RNA extraction.
A previous study demonstrated that a Vitamin D deficient diet leads to increased weight loss and colitis scores in mice [44]. Other studies have shown that VDR−/− mice challenged with DSS exhibit early mortality [22], that VDR deficiency results in a worsened colitis in the CD45RB transfer model [45] and that VDR/− mice have a loss of intestinal transepithelial resistance post DSS challenge [46]. We attempted, in this study, to make our model clinically relevant by starting mice on a Vitamin D deficient diet three weeks prior to colitis induction to model a human with low serum Vitamin D. Both AOM/DSS challenged mice and saline/water control mice on the Vitamin D deficient diet demonstrated systemic Vitamin D levels that were decreased as compared to AOM/DSS challenged mice and saline/water control mice on normal chow. However, mice on the Vitamin D deficient diet did not demonstrate observable symptoms of Vitamin D deficiency, such as osteomalacia. We did not expect systemic signs of Vitamin D deficiency due to the short nature of our experiment. It was shown by Lagishetty et al. 2010 [44] that a one week course of DSS after time on a Vitamin D deficient diet did not lead to clinical symptoms of Vitamin D deficiency, such as hypocalcemia. However, a Vitamin D deficient diet fed to mice, subsequently challenged with AOM/DSS, resulted in an intensity of colitis that was more severe compared to treated mice on a normal chow, measured by increased weight loss, enhanced colitis scores and mortality. A Vitamin D deficient diet also resulted in the downregulation of RXRα mRNA and protein that was not observed in treated mice on normal chow. While complex, we suggest that a Vitamin D deficient diet, without adequate circulating Vitamin D, although not chronic enough in these experiments to lead to clinical symptoms of Vitamin D deficiency, would be highly inflammatory and would impair the regulatory anti-inflammatory pathways. Likewise, although chronic Vitamin D deficiency in the diet would lead to parathyroid hormone upregulation and the subsequent production of 1,25-dihydroxyvitamin D, if there is not adequate VDR expression, the increased levels of active hormone may not have physiological significance. Perhaps these events also trigger the epigenetic silencing of VDR’s heterodimerization partner, RXRα, thus, accelerating inflammation. It became clear that inflammatory markers were not confined to distal, ulcerated tissue as in treated mice on a normal chow, but were widespread throughout the entire colon. This suggests a more systemic inflammatory process that is not confined to the distal colon. There was also a significant upregulation of Snail and Snail2 as compared to treated mice on a normal chow that only had a statistically significant upregulation of Snail.
This study demonstrates the importance of VDR and Vitamin D status in controlling murine colitis development. Although a definitive mechanism for VDR downregulation could not be approached due to the lack of an accepted in vitro model for colitis, our work suggests the transcription factors Snail and Snail2 may be, in part, responsible. The downregulation of RXRα in the Vitamin D deficient mice suggests that severe colitis allows for an unknown mechanism to downregulate RXRα expression and a plausible explanation is through epigenetic silencing. More work must be done to elucidate the mechanism(s) responsible for the downregulation of these receptors with the hopes that therapy could be targeted at restoring the expression of VDR and RXRα to reduce colitis progression and CAC development.
Acknowledgments
Financial support: National Institutes of Health
The present work benefited from the input of Elizabeth Garrett-Mayer, a statistician at Medical University of South Carolina (MUSC) and from Bruce Hollis, PhD and Renee Washington (MUSC) who provided systemic Vitamin D quantification.
Footnotes
Disclosure of Potential Conflicts of Interest
There are no potential conflicts of interest to list.
References
- 1.D’Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harant H, Wolff B, Lindley IJ. 1Alpha,25-dihydroxyvitamin D3 decreases DNA binding of nuclear factor-kappaB in human fibroblasts. FEBS Lett. 1998;436:329–34. doi: 10.1016/s0014-5793(98)01153-3. [DOI] [PubMed] [Google Scholar]
- 3.Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65:7917–25. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol Dial Transplant. 2006;21:889–97. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 5.Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, et al. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291:E315–22. doi: 10.1152/ajpendo.00590.2005. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 7.Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989;320:980–91. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- 8.Zhou JY, Norman AW, Chen DL, Sun GW, Uskokovic M, Koeffler HP. 1,25-Dihydroxy-16-ene-23-yne-vitamin D3 prolongs survival time of leukemic mice. Proc Natl Acad Sci U S A. 1990;87:3929–32. doi: 10.1073/pnas.87.10.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhmann A, Niemann H, Lammering B, Henkel C, Hess I, Nitzki F, et al. Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of hedgehog signaling and induction of vitamin D receptor signaling and differentiation. Mol Cancer Ther. 2011;10:2179–88. doi: 10.1158/1535-7163.MCT-11-0422. [DOI] [PubMed] [Google Scholar]
- 10.Ma J, Stampfer MJ, Gann PH, Hough HL, Giovannucci E, Kelsey KT, et al. Vitamin D receptor polymorphisms, circulating vitamin D metabolites, and risk of prostate cancer in United States physicians. Cancer Epidemiol Biomarkers Prev. 1998;7:385–90. [PubMed] [Google Scholar]
- 11.Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–8. [PubMed] [Google Scholar]
- 12.Jacobs ET, Giuliano AR, Martinez ME, Hollis BW, Reid ME, Marshall JR. Plasma levels of 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and the risk of prostate cancer. J Steroid Biochem Mol Biol. 2004;89–90:533–7. doi: 10.1016/j.jsbmb.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 13.Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, et al. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41:1164–9. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali MM, Vaidya V. Vitamin D and cancer. J Cancer Res Ther. 2007;3:225–30. doi: 10.4103/0973-1482.38998. [DOI] [PubMed] [Google Scholar]
- 16.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30:113–25. doi: 10.1111/j.1365-2036.2009.04022.x. [DOI] [PubMed] [Google Scholar]
- 17.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32:210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97:179–94. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, Mendoza-Cruz AC, et al. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011;56:830–6. doi: 10.1007/s10620-010-1544-3. [DOI] [PubMed] [Google Scholar]
- 21.Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308–16. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 22.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, et al. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep. 2009;22:1021–5. doi: 10.3892/or_00000530. [DOI] [PubMed] [Google Scholar]
- 24.Larriba MJ, Bonilla F, Munoz A. The transcription factors Snail1 and Snail2 repress vitamin D receptor during colon cancer progression. J Steroid Biochem Mol Biol. 2010;121:106–9. doi: 10.1016/j.jsbmb.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Palmer HG, Larriba MJ, Garcia JM, Ordonez-Moran P, Pena C, Peiro S, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917–9. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 26.Pena C, Garcia JM, Silva J, Garcia V, Rodriguez R, Alonso I, et al. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: clinicopathological correlations. Hum Mol Genet. 2005;14:3361–70. doi: 10.1093/hmg/ddi366. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–28. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbera MJ, Puig I, Dominguez D, Julien-Grille S, Guaita-Esteruelas S, Peiro S, et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–54. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 29.Hotz B, Visekruna A, Buhr HJ, Hotz HG. Beyond epithelial to mesenchymal transition: a novel role for the transcription factor Snail in inflammation and wound healing. J Gastrointest Surg. 2010;14:388–97. doi: 10.1007/s11605-009-1068-3. [DOI] [PubMed] [Google Scholar]
- 30.Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem. 2008;283:33437–46. doi: 10.1074/jbc.M802016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175–83. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy HK, Smyrk TC, Koetsier J, Victor TA, Wali RK. The transcriptional repressor SNAIL is overexpressed in human colon cancer. Dig Dis Sci. 2005;50:42–6. doi: 10.1007/s10620-005-1275-z. [DOI] [PubMed] [Google Scholar]
- 33.Pena C, Garcia JM, Larriba MJ, Barderas R, Gomez I, Herrera M, et al. SNAI1 expression in colon cancer related with CDH1 and VDR downregulation in normal adjacent tissue. Oncogene. 2009;28:4375–85. doi: 10.1038/onc.2009.285. [DOI] [PubMed] [Google Scholar]
- 34.Larriba MJ, Martin-Villar E, Garcia JM, Pereira F, Pena C, de Herreros AG, et al. Snail2 cooperates with Snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis. 2009;30:1459–68. doi: 10.1093/carcin/bgp140. [DOI] [PubMed] [Google Scholar]
- 35.Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87–92. doi: 10.1136/gut.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450–9. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 37.Moolenbeek C, Ruitenberg EJ. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15:57–9. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 38.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–33. [PubMed] [Google Scholar]
- 39.Osburn WO, Karim B, Dolan PM, Liu G, Yamamoto M, Huso DL, et al. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer. 2007;121:1883–91. doi: 10.1002/ijc.22943. [DOI] [PubMed] [Google Scholar]
- 40.Goff JP, Koszewski NJ, Haynes JS, Horst RL. Targeted delivery of vitamin D to the colon using beta-glucuronides of vitamin D: therapeutic effects in a murine model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2012;302:G460–9. doi: 10.1152/ajpgi.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–70. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35:217–24. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]
- 43.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 44.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–32. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–92. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 46.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]