Abstract
Neuroglobin (Ngb) is an endogenous neuroprotective molecule against hypoxic/ischemic brain injury, but the underlying mechanisms remain largely undefined. Our recent study revealed that Ngb can bind to voltage-dependent anion channel (VDAC), a regulator of mitochondria permeability transition (MPT). In this study we examined the role of Ngb in MPT pore (mPTP) opening following oxygen-glucose deprivation (OGD) in primary cultured mouse cortical neurons. Co-immunoprecipitation (Co-IP) and immuocytochemistry showed that the binding between Ngb and VDAC was increased after OGD compared to normoxia, indicating the OGD-enhanced Ngb-VDAC interaction. Ngb overexpression protected primary mouse cortical neurons from OGD-induced neuronal death, to an extent comparable to mPTP opening inhibitor, cyclosporine A (CsA) pretreatment. We further measured the role of Ngb in OGD-induced mPTP opening using Ngb overexpression and knockdown approaches in primary cultured neurons, and recombinant Ngb exposure to isolated mitochondria. Same as CsA pretreatment, Ngb overexpression significantly reduced OGD-induced mPTP opening markers including mitochondria swelling, mitochondrial NAD+ release, and cytochrome c (Cyt c) release in primary cultured neurons. Recombinant Ngb incubation significantly reduced OGD-induced NAD+ release and Cyt c release from isolated mitochondria. In contrast, Ngb knockdown significantly increased OGD-induced neuron death, and increased OGD-induced mitochondrial NAD+ release and Cyt c release as well, and these outcomes could be rescued by CsA pretreatment. In summary, our results demonstrated that Ngb overexpression can inhibit OGD-induced mPTP opening in primary cultured mouse cortical neurons, which may be one of the molecular mechanisms of Ngb's neuroprotection.
Introduction
Neuroglobin (Ngb) is an oxygen binding globin protein that is highly expressed in brain neurons (Wystub et al., 2003). Since its discovery in 2000, a large volume of evidence has proven Ngb is an endogenous protective molecule for neurons against hypoxic/ischemic insults both in vitro and in vivo (Burmester and Hankeln, 2009; Greenberg et al., 2008; Yu et al., 2009a). A more recent study showed that Ngb was upregulated in the peri-infarct area of ischemic human brain tissues, suggesting the clinical relevance of Ngb (Jin et al., 2010). Moreover, emerging evidence has demonstrated that Ngb may have broad translational implications in other neurological disorders. For instance, Ngb overexpression was also found to be protective against beta-amyloid-induced neurotoxicity and Alzheimer's phenotype in mice (Khan et al., 2007) and glaucomatous retinal ganglion cell damage (Wei et al., 2011).
Given the neuroprotective effect of Ngb, strategies to develop Ngb-targeted therapeutics against stroke and related neurological disorders have been proposed (Greenberg et al., 2008; Yu et al., 2012a). However, the molecular mechanisms of Ngb neuroprotection remain poorly understood. Previous reports suggested that Ngb may play a role in scavenging reactive oxygen species (ROS) (Fordel et al., 2007) and modulating nitric oxide homeostasis (Brunori et al., 2005), and that Ngb may serve as a hypoxia sensor (Wakasugi and Morishima, 2005) in neurons. Furthermore, Ngb was found to be closely related to mitochondria (Schmidt et al., 2003). Our lab has demonstrated that Ngb overexpression preserves mitochondrial function, including ATP production and mitochondria membrane potential in primary cultured neurons after hypoxia (Liu et al., 2009). These findings suggest an important role of mitochondria in Ngb neuroprotection.
Mitochondrial inner membrane is impermeable to small molecules under normal resting conditions, but its permeability increases in response to insults resulting in mitochondria swelling and eventual rupture of the outer membrane, a process known as mitochondria permeability transition (MPT) (Garrido et al., 2006). MPT is generally believed to be mediated by the MPT pore (mPTP), a high conductance channel formed between the outer and inner membranes (Crompton, 1999). The opening of mPTP plays a key role in cell death caused by various stimuli including hypoxia/ischemia (Honda and Ping, 2006; Sims and Muyderman, 2010). Inhibition of mPTP opening using specified inhibitors, such as CsA, has been shown to be neuroprotective and cardioprotective (Hausenloy et al., 2002; Khaspekov et al., 1999; Uchino et al., 2002). Importantly, our recent study demonstrated that Ngb can physically localize in mitochondria (Yu et al., 2012c). We further found that Ngb can bind to VDAC (Yu et al., 2012b). Although there is an argument about whether VDAC is a component of mPTP (Baines et al., 2007; Crompton et al., 1998), it is well accepted that VDAC functionally associates with MPT (Crompton, 1999; Shimizu et al., 2001), thus our findings provide a strong rationale to investigate the relationship between Ngb and mPTP opening. In this study we examined the role of Ngb in OGD-induced mPTP opening in primary cultured mouse cortical neurons.
Methods
Animals
All animal experiments were performed following protocols approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Primary mouse cortical neuron culture and oxygen-glucose deprivation (OGD) insult
Primary mouse cortical neuron culture was performed as previously described (Liu et al., 2009). Briefly, primary mouse cortical neurons were isolated from 15 day embryonic cortex obtained from the pregnant C57 BL/6 female mouse. Neurons from each embryo cortex were isolated and seeded into multiple well plates with equal cell amounts (1.5×105 cells/well in 24-well plates, 6×105 cells/well in 6-well plate). The neurons were maintained in Neurobasal medium (NBM) with 3% B27 and 0.3 mM glutamine (Invitrogen). Medium was half changed in each 3-4 days.
OGD was performed following the method as previous described (Yu et al., 2009b). Briefly, at day 8-9 of primary neuron culture, NBM was replaced with deoxygenated, glucose-free extracellular solution–Locke's medium (154 mM NaCl, 5.6 mM KCl, 2.3 mM CaCl2, 1.0 mM MgCl2, 3.6 mM NaHCO3, 5 mM Hepes, pH 7.2), and the cells were then put in a specialized, humidified chamber (Heidolph, incubator 1000, Brinkmann Instruments, Westbury, NY, USA) at 37 °C, which contained an anaerobic gas mixture (90% N2, 5% H2, and 5% CO2). After 4 h incubation, the cultures were removed from the anaerobic chamber, and the OGD solution in the cultures was replaced with the previously saved NBM. Neurons were then allowed to recover for 20 hr (for neurotoxicity assay) or 4 hr (for mPTP opening and Cyt c release assay) in a regular incubator. Neurons with medium change only were used as controls (replace culture medium with fresh NBM, incubate at normoxia for 4 hrs, then replace with previously saved old medium). Unless otherwise specified, the “OGD” in the text refers to 4 hr OGD plus 4 hr reoxygenation. For mPTP blockage, neurons were pre-treated with CsA (10 μM) for 1 hr before OGD according to a previous report (Dubinsky and Levi, 1998).
Assessment of neurotoxicity
Lactate dehydrogenase (LDH) release assay was used to measure neurotoxicity. After 4 hr OGD plus 20 hr rexoxygenation, neuron culture medium was collected and LDH activity (experimental value) was measured using the cytotoxicity detection kit (LDH) following the manufacturer's instruction (Roche Applied Science, Indianapolis, IN). LDH level in the untreated normal cell culture medium was measured as “low control”. Additionally, the maximal LDH level in untreated control neurons was measured as “high control” when the neurons were completely lysed with 0.5% Triton X-100. The neurotoxicity was calculated as neurotoxicity (%) = (experimental value – low control) / (high control - low control) × 100.
Co-immunoprecipitation
Co-immunoprecipitation (Co-IP) was performed using Immunoprecipitation Kit-Dynabeads® Protein A (Life Technologies, Grand Island, NY) following the manufacturer's instructions. Briefly, 10 μg of anti-Ngb or anti-VDAC antibody (Ab) was incubated with Dynabeads® for 10 min for antibody binding. IgG was used as a negative control. The Ab-bound Dynabeads were washed with PBS w/Tween by gentle pipetting, and then placed on a magnet to remove the supernatant. Neuronal cell lysate containing the antigen was then mixed with the Ab-bound Dynabeads, and incubated by rotation at 4°C for overnight. The supernatant was then removed by a magnet, and the Dynabeads-Ab-antigen was washed 3 times with PBS/Tween, then subjected to SDS-PAGE and Western blot.
Mitochondria isolation
Mitochondria were isolated with Mitochondria Isolation Kit (ThermoScientific) following the manufacturer's instructions. Briefly, primary cultured neurons were trypsinized and washed with PBS, then re-suspended in isolation buffer. The cells were then homogenized in Dounce Tissue Grinder, and centrifuged at 700 g for 10 min at 4°C. The supernatant was transferred to a new 1.5 mL tube and centrifuged at 12,000 × g for 15 minutes at 4°C. The supernatant (cytosol fraction) was transferred to a new tube. The isolated mitochondria in the pellet were re-suspended for experimental use.
Western blot
To test the total intracellular Ngb level, cortical neurons at day 9 of culture were subjected to OGD and collected for protein extraction. Total intracellular Ngb levels were examined with Western blot by following a previously described protocol (Wang et al., 2008). For Cyt c release assay, isolated mitochondria and cytosol fraction were subjected to SDS–PAGE, Cyt c levels were examined by Western blot using anti-cytochrome c antibody (mouse monoclonal, Abcam, Cambridge, MA). VDAC, an outer membrane protein of mitochondria, was used as mitochondrial protein marker and detected by anti-VDAC antibody (rabbit polyclonal, Abcam) (Monick et al., 2008). GAPDH was used as a cytosol protein marker to verify the purity of isolated mitochondria using anti-GAPDH antibody (rat polyclonal, Thermo Scientific, Rockford, IL). Optical density of protein bands was quantified with Image J.
Immunocytochemistry
Cultured neurons were washed with cold PBS (pH 7.4), and fixed with 4% paraformaldehyde for 30 min. The cells were then washed with PBS containing 0.1% Tween, and further incubated with 5% FBS for 1 hr. Next the cells were incubated with primary antibodies against Ngb (mouse poly Ab, 1:100; Abnova) or VDAC (rabbit poly Ab, 1:200; Abcam) at 4°C overnight. After PBS washing, they were incubated with donkey anti mouse IgG conjugated with Tritc (1:150; Jackson ImmunoResearch) and goat anti rabbit IgG conjugated with FITC (1:150; Jackson ImmunoResearch), respectively, for 1 hr at room temperature. Vectashield (Vector Laboratory, Burlingame, CA) was used to coverslip the immunocytochemic slides. Immunostaining was analyzed using a fluorescence microscope (Olympus BX51).
Mitochondria swelling
Mitochondria swelling was measured following a previously published protocol (Ananthakrishnan et al., 2009). Briefly, isolated mitochondria were suspended in fresh swelling buffer (0.2 M sucrose, 10 mM Tris-MOPS, pH 7.4, 5 mM succinate, 1 mM phosphate, 2 μM rotenone, and 1.0 μM EGTA-Tris, pH 7.4) at 0.5 mg/ml, and swelling of mitochondria was monitored by decrease in absorbance at 540 nm in the presence of CaCl2 (200 μM).
Determination of NAD+ content
NAD+ content in mitochondria was used as an index for NAD+ release from mitochondria. Briefly, Mitochondria were isolated from neurons and homogenized by sonication in 0.5 M HClO4. NAD+ concentration was determined fluorometrically with alcohol dehydrogenase. Absorbance at 565 nm was assessed using SpectraMax M5 (Molecular Devices). Data are expressed as relative NAD+ levels compared to normal control.
Adeno-associated virus (AAV) construction and packaging
Construction and packaging of AAV were conducted as we previously described (Xu et al., 2005; Yu et al., 2012c). Briefly, mouse Ngb cDNA was cloned into an AAV-based vector, pACP, with a 6x His tag added at the C terminus of Ngb sequence. The vector was packaged in a 3 plasmid system by co-complementation of the AAV vector plasmid with another two plasmids, pXX2 and pXX6. Purification of the vector preparations was achieved by a combination of passage over an iodixanol gradient followed by ion exchange chromatography using a 1- or 5-ml HiTrap Q column (Amersham Bioscience, Piscataway, NJ). rAAV vector stocks were titered by real-time PCR using the ABI Prism 7700 Sequence Detection System from Perkin-Elmer Applied Biosystems (Foster City, CA, USA). Functional titers of rAAV vector preparations after purification were on the order of 1010 per ml.
AAV transduction
At day 5-6 of primary neuron culture, half of the culture medium was removed from culture wells, and 20 μl of AAV-Ngb was added to each well of 24-well plates (or 50 μl AAV per well of 6-well plates). The cells were incubated for 3 hr at 37°C, and the removed medium was then returned to the well, followed by continuous incubation for 48 hr. Transduction efficiency was examined by fluorescence imaging for eGFP or Western blot for Ngb protein level. AAV-eGFP was used as a transduction control in each experiment to compare with AAV-Ngb transduction.
Recombinant Ngb
Mouse Ngb gene was cloned into bacterial expression vector pET-30b between NcoI and BamH sites, generating the plasmid pET-Ngb, with His-tag added at the N terminus of Ngb gene. pET-Ngb was then transformed into bacterial strain BL21(de3) plysS, and protein induction was performed using IPTG (1mM) at 30°C for 4-5 hours. The bacteria was collected by centrifugation and lysed by sonication, and the His-tagged Ngb protein was purified using HisTrap HP columns (GE healthcare) following the manufacturer's instruction. The purified protein was then characterized with SDS-PAGE and Western blot.
Ngb knockdown using Ngb-shRNA
The shRNA against mouse Ngb (Ngb shRNA) was inserted in retroviral plasmid pGFP-V-RS, and the plasmid was transfected into primary cultured neurons following the manufacturer's instruction (OriGene, Rockville, MD). Briefly, for every 3× 105 cells, 1 μg of Ngb shRNA expression plasmid DNA (in 100ng/μl solution) was pre-mixed with 3 μl TurboFection Solution and 100 μl neural basal medium, and incubated at room temperature for 30 min. The mixture was then added to the medium of neurons in culture dishes and incubated in 5% CO2 incubator for 48 hrs before OGD. The transfection efficiency was monitored by GFP expression. A Non-effective 29-mer scrambled shRNA was used as a negative control.
Incubation of mitochondria with recombinant Ngb (rNgb)
Isolated mitochondria (1mg protein/ml) from primary cultured neurons were subjected to OGD for 30 min plus reoxygenation for 10 min by incubating with either rNgb (90 μg/ml), CsA (10μM) or rNgb+CsA in a medium containing 10 mM KH2PO4, 0.5 mM EGTA, 60 mM KCl, 60 mM Tris, 110 mM mannitol, and 1 mM free Mg2+ (pH 7.4) at 30 °C. NAD+ content in mitochondria was measured. The dose selection of rNgb was based on Ngb protein to total brain protein ratio is about 0.01% (Burmester et al., 2000), neuron to brain mass ratio is about 5% (Gasser and Hatten, 1990), and neuronal mitochondria to total protein ratio is about 11% (unpublished data), thus the estimated Ngb to mitochondria protein ratio is about 1: 55 under normal physiological condition. As about 5 fold increase of total intracellular Ngb protein level has been achieved by AAV-Ngb transduction (Figure 1), therefore we used Ngb to mitochondria protein ratio 1:11 to mimic overexpressed intracellular Ngb in neurons.
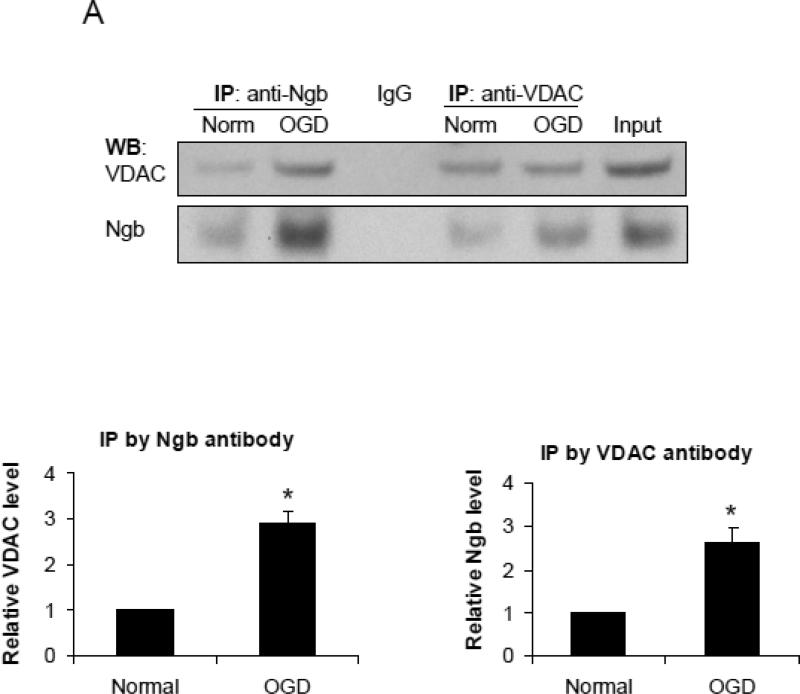
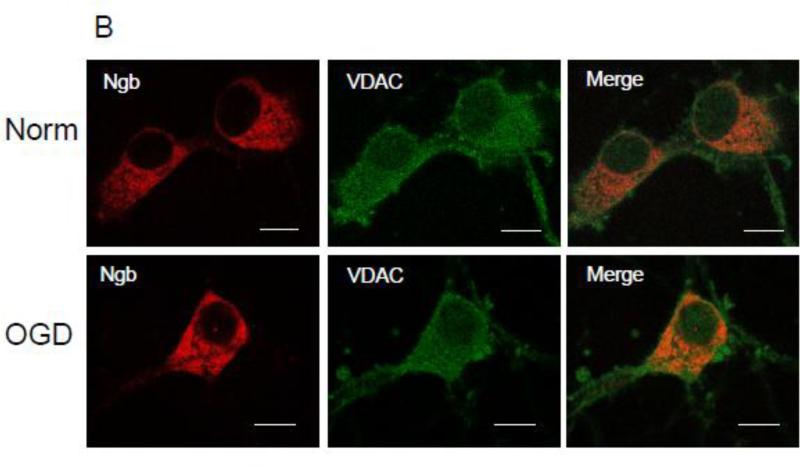
Figure 1. Interaction between Ngb and VDAC.
(A) Co-immunoprecipitation of Ngb and VDAC. Cell lysates from neurons under normal condition or OGD were incubated with anti-Ngb and anti-VDAC antibody. VDAC and Ngb in precipitates were detected by Western blot. IgG was used as a negative control. The Ngb was precipitated by anti-VDAC antibody and VDAC was precipitated by anti-Ngb antibody, and the relative amount of precipitated protein before and after OGD was compared (n=3, * P<0.05). WB: Western blot; IP: Immunoprecipitation. (B) Immuno-cytochemistry and confocal imaging for Ngb and VDAC of primary neurons in normal and OGD conditions. Scale bar = 10μm.
Statistical analysis
Results were expressed as mean ± SEM. Multiple comparisons were evaluated by one-way ANOVA followed by Tukey–Kramer's tests between all groups. P < 0.05 was considered statistically significant.
RESULTS
OGD increased protein binding of Ngb to VDAC in primary cultured mouse cortical neurons
To study the interaction between Ngb and VDAC, the key regulator of MPT, we performed co-immunoprecipitation for Ngb and VDAC in primary cultured mouse cortical neurons under both normal resting condition and after 4 hr OGD plus 4 hr reoxygenation. Our data showed that VDAC can be precipitated by anti-Ngb antibody, and the amount of precipitated VDAC was significantly increased in OGD-treated neurons (2.9 fold increase) (F(1,4) = 43.2, p = 0.003, Figure 1A). Ngb can also be precipitated by anti-VDAC antibody, and the precipitated Ngb was significantly increased in OGD-treated neurons (2.6 fold increase) (F(1,4) = 21.08, p = 0.01, Figure 1A). These data implied the increased protein binding of Ngb with VDAC after OGD. To further define the protein interaction between Ngb and VDAC, confocal imaging was applied following immuno-cytochemistry analysis. We found that Ngb and VDAC were co-localized in primary cultured mouse cortical neurons under both normal resting and OGD conditions (Figure 1B).
Ngb overexpression decreased OGD-induced neuronal cell death
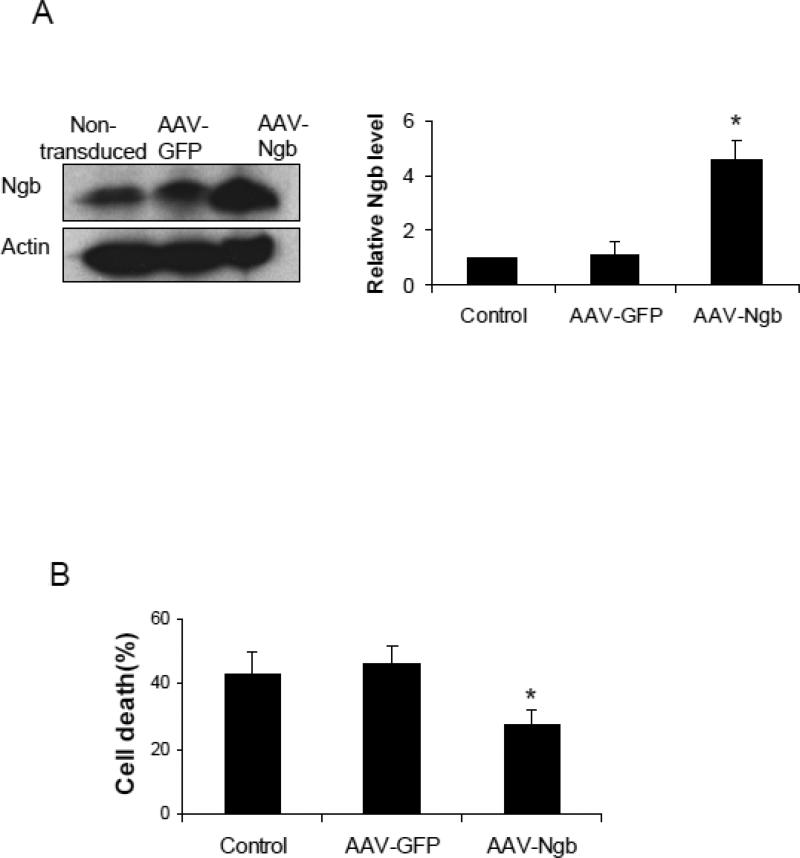
To explore the role of Ngb in mitochondria function and mPTP opening following OGD, we over-expressed Ngb in primary cultured mouse cortical neurons by transduction with AAV-Ngb. The transduction efficiency was about 40% as examined by AAV-GFP transduction as a control (supplemental Figure 1). AAV-Ngb transduction resulted in about 4.6 fold increase of total intracellular Ngb level (F(2,6) = 64.05, p = 0.001, Figure 2A). Neuronal cell death was measured after 4 hr OGD plus 20 hr reoxygenation in non-transduced, AAV-GFP or AAV-Ngb transduced neurons. OGD-induced neuron death was not significantly altered by AAV-GFP transduction compared with non-transduced controls (Figure 2B), whereas the OGD-induced neuron death was significantly reduced in AAV-Ngb transduced neurons (29%) compared to AAV-GFP transduced neurons (45.4%) (F(2,9) = 9.09, p = 0.008, Figure 2B), or as compared to non-transfected control neurons (43.2%) (p = 0.021, Figure 2B).
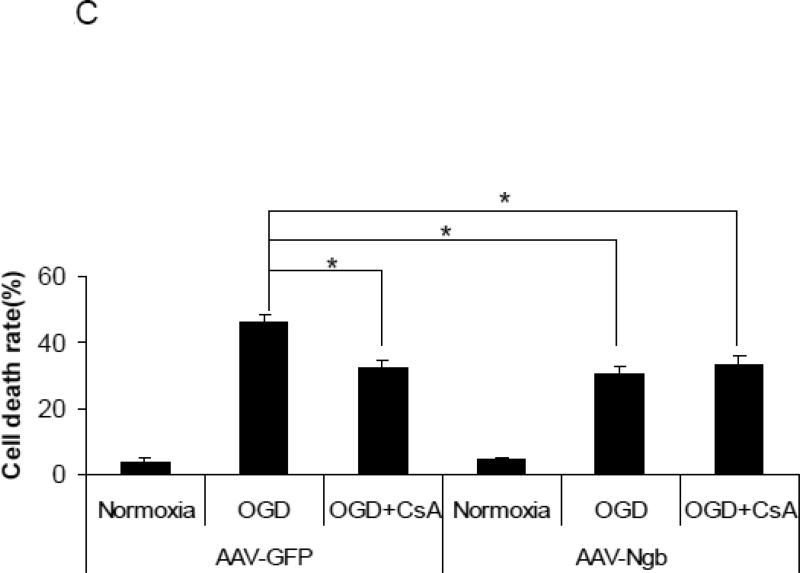
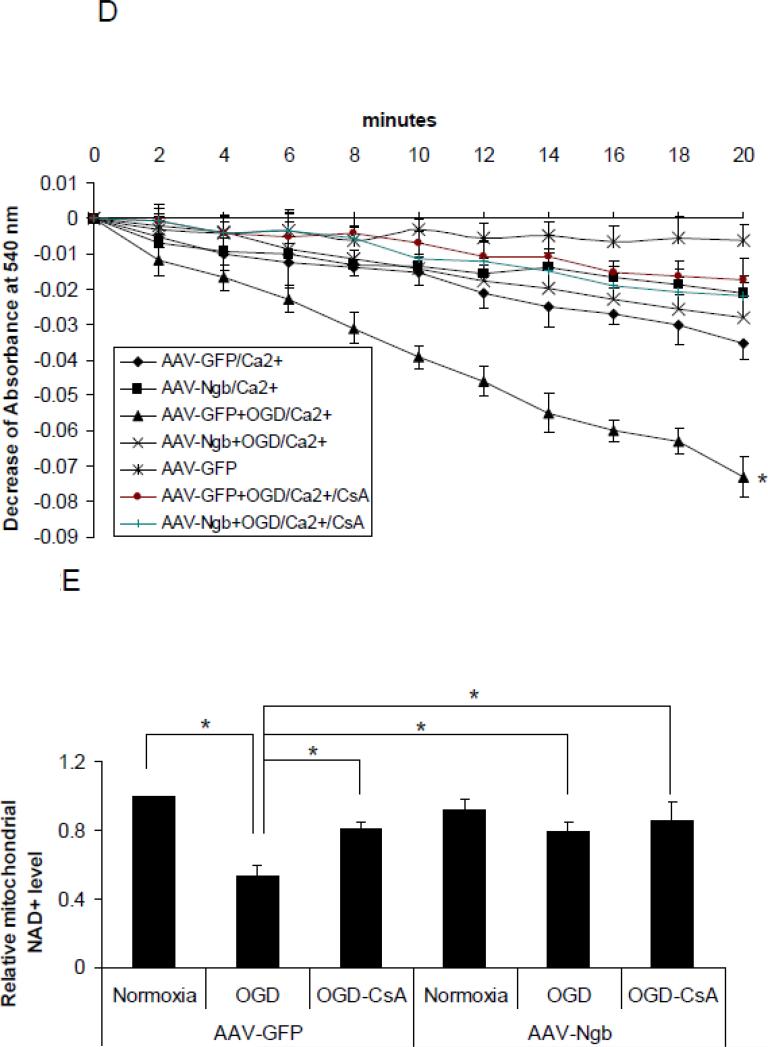
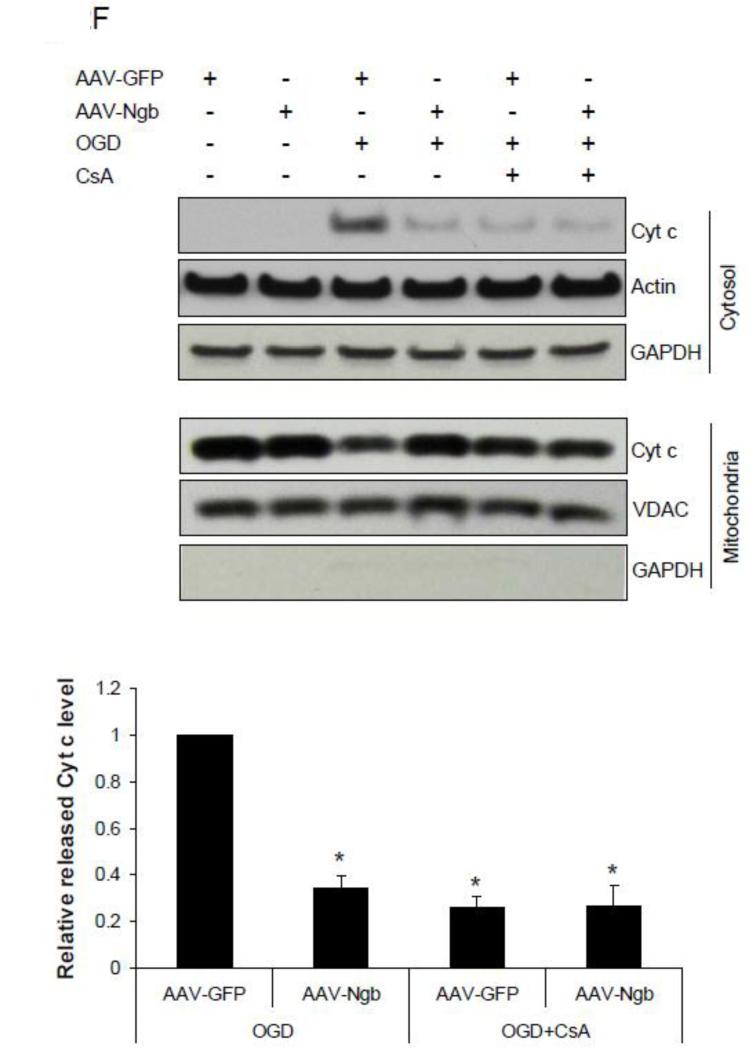
Figure 2. Effects of Ngb overexpression and CsA pre-treatment on OGD-induced mPTP opening.
(A) Total Ngb level in non-transduced neurons or neurons transduced with AAV-GFP and AAV-Ngb. (B) OGD-induced neuron death for non-transduced, AAV-GFP or AAV-Ngb transduced neurons (n=4, * P<0.05 vs non-transduced or AAV-GFP transduced neurons). (C) OGD-induced neuron death for AAV-GFP or AAV-Ngb transduced neurons with CsA pre-treatment. After 4 hr OGD plus 20 hr reoxygenation, cell death was measured by LDH release assay (n=4, * P<0.05). (D) Mitochondria swelling examined by monitoring the absorbance at 540 nm induced by Ca2+ (200 μM). Mitochondria were isolated from AAV-Ngb or AAV-GFP transduced neurons followed by OGD. Baseline absorbance was measured without Ca2+. (E) Mitochondrial NAD+ release measured by relative NAD+ content in mitochondria from AAV-Ngb transduced or control neurons after OGD (n=3, * P< 0.05). (F) Cyt c levels in mitochondria and cytosol fractions ware measured by Western blot (n=3, * P< 0.05).
mPTP inhibitor CsA decreased OGD-induced neuronal cell death
To determine the effects of mPTP opening in OGD-induced neuron death, primary cultured mouse cortical neurons were pretreated with CsA (10 μM), a well-defined inhibitor of mPTP opening, followed by 4 hr OGD plus 20 hr reoxygenation, and then neurotoxicity was measured. Our data showed that OGD-induced neuron death was significantly decreased by both CsA pre-treatment (~33%) (F(3,12) = 8.06, p = 0.026, Figure 2C) and by Ngb-overexpression plus CsA pre-treatment (~34%) (p = 0.028, Figure 2C) compared to AAV-GFP transduced neurons under OGD without CsA pre-treatment (45.4%). We noted that, CsA combined with Ngb overexpression did not further reduce OGD-induced neuron death compared to CsA or Ngb overexpression alone, suggesting that inhibition of mPTP opening might be a dominant mechanism for Ngb's neuroprotection against OGD-induced neuron death.
Ngb overexpression inhibited OGD-induced mPTP opening in cultured neurons
To investigate whether Ngb overexpression alters OGD-induced mPTP opening in primary cultured neurons, we tested the effects of Ngb overexpression on two common biomarkers of mPTP opening, mitochondria swelling and NAD+ release, after 4 hr OGD followed by 4 hr reoxygenation. We found that OGD-induced mitochondria swelling was significantly decreased in AAV-Ngb transduced neurons compared to AAV-GFP transduced neurons (p = 0.027, Figure 2D). For NAD+ measurement, the lower NAD+ content in mitochondria reflects the increased release of NAD+ from mitochondria via mPTP opening. Our data showed that OGD-induced mitochondrial NAD+ content decline (NAD+ release from mitochondria) was significantly diminished by Ngb overexpression (0.79 fold mitochondrial NAD+) (relative to normoxia, same as following) compared with AAV-GFP transduced neurons (0.53 fold) (F(5,12) = 8.95, p = 0.045, Figure 2E). Furthermore, OGD-induced NAD+ release was also significantly diminished by CsA pre-treatment (0.81 fold) (p = 0.028, Figure 2E) or CsA plus Ngb overexpression (0.86 fold) (p = 0.011, Figure 2E). These results indicated a causal relation between Ngb-overexpression and a decrease in OGD-induced mPTP opening.
Ngb overexpression reduced OGD-induced Cyt c release from mitochondria in cultured neurons
Cyt c release from mitochondria is a key step in mitochondria-mediated cell death signaling, and MPT/mPTP opening is one of the mechanisms for Cyt c release (Garrido et al., 2006). Here we examined the role of Ngb in Cyt c release from mitochondria after 4 hr OGD plus 4 hr reoxygenation in AAV-Ngb and AAV-GFP transduced neurons. We found that OGD-induced Cyt c release from mitochondria was significantly reduced in AAV-Ngb transduced neurons (0.34 fold) compared with AAV-GFP transduced neurons (F(3,8) = 40.3, p = 0.001, Figure 2F). Furthermore, OGD-induced Cyt c release was also significantly reduced by CsA (0.26 fold vs. AAV-GFP) (p = 0.001, Figure 2F) and CsA+Ngb overexpression (0.27 fold vs. AAV-GFP) (p = 0.001, Figure 2F). These results suggested that Ngb overexpression may block mPTP opening-associated cell death signaling after OGD, at least in Cyt c release.
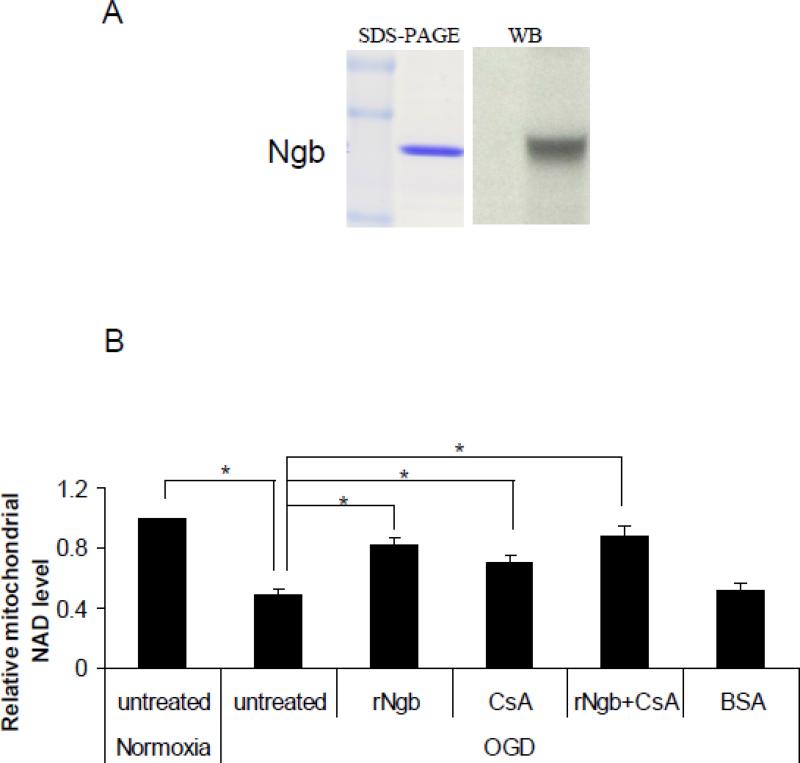
Recombinant Ngb exposure inhibited OGD-induced NAD+ release from isolated mitochondria
To ask whether increasing Ngb concentration in the surrounding environment of mitochondria may alter OGD-induced mPTP opening, rNgb was used to co-incubate with isolated mitochondria. The mouse rNgb was produced in bacteria and characterized with SDS-PAGE and Western blot (Figure 3A). To test the direct and specific effect of Ngb in mPTP opening, isolated mitochondria (1 mg protein/ml) were incubated with rNgb (90 μg/ml), CsA (10μM) or rNgb plus CsA, followed by 30 min OGD plus 10 min reoxygenation. Our data showed that OGD caused significant NAD+ release from isolated mitochondria compared with normoxia (0.49 fold mitochondrial NAD+) (compared with normoxia, same as following) (F(4,10) = 17.85, p = 0.001, Figure 3B), but the NAD+ release was significantly inhibited by rNgb incubation (0.82 fold) (p = 0.003, Figure 3B). Furthermore, CsA pretreatment significantly inhibited OGD-induced NAD+ release (0.70 fold) (p = 0.047 vs OGD alone), while the CsA and rNgb combination did not further enhance the inhibitory effects on NAD+ release (0.89 fold) (p = 0.001 vs OGD alone, Figure 3B). These results suggested that the decreased NAD+ release from mitochondria by rNgb might be primarily via inhibition of mPTP opening.
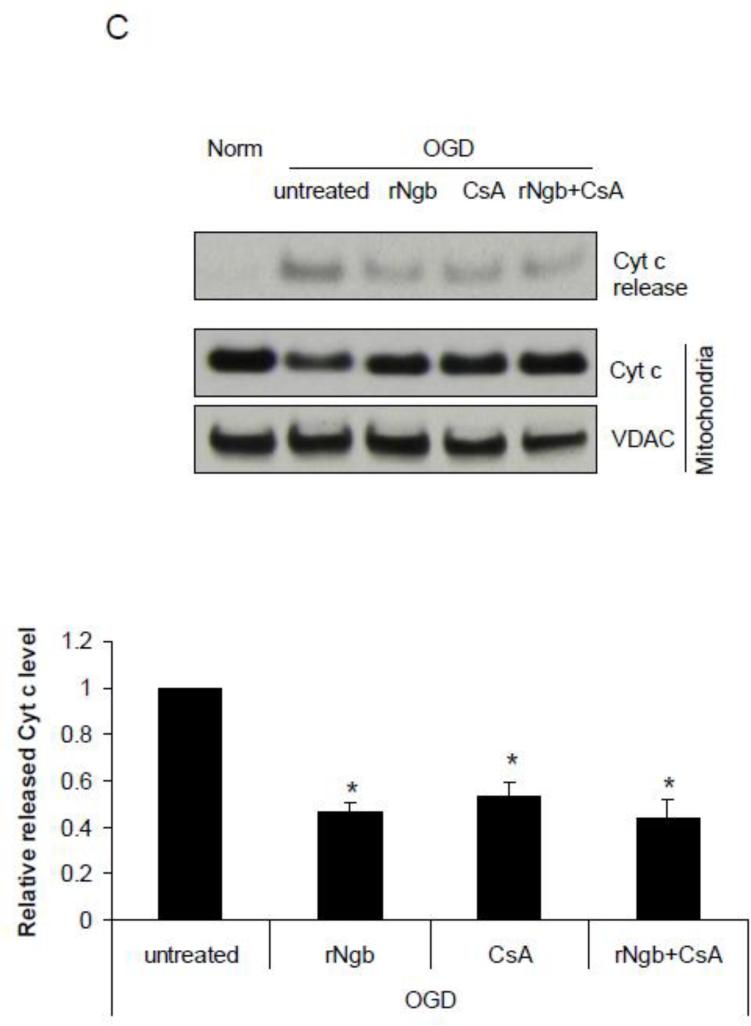
Figure 3. Effect of recombinant Ngb (rNgb) on OGD-induced mPTP opening in isolated mitochondria.
(A) Characterization of rNgb with SDS-PAGE and Western blot. (B) Isolated mitochondria was incubated with rNgb (90 ug/ml), CsA (10μM) or rNgb+CsA for 30 min followed by 30 min OGD plus 10 min reoxygenation. Relative NAD+ content in mitochondria was measured in each group (n=4, * P<0.05). (C) Cyt c contents in isolated mitochondria and releases into media after OGD were measured by Western blot (n=3, * P<0.05).
Recombinant Ngb exposure inhibited OGD-induced Cyt c release from isolated mitochondria
We further measured the effect of rNgb exposure on OGD-induced Cyt c release from isolated mitochondria. OGD (30 min OGD plus 10 min reoxygenation) caused dramatic Cyt c release from isolated mitochondria compared with normoxia, but Cyt c release was significantly inhibited by rNgb incubation (0.47 fold) (relative to OGD alone, same as following) (F(3,8) = 24.99, p = 0.001, Figure 3C). Furthermore, CsA pretreatment significantly inhibited OGD-induced Cyt c release (0.53 fold) (p = 0.002 vs OGD alone, Figure 3C), while the CsA and rNgb combination did not further enhance the inhibitory effects of CsA on Cyt c release (0.44 fold) (p = 0.001 vs OGD alone, Figure 3C). These results suggested that rNgb may inhibit OGD-induced Cyt c release from mitochondria by inhibition of mPTP opening.
Ngb knockdown potentiated OGD-induced mPTP opening in cultured neurons
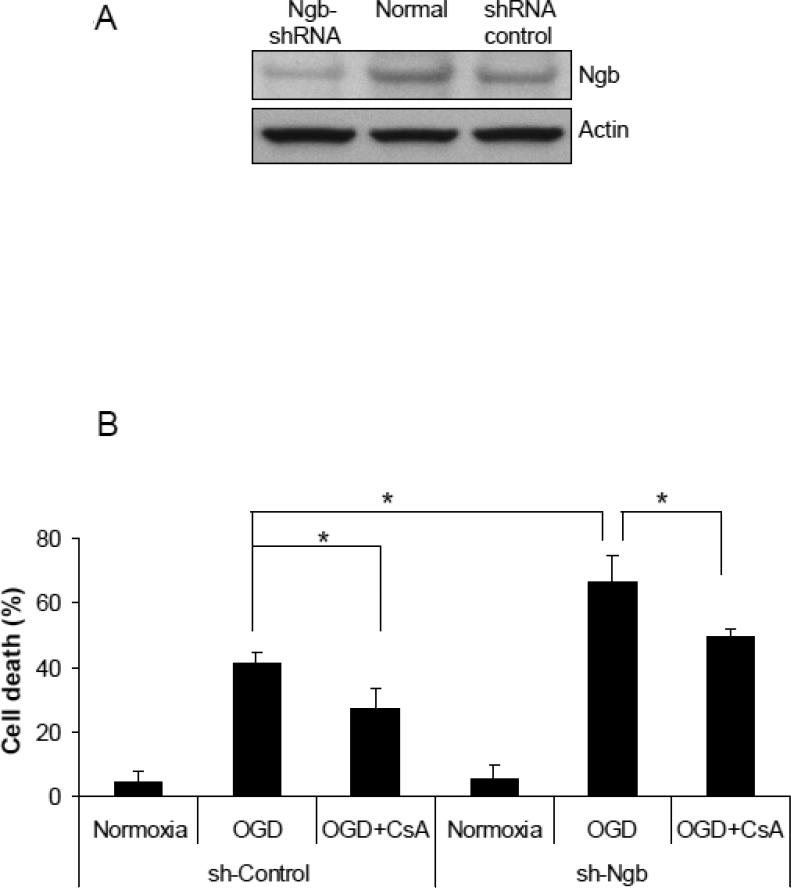
To further validate the role of Ngb in OGD-induced mPTP opening, we used Ngb-shRNA to knockdown Ngb expression in primary cultured cortical neurons. The transfection efficiency was about 40% measured by GFP expression in comparison with DAPI staining (Supplemental Figure 3). Ngb protein level was significantly reduced in neurons transfected with Ngb-shRNA compared to normal neurons or neurons transfected with control-shRNA (Figure 4A). Ngb knockdown significantly increased neuron death after 4 hr OGD plus 20 hr reoxygenation (65.3%) compared with control-shRNA (41.8%) (F(3,8) = 26.72, p = 0.003, Figure 4B). Furthermore, CsA pretreatment significantly decreased OGD-induced neuron death in both control-shRNA group (27.8%) (p = 0.046, Figure 4B) and Ngb-shRNA group (49.8%) (p = 0.028, Figure 4B).
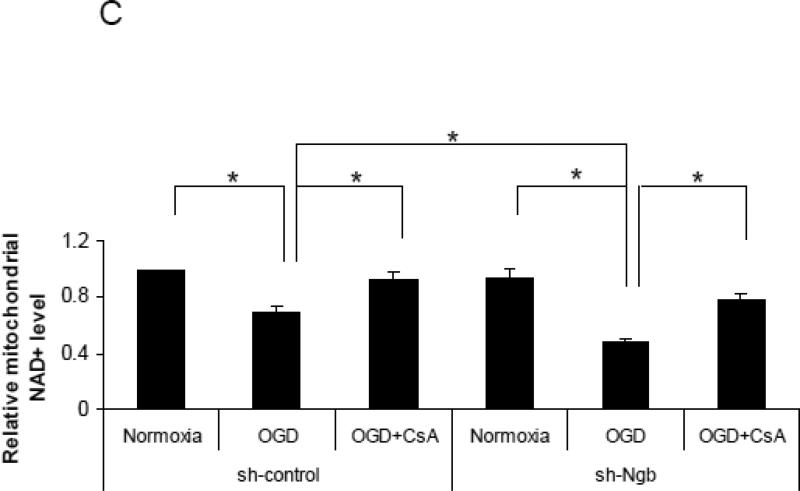
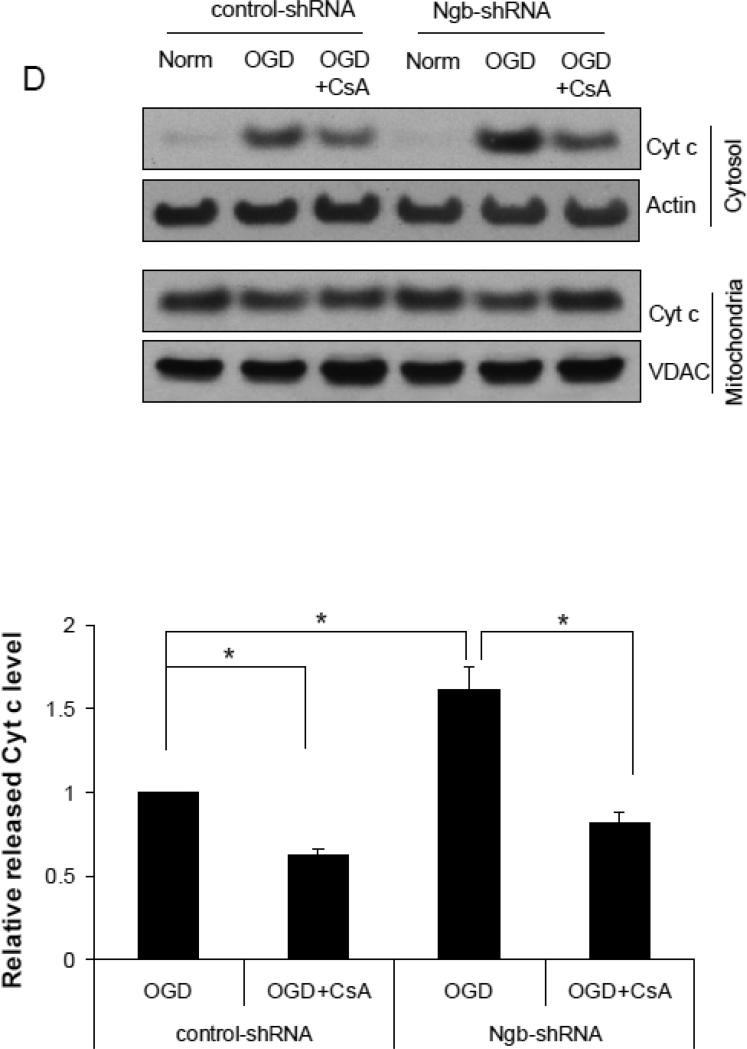
Figure 4. Effect of Ngb knockdown on OGD-induced mPTP opening in isolated mitochondria.
(A) Ngb protein levels measured in normal neuron and neurons transduced with Ngb-shRNA or shRNA control. (B) OGD-induced neuronal cell death for neurons transduced with Ngb-shRNA or shRNA control. Neurons were pretreated with CsA (10μM) before OGD. Cell death was measured by LDH release at 4 hr OGD plus 20 hr reoxygenation (n=3, *P<0.05). (C) Relative NAD+ content in mitochondria was measured after OGD for neurons transduced with Ngb-shRNA or shRNA control, and combined with CsA (10μM) pretreatment (n=3, * P<0.05). (D) Cyt c levels in mitochondria and cytosol fractions were measured by Western blot (n=3, * P<0.05).
We further measured the effect of Ngb knockdown on mPTP opening markers in neurons after 4 hr OGD plus 4 hr reoxygenation. Our results showed that OGD-induced mitochondrial NAD+ release in Ngb knockdown group (0.48 fold mitochondrial NAD+) (compared with normoxia, same as following) was significantly potentiated compared with control-shRNA group (0.7 fold) (F(5,12) = 18.28, p = 0.044, Figure 4C). Furthermore, CsA pretreatment significantly attenuated OGD-induced NAD+ release in both control-shRNA group (0.93 fold) (p=0.035, Figure 4C) and Ngb-shRNA group (0.79 fold) (p = 0.001, Figure 4C).
Moreover, we measured the effect of Ngb knockdown on OGD-induced Cyt c release in neurons after 4 hr OGD plus 4 hr reoxygenation. OGD-induced mitochondrial Cyt c release was significantly increased by Ngb knockdown (1.61 fold increase) compared with control-shRNA (F(3,8) = 28.56, p = 0.003, Figure 4D). Furthermore, CsA pretreatment significantly inhibited OGD-induced Cyt c release in both control-shRNA group (0.62 fold vs. control-shRNA) (p = 0.042, Figure 4D) and Ngb-shRNA group (0.81 fold vs. control-shRNA) (p = 0.001, Figure 4D). These results further indicated the critical role of Ngb in OGD-induced mPTP opening and the associated neuron death.
DISCUSSION
The physical and functional association between Ngb and mitochondria has long been hypothesized as one of the mechanisms of Ngb neuroprotection, but has not been experimentally defined. Results from this study provided strong evidence in support of this hypothesis. We first showed that Ngb protein can bind to VDAC, a key regulator of mPTP, and this binding was increased after OGD. We further showed that Ngb overexpression inhibited OGD-induced mitochondria swelling and NAD+ release, the biomarkers of mPTP opening, to an extent comparable to CsA, an inhibitor of mPTP opening. Ngb overexpression also inhibited OGD-induced Cyt c release, a critical event in OGD-induced cell death signaling. Additionally, Ngb knockdown increased OGD-induced mPTP opening and neuron death. Finally, recombinant Ngb exposure to isolated mitochondria also inhibited OGD-induced mitochondrial NAD+ release and Cyt c release. These results suggest Ngb may protect neurons against OGD by inhibiting OGD-induced mPTP opening.
Since the discovery of Ngb gene in 2000, a large array of studies have confirmed that Ngb is an endogenous neuroprotective molecule against hypoxia/ischemia and other related neurological disorders in both in vitro cell culture and in vivo animal models, however the protection mechanisms remain largely undefined. Although previous studies have shown the association of Ngb expression with highly metabolically active and mitochondria-rich cell types in the brain (Fuchs et al., 2004), these observations in linking Ngb's neuroprotection with mitochondria have not been experimentally well defined and characterized. We previously found that Ngb overexpression prevented mitochondria dysfunction caused by hypoxia (Liu et al., 2009), which is further endorsed by our recent data that Ngb physically interacts with proteins involved in mitochondrial respiration and ATP metabolism, including VDAC, mitochondrial complex III, and Na/K ATPase (Yu et al., 2012b). We also found that Ngb is physically localized in mitochondria (Yu et al., 2012c). All these findings established a convincing connection between Ngb and mitochondria.
In this study, by assessing the changes of multiple mPTP biomarkers with/without the pretreatment of mPTP inhibitor CsA (Schinzel et al., 2005) or in combination with/without Ngb overexpression or knockdown, for the first time we demonstrated that inhibition of OGD-induced mPTP opening might be one important molecular mechanism for Ngb's neuroprotection against acute ischemic/hypoxic brain injuries. Since mPTP opening is a key factor involved in cell death caused by various stimuli, not limited to hypoxia/ischemia (Honda and Ping, 2006; Sims and Muyderman, 2010), our finding suggests that Ngb may also have broad implications in neurological disorders through inhibiting mPTP opening. For example, experimental investigations have demonstrated that mPTP opening also plays a crucial role in aging-associated chronic neurodegenerative diseases (Abou-Sleiman et al., 2006; Toman and Fiskum, 2011). Interestingly, Ngb may also be involved in aging-related neurodegeneration, as Ngb protein in aged rats was significantly decreased (Sun et al., 2005). These findings suggest that upregulation of Ngb may be a novel therapeutic strategy against cerebrovascular diseases and other neurological disorders, possibly through maintaining mitochondrial homeostasis and inhibiting mPTP opening.
We also found that Ngb overexpression significantly inhibited OGD-induced Cyt c release from mitochondria. Cyt c release and the subsequent caspase activation are typical responses of neurons to death-inducing stimuli (Brunelle and Chandel, 2002; Chan, 2004). It has been well demonstrated that Cyt c can be released from mitochondria via mPTP opening-dependent and -independent pathways (De Giorgi et al., 2002; Petrosillo et al., 2003; Shimizu et al., 2001). The reduced Cyt c release by Ngb overexpression may be partially caused by Ngb inhibition of mPTP opening. However, other mechanisms may also be involved. For example, previous reports showed that ferrous Ngb can reduce ferric Cyt c, which may play a role in preventing initiation of apoptosis (Brittain et al., 2010; Fago et al., 2006). It is possible that Ngb-Cyt c interaction is also causative for decreased Cyt c release from mitochondria, but this remains to be investigated further.
The binding between Ngb with VDAC and its increase after OGD might have important pathophysiological implications due to the critical roles of VDAC in mitochondrial function. VDAC has long been recognized as a component of mPTP (Crompton, 1999), which is supported by multiple experimental data. For example, VDAC can be pulled down by GST-CypD (Cyclophilin D) along with ANT (adenine nucleotide translocase) as CypD and ANT are well defined mPTP components (Crompton et al., 1998). Furthermore, anti-VDAC antibodies can prevent Ca2+-induced MPTP opening in liver mitochondria (Shimizu et al., 2001). However, this notion was argued by a recent genetics study suggesting that VDAC may not be an essential component of mPTP (Baines et al., 2007). Although this issue has not been clearly defined, it is indeed well accepted that VDAC plays an important role in mPTP function (McCommis and Baines, 2012), and it is possible that the protein binding of Ngb with VDAC participates in mPTP pathophysiological responses to OGD in cultured cortical neurons.
Additionally, it has been demonstrated that VDAC is an important mediator of mitochondria outer membrane permeabilization and Cyt c release, although it could be mPTP-dependent or -independent based on stimulus conditions and cell types (S. Shimizu et al., 2001; F. De Giorgi et al., 2002; G. Kroemer et al., 2007). Therefore the Ngb-VDAC interaction might be one of the underlying mechanisms of Ngb neuroprotection. However, this hypothesis has to be validated by specifically blocking the Ngb-VDAC binding in the future. It should be noted that VDAC may also be directly involved in regulation of mitochondria-mediated apoptosis via interaction with anti-apoptotic proteins, namely Bcl-2 family members, and a pro-survival protein hexokinase (Shoshan-Barmatz and Ben-Hail, 2012). How Ngb is implicated in this process might be worth investigating. Furthermore, the detailed mode of Ngb-VDAC interaction remains to be clarified. Our previous yeast two-hybrid assay (Yu et al., 2012b) suggested that the 10-160 aa (from N terminus) of VDAC may be the region responsible for Ngb binding. However, in this study we have not made VDAC and Ngb protein truncation mutants to identify the exact interaction regions. Further investigations would be fundamentally important to dissect the detailed mode of Ngb-VDAC interaction, and its role in modulating mPTP and other mitochondrial functions.
There are a few caveats in our study. One caveat is that we were unable to elucidate the molecular mechanisms on how Ngb inhibits OGD-induced mPTP opening, mainly due to limited technical approaches, for example, methods to specifically block Ngb-VDAC interaction. Another caveat relates to the specificity of mPTP inhibitor CsA used in this study. CsA pretreatment significantly decreased OGD-induced neuron cell death compared to no CsA treatment control, indicating that CsA-caused inhibition of mPTP opening might contribute to the protection against OGD-induced cell death. However, CsA pretreatment may not completely block OGD-induced mPTP opening since it only targets Cyp-D, one of the components of mPTP (Nicolli et al., 1996), thus it can only partially prevent OGD-induced cell death (Clarke et al., 2002; Waldmeier et al., 2003). Another reason for the incomplete cell death inhibition by CsA might be that mPTP opening is not the only mechanism of OGD-induced cell death; other mechanisms, such as excitotoxicity and Na+/Ca2+ homeostasis pathways (Martinez-Sanchez et al., 2004; Snider et al., 1998; Wang et al., 2002), may also be involved. Therefore the effect of CsA on mPTP opening and cell death might be a mixed outcome of mPTP-dependent and -independent mechanisms. One more caveat is that besides mPTP opening inhibition, we did not dissect other neuroprotective mechanisms of Ngb in OGD-induced neuron death. Findings from this study suggested that Ngb may protect against OGD-induced neuron death at least partially through inhibition of mPTP opening. However, Ngb may also function against other pathogenic signaling pathways such as ROS scavenging (Fordel et al., 2007), nitric oxide homeostasis (Brunori et al., 2005), and serving as hypoxia sensor (Wakasugi and Morishima, 2005). These protective mechanisms of Ngb might work in concert with mPTP opening inhibition, which will be studied in both in vitro cell culture and in vivo animal models in the future.
In conclusion, experimental findings from this study demonstrate that Ngb overexpression can inhibit OGD-induced mPTP opening in primary cultured mouse cortical neurons, which may be one of the important mechanisms in Ngb neuroprotection.
Supplementary Material
Highlights.
Neuroglobin(Ngb) can bind to voltage dependent anion channel in cultured neurons.
Oxygen/glucose deprivation-induced mPTP opening was reduced by Ngb overexpression.
Ngb overexpression decreased OGD-induced cytochrome c release from mitochondria.
Recombinant Ngb blocked OGD-induced mPTP opening in isolated mitochondria.
Ngb knockdown in neurons showed enhanced mPTP opening following OGD.
Acknowledgments
This work was supported in part by NIH grant R01-NS049476 (to X.W.) and postdoctoral fellowship (12POST9720007) from American Heart Association (to Z.Y.). We thank Ms. Jessica Poppe for language editorial assistance. We appreciate Dr. Eng H. Lo for his very helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
Reference
- Abou-Sleiman PM, et al. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–19. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan R, et al. Aldose reductase mediates myocardial ischemia-reperfusion injury in part by opening mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2009;296:H333–41. doi: 10.1152/ajpheart.01012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, et al. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–5. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain T, et al. An antiapoptotic neuroprotective role for neuroglobin. Int J Mol Sci. 2010;11:2306–21. doi: 10.3390/ijms11062306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle JK, Chandel NS. Oxygen deprivation induced cell death: an update. Apoptosis. 2002;7:475–82. doi: 10.1023/a:1020668923852. [DOI] [PubMed] [Google Scholar]
- Brunori M, et al. Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc Natl Acad Sci U S A. 2005;102:8483–8. doi: 10.1073/pnas.0408766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester T, Hankeln T. What is the function of neuroglobin? J Exp Biol. 2009;212:1423–8. doi: 10.1242/jeb.000729. [DOI] [PubMed] [Google Scholar]
- Burmester T, et al. A vertebrate globin expressed in the brain. Nature. 2000;407:520–3. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem Res. 2004;29:1943–9. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- Clarke SJ, et al. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–9. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–49. [PMC free article] [PubMed] [Google Scholar]
- Crompton M, et al. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem. 1998;258:729–35. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- De Giorgi F, et al. The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. FASEB J. 2002;16:607–9. doi: 10.1096/fj.01-0269fje. [DOI] [PubMed] [Google Scholar]
- Dubinsky JM, Levi Y. Calcium-induced activation of the mitochondrial permeability transition in hippocampal neurons. J Neurosci Res. 1998;53:728–41. doi: 10.1002/(SICI)1097-4547(19980915)53:6<728::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Fago A, et al. The reaction of neuroglobin with potential redox protein partners cytochrome b5 and cytochrome c. FEBS Lett. 2006;580:4884–8. doi: 10.1016/j.febslet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Fordel E, et al. Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene. 2007;398:114–22. doi: 10.1016/j.gene.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Fuchs C, et al. Zebrafish reveals different and conserved features of vertebrate neuroglobin gene structure, expression pattern, and ligand binding. J Biol Chem. 2004;279:24116–22. doi: 10.1074/jbc.M402011200. [DOI] [PubMed] [Google Scholar]
- Garrido C, et al. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–33. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- Gasser UE, Hatten ME. Central nervous system neurons migrate on astroglial fibers from heterotypic brain regions in vitro. Proc Natl Acad Sci U S A. 1990;87:4543–7. doi: 10.1073/pnas.87.12.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, et al. Neuroglobin: an endogenous neuroprotectant. Curr Opin Pharmacol. 2008;8:20–4. doi: 10.1016/j.coph.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, et al. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–43. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- Honda HM, Ping P. Mitochondrial permeability transition in cardiac cell injury and death. Cardiovasc Drugs Ther. 2006;20:425–32. doi: 10.1007/s10557-006-0642-0. [DOI] [PubMed] [Google Scholar]
- Jin K, et al. Neuroglobin expression in ischemic stroke. Stroke. 2010;41:557–9. doi: 10.1161/STROKEAHA.109.567149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, et al. Neuroglobin attenuates beta-amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo. Proc Natl Acad Sci U S A. 2007;104:19114–9. doi: 10.1073/pnas.0706167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaspekov L, et al. Cyclosporin A and its nonimmunosuppressive analogue N-Me-Val-4-cyclosporin A mitigate glucose/oxygen deprivation-induced damage to rat cultured hippocampal neurons. Eur J Neurosci. 1999;11:3194–8. doi: 10.1046/j.1460-9568.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Effects of neuroglobin overexpression on mitochondrial function and oxidative stress following hypoxia/reoxygenation in cultured neurons. J Neurosci Res. 2009;87:164–70. doi: 10.1002/jnr.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Sanchez M, et al. Na(+) and Ca(2+) homeostasis pathways, cell death and protection after oxygen-glucose-deprivation in organotypic hippocampal slice cultures. Neuroscience. 2004;128:729–40. doi: 10.1016/j.neuroscience.2004.06.074. [DOI] [PubMed] [Google Scholar]
- McCommis KS, Baines CP. The role of VDAC in cell death: Friend or foe? Biochim Biophys Acta. 2012;1818:1444–50. doi: 10.1016/j.bbamem.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monick MM, et al. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J Immunol. 2008;180:7485–96. doi: 10.4049/jimmunol.180.11.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolli A, et al. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J Biol Chem. 1996;271:2185–92. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, et al. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17:2202–8. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–10. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, et al. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem. 2003;278:1932–5. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- Shimizu S, et al. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J Cell Biol. 2001;152:237–50. doi: 10.1083/jcb.152.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Ben-Hail D. VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion. 2012;12:24–34. doi: 10.1016/j.mito.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Snider BJ, et al. Conditioning heat stress reduces excitotoxic and apoptotic components of oxygen-glucose deprivation-induced neuronal death in vitro. J Neurochem. 1998;70:120–9. doi: 10.1046/j.1471-4159.1998.70010120.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, et al. Effect of aging on neuroglobin expression in rodent brain. Neurobiol Aging. 2005;26:275–8. doi: 10.1016/j.neurobiolaging.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Toman J, Fiskum G. Influence of aging on membrane permeability transition in brain mitochondria. J Bioenerg Biomembr. 2011;43:3–10. doi: 10.1007/s10863-011-9337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino H, et al. Differential neuroprotection by cyclosporin A and FK506 following ischemia corresponds with differing abilities to inhibit calcineurin and the mitochondrial permeability transition. Neurobiol Dis. 2002;10:219–33. doi: 10.1006/nbdi.2002.0514. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Morishima I. Preparation and characterization of a chimeric zebrafish-human neuroglobin engineered by module substitution. Biochem Biophys Res Commun. 2005;330:591–7. doi: 10.1016/j.bbrc.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, et al. Cyclophilin D as a drug target. Curr Med Chem. 2003;10:1485–506. doi: 10.2174/0929867033457160. [DOI] [PubMed] [Google Scholar]
- Wang C, et al. Role of intracellular calcium stores in cell death from oxygen-glucose deprivation in a neuronal cell line. J Cereb Blood Flow Metab. 2002;22:206–14. doi: 10.1097/00004647-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–74. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, et al. Neuroglobin is an endogenous neuroprotectant for retinal ganglion cells against glaucomatous damage. Am J Pathol. 2011;179:2788–97. doi: 10.1016/j.ajpath.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wystub S, et al. Localization of neuroglobin protein in the mouse brain. Neurosci Lett. 2003;346:114–6. doi: 10.1016/s0304-3940(03)00563-9. [DOI] [PubMed] [Google Scholar]
- Xu J, et al. A combination of mutations enhances the neurotropism of AAV-2. Virology. 2005;341:203–14. doi: 10.1016/j.virol.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Yu Z, et al. Neuroprotective roles and mechanisms of neuroglobin. Neurol Res. 2009a;31:122–7. doi: 10.1179/174313209X389866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, et al. Neuroglobin-overexpression alters hypoxic response gene expression in primary neuron culture following oxygen glucose deprivation. Neuroscience. 2009b;162:396–403. doi: 10.1016/j.neuroscience.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, et al. Neuroglobin, a Novel Target for Endogenous Neuroprotection against Stroke and Neurodegenerative Disorders. Int J Mol Sci. 2012a;13:6995–7014. doi: 10.3390/ijms13066995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, et al. Identification of neuroglobin-interacting proteins using yeast two-hybrid screening. Neuroscience. 2012b;200:99–105. doi: 10.1016/j.neuroscience.2011.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, et al. Mitochondrial distribution of neuroglobin and its response to oxygen-glucose deprivation in primary-cultured mouse cortical neurons. Neuroscience. 2012c;218:235–42. doi: 10.1016/j.neuroscience.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.