Abstract
Purpose
To describe visual impairment (VI) over a 20-year period and its associations with age-related eye diseases and socioeconomic factors in the Beaver Dam Eye Study.
Design
Population-based cohort study.
Participants
4926 persons aged 43-86 years participated in the baseline examination phase in 1988-1990, and 3721, 2962, 2375, and 1913 participated in follow-up examinations each spaced 5 years apart in 1993-1995, 1998-2000, 2003-2005, and 2008-2010, respectively.
Methods
Best-corrected visual acuity after refraction, assessed by the Early Treatment of Diabetic Retinopathy Study protocol.
Main Outcome Measures
Incidence of VI, defined as best-corrected visual acuity of poorer than 20/40 in the better eye in persons with one or both eyes 20/40 or better at the beginning of a 5-year interval, and severe VI as 20/200 or worse in the better eye in persons with one or both eyes better than 20/200 at the beginning of a 5-year interval.
Results
Overall incidence of VI between examinations (5-year interval) was 1.4% (varying from 0.1% in persons aged 50-54 years to 14.6% in those aged 85 years and older) while for severe VI it was 0.4% (varying from 0.0% in persons aged 50-54 years to 6.9% in those aged ≥85 years). The incidence of VI decreased for each period, after adjustment for age, from the first 5-year interval between examinations (1988-1990 to 1993-1995) to the fourth and most recent 5-year interval (2003-2005 to 2008-2010; odds ratio [OR] fourth interval vs first interval 0.53, 95% confidence interval [CI] 0.32-0.87, P=0.01). This period effect was no longer significant after adjustment for age-related macular degeneration (AMD). AMD remained the leading cause of incident severe VI (54% of eyes with incident severe VI, which was as low as 40% and as high as 57% for specific visits) with no evidence of a trend across visits. The overall frequency of VI correctable with new refraction was 38% of all eyes with VI.
Conclusions
These data provide population-based estimates that show a high (15%) 5-year incidence of VI in persons aged 85 years and older. AMD remained the leading cause of severe VI in this population over the 20 years of the study.
Few population-based studies have provided estimates of the incidence of visual impairment over a long period of time.1-3 This information is important for assessing temporal trends as well as for projecting the long-term risk of developing visual impairment (VI) and the need for resources to care for visually impaired persons. Currently, this information is especially relevant because of the increasing number of persons in the United States projected to reach their 8th, 9th, and 10th decades of life. The number of persons in the United States aged 75 years and older, who are most vulnerable to loss in vision due to age-related eye diseases, is expected to increase from approximately 18.8 million in 2010 to 48.4 million in 2050.4 At the same time, the treatment and prevention of diabetic retinopathy and age-related macular degeneration (AMD), two of the leading causes of VI in the United States, have changed significantly over the past 20 years.5-7 This report extends our previous findings8-11 to describe the incidence of VI and its relationship to sociodemographic factors and the presence of AMD and cataract over a 20-year period in the population-based Beaver Dam Eye Study (BDES).
Patients and Methods
Population
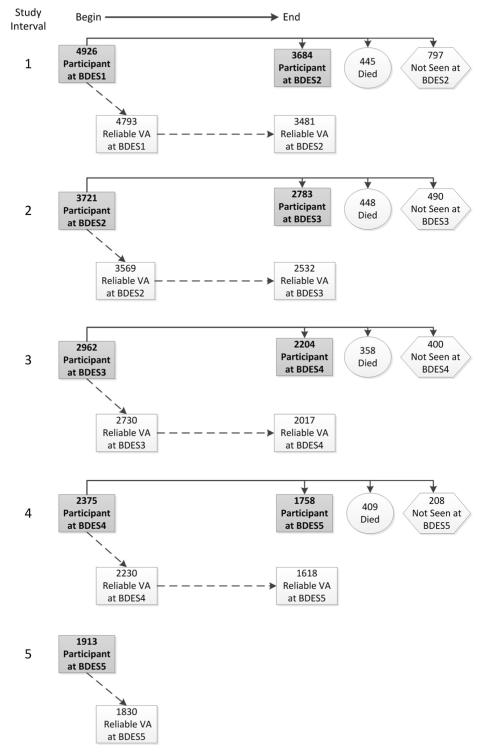
Descriptions of the methods used to identify the population have appeared previously.8,12 In brief, a private census of the population of Beaver Dam, Wisconsin, was performed from September 15, 1987 to May 4, 1988. Eligibility requirements for the study included living in the city or township of Beaver Dam and being 43-84 years of age at the time of the census. All persons eligible at the time of the census who were alive at follow-up were invited to participate at each examination regardless of whether they had participated in a previous examination. No additional individuals outside of the group eligible for the study at the time of the census were invited to participate at follow-up examinations. There were 5924 eligible individuals, of whom 4926 participated in the baseline examination between March 1, 1988 and September 15, 1990 (BDES1); 3721 participated in the 5-year follow-up examination between March 1, 1993 and June 15, 1995 (BDES2); 2962 participated in the 10-year follow-up examination between March 16, 1998 and June 9, 2000 (BDES3); 2375 participated in the 15-year follow-up examination between March 31, 2003 and June 1, 2005 (BDES4); and 1913 participated in the 20-year follow-up examination between November 5, 2008 and November 16, 2010 (BDES5) (Figure 1). Ninety-nine percent of the cohort was Caucasian.
Figure 1.
Participation and measurement of visual acuity (VA) from each examination interval of the Beaver Dam Eye Study (BDES). The participants at the beginning of the analysis interval (left side of figure) included all individuals that had been identified at the time of the census and were still alive regardless of prior participation. The right side of the figure shows what happened to the participants of each examination by the end of the 5-year analysis interval. Numbers after BDES abbreviation indicate specific examination phase (i.e., BDES1 = first examination, BDES2 = second examination [first follow-up], and so on).
Approval was granted by the Institutional Review Board at the University of Wisconsin. Written informed consent for the use and disclosure of protected health information was obtained from all subjects before being enrolled in the study and before each examination. The study was performed in accordance with the tenets of the Declaration of Helsinki.
The follow-up participation rate was high and similar for each examination. The main reason for nonparticipation was death (Figure 1). Unlike previous reports in which we reported the characteristics of participants and nonparticipants at a particular examination phase among those that had previously participated (consistent with analyses in those papers),9-11 we accumulated the participation information for each examination phase into a single analysis, consistent with analyses reported in this paper. Participation occurred at consecutive examinations for 10429 (75%) of the 13984 person-visits accumulated across all 4 examination intervals (3684 of 4926 participants at BDES1 returned for BDES2, 2783 of 3721 participants at BDES2 returned for BDES3, 2204 of 2962 participants at BDES3 returned for BDES4, and 1758 of 2375 participants at BDES4 returned for BDES5). The remaining 3555 person-visits were accumulated from 1660 (12%) person-visits where a participant at one examination died before the next follow-up (445 BDES1 participants died before BDES2, 448 BDES2 participants died before BDES3, 400 BDES3 participants died before BDES4, and 409 BDES4 participants died before BDES5) and 1895 (13%) person-visits where participant participated at one examination but refused participation or only completed an interview at the next (797 BDES1 participants were alive and nonparticipants at BDES2, 490 BDES2 participants were alive and nonparticipants at BDES3, 400 BDES3 participants were alive and nonparticipants at BDES4, and 208 BDES4 participants were alive and nonparticipants at BDES5). Those who participated at follow-up were more likely than nonparticipants who were alive or those who died before follow-up to be younger, and while adjusting for age were more likely to have a higher annual household income, to have more education, to not be institutionalized, to not be visually impaired, to not have a central cataract, and to not have AMD (Table 1, available at http://aaojournal.org). The mean and median times between the baseline and BDES5 examinations were 20.4 years (standard deviation=0.59) and 20.3 years, respectively.
At each examination, we used the visual acuity (VA) from the better-seeing eye for analyses. Visits with unreliable (or unmeasured) VA in one of the eyes were not used for analyses. To be eligible for incidence, a participant had to be seen at two consecutive visits and have reliable VA measured in both eyes at both visits. During each examination phase, at least 95% of participants had reliable VA in both eyes (Figure 1). During BDES1, 4793 of the 4926 participants had reliable VA measured. Of these 4793 persons, 1186 did not return for the BDES2 examination and 126 returned but reliable VA was not obtainable in at least one eye, leaving 3481 persons with reliable VA measured in both eyes at both the BDES1 and BDES2 examinations. Similarly, 3569 of 3721 persons seen at BDES2 had reliable VA in both eyes, of which 2532 had reliable VA measured in both eyes at BDES3. During BDES3, 2730 of 2962 persons had reliable VA, of which 2017 had reliable VA at BDES4. During BDES4, 2230 of 2375 persons had reliable VA, of which 1618 had reliable VA at BDES5. Thus, over the 20 years of follow-up there were a total of 9648 person-visits with reliable VA data available for change analyses.
Measurements
Similar procedures were used at all examinations. Participants underwent a standardized interview and examination at each visit. Information on demographic characteristics was obtained from a questionnaire. Street address was recorded for classification as a nursing home or group home. At baseline this was almost exclusively nursing homes, but by BDES5 there were many residential communities that provided various levels of care, including some areas within these communities where residents were still completely independent. We did not distinguish among areas within facilities, so persons were classified as institutionalized if any part of their living facility provided some level of care. Photographs of the ocular fundus13 and the lens14 were taken after pharmacological dilation of the pupil according to protocol and were graded in masked fashion by experienced graders. Slit-lamp photographs were taken to grade the degree of nuclear sclerosis. Retroillumination photographs were taken to grade presence and severity of cortical and posterior subcapsular cataracts. The protocols for photography and the grading procedures have been previously described.15 Scores for nuclear sclerosis were based on comparisons with standard photographs, which resulted in a 5-step scale of severity based on degree of opacity of the nucleus. Scores for cortical and posterior subcapsular cataracts were based on estimated amount of involvement within grid segments. Central cataract was defined as presence of nuclear cataract (standard 3 or greater) or 25% or more of the central circle of the grid with cortical or posterior subcapsular opacity present. History of cataract surgery was obtained by grading of red reflex photographs corroborated by clinical assessment during the slit lamp examination.
The Wisconsin Age-Related Maculopathy Grading System15-17 was used to assess the presence and severity of lesions associated with AMD from the fundus photographs. Grading procedures, lesion descriptions, and detailed definitions for their presence and severity appear elsewhere.15 Early AMD was defined as the presence of soft drusen and/or any drusen with retinal pigmentary abnormalities (increased retinal pigment and/or retinal pigment epithelium depigmentation) in the absence of signs of late AMD. Late AMD was defined as the presence of exudative macular degeneration and/or pure geographic atrophy.
At all follow-up examinations, before refraction, participants were asked to read the Early Treatment Diabetic Retinopathy Study (ETDRS) chart R modified for a 2 meter distance with their current prescription without covering either eye. The number of letters correctly read was recorded. At all examinations, the refraction from a Humphrey 530 refractor (Carl Zeiss Inc., Oberkochen, Germany) was placed in a trial lens frame and the best-corrected VA was re-measured for each eye by means of the ETDRS protocol with charts R 1 and 2 modified for a 2 meter distance.8,18 If the best-corrected VA was 20/40 or worse in either eye, an ETDRS refraction was performed for that eye and the VA was measured. The inter-observer difference among the examiners for the refraction or the best-corrected VA was low and not clinically appreciable (data not shown). Three levels of impairment in visual function were defined by the best-corrected VA in the better-seeing eye: no impairment (20/40 or better), any VI (worse than 20/40), and severe VI (legal blindness, 20/200 or worse).
All persons with vision of 20/200 or worse in at least one eye were reviewed by the principal investigator (RK) and the primary cause of the severe VI was noted based on disease status ascertained from photographs and observations from the study examiners. When the cause was unclear, the participant’s eye doctor was contacted.
Incidence of any VI was calculated within each 5-year interval between examinations. Persons with VA of 20/40 or better in one or both eyes at the beginning of a 5-year examination interval were considered at risk for incidence of any VI. Similarly, persons considered at risk for developing severe VI were those with VA better than 20/200 in one or both eyes at the beginning of a 5-year interval. For most analyses, age and other characteristics were defined at the beginning of an interval. Because all analyses use one VA measure (from the better-seeing eye) for a person, the corresponding AMD and central cataract status from this eye were used for analyses. When the VA was the same in both eyes, the AMD and central cataract status from the worse eye were used. For persons in which the AMD and central cataract status for the analysis eye was not gradable, if the VA in the other eye was similar (< 10 letters different), the AMD/central cataract status from the other eye was used.
Statistical Analysis
Comparisons of participants and nonparticipants were performed using the main effect F-test in a two-way analysis of variance model and the Cochran-Mantel-Haenszel test of independence to adjust for age groups with continuous (e.g., education level attained) and categorical (e.g., VA) characteristics, respectively. Unless otherwise noted, all models include information from each 5-year interval with appropriate adjustments for correlation from multiple visits.
Risk factor information was updated at each examination. Model selection began with examination of the age relationship. Once the appropriate age model was determined, age-period interactions were evaluated. Then all other factors were considered separately, and interactions between the factor and age and period were evaluated. Statistically significant (P<0.05) “univariate” relationships were entered into a multivariate model. Cumulative incidence estimates were obtained from models with age and period alone. Backward selection was used to obtain a final parsimonious model. SAS version 9 (SAS Institute, Cary, NC) was used for all analyses.
Results
Characteristics of the Cohort
Characteristics of the cohort participating at each examination are found in Table 2. The mean age increased from 62.0 years at BDES1 to 75.4 years at BDES5, with the proportion of those aged 85 years and older increasing from 1% at BDES1 to 15% at BDES5. The prevalence of central cataract and cataract surgery and the severity of AMD in right eyes (Table 2) and left eyes (data not shown) at the beginning of each interval increased over the 20-year period, as did the prevalence of more education, higher annual household income, and institutionalization.
Table 2.
Characteristics of Participants in the Beaver Dam Eye Study at the Beginning of Each Examination Phase.
| Baseline Examination (N=4926) |
5 Year Follow-Up (N=3722) |
10 Year Follow-Up (N=2962) |
15 Year Follow-Up (N=2375) |
20 Year Follow-Up (N=1913) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Characteristic | %* | Mean | %* | Mean | %* | Mean | %* | Mean | %* | Mean |
| Age (years) | 62.0 | 65.3 | 69.0 | 71.9 | 75.4 | |||||
| 43-54 | 30.9 | 19.8 | 2.4 | 0.0 | 0.0 | |||||
| 55-64 | 26.7 | 29.0 | 36.3 | 26.0 | 2.4 | |||||
| 65-74 | 26.0 | 29.7 | 30.9 | 36.6 | 49.9 | |||||
| 75-84 | 15.4 | 17.3 | 23.0 | 27.2 | 32.3 | |||||
| ≥85 | 1.0 | 4.2 | 7.5 | 10.2 | 15.4 | |||||
| Sex | ||||||||||
| Women | 56.1 | 56.7 | 58.1 | 58.7 | 58.4 | |||||
| Men | 43.9 | 43.3 | 41.9 | 41.3 | 41.6 | |||||
| Education (years) | 12.0 | 12.3 | 12.5 | 12.7 | 12.9 | |||||
| Less than high school | 29.3 | 24.3 | 20.2 | 16.7 | 14.1 | |||||
| High school or more | 70.8 | 75.7 | 79.8 | 83.3 | 85.9 | |||||
| Annual income (USD) (Missing information) |
(N=254) | (N=337) | (N=348) | (N=491) | (N=406) | |||||
| ≤ 9,000 | 16.3 | 10.9 | 6.8 | 4.7 | 3.3 | |||||
| $10,000-$19,000 | 27.6 | 24.1 | 20.9 | 20.8 | 18.8 | |||||
| $20,000-$29,000 | 20.3 | 20.1 | 19.9 | 19.5 | 20.4 | |||||
| $30,000-$44,000 | 20.5 | 21.6 | 21.1 | 21.9 | 22.6 | |||||
| ≥$45,000 | 15.2 | 23.3 | 31.4 | 33.2 | 34.8 | |||||
| Institutionalized | ||||||||||
| No | 98.2 | 95.8 | 93.6 | 93.3 | 91.7 | |||||
| Yes | 1.8 | 4.2 | 6.4 | 6.7 | 8.3 | |||||
| Visual acuity† (Missing information) |
(N=89) | (N=135) | (N=205) | (N=119) | (N=103) | |||||
| 20/40 and better | 93.1 | 94.7 | 94.0 | 93.3 | 92.7 | |||||
| 20/50 – 20/160 | 5.2 | 3.3 | 3.8 | 4.0 | 4.4 | |||||
| 20/200 and worse | 1.7 | 2.0 | 2.2 | 2.7 | 2.9 | |||||
| N letters read correctly | 51.6 | 51.8 | 50.6 | 48.8 | 49.3 | |||||
| AMD status† (Missing information) |
(N=336) | (N=312) | (N=268) | (N=204) | (N=199) | |||||
| None | 85.8 | 82.6 | 82.7 | 82.4 | 78.8 | |||||
| Early | 13.0 | 15.9 | 15.5 | 14.7 | 17.2 | |||||
| Late | 1.2 | 1.4 | 1.9 | 3.0 | 4.0 | |||||
| Central cataract status† (Missing information) |
(N=116) | (N=208) | (N=308) | (N=100) | (N=101) | |||||
| Absent | 82.0 | 73.3 | 70.1 | 65.1 | 54.5 | |||||
| Present | 13.7 | 18.9 | 15.9 | 16.1 | 15.8 | |||||
| Cataract surgery | 4.4 | 7.8 | 14.0 | 18.8 | 29.7 | |||||
AMD, age-related macular degeneration; USD, United States dollars.
Percentage calculated based on total number of responses to individual questions (not all participants answered every question). Percentages may not always add to 100% due to rounding.
In right eye.
Incidence of VI
Incidence of VI was calculated among those without any impairment (in at least one eye) at the beginning of each interval. VI was present at the beginning of the interval in 42 of the 3481 persons with reliable VA at both BDES1 and BDES2, 21 of the 2532 persons with reliable VA at both BDES2 and BDES3, 18 of the 2017 persons with reliable VA at both BDES3 and BDES4, and 19 of the 1618 persons with reliable VA at both BDES4 and BDES5. Thus, incidence of VI was evaluated in 9548 person-visits. Incidence of VI occurred in 38 of the 3439 persons at risk from BDES1 to BDES2, 37 of the 2511 persons at risk from BDES2 to BDES3, 28 of the 1999 persons at risk from BDES3 to BDES4, and 28 of the 1599 persons at risk from BDES4 to BDES5. In total, VI developed over a 5-year period in 131 of 9548 (1.4%, 95% CI 1.1-1.6) person-visits. Similarly, severe VI developed over a 5-year period in 34 of 9620 (0.4%, 95% confidence interval [CI] 0.2-0.5%) person-visits.
Incidence of any VI over a 5-year period was linearly associated (log-odds in logistic models) with age and ranged from 0.1% in persons aged 50-54 years to 14.6% in persons aged 85 years and older. From the model, cumulative incidences of VI over 5, 10, 15, and 20 years starting at age 45 years with no impairment were computed (Table 3, available at http://aaojournal.org). The estimated 20-year cumulative incidence varied from 0.6% in persons aged 45 years to 30.3% in persons aged 75 years with no impairment.
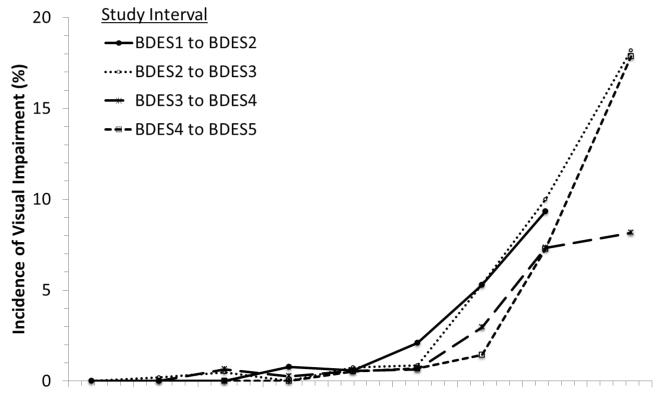
For specific age groups, the incidence of VI was slightly lower at more recent visits compared to earlier ones (Figure 2). For example, at age 65-69 years, incidence of VI occurred in 0.6% from BDES1 to BDES2 and in 0.5% from BDES4 to BDES5; at age 80-84 years, incidence of VI occurred in 9.3% from BDES1 to BDES2 and 7.3% from BDES4 to BDES5. There were no statistically significant interactions between period and age. Differences in incidence of VI from BDES1 to BDES2, BDES2 to BDES3, or BDES3 to BDES4 were not statistically significant after adjusting for age, but the incidence from BDES4 to BDES5 was statistically significantly lower (53% lower) than for BDES1 to BDES2 (OR for last period vs. first period=0.47, 95% CI 0.31-0.72, P<0.001). The period effect was no longer significant after adjustment for other factors such as AMD (data not shown).
Figure 2.
Incidence of visual impairment in the better eye by age and period for each of the 4 examination intervals in the Beaver Dam Eye Study (BDES), 1988-2010. Numbers after BDES abbreviation indicate specific examination phase (i.e., BDES1 = first examination, BDES2 = second examination [first follow-up], and so on).
Figure 3 A-F (available at http://aaojournal.org) and Table 4 show the incidence of any VI and severe VI by age, sex, education level, income, institutionalization status, central cataract status, and AMD status at the beginning of an interval over the 20 years of the study. With just age and visit in the model, there were no associations (Table 5) with gender (Figure 3A), more education (Figure 3B), higher income (Figure 3C), or institutionalization (Figure 3F). Persons in an institution had a different pattern of incidence of VI, but the numbers of institutionalized persons that were at risk for incidence (not visually impaired and returned for follow-up visit) were very low. Persons with late AMD (Figure 3E) were more likely to have incident VI than those without AMD or those with early AMD. There was no interaction of late AMD with age or visit. The relationship between cataract status (Figure 3D) and incident VI varied with age but was not statistically significant in person-specific models. The cataract relationship is more apparent when looking at each eye separately (data not shown).
Table 4.
Distribution of Incident Visual Impairment and Incident Severe Visual Impairment by Risk Factors in the Beaver Dam Eye Study (BDES).
| Any Visual Impairment | Severe Visual Impairment | |||
|---|---|---|---|---|
|
|
||||
| Risk Factor | N at risk | % Incidence | N at risk | % Incidence |
| Study interval | ||||
| BDES1 to BDES2 | 3439 | 1.1 | 3473 | 0.3 |
| BDES2 to BDES3 | 2511 | 1.5 | 2528 | 0.4 |
| BDES3 to BDES4 | 1999 | 1.4 | 2008 | 0.2 |
| BDES4 to BDES5 | 1599 | 1.8 | 1611 | 0.6 |
| Age (years) | ||||
| <50 | 768 | 0.0 | 768 | 0.0 |
| 50-54 | 1105 | 0.1 | 1106 | 0.0 |
| 55-59 | 1452 | 0.3 | 1454 | 0.1 |
| 60-64 | 1759 | 0.3 | 1762 | 0.0 |
| 65-69 | 1632 | 0.6 | 1641 | 0.0 |
| 70-74 | 1330 | 1.1 | 1336 | 0.2 |
| 75-79 | 863 | 3.7 | 879 | 1.1 |
| 80-84 | 482 | 8.3 | 500 | 1.6 |
| ≥85 | 157 | 14.6 | 174 | 6.9 |
| Sex | ||||
| Women | 5475 | 1.6 | 5526 | 0.5 |
| Men | 4073 | 1.1 | 4094 | 0.2 |
| Education | ||||
| Less than high school | 1814 | 2.8 | 1843 | 0.7 |
| High school or more | 7712 | 1.0 | 7754 | 0.3 |
| Annual income (USD) | ||||
| ≤$9,000 | 747 | 5.1 | 771 | 1.4 |
| $10,000-$19,000 | 1908 | 1.8 | 1934 | 0.4 |
| $20,000-$29,000 | 1817 | 1.0 | 1821 | 0.2 |
| $30,000-$44,000 | 2010 | 0.5 | 2011 | 0.2 |
| ≥$45,000 | 2375 | 0.4 | 2380 | 0.1 |
| Institutionalized | ||||
| No | 9502 | 1.3 | 9568 | 0.3 |
| Yes | 46 | 19.6 | 52 | 1.9 |
| AMD status* | ||||
| None | 7997 | 0.6 | 8026 | 0.0 |
| Early | 1329 | 3.9 | 1341 | 0.8 |
| Late | 38 | 42.1 | 54 | 31.5 |
| Central cataract status* | ||||
| Absent | 7616 | 0.7 | 7633 | 0.1 |
| Present | 1166 | 3.6 | 1196 | 0.8 |
| Cataract surgery | 637 | 4.1 | 652 | 1.8 |
AMD, age-related macular degeneration; USD, United States dollars. Ns indicate person-visits.
In the same eye which determined visual acuity.
Table 5.
Model Results for Incidence of Any Visual Impairment in the Beaver Dam Eye Study (BDES).
| Univariate Models | Multivariate Models | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| OR* | 95% CI | Overall P value |
OR* | 95% CI | Overall P value |
|
|
|
||||||
| Study interval | 0.03 | |||||
| BDES1 to BDES2 | 1.77 | (1.05, 3.00) | ||||
| BDES2 to BDES3 | 1.60 | (0.95, 2.69) | ||||
| BDES3 to BDES4 | 1.00 | (referent) | ||||
| BDES4 to BDES5 | 0.93 | (0.54, 1.59) | ||||
| Age, per 5 years | 2.34 | (2.07, 2.65) | <.001 | 1.96 | (1.70, 2.26) | <.001 |
| Sex, men vs. women | 1.27 | (0.81, 1.98) | 0.30 | |||
| Education, per year | 0.93 | (0.86, 1.01) | 0.07 | |||
| Yearly income (USD) | 0.13 | 0.04 | ||||
| ≤ $9,000 | 1.00 | (referent) | 1.00 | (referent) | ||
| $10,000-$19,000 | 0.54 | (0.31, 0.93) | 0.51 | (0.29, 0.90) | ||
| $20,000-$29,000 | 0.47 | (0.24, 0.91) | 0.35 | (0.18, 0.67) | ||
| $30,000-$44,000 | 0.36 | (0.16, 0.82) | 0.38 | (0.17, 0.82) | ||
| ≥$45,000 | 0.41 | (0.16, 1.06) | 0.35 | (0.15, 0.80) | ||
| Institutionalized, yes vs. no | 0.85 | (0.10, 7.40) | 0.88 | |||
| AMD status† | <.001 | <.001 | ||||
| None | 1.00 | (referent) | 1.00 | (referent) | ||
| Early | 3.25 | (2.04, 5.17) | 3.09 | (1.97, 4.85) | ||
| Late | 46.34 | (20.57, 104.40) | 49.55 | (21.49, 114.23) | ||
| Central cataract status† | 0.79 | |||||
| Absent | 1.00 | (referent) | ||||
| Present | 1.18 | (0.70, 2.00) | ||||
| Cataract surgery | 0.98 | (0.54, 1.78) | ||||
AMD, age-related macular degeneration; CI, confidence interval; OR, odds ratio; USD, United States dollars.
Adjusted for age.
In the same eye which determined visual acuity.
Model building began with all significant factors from the univariate associations (Table 5), which was just AMD. From this full model, non-significant terms were removed (visit was no longer statistically significant). With the new full model (age and AMD status), all other factors were once again evaluated, and income and institutionalization (including an interaction with age) became significantly associated with the incidence of VI. Both terms did not contribute independently, and income was a better predictor than institutionalization. Therefore, the final (most parsimonious) model contained age, income, and AMD severity level (Table 5). The presence of early (OR early AMD vs. none=3.1) and late AMD (vs. none OR=50) were statistically significantly associated with higher incidence of VI. For every income category, risk of incident VI was lower compared to persons with annual income of less than $9,000. However, all the other income categories did not differ significantly from each other for the incidence of VI. None of the interactions tested were statistically significant.
Because AMD and central cataract might be better evaluated in eye-specific models, we also explored these. The eye-specific models also showed age, income, and AMD status to be associated with incident VI, but in addition, central cataract status and cataract-age interaction terms were significant (data not shown). At younger ages, persons with cataract were more likely to have incident VI, but around age 70 years or older the incidence of VI was similar for persons with cataract compared to persons without. The opposite was true for cataract surgery. At younger ages, persons with cataract surgery had a similar incidence of VI as persons without any cataract, but at older ages, persons with cataract surgery had a statistically significantly lower incidence of VI than persons without any cataract.
Incidence of Severe VI
Incidence of severe VI was 0.35% overall (34 of 9620 person-visits) and varied with age from 0.0% at age 50-54 years to 0.3% at age 60-64 years, 1.3% at age 70-74 years, 4.3% at age 80-84 years, and 9.7% at age ≥85 years (Table 4). Only age and AMD were significantly associated with incidence of severe VI. In multivariate models, age was linearly related to incidence of severe VI and AMD was associated with higher incidence of severe VI. With few events, models with institutionalization and cataract status failed to converge and no interaction terms reached statistical significance. There was no period effect for severe VI (data not shown).
Visual Impairment in Persons Aged 90 Years and Older
One hundred and one persons aged 90 years and older at BDES5 had reliable VA measured in both eyes, of whom 27% (N=27) were visually impaired in their better eye, including 10 with severe VI. Similarly, 25% (3/12), 22% (14/63), and 26% (18/70) of persons aged 90 years and older at the time of BDES2, BDES3, and BDES4, respectively, were visually impaired.
Follow-up information for participants aged 90 years and older was limited because on average 64% of them died before the next visit and 44% of those who survived did not participate or did not have VA measured in both eyes at follow-up. This loss of follow-up was higher for persons with VI. Furthermore, among the 29 persons aged ≥90 years during BDES2, BDES3, and BDES4 with VA measured at both visits, 10% already had VI. Among the 26 people aged ≥90 years without any VI, 28% (N=8) became visually impaired by follow-up.
Causes of Severe VI
Of the 184 eyes with severe VI at their first visit and the 256 that developed severe VI between visits, late AMD was the primary cause in 44% of eyes, branch or central retinal vein occlusion in 8%, and cataract in 10% (Table 6). The percentage of eyes with severe VI caused by AMD changed little over the 20 years of the study. Other less frequent causes of severe VI included macular hole (4%), diabetic retinopathy (3%), retinal detachment (3%), and trauma (8%). Some causes (e.g., trauma) were much more common causes for severe impairment at the first visit than for incident severe VI between visits, which diminished the apparent impact of other causes, e.g., AMD. When eyes with severe VI caused by trauma, amblyopia, and congenital conditions were removed, AMD was the primary cause of severe VI for persons first seen with impairment in 43% (of 128) eyes (rather than 30% of the 184 eyes).
Table 6.
Causes of Severe Visual Impairment Among Participants of the Beaver Dam Eye Study.
| Severe Impairment at Baseline |
Developed Severe Impairment |
Total Severe Impairment |
||||
|---|---|---|---|---|---|---|
|
|
||||||
| Cause | N | % | N | % | N | % |
| Retina | ||||||
| Age-related macular degeneration | 55 | 29.9 | 138 | 53.9 | 193 | 43.9 |
| Diabetic retinopathy | 7 | 3.8 | 5 | 2.0 | 12 | 2.7 |
| Macular edema | 0 | 1 | 0.4 | 1 | 0.2 | |
| Macular hole | 3 | 1.6 | 14 | 5.5 | 17 | 3.9 |
| Occlusion | 10 | 5.4 | 25 | 9.8 | 35 | 8.0 |
| Detachment | 9 | 4.9 | 5 | 2.0 | 14 | 3.2 |
| Other | 11 | 6.0 | 8 | 3.1 | 19 | 4.3 |
| Lens (Cataract) | 19 | 10.3 | 26 | 10.2 | 45 | 10.2 |
| Cornea | 4 | 2.2 | 7 | 2.7 | 11 | 2.5 |
| Optic nerve | ||||||
| Glaucoma | 1 | 0.5 | 9 | 3.5 | 10 | 2.3 |
| Ischemic optic neuropathy | 0 | 5 | 2.0 | 5 | 1.1 | |
| Other | 6 | 3.3 | 2 | 0.8 | 8 | 1.8 |
| Enucleated | 1 | 0.5 | 3 | 1.2 | 4 | 0.9 |
| Trauma | 35 | 19.0 | 2 | 0.8 | 37 | 8.4 |
| Congenital | 8 | 4.4 | 0 | 8 | 1.8 | |
| Amblyopia | 13 | 7.1 | 0 | 13 | 3.0 | |
| Unknown | 2 | 1.1 | 6 | 2.3 | 8 | 1.8 |
| Total | 184 | 256 | 440 | |||
Correctable VI by New Refraction
In 19.3% (n=1986) person-visits, there was an improvement of ≥5 letters read after refraction compared with their current refraction. Among the 406 visits in which persons were visually impaired with their current correction, 26% (N=104) persons improved by at least 1 line but were still impaired and 38% (N=153) improved by at least 1 line and were no longer impaired after refraction. The percentage of persons with VI correctable by new refraction changed little over the 20 years of the study. Among persons with impairment with their current prescription, improvement of 1 line of vision or more upon refraction that resulted in VA 20/40 or better was 73% less common in those institutionalized (OR=0.27, P<0.001) and 90% less common in those who had late AMD in either eye (OR=0.10, P<0.001). With institutionalization and AMD present, there were no differences with age. There were no differences by examination phase, education, income, sex, or cataract status.
Discussion
The incidence of VI over a 5-year period increased with age in the BDES, reaching 15% in persons aged 85 years and older. There was an age-period effect in VI in the population which was no longer significant after adjusting for AMD status. AMD remained the most important cause of severe VI. The prevalence of VI correctable by new refraction remained unchanged throughout the 20 years of the study.
There was a small decrease in the odds of incident VI from the beginning of our study to the end (OR for incidence between BDES4 to BDES5 vs BDES1 to BDES2 = 0.53; 95% CI 0.32, 0.87). While this was no longer statistically significant after adjustment for AMD, it still suggests lower rates of incidence in the last 5 years of the study. This may be due, in part, to new treatment interventions for AMD.7
To our knowledge, there are no other population-based studies that have examined and reported changes in the incidence of VI over a 20-year period. The National Health Interview Survey, based on self-reported response to 2 questions about vision, showed a non-significant annual downward trend of −0.04% per year from 1997-2005.2 In those data, there were statistically significant downward trends in self-reported VI in those who reported being seen by an eye care provider in the previous 12 months (−0.18% per year), in those with diabetes (−0.81% per year) and in persons aged ≥80 years (−0.46% per year). Lee et al.2 speculated that these downward trends may be due to improvements in the treatment of ocular disease, increased eye care utilization in those with VI, or both.
While the decline in incidence of impairment is not statistically significant after adjustment for AMD, our suggestion of a period effect may have public health implications. If a decline in the incidence of VI is occurring in the United States population, it would result in lower estimates than those expected based on the 2004 prevalence report.19 In the BDES, 13.3% of persons aged ≥75 years developed VI and 2.4% developed severe VI over a 10-year period using information collected from 1988-1990 to 1998-2000, with the corresponding incidence during the last 10-year period of 9.9% and 2.7%, respectively. Projecting these estimates to the current US population aged ≥75 years (18,766,000) would result in 26% fewer persons developing VI over the next 5 years using the current estimates (N=1,851,355) compared to the older estimates (N=2,502,134). However, it is possible that selective survival and participation and uncontrolled confounding may explain these changes in incidence of VI over time. At every visit, those with VI or with AMD were less likely to participate, and nonparticipation of those at risk rather than a true decline in VI over the course of the study may, in part, explain the decrease in the incidence of VI in more recent period.
In the BDES, the 20-year cumulative incidence of any VI and severe VI for persons aged 60 years without prior impairment was 3.0% and 0.3%, respectively, and for persons aged 70 years without prior impairment it was 14.8% and 2.7%, respectively. If the BDES estimates are extrapolated to the future, then approximately 3% of people aged 60 years (with a current average life expectancy of 21 more years for men and 24 for women) are at risk of developing VI over their remaining lifetime.20 There are no similar long-term population-based incidence data from the United States and limited data from elsewhere with which to compare these estimates. For example, in a study in Italy, severe VI developed over a 12-year period in 0.7% of those aged 40 years and older at baseline.3 In the Barbados Eye Study, the 9-year cumulative incidence in persons aged 40-84 years at baseline for any VI was 10.1% and for severe VI it was 2.1%.1 These differences among studies may reflect differences in age-related ocular disease distributions that may be related to racial/ethnic and environmental factors, in methodologies used to estimate VI, and to the decreasing incidence of VI in more recent times that might be as suggestive in the BDES, which extends more than 10 years beyond the last examination of the other 2 studies.
In this report, we use the expected 5-year incidence to calculate the 20-year cumulative incidence. While it is possible to directly calculate the incidence with life-table approaches to the observed data, the direct approach over long follow-up, with the loss to follow-up in the oldest ages, limits the stability of 20-year incidence estimates. For example, to directly calculate the 20-year cumulative incidence for a 75-year-old, we need enough people aged 75 years at the baseline examination to return for all examinations (and still be at risk for incidence). In the BDES, as in other studies, there are usually a small number of persons in this group. Instead, we use data from persons aged 75, 80, 85, and 90 years at BDES1 along with the persons aged 75, 80, 85, and 90 years at the time of BDES2, BDES3, and BDES4 to identify the 5-year incidence. We then use the life-table approaches with those estimates to calculate the expected 20-year cumulative incidence. Because these 5-year incidences were estimated with more data, the calculated 20-year incidences provide a more precise estimate than what could be obtained directly. If we directly calculate the 10-, 15- and 20-year cumulative incidence of any impairment for a 60-year-old, we observe 1.4%, 1.6% and 2.5%, respectively, whereas the expected cumulative incidence from our models predicts 0.5%, 1.2%, and 3.0% for the 10-, 15-, and 20-year cumulative incidence, respectively.
AMD remained the leading cause of severe VI in the BDES, affecting a similar proportion of eyes throughout the 20 years. Retinal vein occlusion was a more frequent cause of severe VI than diabetic retinopathy. This is consistent with the decrease in proliferative diabetic retinopathy and macular edema reported in persons with type 2 diabetes through better glycemic control and timely detection, as well as treatment of proliferative retinopathy and clinically significant macular edema with laser photocoagulation.5 Retinal vein occlusions and other conditions (e.g., ischemic optic neuropathy), while less frequent in the population than proliferative retinopathy and clinically significant macular edema, are more likely to affect vision because there are no prevention programs and limited treatment strategies to restore vision. Other conditions, e.g., trauma and amblyopia, which account for 9% and 3% of severe VI, are infrequent causes of incident severe VI in persons aged ≥40 years; approaches to prevent and treat them must be focused on persons in early years of life when these conditions occur.
Visual impairment that can be corrected by refraction has been estimated cross-sectionally to involve approximately 25-50% of those with VI in population-based studies.11,21-25 Over the 20 years of the BDES, 38% of eyes with VI were correctable by new refraction. It is important to correct VI because it has been shown to affect self-rated quality of life and be associated with a higher frequency of falls than in persons without VI.23 In the BDES, while adjusting for age, having AMD and not being institutionalized were associated with having correctable VI (compared to those with no improvement in VI). In the Blue Mountains Eye Study, older age, and after adjusting for age, being married, home ownership, high job prestige, having more than a high school education, and being a current driver were factors associated with being less likely to have correctable VI.26 In the BDES, frequency of correctable VI varied little over the 20-year period. This was similar to the lack of variation in correctable VI in the Blue Mountains Eye Study in which approximately 68% of those visually impaired had correctable VI. Similar sociodemographic measures were associated with correctable VI in both studies.
In the BDES, only higher income was associated with less VI after adjustment for age and AMD status. This has been previously reported and may be due, in part, to having better access to care and information and better overall health.27-29 However, the effect of income on VI was small.
With just AMD and age in the model, institutionalization was also associated with incident VI, but did not remain in the model once income was also added. Income and institutionalization are highly correlated, so each can be significant, but not independently. We chose to report the model with income because it had a better overall fit than the model with institutionalization. Institutionalization was also associated with less VI correctable by new refraction. This may be due to a lowered likelihood of surgically indicated interventions for treatable conditions, e.g., cataract surgery for central cataract in persons institutionalized because of higher frequency of cognitive dysfunction and other comorbidities, or to the lack of access to ophthalmologic care.
While our study has many strengths, there are limitations that might affect its outcomes and generalizability. First, selective survival may have affected our findings. Second, as people age into their 10th decade of life, measuring vision reliably becomes more difficult due to cognitive decline and co-morbidities.
In summary, these data provide unique information regarding changes in VI over a period of significant change in medical and ophthalmological care. They show a relatively high long-term incidence of VI (21%) and severe VI (7%) in persons aged 85 years and older. There were significant age-period and cohort effects, with lower prevalence of VI in those most recently examined that is explained, in part, by AMD. This has important public health care implications in estimating the projected burden in the United States population of the number of those expected to become visually impaired. Continued national epidemiological surveillance is needed to monitor changes in the incidence and prevalence of VI and the diseases that cause them in order to estimate the costs and benefits of new ophthalmological interventions after they are introduced.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by National Institutes of Health grant EY06594 (BEK Klein and R Klein) and, in part, by Research to Prevent Blindness (R Klein and BEK Klein, Senior Scientific Investigator Awards), New York, NY. The National Eye Institute provided funding for entire study including collection and analyses of data; RPB provided additional support for data analyses. Neither funding organization had a role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
Conflict of Interest: No conflicting relationship exists for any author.
Contributor Information
Ronald Klein, Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health, Madison, WI.
Kristine E. Lee, Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health, Madison, WI.
Ronald Gangnon, Departments of Biostatistics and Medical Informatics and Population Health Sciences, University of Wisconsin School of Medicine and Public Health, Madison, WI.
Barbara E. K. Klein, Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health, Madison, WI.
References
- 1.Hennis AJ, Wu SY, Nemesure B, et al. Barbados Eye Study Group. Nine-year incidence of visual impairment in the Barbados Eye Studies. Ophthalmology. 2009;116:1461–8. doi: 10.1016/j.ophtha.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DJ, Arheart KL, Lam BL, et al. Trends in reported visual impairment in United States adults. Ophthalmic Epidemiol. 2009;16:42–9. doi: 10.1080/09286580802624434. [DOI] [PubMed] [Google Scholar]
- 3.Nucci C, Cedrone C, Culasso F, et al. Incidence of visual loss in the Ponza Eye Study, Italy. Eye (Lond) 2005;19:175–82. doi: 10.1038/sj.eye.6701444. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Census Bureau . Projections of the Population by Age and Sex for the United States: 2010 to 2050. [Accessed November 13, 2012]. 2008. National Population Projections. Summary Tables. Table 12. Available at: http://www.census.gov/population/projections/data/national/2008/summarytables.html. [Google Scholar]
- 5.Klein R, Klein BE. Are individuals with diabetes seeing better? A long-term epidemiological perspective. Diabetes. 2010;59:1853–60. doi: 10.2337/db09-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong IY, Koo SC, Chan CW. Prevention of age-related macular degeneration. Int Ophthalmol. 2011;31:73–82. doi: 10.1007/s10792-010-9397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang A, Regillo CD. Preferred therapies for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2011;22:199–204. doi: 10.1097/ICU.0b013e32834597d9. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–5. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–78. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 10-year period: the Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–66. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–49. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Campbell JA, Palit CD. Total digit dialing for a small area census by phone; Proceedings of the Section on Survey Research Methods: papers presented at the Annual Meeting of the American Statistical Association; New Orleans, Louisiana. 1988; Alexandria, VA: American Statistical Association; Aug 22-25, 1988. pp. 549–51. [Google Scholar]
- 13.Klein BE, Klein R. Report for 16 Jun 87-31 May 92. U.S. Dept of Commerce; Springfield, VA: 1991. Beaver Dam Eye Study: Manual of Operations (Revised) pp. xx–xx. NTIS Publication PB91-149823. AQ: document is over 400 pages; must provide specific, inclusive pagination for material being cited. [Google Scholar]
- 14.Cruickshanks KJ, Klein BE, Klein R. Ultraviolet light exposure and lens opacities: the Beaver Dam Eye Study. Am J Public Health. 1992;82:1658–62. doi: 10.2105/ajph.82.12.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein BE, Klein R, Linton KL, et al. Assessment of cataracts from photographs in the Beaver Dam Eye Study. Ophthalmology. 1990;97:1428–33. doi: 10.1016/s0161-6420(90)32391-6. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 18.Early Treatment Diabetic Retinopathy Study (ETDRS) Manual of Operations. U.S. Dept of Commerce; Springfield, VA: 1985. pp. 101–19. NTIS Publication PB85-223006. [Google Scholar]
- 19.Eye Diseases Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Social Security Administration . Period Life Table, 2007. [Accessed July 9, 2012]. Available at: http://www.ssa.gov/oact/STATS/table4c6.html. [Google Scholar]
- 21.Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the United States. JAMA. 2006;295:2158–63. doi: 10.1001/jama.295.18.2158. [DOI] [PubMed] [Google Scholar]
- 22.Munoz B, West SK, Rubin GS, et al. SEE Study Team Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118:819–25. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 23.Wang JJ, Mitchell P, Smith W, et al. Impact of visual impairment on use of community support services by elderly persons: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 1999;40:12–9. [PubMed] [Google Scholar]
- 24.Tielsch JM, Sommer A, Witt K, et al. Baltimore Eye Survye Research Group. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol. 1990;108:286–90. doi: 10.1001/archopht.1990.01070040138048. [DOI] [PubMed] [Google Scholar]
- 25.Varma R, Wang MY, Ying-Lai M, et al. Los Angeles Latino Eye Study Group. The prevalence and risk indicators of uncorrected refractive error and unmet refractive need in Latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2008;49:5264–73. doi: 10.1167/iovs.08-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foran S, Rose K, Wang JJ, Mitchell P. Correctable visual impairment in an older population: the Blue Mountains Eye Study. Am J Ophthalmol. 2002;134:712–9. doi: 10.1016/s0002-9394(02)01673-2. [DOI] [PubMed] [Google Scholar]
- 27.Tielsch JM, Sommer A, Katz J, et al. Baltimore Eye Survey Research Group. Socioeconomic status and visual impairment among urban Americans. Arch Ophthalmol. 1991;109:637–41. doi: 10.1001/archopht.1991.01080050051027. [DOI] [PubMed] [Google Scholar]
- 28.Dandona R, Dandona L. Socioeconomic status and blindness. Br J Ophthalmol. 2001;85:1484–8. doi: 10.1136/bjo.85.12.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein R, Klein BE, Jensen SC, et al. The relation of socioeconomic factors to age-related cataract, maculopathy, and impaired vision. The Beaver Dam Eye Study. Ophthalmology. 1994;101:1969–79. doi: 10.1016/s0161-6420(13)31077-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.