Abstract
The imatinib paradigm in chronic myeloid leukemia (CML) established continuous BCR-ABL inhibition as a design principle for ABL tyrosine kinase inhibitors (TKIs). However, clinical responses seen in patients treated with the ABL TKI dasatinib despite its much shorter plasma half-life and the apparent rapid restoration of BCR-ABL signaling activity following once-daily dosing suggested acute, potent inhibition of kinase activity may be sufficient to irrevocably commit CML cells to apoptosis. To determine the specific requirements for ABL TKI-induced CML cell death for a panel of clinically important ABL TKIs (imatinib, nilotinib, dasatinib, ponatinib, and DCC-2036), we interrogated response of CML cell lines and primary CML cells following acute drug exposure using intracellular FACS and immunoblot analyses of BCR-ABL signaling, apoptosis measurements, liquid chromatography/tandem mass spectrometry of intracellular drug levels, and biochemical TKI dissociation studies. Importantly, significant intracellular TKI stores were detected following drug washout, levels of which tracked with onset of apoptosis and incomplete return of BCR-ABL signaling, particularly pSTAT5, to baseline. Among TKIs tested, ponatinib demonstrated the most robust capacity for apoptotic commitment showing sustained suppression of BCR-ABL signaling even at low intracellular levels following extensive washout, consistent with high-affinity binding and slow dissociation from ABL kinase. Together, our findings suggest commitment of CML cells to apoptosis requires protracted incomplete restoration of BCR-ABL signaling mediated by intracellular retention of TKIs above a quantifiable threshold. These studies refine our understanding of apoptotic commitment in CML cells and highlight parameters important to design of therapeutic kinase inhibitors for CML and other malignancies.
INTRODUCTION
The clinical success of imatinib in chronic myeloid leukemia (CML) represents a hallmark in tyrosine kinase inhibitor (TKI) therapy for the treatment of cancer. Design and development efforts of additional TKIs in CML (1-5) and other cancers (6, 7) have emulated and attempted to improve upon imatinib’s favorable specificity, tolerability, and pharmacokinetics properties. Among those properties, the rationale behind dosing requirements for TKIs has received recent attention. Pre-clinical studies with imatinib established concentrations of at least 1 μM sustained for at least 16 h as threshold conditions for irreversibly committing CML cell lines to apoptotic death (8). Coupled with subsequent data from phase 1 clinical trials of imatinib which identified a plasma half-life of ~18 h and found significant responses in patients with plasma trough levels greater than 1 μM (9), the imatinib paradigm suggested continuous complete BCR-ABL inhibition as a design principle for ABL TKIs.
In contrast, pre-clinical and subsequent clinical evaluation of the second-generation ABL TKI dasatinib found impressive, durable responses with once-daily dosing regimens, despite a much shorter plasma half-life (3-5 h) and rapid restoration of BCR-ABL activity in vivo (10, 11). A further phase 3 comparison of once- versus twice-daily dasatinib in CML revealed comparable cytogenetic and molecular response rates, with the benefit of reduced incidence of toxicity with the once-daily schedule (12). The finding that clinical efficacy can be maintained despite only transiently inhibiting BCR-ABL signaling opens an opportunity to study the mechanistic requirements for ABL TKI-induced CML cell death.
We and others have previously shown commitment of CML cells to apoptosis following potent, transient target inhibition with ABL TKIs in vitro (13-15), although differences between concentrations required to produce this effect and their relative activity against BCR-ABL kinase suggest potential involvement of previously unrecognized factors. One hypothesis, referred to as the oncogenic shock premise, holds that intense, temporary disruption of BCR-ABL activity sets up a kinetic imbalance between prosurvival and proapoptotic signaling favoring the latter, the consequence of which is irreversible commitment to apoptosis (16, 17).
We report a mechanistic evaluation encompassing transient exposure of CML cells to a panel of FDA-approved ABL TKIs (imatinib, nilotinib, dasatinib, ponatinib (AP24534) (2, 18), as well as DCC-2036 (rebastinib), which is entering Phase 2 trials (3, 19). After transient exposure of cells to each of these agents, we interrogate response using multi-parameter intracellular FACS and immunoblot analyses, apoptosis measurements, liquid chromatography tandem mass spectrometry (LC/MS/MS), and biochemical dissociation studies of ABL from ABL TKIs. In aggregate, our findings reveal that attenuated restoration of BCR-ABL signaling correlates with apoptosis commitment and that intracellular retention of ABL TKIs above a quantifiable threshold is a critical, previously unrecognized parameter mediating this effect.
MATERIAL AND METHODS
Inhibitors
All inhibitors were prepared as 10 mM stock solutions in DMSO and stored at −20 °C. Serial dilutions of stock solutions were carried out just prior to use in each experiment.
Cell lines
Certified BCR-ABL-positive human CML blast-crisis-derived K562 (ATCC) and LAMA-84 cells (DSMZ) were maintained in RPMI 1640 supplemented with 10% FBS, 1 unit/mL penicillin G, and 1 mg/mL streptomycin (complete media) at 37 °C and 5% CO2. Neither of the cell lines used in this study was cultured for longer than 6 months from initial purchase or characterization. No further authentication of cell lines characteristics was done.
Collection of patient samples
Clinical samples were obtained with informed consent and under the approval of the OHSU Institutional Review Board. Bone marrow from patients was separated on a Ficoll gradient (GE Healthcare) for isolation of mononuclear cells.
Inhibitor washout protocol for CML cell lines
K562 and LAMA-84 cells (5 × 105/mL) were incubated in complete media alone or with dasatinib (10 or 100 nM), ponatinib (10 or 100 nM), nilotinib (500 or 5000 nM), imatinib (500 or 5000 nM), or DCC-2036 (500 or 5000 nM) for 2 h at 37 °C. Following drug exposure, cells were subjected to a series of one of two types of wash steps: resuspension in fresh inhibitor-free complete media only (quick wash) or resuspension in fresh media followed by a 5 min incubation at 37 °C (long wash). The standard washout protocol (defined to match our previously reported studies (13)) consisted of two quick washes followed by one long wash step. The expanded washout protocol involved an additional five wash steps (alternating long and quick washes) beyond the standard washout. Samples for apoptosis and inhibitor LC/MS/MS analyses were collected from each treatment condition at the end of the 2 h exposure, immediately following standard washes, and immediately following extra washes. Samples for immunoblot and FACS analyses were collected prior to and at multiple time points following standard and expanded washout.
Inhibitor washout protocol for primary CML cells
Mononuclear cells (5 × 105 cells/mL) from CML patients were incubated in complete media (supplemented with 100 μM β-mercaptoethanol) alone or with ABL TKIs (same concentrations as for cell line protocol above) for 4 h at 37 °C. Following drug exposure, cells were subjected to the standard or expanded washout protocol (identical to those performed in cell lines). Samples for apoptosis and inhibitor LC/MS/MS analyses were collected from each treatment condition at the end of the 4 h exposure and immediately following standard and expanded washout. Samples for immunoblot and FACS analyses were collected at the end of the 4 h exposure and at 24 h post standard and expanded washout.
Apoptosis assay
At each relevant time point within the washout protocols described above, cells from each treatment condition were plated in triplicate in a 96-well plate (5 × 104/well), incubated at 37 °C, and apoptosis was measured at 72 h after the start of the experiment using the Guava Nexin assay (Millipore). Results are reported as the mean percent annexin-V positivity; error bars represent S.E.M.
Immunoblot analysis for CrkL phosphorylation
For CML cell lines, cells were collected for each treatment condition at 0, 0.5, 2, 4, 8, 12, 24, 30, and 36 h post either standard or expanded washout (as described above) and lysed (5 × 105 cells/30 μL) in boiling SDS-PAGE loading buffer supplemented with protease and phosphatase inhibitors for 10 min. All lysates were subjected to SDS-PAGE and immunoblotted with anti-CrkL antibody C20 (Santa Cruz). Phosphorylated and nonphosphorylated CrkL forms were distinguished based on differential band migration, and band signal intensities were quantified by densitometry on a Lumi Imager (Roche) and expressed as percent phosphorylated CrkL (calculated as [pCrkL]/[pCrkL + non-pCrkL] × 100%).
Phosflow analysis
For CML cell lines, cells were collected for each treatment condition at the end of the 2 h drug exposure and at 0, 0.5, 2, 4, and 8 h post either standard or expanded washout (as described above). For experiments involving primary CML mononuclear cells, where sufficient cell numbers were available, samples were collected at the end of the 4 h drug exposure and at 24 h post standard and expanded washout (see above protocol). Samples were prepared by incubating cells (1 × 106) in Cytofix buffer (BD) at 37 °C for 15 min and resuspending in PBS (Gibco) supplemented with 10% DMSO for storage at −80 °C prior to analysis. Upon thawing of samples, fixed cells were permeabilized with Perm Buffer III (BD) for 30 min on ice. Samples were stained for pCrkL (Alexa647) and pSTAT5 (Alexa488) and analyzed using a Canto II instrument (BD) and FlowJo or FCS Express (De Novo Software).
Detection of inhibitors by LC/MS/MS
For CML cell lines, cellular and media fractions were separated by centrifugation for each treatment condition at the end of the 2 h drug exposure, immediately following standard and expanded washout (as described above), and at 2 h after the expanded washout. For experiments involving primary CML mononuclear cells, where sufficient cell numbers were available, cellular and media fraction samples were collected at the end of the 4 h drug exposure and immediately following standard and expanded washout. Cellular fraction lysate samples were prepared by washing cells (1 × 106) once with PBS, resuspending in hypotonic lysis buffer (5 mM Tris, 5 mM EDTA, 5 mM EGTA in ddH2O; pH 7.0), shearing with a 27-gauge needle, and incubating on ice for 15 min. Samples were spun down and clarified lysates isolated. All samples (both cellular and media fractions) were stored at −80 °C prior to analysis. Amounts of dasatinib, ponatinib, nilotinib, imatinib, and DCC-2036 in cellular and media fractions were determined by LC/MS/MS using an adaption of the method of Haouala and colleagues (20); for full details, see Supplementary Material and Methods. Briefly, samples were thawed and extracted in acetonitrile containing internal standard (sorafenib) and analyzed using an Applied Biosystems/MDS SCIEX 5500QTRAP triple-quadrupole hybrid linear ion trap mass spectrometer. Compounds were quantified using multiple reaction monitoring (MRM). Results for intracellular inhibitor levels are reported in ng per 1 × 106 cells and concentrations of inhibitor in media are reported in nM.
ABL kinase:inhibitor dissociation studies
The rate of dissociation of dasatinib, ponatinib, nilotinib, imatinib, and DCC-2036 from ABL kinase was determined using the LanthaScreen® Eu Kinase Binding Assay (Life Technologies); staurosporine was also included as a control. For full details, see Supplementary Material and Methods. Briefly, full-length purified, recombinant His-tagged ABL kinase protein (either unaltered or tyrosine dephosphorylated) was pre-incubated with a biotin-labeled anti-His-tag antibody and Europium chelate-labeled streptavidin in saturating concentrations of dasatinib, ponatinib, nilotinib, imatinib, DCC-2036, or staurosporine for 2 h at room temperature. This mix was subsequently diluted into solution containing excess of Alexa647-labeled kinase ligand (Kinase Tracer 178), where dissociation of the inhibitor is followed by rapid binding of tracer. Upon tracer binding, productive TR-FRET signal was measured over time using a PHERAStar Plus instrument (BMG Labtech, Germany) to establish dissociation curves. Off-rate data are reported as t1/2 values in hours.
RESULTS
Commitment of BCR-ABL-Positive Cells to Apoptosis Following Acute Exposure to ABL TKIs Is Not Fully Explained By Relative Potency for BCR-ABL Inhibition
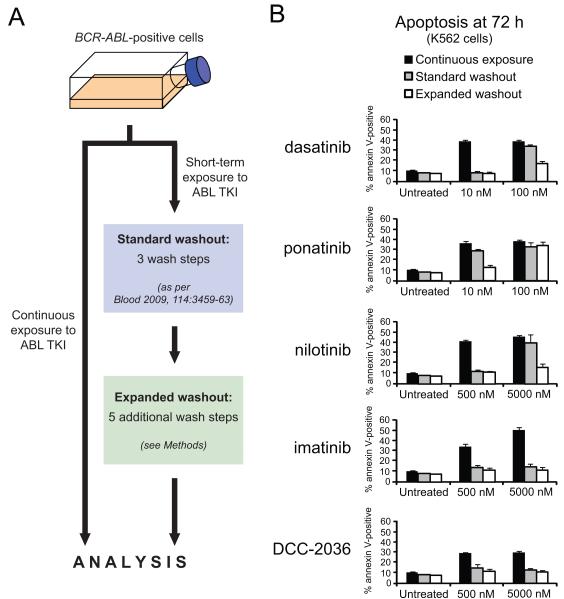
We established a two-tier washout system (Fig. 1A) to evaluate whether acute exposure to ABL kinase inhibitors is necessary and sufficient to irrevocably commit BCR-ABL-positive cells to apoptosis. In line with previous reports from our laboratory and others, continuous exposure of K562 cells to dasatinib at 10 nM or 100 nM resulted in substantial onset of apoptosis as measured by percentage of annexin V-positive cells (Fig. 1B) (13, 14). Implementation of our previously established in vitro standard washout procedure after two hours of exposure to dasatinib, designed to emulate the clinical situation of acute dasatinib exposure (serum half-life: 3-5 h) (13), abolished onset of apoptosis for cells treated with 10 nM dasatinib but only slightly diminished the extent of apoptosis induced in 100 nM dasatinib-treated cells compared to continuous exposure (Fig. 1B).
Figure 1. Commitment of BCR-ABL-positive cells to apoptosis following acute exposure to ABL TKIs varies by inhibitor, concentration, and extent of washout.
(A) Schematic outlining general experimental washout protocol. Briefly, BCR-ABL-positive cells were incubated in complete media alone or in the presence of each of five clinically relevant ABL TKIs either continuously (72 h) or for a short exposure (2 h for cell lines, 4 h for primary cells). For acute exposure conditions, cells were subjected to our previously published 3-wash-step protocol (standard washout) followed by an additional 5 wash steps (expanded washout) and cultured in fresh complete media for the remainder of the 72 h experiment. (B) Levels of apoptosis in K562 cells following continuous or acute exposure to ABL kinase inhibitors. Cells were incubated in the presence of the indicated inhibitor concentrations for 2 h, and samples were collected just prior to washout and immediately following standard and expanded washout. Annexin V-positivity was measured at 72 h after the start of the experiment, and bars represent the mean of at least three independent experiments performed in triplicate ± S.E.M.
Although dasatinib’s short 3-5 h plasma half-life is in sharp contrast to that of other clinically available ABL TKIs (t1/2 > 15 h) (9, 21), the variation in potency, target selectivity, and drug binding properties among these different inhibitors provides a valuable means for further interrogating the mechanisms responsible for apoptotic commitment following acute ABL TKI exposure in CML cells. Dasatinib and ponatinib represent highly potent, multi-kinase TKIs, whereas nilotinib, imatinib, and DCC-2036 are less potent TKIs with reduced off-target kinase profiles (2, 3, 22) (Supplementary Fig. S1). Thus, we carried out parallel washout experiments at physiologically relevant concentrations to examine the properties of each of these five clinically relevant ABL TKIs with respect to triggering of apoptosis. Among these, ponatinib is approximately equipotent with dasatinib as an ABL TKI (2) (Supplementary Fig. S1), suggesting that if absolute potency against BCR-ABL were the sole determinant of irrevocable commitment to apoptosis, the two TKIs would be expected to elicit similar concentration-dependent levels of apoptosis. However, we found acute exposure to 10 or 100 nM ponatinib followed by standard washout resulted in minimal or no reduction in apoptosis, respectively, compared to continuous exposure at these same concentrations (Fig. 1B). These differences between dasatinib and ponatinib were also observed for a second BCR-ABL-positive cell line (LAMA-84; Supplementary Fig. S2A).
Consistent with previous studies (13, 15), experiments involving the less potent, more restricted kinase off-target profile ABL TKI nilotinib confirmed a pattern of concentration-dependent commitment to apoptosis similar to dasatinib following standard washout protocol (Fig. 1B). In contrast, imatinib- and DCC-2036-induced apoptosis returned to approximately baseline levels observed in untreated cells following standard washout conditions even at high concentration (5000 nM); similar results and trends were obtained using LAMA-84 cells (Supplementary Fig. S3A). Notably, DCC-2036 is roughly comparable to nilotinib in terms of relative potency against BCR-ABL (19, 23) (Supplementary Fig. S1).
The intriguing differences in apoptosis-triggering capacity between TKIs with nearly identical equimolar potency against BCR-ABL led us to hypothesize that these distinct properties could potentially be attributable to fortuitous off-target effects that reinforce ABL TKI effectiveness in a multifactorial way or to differences in the extent of TKI removal from the cells following implementation of the same standard washout protocol. To investigate whether differences not addressed by the standard washout protocol could be discerned, an expanded washout protocol consisting of five additional wash steps beyond the standard three washouts was implemented (Fig. 1A). Strikingly, when the additional expanded washout protocol was applied to K562 cells initially treated with 100 nM dasatinib, a moderate reduction in the extent of apoptosis was achieved (Fig. 1B). This was also true for 10 nM ponatinib and 5000 nM nilotinib, where apoptosis levels were reduced to background levels matching the untreated control. By contrast, levels of apoptosis in cells treated with 100 nM ponatinib were unaffected by inclusion of the expanded washout steps (Fig. 1B). Similar results and trends were obtained for LAMA-84 cells (Supplementary Figs. S2A and S3A). These results suggest that a pool of drug remains sequestered within CML cells following acute exposure, and in the case of exposure to 100 nM ponatinib these levels remain above a threshold required to induce maximal apoptosis even after implementing the two-tier washout protocol. Taken together these data suggest that commitment of CML cells to apoptosis following acute exposure to ABL TKIs is 1) dependent upon the extent of drug washout and 2) not fully explained by absolute potency against BCR-ABL.
BCR-ABL Signaling is Not Fully Restored Under Conditions That Commit CML Cells to Apoptosis Following ABL TKI Exposure
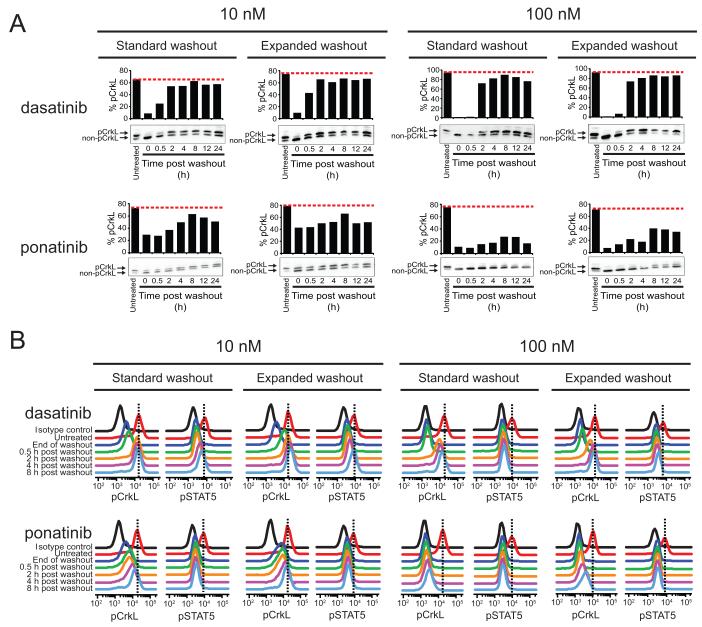
To explore this situation further in the context of BCR-ABL signaling, we conducted an assessment of each of these five ABL TKIs using a sensitive immunoblot assay that allows direct, same-lane comparison of tyrosine-phosphorylated and non-phosphorylated levels of the direct BCR-ABL substrate CrkL (24) and two intracellular FACS-based readouts of BCR-ABL tyrosine phosphorylation substrates (pSTAT5 and pCrkL).
Comparison of signaling events following acute exposure to dasatinib and ponatinib revealed fundamentally different situations (Fig. 2). In K562 cells exposed to dasatinib, treatment for 2 h with concentrations of 10 nM and 100 nM reduced pCrkL to <10% and nearly undetectable levels compared to untreated cells, respectively, as determined by immunoblot analysis (Fig. 2A). However, while both the standard and expanded washout procedures restored pCrkL signaling in cells treated with 10 nM dasatinib to levels comparable to pretreatment, the 100 nM dasatinib treatment followed by standard washout resulted in less rapid return of pCrkL signaling to levels approaching those of pretreatment. In all cases, the two-tier washout gave more consistent, slightly higher levels of pCrkL restoration compared to the standard washout protocol (though differences were not dramatic), with the substantial recovery of pCrkL signal occurring as early as the 2 h post-washout timepoint. Although pCrkL signaling differences were slight for the standard versus expanded washout for 100 nM dasatinib (Fig. 2A), given the apparent difference in the extent of apoptosis (Fig. 1B) we more quantitatively explored the relationship between even slightly attenuated signal restoration and extent of apoptosis using intracellular FACS analysis of pCrkL and pSTAT5. The tyrosine-phosphorylation status of STAT5 provides a more sensitive surrogate measure of extent of BCR-ABL signaling restoration (25), and by extension, is a better indicator than pCrkL for low levels of residual TKI. Importantly, we found pSTAT5 signal in the 100 nM dasatinib treated cells showed a greater degree of restoration at every timepoint post-expanded washout than the corresponding standard washout timepoint (Fig. 2B and Supplementary Table S1), and yet signal was never fully restored, consistent with results in Fig. 1B.
Figure 2. BCR-ABL signaling activity is not fully restored to baseline levels in treatment/washout conditions of dasatinib and ponatinib that commit cells to apoptosis.
K562 cells were incubated alone or in the presence of 10 and 100 nM dasatinib or ponatinib for 2 h, subjected to standard and expanded washout, and collected at the indicated timepoint post washout for analysis by (A) immunoblot and (B) Phosflow FACS analyses. For immunoblot analyses, the phosphorylated and non-phosphorylated forms of CrkL were resolved by SDS-PAGE, blotted using a total CrkL antibody, and results are expressed as % pCrkL with the red, dashed line indicating the level of % pCrkL in untreated K562 cells. For Phosflow FACS analyses, cells were fixed, permeabilized, and stained using Alexa647-pCrkL and Alexa488-pSTAT5 conjugated antibodies. Results are displayed, for comparison purposes, as the overlaid signal peak traces of isotype control, untreated cells, and each indicated timepoint post washout. Vertical, black dashed lines highlight the peak signal in untreated K562 cells for reference.
In the case of ponatinib, pCrkL signal was only partially reduced (~25-50% of pretreatment levels) following 2 h treatment at a concentration of 10 nM, whereas 100 nM treatment inhibited pCrkL signal to essentially undetectable levels by immunoblot (Fig. 2A). Upon washout under the standard or expanded protocol, limited restoration of pCrkL levels was achieved in cells treated with 10 nM ponatinib despite substantial reduction in the extent of apoptosis in the case of the expanded washout compared to the standard washout protocol. Once again, however, this subtle difference was most discernible in the levels of pSTAT5 (Fig. 2B and Supplementary Table 1). In marked contrast to findings for dasatinib, upon washout of 100 nM ponatinib under the standard or expanded protocol, minimal restoration was observed as monitored by pCrkL immunoblot or FACS measurements of intracellular pCrkL and pSTAT5 (Fig. 2 and Supplementary Table 1), suggesting that TKI cannot be washed out readily and that BCR-ABL remains inhibited.
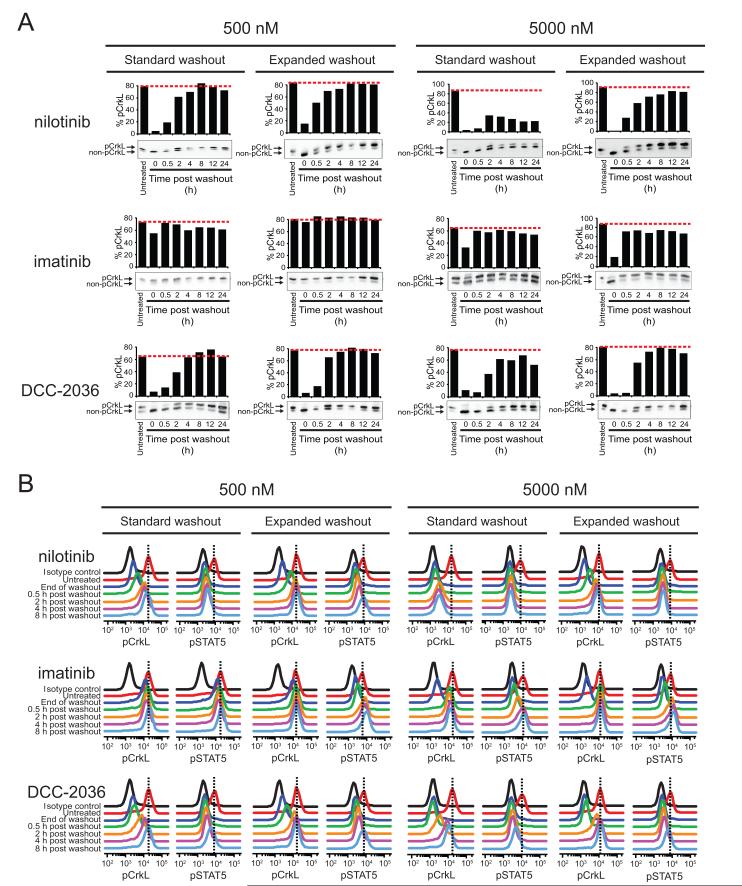
Exposure of K562 cells to nilotinib for 2 h resulted in near complete pCrkL signal blockade with 500 nM and complete interruption with 5000 nM, although substantial restoration of pCrkL was only apparent in cells treated with 500 nM nilotinib following standard washout protocol as gauged by immunoblot analysis (Fig. 3A). Consistent with apoptosis data, the expanded washout protocol resulted in pCrkL levels approximating pretreatment levels as judged by FACS for both concentrations tested (Fig. 3B). In contrast to nilotinib but consistent with our earlier apoptosis findings, acute exposure to either 500 or 5000 nM imatinib demonstrated minimal or partial interruption of pCrkL signal, respectively, which was subsequently restored to levels at or near pretreatment following standard and expanded washout protocols (Fig. 3A); complete restoration was apparent for both pCrkL and pSTAT5 by FACS (Fig. 3B and Supplementary Table 1). Lastly, despite showing significant signal blockade at the end of drug exposure, cells treated with 500 nM DCC-2036 showed rapid and complete restoration following both wash regimens. However, cells treated with 5000 nM DCC-2036 showed similar trends in signaling inhibition as nilotinib. The extent of signaling recovery after standard washout conditions was considerably greater for DCC-2036 than for nilotinib by immunoblot and FACS (Fig. 3), consistent with differences in apoptotic commitment (Fig. 1B). Similar results and trends were seen using LAMA-84 cells (Supplementary Figs. S2A,C and S3A,C). Together, these findings suggest that apoptotic commitment in CML cells tracks with incomplete restoration of BCR-ABL signaling and, for certain TKIs, can be rescued with a more thorough washout protocol, implicating drug retention properties of ABL TKIs as a mediator of this effect.
Figure 3. Extent of restoration of BCR-ABL signaling activity following washout of less potent, more selective ABL TKIs tracks with conditions committing cells to apoptosis.
K562 cells were incubated alone or in the presence of 500 and 5000 nM nilotinib, imatinib, or DCC-2036 for 2 h, subjected to standard and expanded washout, and collected at the indicated timepoints post washout for analysis by (A) immunoblot and (B) Phosflow FACS analyses. For immunoblot analyses, the phosphorylated and non-phosphorylated forms of CrkL were resolved by SDS-PAGE, blotted using a total CrkL antibody, and results are expressed as % pCrkL with the red, dashed line indicating the level of % pCrkL in untreated K562 cells. For Phosflow FACS analyses, cells were fixed, permeabilized, and stained using Alexa647-pCrkL and Alexa488-pSTAT5 conjugated antibodies. Results are displayed, for comparison purposes, as the overlaid signal peak traces of isotype control, untreated cells, and each indicated timepoint post washout. Vertical, black dashed lines highlight the peak signal in untreated K562 cells for reference.
ABL TKIs Are Retained Intracellularly Following Drug Washout and Intracellular TKI Levels Track With Apoptosis and Signaling Inhibition
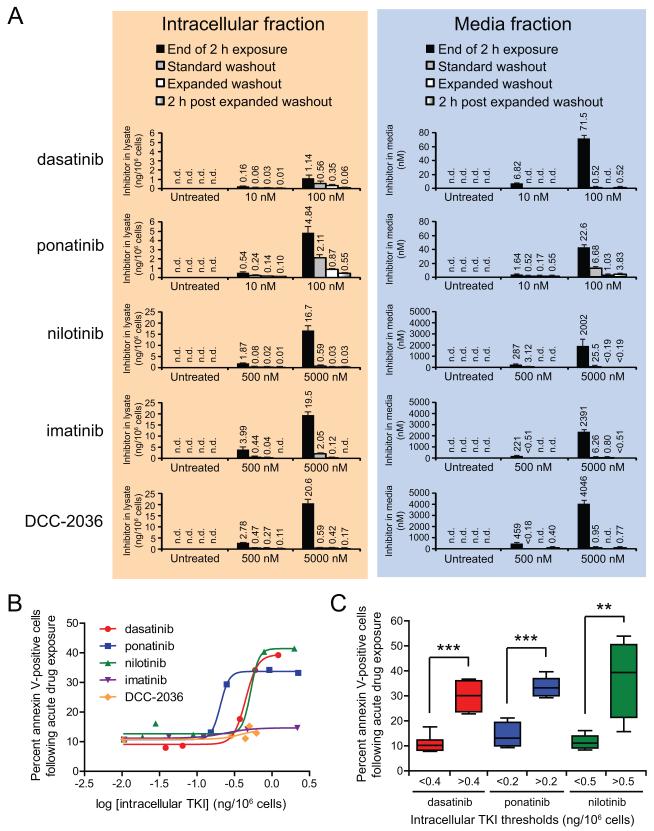
The potential existence of a functionally important, difficult-to-remove residual intracellular drug pool has major consequences for inhibitor design. To detect and quantitatively determine the amount of residual TKI present in CML cells following acute drug exposure, we developed a highly sensitive liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS)-based assay for each of the five ABL TKIs under investigation and collected intracellular and media fractions for evaluation. This analysis revealed dramatic variance in levels of retained TKI in K562 cells among the five inhibitors following both standard and expanded washout after acute drug exposure (Fig. 4A; left panel). For 10 nM dasatinib treatment, intracellular levels were 0.06 and 0.04 ng/106 cells after standard and expanded washout protocols, respectively (Fig. 4A; left panel), and these both corresponded with background levels of apoptosis (Figs. 1B). In contrast, cells exposed for 2 h to 100 nM dasatinib demonstrated intracellular levels of 0.56 and 0.35 ng/106 cells after standard and expanded washout protocols, respectively, with both conditions associated with concentration-dependent increased levels of apoptosis (Fig. 1B). Further regression analysis of the association between intracellular TKI levels post-washout and levels of apoptosis revealed a non-linear, concentration-dependent relationship consistent with intracellular TKI levels above a critical threshold being necessary for commitment to apoptosis (Fig. 4B). Thus, the LC/MS/MS technique is capable both of establishing a threshold for onset of apoptosis and of quantitating and distinguishing differences in amounts of functionally important residual TKI.
Figure 4. ABL TKIs are retained intracellularly following washout of drug from culture media and define thresholds of apoptotic commitment.
(A) Residual levels of ABL TKIs detected post washout in isolated intracellular and media fractions. Following acute (2 h) exposure to ABL TKIs, K562 cells were collected just prior to washout, immediately following standard and expanded washout, and 2 h after completion of expanded washout. Cellular and media fractions were isolated by centrifugation, and cells were washed in PBS and subjected to hypotonic lysis on ice. Clarified intracellular lysate and culture media samples were analyzed for levels of ABL TKIs by LC/MS/MS and results are reported as the mean ng/106 cells and nM in media, respectively, of at least three replicate experiments ± S.E.M. Data labels of “n.d.” indicate “not detected” values preceded by a “<” symbol indicate detection of a low level peak, but below the lower limit of quantitation (LLOQ). (B) Relationship between intracellular TKI levels and levels of apoptosis following drug washout. Intracellular levels of ABL TKIs detected in post-standard or post-expanded washout samples were log-transformed and plotted against percent of annexin V-positive cells measured at 72 h after the start of the experiment. Non-linear regression curve-fitting was done using Graphpad Prism software. (C) Intracellular threshold levels of dasatinib, ponatinib, and nilotinib following drug washout. Intracellular TKI levels and apoptosis data for all samples collected post-standard or post-expanded washout were divided according to the indicated intracellular TKI threshold values and compared using a two-tailed Student’s t-test. Statistically significant p-values less than 0.01 or 0.001 are denoted by 2 or 3 asterisks, respectively.
Given ponatinib’s pronounced capacity for inducing apoptosis and for sustaining marked inhibition of BCR-ABL signaling despite extensive drug washout, ponatinib intracellular drug retention was compared with dasatinib (which features comparable molecular weight and potency for BCR-ABL to that of ponatinib), revealing multiple differences. First, despite a 10-fold difference in initial exposure concentration, results with 10 nM ponatinib treatment were similar to those achieved with 100 nM dasatinib: ponatinib intracellular levels were 0.24 and 0.14 ng/106 cells following standard and expanded washout, respectively (Fig. 4A; left panel), with the latter inducing apoptosis only slightly above background (Fig. 1B). Second, cells treated with 100 nM ponatinib retained substantially more drug following the acute exposure period compared to 100 nM dasatinib treatment such that reduced intracellular ponatinib levels achieved with the standard and expanded wash protocols (2.11 and 0.87 ng/106 cells, respectively) did not produce a significant reduction in the level of apoptosis compared to continuous treatment and only minimally restored BCR-ABL signaling (Figs. 1B and 2).
Among the three tested less potent, more selective ABL TKIs, K562 cells most readily retained imatinib following standard and expanded washout of 5000 nM treatment (Fig. 4A; left panel), although given the approximately 5- to 20-fold reduced potency of imatinib compared to either nilotinib or DCC-2036 (19, 23) (Supplementary Fig. S1), these intracellular levels of imatinib were not sufficient to induce significant apoptosis above background (Figs. 1B and 4B). Comparison of nilotinib and DCC-2036 also highlighted fundamental differences in the properties of these two inhibitors in that treatment with 5000 nM of either TKI resulted in equal amounts of drug retained after standard washout (Fig. 4A; left panel), yet only nilotinib under these conditions resulted in substantial apoptosis (Fig. 1B). Furthermore, intracellular nilotinib levels were markedly reduced after expanded versus standard washout (0.03 vs. 0.59 ng/106 cells, respectively), which tracked with abrogation of apoptosis induction. Similar intracellular TKI retention findings were obtained with all tested inhibitors using LAMA-84 cells (Supplementary Figs. S2B and S3B). These findings suggest that either the majority of the residual TKI pool retained within a CML cell following washout is not bound to BCR-ABL or that auxiliary, off-target kinase pathways may also help mediate commitment to apoptosis.
To rule out the possibility that, either by equilibration with intracellular stores and/or despite extensive washout and several cycles of media and tissue culture plasticware exchange, the media still contained appreciable concentrations of TKI sufficient to produce an unrecognized continuous exposure situation, we determined the minimum media concentration of each TKI sufficient to trigger apoptosis under continuous exposure conditions (Supplementary Fig. S4A). Thresholds for continuous media concentrations triggering substantial apoptosis (>50% that for the highest concentration tested) were: 1 nM dasatinib, 2.5 nM ponatinib, 25 nM nilotinib, 500 nM imatinib, and 50 nM DCC-2036. For dasatinib, imatinib, and DCC-2036, TKI in the media fraction was either not detected or below the lower limit of quantitation (LLOQ) of the LC/MS/MS-based assay subsequent to either wash protocol (Fig. 4A; right panel). In conditions following standard or expanded washout where media TKI was detected, concentrations were well below the aforementioned thresholds for triggering substantial apoptosis (Supplementary Fig. S4A), consistent with residual intracellular stores serving as the source of functionally important TKI. In contrast, while media TKI concentrations detected following washout of lower treatment concentrations of ponatinib and nilotinib were below thresholds inducing substantial apoptosis, media levels of TKI for 100 nM ponatinib and 5000 nM nilotinib following standard washout protocol were 6.68 and 25.5 nM, respectively, likely reflecting slow equilibration of high-level intracellular stores (Fig. 4A; right panel). Media TKI levels for both drugs were reduced to below apoptosis-inducing thresholds after implementing the expanded washout.
The substantial intracellular stores of TKI we observed following acute drug exposure and the media concentrations of ponatinib and nilotinib detected post-standard washout suggested that drug retained intracellularly may also continue to leach out of the cells after washout completion. To confirm this, we also collected intracellular and media fractions from each treatment condition at 2 h after completion of the expanded washout protocol. In every case, intracellular concentration of TKI was decreased relative to that immediately after washout completion (Fig. 4A; left panel) and paired with a concomitant slight increase in TKI levels in the media (Fig. 4A; right panel). These data suggest that detectable concentrations of ABL TKIs in the media following washout are likely attributable to variable kinetics of drug slowly escaping from intracellular stores, possibly as apoptosis takes place.
Primary CML Cells Demonstrate Intracellular TKI Retention-Dependent Commitment to Apoptosis and Highlight Prolonged Efficacy of Ponatinib
To confirm that our findings from CML cell lines regarding intracellular TKI retention and commitment to apoptosis extend to primary CML cells, we performed similar washout studies using mononuclear cells isolated from newly diagnosed CML patients. Absolute levels of apoptosis achieved with continuous exposure were slightly less in primary cells compared to those observed with cell lines. Consistent with trends observed with K562 and LAMA cells, only dasatinib, ponatinib, and nilotinib triggered substantial apoptosis over untreated following standard washout, and among these, only ponatinib (100 nM) induced substantial, though slightly reduced, levels of apoptosis following the expanded washout protocol (Supplementary Fig. S5). Also in register with CML cell line findings, levels of each TKI retained intracellularly were serially reduced following the standard and expanded washout protocols, which paralleled ultimate rescue of apoptotic commitment for all inhibitors except ponatinib (Supplementary Fig. S5).
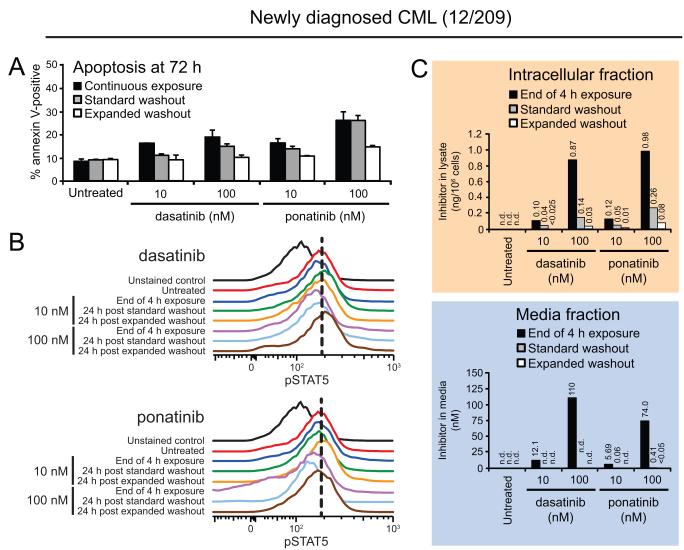
Based on the informative differences we observed between equimolar treatments of dasatinib and ponatinib in cell lines, we further examined this specific comparison in washout experiments using primary cells from an additional newly diagnosed CML patient (12/209; Fig. 5). Findings for 10 nM ponatinib mirrored those of 100 nM dasatinib with a modest decrease in apoptosis levels following standard washout and subsequent return to baseline levels following expanded washout. Cells treated with 100 nM ponatinib showed increased levels of apoptosis overall that were only reduced following expanded washout, and even then not completely back to baseline (Fig. 5A).
Figure 5. Commitment to apoptosis in primary CML cells depends on intracellular retention and extent of washout of ABL TKIs, underscoring prolonged efficacy of ponatinib.
Mononuclear cells from a patient with newly diagnosed CML (12/209) were incubated for 4 h in the presence 10 and 100 nM dasatinib or ponatinib. Cells were collected prior to washout and following standard and expanded washout and analyzed for (A) apoptosis, (B) BCR-ABL signaling inhibition, and (C) residual TKI levels in the cells and culture media. Apoptosis was measured by annexin V-positivity at 72 h after the start of the experiment, and results represent the mean of three replicates ± S.E.M. For Phosflow FACS analyses, cells were fixed, permeabilized, and stained using an Alexa488-pSTAT5 conjugated antibody. Results are displayed, for comparison purposes, as the overlaid signal peak traces of unstained control, untreated cells, and 24 h post standard or expanded washout. Vertical, black dashed lines highlight the peak signal in untreated K562 cells for reference. Cellular and media fractions were isolated by centrifugation, and cells were washed in PBS and subjected to hypotonic lysis on ice. Clarified intracellular lysate and culture media samples were analyzed for TKI levels by LC/MS/MS and results are reported as ng/106 cells and nM in media, respectively. Data labels of “n.d.” indicate “not detected” values preceded by a “<” symbol indicate detection of a low level peak, but below the lower limit of quantitation (LLOQ).
As our previous findings in K562 and LAMA cells suggested pSTAT5 represents a more sensitive biomarker for resolving subtle differences in BCR-ABL signal attenuation and given that absolute levels of apoptosis following continuous dasatinib or ponatinib treatment in primary CML samples were slightly lower at 72 h compared to those seen with CML cell lines, we focused our FACS-based analysis on pSTAT5 levels. As expected, absolute differences in signal shift were reduced compared with CML cell lines but trends were consistent, with pSTAT5 inhibition following acute 100 nM dasatinib treatment (4 h) partially and fully rescued to pretreatment levels by 24 h post standard and expanded washouts, respectively (Fig. 5B; upper panel). In contrast, in cells treated with 100 nM ponatinib, pSTAT5 remained inhibited at 24 h after standard washout at levels comparable to those just prior to washout under treatment. Levels of pSTAT5 approaching but not quite reaching those of untreated cells were observed by 24 h post expanded washout (Fig. 5B; lower panel).
Consistent with the slightly reduced absolute numerical differences but preserved data trends between CML cell lines and primary CML cells, we observed slightly increased intracellular retention of ponatinib versus dasatinib, with post-washout reduction in intracellular levels of both TKIs tracking with varying levels of pSTAT5 signal restoration and apoptosis (Fig. 5C). Notably, even intracellular levels of ponatinib as low as 0.05 ng/106 cells post-washout were sufficient to induce a substantial increase in apoptosis; in contrast, this threshold for dasatinib was approximately 2- to 3-fold higher post-washout (Fig. 5C). Taken together, these findings validate the relationship between apoptotic commitment, intracellular retention of ABL TKIs, and incomplete restoration of BCR-ABL signaling in primary patient cells and suggest that among the inhibitors tested, ponatinib demonstrates the most robust prolonged efficacy extending to intracellular levels of drug below the apoptosis-triggering thresholds of all four other ABL TKIs. This may suggest that a higher proportion of retained ponatinib is bound to BCR-ABL target than for other inhibitors.
Differences in Kinase:Inhibitor Dissociation Rates Among ABL TKIs Largely Parallel Acute Exposure Apoptosis Trends
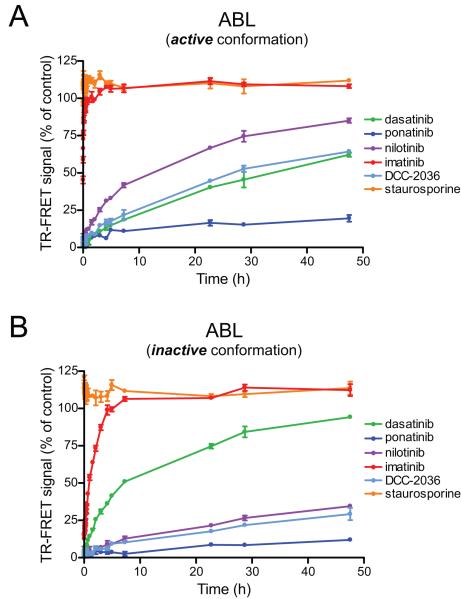
We observed through sensitive LC/MS/MS-based assays and apoptotic quantitation in both CML cell lines and primary CML cells that 1) different ABL TKIs are retained intracellularly to different extents post washout and 2) comparable intracellular amounts of approximately equipotent TKIs with respect to BCR-ABL do not ensure comparable apoptotic induction. One possible factor contributing to the variability in degree of washout efficiency between different TKIs is differences in binding properties of each TKI to the BCR-ABL kinase domain, with the presumption that kinase-bound inhibitor is less readily washed out of the cells. To explore this question, we utilized a time-resolved fluorescence energy transfer (TR-FRET)-based assay (see Methods) to evaluate relative rates of dissociation of all five ABL TKIs from purified ABL kinase (26). Among these inhibitors, imatinib and ponatinib represented the two extremes of very fast and extremely slow off-rates, respectively (Fig. 6), with ponatinib’s remarkably long residence time profile resembling that of irreversible kinase inhibitors. Dasatinib, nilotinib, and DCC-2036 dissociation kinetics were intermediate between imatinib and ponatinib. Differences in dissociation rates were observed between the catalytically active and inactive conformations of the ABL kinase domain, consistent with previously characterized crystallographic binding evidence for each compound (3, 27). These findings suggest that certain ABL TKIs, most notably ponatinib, may remain bound to BCR-ABL kinase for long periods following acute drug exposure, enabling prolonged intracellular retention and partial signal disruption after washout.
Figure 6. Dissociation parameters for inhibitor:ABL kinase binding suggest slower off-rates for ABL kinase inhibitors capable of committing cells to apoptosis following acute exposure and washout.
Purified recombinant ABL kinase protein (either unaltered or tyrosine dephosphorylated to represent the (A) active and (B) inactive conformations, respectively) was pre-incubated with a biotin-labeled antibody and Europium-labeled streptavidin in saturating concentrations of dasatinib, ponatinib, nilotinib, imatinib, DCC-2036, or staurosporine and diluted into solution containing excess Alexa-647-labeled kinase tracer. Dissociation of the inhibitor is followed by rapid binding of tracer, resulting in productive TR-FRET signal measured over time to establish dissociation curves.
DISCUSSION
The clinical success of the TKI paradigm in CML has motivated analogous strategies for many malignancies. As such, the ability of dasatinib to induce durable responses with once-daily dosing despite a much shorter plasma half-life than imatinib (3-5 h vs. >18 h) and subsequent rapid restoration of BCR-ABL activity (10, 11) has prompted investigation into the mechanistic requirements for ABL TKI-induced CML cell death.
In vitro drug washout protocols have previously demonstrated apoptotic commitment in CML cells following acute exposure to ABL TKIs despite only transient inhibition of BCR-ABL activity (13-15). Among the potential explanations offered for this phenomenon is the oncogenic shock hypothesis, which holds that temporary disruption of prosurvival and proapoptotic signaling sets up a kinetic imbalance that guarantees irreversible commitment to apoptosis (16, 17). Much like a tightrope walker who loses contact with the high wire, the cell’s fate is sealed upon satisfaction of a single condition. Enforcement of the concept of oncogenic shock as applied to ABL TKIs requires only that transient, potent interruption of BCR-ABL signaling is both necessary and sufficient to irrevocably commit CML cells to apoptosis.
In our studies in CML cells, the condition of complete interruption of BCR-ABL signaling was met following 2 h exposure to physiologically relevant concentrations of all TKIs except imatinib (at both 500 and 5000 nM for nilotinib and DCC-2036; at 100 nM for dasatinib and ponatinib; Figs. 2 and 3), yet this did not result in irrevocable onset of apoptosis in all cases, particularly following more extensive washout protocol (Fig. 1B). This suggests that the extent of ABL TKI-induced apoptosis can indeed be modulated following acute, potent target inhibition, thus calling the oncogenic shock explanation into question. Moreover, the oncogenic shock concept implies that levels of BCR-ABL signaling restoration following acute, potent target inhibition do not alter commitment to programmed cell death. In contrast, we found that sensitive monitoring of BCR-ABL activity (particularly as measured by levels of pSTAT5;Supplementary Table S1) revealed an association for all ABL TKIs between incomplete restoration of signaling and increased levels of apoptosis (Figs. 1B, 2, and 3). Importantly, while previous studies of acute dasatinib-induced cell death reported full (14) or approaching full (13) restoration of pCrkL levels rapidly following washout, extent of pSTAT5 signal restoration following washout was not evaluated. Notably, FACS analysis of pSTAT5 and pCrkL in ponatinib-treated cells at 24 h following washout revealed in select cases a subpopulation of cells undergoing early apoptosis that demonstrated reduced levels of both phospho-proteins (data not shown), resulting in a reduction in mean signal for the sample (Supplementary Table S1). However, we did not observe this to be a robust difference and this was less pronounced or not observed for other inhibitors at the same timepoint. Given our observations that for select TKI treatment conditions (including 100 nM dasatinib) pCrkL but not pSTAT5 signal was completely restored following washout (Fig. 2B and Supplementary Table S1), this suggests that STAT5 phosphorylation may serve as a more sensitive metric for monitoring low level changes in BCR-ABL signaling activity.
Investigation into the source of incomplete BCR-ABL signaling restoration following drug washout revealed varying levels of TKI retained within CML cells as detected by sensitive LC/MS/MS assay (Fig. 4A). Intracellular levels of TKI were reduced for all ABL TKIs after our standard washout protocol and reduced further following expanded washout, which tracked with rescue of BCR-ABL signaling activity and reduction in apoptosis (Figs. 1B, 2, 3, and Supplementary Table S1). These findings provide evidence for a functionally significant pool of residual TKI following drug washout capable of attenuating BCR-ABL signaling, consistent with results for low-level continuous inhibition of BCR-ABL (Supplementary Fig. S4B). Additionally, the non-linearity of the relationship between specific levels of intracellular ABL TKI and levels of apoptosis after washout suggests a threshold-based requirement for triggering apoptotic commitment in CML cells (Fig. 4B). In fact, for the three TKIs for which we observed a capacity for apoptotic induction following acute drug exposure at the physiologically-relevant concentrations tested (dasatinib, ponatinib, nilotinib), we used receiver-operator characteristic (ROC) analysis based on induction of apoptosis comparable to that of continuous drug exposure to help guide selection of threshold values and found post-washout samples with intracellular TKI levels above these thresholds to have significantly increased levels of apoptosis compared to those below the threshold (Fig. 4C). For example, standard washout of 10 nM dasatinib-treated cells apparently reduced the intracellular stores of TKI below an apoptosis-triggering threshold (Figs. 1B and 4). In contrast, standard washout following acute exposure to 10 nM ponatinib or 100 nM dasatinib reduced intracellular TKI to levels approaching but still above such a threshold for each drug, and 100 nM ponatinib-treated cells retained TKI at levels well above a threshold for apoptosis commitment, such that no reduction in apoptosis was discernible relative to continuous ponatinib treatment (Figs. 1B and 4). An inhibitory threshold that must be exceeded to commit CML cells to apoptosis is consistent with the oncogenic shock premise. However, our data suggest that the threshold is not dictated by potent, acute inhibition of BCR-ABL alone. At minimum, commitment to apoptosis also requires residual TKI levels sufficient to provide functionally relevant low-level continuous inhibition of BCR-ABL signaling.
Although dasatinib is the only one of the five TKIs we tested which features a short plasma half-life clinically (10, 11), many of the key findings from this study were made based on valuable comparisons to other ABL TKIs. To mimic the plasma pharmacokinetics of dasatinib for all ABL TKIs examined, our standard washout protocol was designed, through use of serial volume exchanges, to reduce initial media TKI levels to femtomolar concentrations, which is well below detection limits of LC/MS/MS. Thus, while for several treatment conditions (including 10 nM dasatinib) washout did reduce levels of TKI in the culture media to undetectable levels, it was somewhat surprising to find TKI present at very low concentrations in the media fraction following washout (Fig. 4, right panel). Although in almost all cases these media concentrations of TKI were alone too low to contribute significantly to increased apoptosis via continuous exposure in K562 cells (Supplementary Fig. 3A), the two exceptions were 100 nM ponatinib and 5000 nM nilotinib (Fig. 4, right panel) which demonstrated levels of 6.68 and 25.5 nM, respectively. Importantly, media levels of both drugs were reduced to sub-apoptosis-contributing levels following expanded washout, suggesting they cannot alone explain the induction of apoptosis under these conditions (Fig. 4, right panel and Supplementary Fig. 3A). Furthermore, rather than inefficiency of the drug washout protocol, the low levels of TKI detected in the media following washout may reflect differences in dynamics of cellular drug efflux mechanisms in response to significant intracellular levels of each drug (28-34) and/or differences in subcellular drug sequestration (35). Indeed, a recent study found that overexpression of the drug transporters ABCB1 or ABCG2 rescued K562 cells from apoptosis following exposure to very high concentrations of imatinib (36). However, while the cellular drug transporter-mediated uptake and efflux of imatinib has been reasonably well-studied (29, 33), application of these findings to newer ABL TKIs including dasatinib, nilotinib, and ponatinib has been limited due to differences in transporter substrate specificity (28, 30-32, 34). Regardless of the specific transport mechanisms, our findings that a measurable decrease in intracellular TKI levels at 2 h after the expanded washout was paralleled by a slight increase in levels of TKI in the media (Fig. 4A) are consistent with the cells as the source of media TKI post washout.
Our findings in CML cell lines of intracellular retention of ABL TKIs tracking with incomplete restoration of BCR-ABL signaling and commitment to apoptosis were also observed in similar drug washout experiments using primary CML cells (Fig. 5 and Supplementary Figs. S5 and S6). Although absolute differences in readouts for apoptosis and pSTAT5 signal were less than those observed with CML cell lines (Figs. 1B, 2B, 3B, and Supplementary Figs. S2A,C and S3A,C), all data trends were consistent. We have also previously shown that a modified standard washout protocol following acute exposure of CD34+ CML cells to dasatinib results in partial reduction in apoptosis and substantial but incomplete restoration of pCrkL and total phosphotyrosine levels (13).
Prolonged efficacy of ABL TKIs in CML cells following drug washout appears to be dependent not only on the amount of drug retained intracellularly but also on the residence time of the TKI bound to the kinase. Upon comparison of all five ABL TKIs in kinase:inhibitor dissociation studies, we observed striking differences in off-rate kinetics for different inhibitors (Fig. 6). Most notably, ponatinib demonstrated extremely slow dissociation from ABL kinase compared to the other inhibitors (Supplementary Table S2), consistent with increased intracellular retention and prolonged substantial inhibition of BCR-ABL signaling (Figs. 2 and 4) particularly as compared to dasatinib (2, 23). Previous crystallographic and in vitro cellular evidence suggests that the native c-ABL protein is normally tightly regulated within the cell by autoinhibitory mechanisms involving the N-terminal SH3, SH2, and cap domains, maintaining the kinase’s catalytically inactive conformation (37, 38). Furthermore, wild-type c-ABL has also been shown to exhibit greater protein stability than constitutively activated, tyrosine phosphorylated c-ABL in vitro (half-life: 18 vs. 7 h, respectively) (39). Interestingly, although imatinib demonstrated very rapid dissociation from ABL kinase, intracellular levels retained following either wash protocol were frequently ~3- to 4-fold greater than those for same tested concentration of nilotinib (Fig. 4A, left panel), suggesting potential differences in either drug efflux kinetics or subcellular sequestration (35). However, the lack of apoptotic induction even at these intracellular imatinib levels (Fig. 4B) is likely reflective of the significantly reduced inhibitory potency of imatinib relative to the other tested TKIs (Supplementary Fig. S1). Indeed, it has been previously shown that acute exposure of CML cells to physiologically non-achievable high concentrations of imatinib in vitro (selected to accommodate the fold difference in potency for BCR-ABL between dasatinib and imatinib) is capable of triggering apoptotic cell death (14, 36). Also intriguing was our observation that DCC-2036 exhibited similar off-rate kinetics from ABL kinase to those of nilotinib (Fig. 6 and Supplementary Table S2) (19, 23), though only nilotinib was capable of inducing substantially increased apoptosis in CML cells following acute drug exposure (Fig. 1B). One possible explanation is that DCC-2036, which binds a unique switch control pocket of ABL formed during the conformational transition between the catalytically active and inactive states (3), may initially be slower to bind BCR-ABL such that the 2 to 4 h drug exposure period was insufficient to resolve potential acute exposure efficacy. By contrast, continuous exposure for 72 h to 25-50 nM DCC-2036 induced substantial apoptosis in K562 cells over untreated (Supplementary Fig. S4A). Importantly, however, washout studies performed using cells from a CML blast crisis patient harboring a dominant BCR-ABLT315I mutation revealed decreased retention of dasatinib, nilotinib, and imatinib (in contrast to relatively unchanged levels of the ABLT315I inhibitors ponatinib and DCC-2036) after standard washout compared to similar experiments in primary CML cells harboring unmutated BCR-ABL (Supplementary Figs. S5 and S6), further highlighting the importance of drug binding in intracellular retention.
The findings of our comprehensive investigation into the requirements of ABL TKIs to commit CML cells to apoptosis following acute drug exposure underscore the importance of intracellular drug residence time. The drive to better understand and exploit the mechanisms of TKI uptake and retention has received considerable recent attention (4, 28-34, 36, 40-42). Our findings provide novel insights into the importance of previously unrecognized intracellular drug retention threshold levels required for apoptotic induction and identify ponatinib as a TKI with a kinase residence time profile reminiscent of irreversible kinase inhibitors (26, 43). This has particular application to drug design and development, where currently many compounds that may feature highly favorable target specificity and potency may be excluded from further development clinically based on plasma pharmacokinetic profiles. The oncogenic shock model would suggest that in order to maximize efficacy and minimize toxicity the key design features of novel compounds should be extreme kinase target potency and rapid clearance from both the plasma and tissues. By contrast, our findings heavily challenge the oncogenic shock concept by arguing strongly that absolute potency is not sufficient to mediate efficacy and that residual intracellular TKI above a critical threshold level is required. Furthermore, exploiting the basis of the extremely slow dissociation properties of TKIs like ponatinib may aid in maximizing efficacy of a small-molecule even at lower doses. While extensive studies have been performed confirming the therapeutic and prognostic value of monitoring drug levels in plasma in CML patients (9, 44, 45), this approach does not provide direct information as to the intracellular stores of drug, which appears to be of critical importance particularly in the case of TKIs featuring short plasma half-lives such as dasatinib. Consideration and monitoring of intracellular drug retention properties will yield improved candidate compounds for clinical development and complementary data for interpreting and defining rationale behind optimal dosing regimens for molecularly targeted therapies in CML and other malignancies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank all members of the Druker and Deininger research laboratories for valuable discussions and input and the University Shared Resource program at OHSU for support of the Bioanalytical Shared Resource/Pharmacokinetics Core facility.
GRANT SUPPORT
This work was supported by Howard Hughes Medical Institute, NIH/NCI MERIT award R37CA065823, and Leukemia & Lymphoma Society award 7005-11 (B.J.D.). We acknowledge support of funds in conjunction with grant P30 CA042014 awarded to Huntsman Cancer Institute (T.O.). J.W.T. is supported by grants from the William Lawrence and Blanche Hughes Fund, the Leukemia & Lymphoma Society, the V Foundation for Cancer Research, and NCI award 4R00CA151457-03.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
OHSU and B.J.D. have a financial interest in MolecularMD. Technology used in this research has been licensed to MolecularMD. This potential conflict of interest has been reviewed and managed by the OHSU Conflict of Interest in Research Committee and Integrity Oversight Council. OHSU also has clinical trial contracts with Novartis and Bristol-Myers-Squibb to pay for patient costs, nurse and data manager salaries, and institutional overhead. B.J.D. does not derive salary, nor does his lab receive funds, from these contracts. S.M.R., B.D.M., and K.W.V. are employees of Life Technologies, Inc. Data analysis by J.A. for this work was performed as an employee of BD Biosciences. M.W.D. served on advisory boards and as a consultant for Bristol-Myers-Squibb, ARIAD, and Novartis and receives research funding from Bristol-Myers-Squibb, Celgene, Genzyme, and YM BioSciences.
REFERENCES
- 1.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 2.O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer cell. 2009;16:401–12. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan WW, Wise SC, Kaufman MD, Ahn YM, Ensinger CL, Haack T, et al. Conformational control inhibition of the BCR-ABL1 tyrosine kinase, including the gatekeeper T315I mutant, by the switch-control inhibitor DCC-2036. Cancer cell. 2011;19:556–68. doi: 10.1016/j.ccr.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer cell. 2005;7:129–41. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 5.O’Hare T, Zabriskie MS, Eiring AM, Deininger MW. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nature reviews Cancer. 2012;12:513–26. doi: 10.1038/nrc3317. [DOI] [PubMed] [Google Scholar]
- 6.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. The Journal of pharmacology and experimental therapeutics. 2005;315:971–9. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 7.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–42. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 8.le Coutre P, Mologni L, Cleris L, Marchesi E, Buchdunger E, Giardini R, et al. In vivo eradication of human BCR/ABL-positive leukemia cells with an ABL kinase inhibitor. Journal of the National Cancer Institute. 1999;91:163–8. doi: 10.1093/jnci/91.2.163. [DOI] [PubMed] [Google Scholar]
- 9.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. The New England journal of medicine. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 10.Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:352–9. doi: 10.1158/1078-0432.CCR-07-4175. [DOI] [PubMed] [Google Scholar]
- 11.Luo FR, Yang Z, Camuso A, Smykla R, McGlinchey K, Fager K, et al. Dasatinib (BMS-354825) pharmacokinetics and pharmacodynamic biomarkers in animal models predict optimal clinical exposure. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:7180–6. doi: 10.1158/1078-0432.CCR-06-1112. [DOI] [PubMed] [Google Scholar]
- 12.Shah NP, Kantarjian HM, Kim DW, Rea D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:3204–12. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 13.Snead JL, O’Hare T, Adrian LT, Eide CA, Lange T, Druker BJ, et al. Acute dasatinib exposure commits Bcr-Abl-dependent cells to apoptosis. Blood. 2009;114:3459–63. doi: 10.1182/blood-2007-10-113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NP, Kasap C, Weier C, Balbas M, Nicoll JM, Bleickardt E, et al. Transient potent BCR-ABL inhibition is sufficient to commit chronic myeloid leukemia cells irreversibly to apoptosis. Cancer cell. 2008;14:485–93. doi: 10.1016/j.ccr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Hiwase DK, White DL, Saunders VA, Kumar S, Melo JV, Hughes TP. Short-term intense Bcr-Abl kinase inhibition with nilotinib is adequate to trigger cell death in BCR-ABL(+) cells. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23:1205–6. doi: 10.1038/leu.2009.45. [DOI] [PubMed] [Google Scholar]
- 16.Sharma SV, Fischbach MA, Haber DA, Settleman J. “Oncogenic shock”: explaining oncogene addiction through differential signal attenuation. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:4392s–5s. doi: 10.1158/1078-0432.CCR-06-0096. [DOI] [PubMed] [Google Scholar]
- 17.Sharma SV, Gajowniczek P, Way IP, Lee DY, Jiang J, Yuza Y, et al. A common signaling cascade may underlie “addiction” to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer cell. 2006;10:425–35. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. The New England journal of medicine. 2012;367:2075–88. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eide CA, Adrian LT, Tyner JW, Mac Partlin M, Anderson DJ, Wise SC, et al. The ABL switch control inhibitor DCC-2036 is active against the chronic myeloid leukemia mutant BCR-ABLT315I and exhibits a narrow resistance profile. Cancer research. 2011;71:3189–95. doi: 10.1158/0008-5472.CAN-10-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haouala A, Zanolari B, Rochat B, Montemurro M, Zaman K, Duchosal MA, et al. Therapeutic Drug Monitoring of the new targeted anticancer agents imatinib, nilotinib, dasatinib, sunitinib, sorafenib and lapatinib by LC tandem mass spectrometry. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2009;877:1982–96. doi: 10.1016/j.jchromb.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. The New England journal of medicine. 2006;354:2542–51. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 22.Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leukemia & lymphoma. 2008;49:615–9. doi: 10.1080/10428190801896103. [DOI] [PubMed] [Google Scholar]
- 23.O’Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer research. 2005;65:4500–5. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 24.La Rosee P, Holm-Eriksen S, Konig H, Hartel N, Ernst T, Debatin J, et al. Phospho-CRKL monitoring for the assessment of BCR-ABL activity in imatinib-resistant chronic myeloid leukemia or Ph+ acute lymphoblastic leukemia patients treated with nilotinib. Haematologica. 2008;93:765–9. doi: 10.3324/haematol.12186. [DOI] [PubMed] [Google Scholar]
- 25.Jacobberger JW, Sramkoski RM, Frisa PS, Ye PP, Gottlieb MA, Hedley DW, et al. Immunoreactivity of Stat5 phosphorylated on tyrosine as a cell-based measure of Bcr/Abl kinase activity. Cytometry A. 2003;54:75–88. doi: 10.1002/cyto.a.10063. [DOI] [PubMed] [Google Scholar]
- 26.Lebakken CS, Riddle SM, Singh U, Frazee WJ, Eliason HC, Gao Y, et al. Development and applications of a broad-coverage, TR-FRET-based kinase binding assay platform. Journal of biomolecular screening. 2009;14:924–35. doi: 10.1177/1087057109339207. [DOI] [PubMed] [Google Scholar]
- 27.Vajpai N, Strauss A, Fendrich G, Cowan-Jacob SW, Manley PW, Grzesiek S, et al. Solution conformations and dynamics of ABL kinase-inhibitor complexes determined by NMR substantiate the different binding modes of imatinib/nilotinib and dasatinib. The Journal of biological chemistry. 2008;283:18292–302. doi: 10.1074/jbc.M801337200. [DOI] [PubMed] [Google Scholar]
- 28.Davies A, Jordanides NE, Giannoudis A, Lucas CM, Hatziieremia S, Harris RJ, et al. Nilotinib concentration in cell lines and primary CD34(+) chronic myeloid leukemia cells is not mediated by active uptake or efflux by major drug transporters. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23:1999–2006. doi: 10.1038/leu.2009.166. [DOI] [PubMed] [Google Scholar]
- 29.Dohse M, Scharenberg C, Shukla S, Robey RW, Volkmann T, Deeken JF, et al. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug metabolism and disposition: the biological fate of chemicals. 2010;38:1371–80. doi: 10.1124/dmd.109.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannoudis A, Davies A, Lucas CM, Harris RJ, Pirmohamed M, Clark RE. Effective dasatinib uptake may occur without human organic cation transporter 1 (hOCT1): implications for the treatment of imatinib-resistant chronic myeloid leukemia. Blood. 2008;112:3348–54. doi: 10.1182/blood-2007-10-116236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiwase DK, Saunders V, Hewett D, Frede A, Zrim S, Dang P, et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:3881–8. doi: 10.1158/1078-0432.CCR-07-5095. [DOI] [PubMed] [Google Scholar]
- 32.Hiwase DK, White D, Zrim S, Saunders V, Melo JV, Hughes TP. Nilotinib-mediated inhibition of ABCB1 increases intracellular concentration of dasatinib in CML cells: implications for combination TKI therapy. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24:658–60. doi: 10.1038/leu.2009.242. [DOI] [PubMed] [Google Scholar]
- 33.White DL, Saunders VA, Dang P, Engler J, Zannettino AC, Cambareri AC, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- 34.White DL, Liu L, Clackson TP, Saunders VA, Hughes TP. ATP dependent efflux transporters ABCB1 and ABCG2 are unlikely to impact the efficacy, or mediate resistance to the tyrosine kinase inhibitor, ponatinib. Blood. 2011;118 abstract 2745. [Google Scholar]
- 35.Chapuy B, Panse M, Radunski U, Koch R, Wenzel D, Inagaki N, et al. ABC transporter A3 facilitates lysosomal sequestration of imatinib and modulates susceptibility of chronic myeloid leukemia cell lines to this drug. Haematologica. 2009;94:1528–36. doi: 10.3324/haematol.2009.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipka DB, Wagner MC, Dziadosz M, Schnoder T, Heidel F, Schemionek M, et al. Intracellular Retention of ABL Kinase Inhibitors Determines Commitment to Apoptosis in CML Cells. PloS one. 7:e40853. doi: 10.1371/journal.pone.0040853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pluk H, Dorey K, Superti-Furga G. Autoinhibition of c-Abl. Cell. 2002;108:247–59. doi: 10.1016/s0092-8674(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 38.Nagar B, Hantschel O, Young MA, Scheffzek K, Veach D, Bornmann W, et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell. 2003;112:859–71. doi: 10.1016/s0092-8674(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 39.Echarri A, Pendergast AM. Activated c-Abl is degraded by the ubiquitin-dependent proteasome pathway. Current biology: CB. 2001;11:1759–65. doi: 10.1016/s0960-9822(01)00538-3. [DOI] [PubMed] [Google Scholar]
- 40.O’Hare T, Eide CA, Adrian LT, Agarwal A, Zabriskie MS, MacKenzie R, et al. Cryptic intracellular retention of ABL tyrosine kinase inhibitors within CML cells mediates apoptosis commitment following acute drug exposure. Blood. 2011;118 abstract 3504. [Google Scholar]
- 41.Hegedus C, Ozvegy-Laczka C, Apati A, Magocsi M, Nemet K, Orfi L, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. British journal of pharmacology. 2009;158:1153–64. doi: 10.1111/j.1476-5381.2009.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copeland RA, Pompliano DL, Meek TD. Drug-target residence time and its implications for lead optimization. Nature reviews Drug discovery. 2006;5:730–9. doi: 10.1038/nrd2082. [DOI] [PubMed] [Google Scholar]
- 43.Yu Z, Cui B, Jin Y, Chen H, Wang X. Novel irreversible EGFR tyrosine kinase inhibitor 324674 sensitizes human colon carcinoma HT29 and SW480 cells to apoptosis by blocking the EGFR pathway. Biochemical and biophysical research communications. 2011;411:751–6. doi: 10.1016/j.bbrc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Larson RA, Druker BJ, Guilhot F, O’Brien SG, Riviere GJ, Krahnke T, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–8. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 45.Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–9. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.