Abstract
Background
Few studies have examined real world effectiveness of integrated buprenorphine maintenance treatment (BMT) programs in federally qualified health centers (FQHCs).
Methods
Opioid dependent patients (N=266) inducted on buprenorphine between July 2007 and December 2008 were retrospectively assessed at Connecticut’s largest FQHC network. Six-month BMT retention and opioid-free time were collected longitudinally from electronic health records; 136 (51.1%) of patients were followed for at least 12 months.
Results
Participants had a mean age of 40.1 years, were primarily male (69.2%) and treated by family practitioners (70.3%). Co-morbidity included HCV infection (59.8%), mood disorders (71.8%) and concomitant cocaine use (59%). Retention on BMT was 56.8% at 6 months and 61.6% at 12 months for the subset observed over 1 year. Not being retained on BMT at 12 months was associated with cocaine use (AOR=2.18; 95% CI=1.35–3.50) while prescription of psychiatric medication (AOR=0.36; 95% CI 0.20–0.62) and receiving on-site substance abuse counseling (AOR=0.34; 95% CI 0.19, 0.59) improved retention. Two thirds of the participants experienced at least one BMT gap of 2 or more weeks with a mean gap length of 116.4 days.
Conclusions
Integrating BMT in this large FQHC network resulted in retention rates similarly reported in clinical trials, but emphasizes the need for providing substance abuse counseling and screening and treating medical and psychiatric comorbidity.
Keywords: buprenorphine, substance abuse, opioid dependence, healthcare utilization, community health centers, federally qualified health centers, implementation science
1. BACKGROUND
Opioid dependence and abuse, including use of heroin or prescription pain killers, affects approximately 2.28 million Americans (SAMHSA, 2009). Opioid substitution therapy, such as methadone and buprenorphine, has documented effectiveness in treating opioid dependence (Connock et al., 2007; Mattick et al., 2008). Access to specially licensed and highly structured methadone maintenance programs, however, is limited, leaving 80–85% of the opioid-dependent population untreated (Friedman et al., 2007). The Drug Addiction Treatment Act of 2000 and the approval of buprenorphine in 2002 allowed certified physicians to prescribe buprenorphine in primary and specialty care settings, making opioid maintenance treatment more available and easier to access (Altice et al., 2006; Basu et al., 2006).
In March 2010, the U.S. Congress passed the Affordable Care Act (ACA) to substantially reduce the number of uninsured Americans and the United States Supreme Court largely upheld the healthcare reform law in June 2012. The ACA seeks to increase access to affordable, high quality healthcare and thus supports expanded healthcare delivery in federally qualified health centers (FQHCs). FQHCs are public or private non-profit health centers governed by a community board that are grant-supported under the Public Health Service Act and provide comprehensive primary care services in communities where there is a need to provide care for the medically underserved. Those individuals who have lower education, are unemployed, live in metropolitan areas or who are on probation or parole have higher rates of substance dependence or abuse (SAMHSA, 2009) and many FQHCs serving these individuals strive to deliver comprehensive and integrated healthcare including mental health services. Nevertheless, despite the capability of primary care physicians (PCPs) to prescribe buprenorphine, barriers persist that prevent expansion of buprenorphine maintenance treatment (BMT; Barry et al., 2009; Netherland et al., 2009).
Aside from efficacy trials conducted in primary care settings, few studies examine the relative effectiveness of BMT that is provided in real-world FHQCs. Most studies examining BMT in primary care centers are either university-affiliated or hospital-based (Alford et al., 2011; Altice et al., 2011; Fiellin et al., 2008; Harris et al., 2005; Mintzer et al., 2007; Soeffing et al., 2009; Stein et al., 2005). Thus, real-world clinical effectiveness studies are likely helpful to support further expansion of BMT. Provision of BMT in FQHCs, which cater to medically underserved populations, is even more critical since vulnerable patients from these centers urgently need opioid substitution therapy.
Patients with opioid dependence often have multiple medical and psychiatric co-morbidities (Altice et al., 2010). Therefore, integrating BMT in healthcare settings allows for the simultaneous treatment of multiple comorbidities (Altice et al., 2010; Korthuis et al., 2011; Sylla et al., 2007). There are currently few clinical effectiveness studies conducted in FQHCs that examine the factors related to buprenorphine’s effectiveness in treating opioid dependence and simultaneously engaging them in routine primary care, including prevention, diagnosis, and treatment of co-morbid medical conditions (e.g. HIV, HCV, HBV, hyperlipidemia, and hypertension). In this paper, we examine real-world substance abuse treatment outcomes in patients within a FQHC network. Such findings have important implications for other countries globally where BMT can be prescribed in primary care settings and reassure countries where BMT remains highly regulated.
2. METHODS
2.1 Source of study population
Community Health Center, Inc. (CHC) is Connecticut’s largest FQHC network, comprised of 13 sites. Two of the largest sites are located in the impoverished cities of New Britain and Meriden and serve over 28,500 patients of whom the majority are people of color [Hispanics (56%), Blacks (10%)] and are on Medicaid (71%), while 17% are uninsured. During the study period, four physicians were certified and providing BMT at these two sites; three were family physicians and one was a psychiatrist.
2.2 Description of the Buprenorphine Maintenance Treatment program
BMT began at CHC in 2006 with one psychiatrist prescribing in New Britain and Meriden. By 2007, two family practitioners in Meriden and one in New Britain initiated BMT resulting in a standardized induction and stabilization protocol based on SAMHSA’s Treatment Improvement Protocol 40 (Center for Substance Abuse Treatment, 2004). Induction was primarily observed within the clinic; however, occasionally home induction occurred (Gunderson et al., 2010). Patients were generally reassessed weekly over the first 2 weeks. Thereafter, patients were usually seen every 1–4 weeks, depending on provider determination. Urine toxicology screens were typically collected at every visit. Supervised urine collection and buprenorphine pill counts were done at the discretion of the provider. Patients were initially referred to either on- or off-site substance abuse counseling, depending on provider preference and availability. Management of urine test results positive for illicit drugs, including heroin and cocaine, remained at the discretion of the individual provider. For patients deemed not succeeding on BMT, referral to off-site intensive outpatient counseling, inpatient treatment, methadone maintenance, or discharge from the program would usually result. Buprenorphine prescriptions were sent to two designated pharmacies that dispensed the medication only upon the patient’s presentation of a validated voucher embossed by an authorized healthcare provider. The CHC protocol served only as a guide to providers and thus inter-provider variability in patient management existed based on differences in style and philosophy of practice.
2.3 Study design and sample
In this retrospective, observational study, persons were included in the cohort if they were ≥18 years, met DSM-IV criteria for opioid dependence, were prescribed at least one prescription for buprenorphine by a CHC provider between July 1, 2007 and December 1, 2008, and received treatment at either the New Britain or Meriden site. Overall, 266 patients met the inclusion criteria and were included in the analysis. Study participants were identified through the electronic health record (EHR) for anyone prescribed buprenorphine. The dates of enrollment were chosen since the BMT protocol had been adopted and the EHR had been fully implemented at all CHC health center sites by July 1, 2007.
All subjects were observed for at least 6 months beyond the date of their first prescription, with a range from 6 to 21.5 months. Observation consisted solely of review of the EHR. For this analysis, outcomes of interest were related to substance abuse treatment outcomes, including retention in care, treatment gaps, and opioid-free time.
2.4 Study procedure
A standardized data collection instrument was created for the electronic chart review. The instrument included age, health care site, type of buprenorphine prescriber (family practitioner or psychiatrist), all FQHC visits, buprenorphine prescription doses and dates, medication lists, problem lists, and laboratory results including urine toxicology screening tests. Two research assistants independently extracted these data and where there was data entry discordance, the lead author (MSH) resolved the discrepancy.
2.5 Measures
2.5.1 Covariate Definitions
The main reason for entry into care at CHC was considered primary care if subjects were engaged at the FQHC for longer than a month prior to being evaluated for BMT. If BMT was requested at the first visit or within one month of enrolling at the FQHC, then BMT was considered their reason for entry.
Co-morbidity data were based on International Classification of Diseases, 9th Revision (ICD-9) coding in the EHR and included: HIV, HCV, and HBV infections; metabolic disorders, including diabetes, hypertension, hyperlipidemia, and coronary artery disease; pulmonary disorders including asthma and chronic obstructive pulmonary disorder; and mood disorders including depression, anxiety, bipolar, and psychotic disorders. Subjects prescribed anti-depressant, anti-anxiety, anti-psychotic, and mood stabilizing medications, were also identified. Either the primary care or behavioral health provider could have prescribed these psychiatric medications.
Co-morbid cocaine use was defined in two ways: 1) positive upon entry if cocaine was detected at baseline or within 1 week after induction and 2) positive if detected in at least one urine sample any time during the observation period.
The number and type of visits to the FQHC were divided into 1) medical visits, which included visits to a medical provider, nurse, nutritionist, or podiatrist, 2) behavioral health visits, which included visits to a psychiatrist, behavioral health prescriber, or clinician, and 3) on-site substance abuse counseling visits, which included individual or group visits with the substance abuse counselor. A subject was considered to have received substance abuse counseling on-site if he or she attended one or more individual or group visits with the substance abuse counselor; these visits were documented in the EHR.
2.5.2 Substance Abuse Outcome Definitions
The primary outcome was retention on buprenorphine. Retention on buprenorphine was defined as being on BMT at the end of a pre-specified time period, similar to other studies which defined retention as the time until initial discontinuation of BMT (Alford et al., 2011; Cunningham et al., 2008; Moore et al., 2007; Parran et al., 2010; Soeffing et al., 2009). Retention was assessed at 1, 3, 6, and 12 months. In recognition that opioid dependence is a chronic relapsing disease, subject data were not censored if they discontinued BMT and were later reinducted.
In addition, subjects were examined for persistence on BMT, a concept that recognizes recurrent treatment episodes (Bae et al., 2011; Ing et al., 2011). Treatment persistence was defined as receiving buprenorphine prescriptions continuously without any gaps in treatment of 2 weeks or more. Non-persistent treatment was defined as experiencing one or more gaps of 2 weeks or more in buprenorphine prescriptions and it included the gap between the end of the last prescription given and the end of observation period. Indeed, patients who missed receiving a buprenorphine prescription for 2 weeks or more may have tapered their dose or obtained buprenorphine outside the health center until they returned to the clinic. Individual charts were not reviewed to see if buprenorphine was continued during their absence from the clinic. Given easy accessibility of appointments at the FQHC, however, choosing a buffer of 2 weeks was assumed to be adequate in limiting potential misclassifications of non-persistence treatment. This approach acknowledged the integrated chronic disease model of care espoused by FQHCs as it is applied to the chronic and relapsing nature of opioid dependence.
A secondary outcome was opioid-free time. This outcome was defined in three distinct ways because of the inconsistency reported in previous studies. The first two definitions, similar to those examined by others to allow for comparison with existing data, were 1) no urine opioids in the last month of observed treatment (Alford et al., 2011, 2007, 2004; Cunningham et al., 2011; Soeffing et al., 2009); and 2) the percentage of all collected opioid-free urines (Fiellin et al., 2008; Kakko et al., 2007, 2003; Moore et al., 2007; O’Connor et al., 1998). In an attempt to correct for the real-world discrepancy in the number of urines collected per person over the variable lengths of time each person was on treatment, we created a third definition, the duration of opioid-free time while on prescribed buprenorphine, by multiplying the percentage of all collected opioid-free urine samples by the length of time the patient was on BMT, but excluding the documented gaps in treatment. Urine screens included in the analysis started with the first urine collected one week after buprenorphine induction, which we defined as baseline.
2.6 Statistical Analysis
To address the issue of missing data, a series of multiple imputations were performed using a Markov Chain Monte Carlo (MCMC) simulation, conditional on variables which were observed (Jackman, 2000). Urine testing data were missing for only 5% and 3% of subjects for opioids and cocaine, respectively. For subjects who had urines collected, 12% and 4% had missing data for opioids and cocaine in the last observation month, respectively. Using MCMC simulation, the propensity of missing urine test results was not statistically related to the number of opioid and cocaine screening tests. Missing at Random (MAR) assumption was therefore invoked, which specifies that the probability of missing values is related to other observed covariates, but not the values of the missing variable itself (Enders, 2010). Sensitivity analysis, conducted using additional simulations, confirm that the results were not sensitive to the departures from the MAR assumption.
Average BMT retention was analyzed by estimating a series of Cox proportional hazard models, where the outcome was defined in terms of whether the subject was on or off BMT based on pharmacy refill data. Consistent with the chronic and relapsing nature of opioid dependence, subjects were not censored from subsequent time points if they experienced a treatment gap and were re-inducted. The covariates in the Cox regression model included age, gender, site, prescriber specialty, cocaine use (both as ‘baseline urine positive’ and as ‘at least one urine positive anytime during observation’), receipt of on-site substance abuse counseling, receipt of psychiatric medication, and presence of co-morbid mood disorder, and HIV and HCV infections.
In univariate analyses, covariates found to be statistically significant at p<0.20 were modeled to determine the subset of the covariates which accounted for most of the variation in the dependent variable and then included in the multivariate analysis. The Wald test was used to assess the significance of the coefficients.
We evaluated the variability in the opioid-free time using several distinct models based on the three definitions of opioid-free time and a set of covariates. Because the first definition of opioid-free time was based on a dichotomous measure assessing whether the urine sample was opioid-free in the last month of treatment, we used logistic regression. Because our second definition was based on a percent of opioid-free urine samples, we employed a generalized linear model with a logit link. Since our third definition was based on a continuous measure expressed in units of days, we used an ordinary least square regression, in which the standard errors were adjusted for heteroskedasticity. In all three cases, the covariates that were found to be significant at p<0.20 were included into the multivariate framework. The final multivariate model estimated in all three cases was based on the Akaike Information Criterion (AIC). All statistical analyses were conducted using STATA v.11.1 (StatCorp, College Station, TX).
3. RESULTS
3.1 Characteristics of the study population
Of the 266 eligible subjects, the mean age was 40.1 years and most were male (69.2%), prescribed buprenorphine by a family practitioner (70.3%) and had established care at the FQHC primarily seeking BMT (80.5%). Thus, most patients entered primary care in search of substance abuse treatment. Subjects had significant co-morbidity including HIV infection (10.9%), HCV infection (59.8%), and a mood disorder (71.8%) with 65.0% being prescribed psychiatric medications. Over a third had a metabolic disorder and 17.7% had a chronic pulmonary disorder (Table 1).
Table 1.
Baseline characteristics of 266 opioid-dependent patients initiating buprenorphine treatment
| Patient Characteristic | N (%) |
|---|---|
| Age, mean (range) | 40.1 (20–64) |
| Gender | |
| Male | 184 (69.2) |
| Female | 82 (30.8) |
| Clinical Site | |
| Site 1 | 157 (59.0) |
| Site 2 | 109 (41.0) |
| Specialty of Buprenorphine Prescriber | |
| Primary Care | 187 (70.3) |
| Psychiatry | 79 (29.7) |
| Main reason for entry into federally qualified health center | |
| Buprenorphine maintenance treatment | 214 (80.5) |
| Primary care treatment | 52 (19.5) |
| Co-morbidities (based on ICD-9 coding in electronic medical record) | |
| HIV infection | 29 (10.9) |
| HCV infection | 159 (59.8) |
| HBV infection | 3 (1.1) |
| Metabolic disorder (diabetes, hypertension, hyperlipidemia, coronary artery disease) | 94 (35.3) |
| Pulmonary disorder (chronic obstructive pulmonary disorder, asthma) | 47 (17.7) |
| Mood disorder (depression, bipolar disorder, anxiety disorder) | 191 (71.8) |
| Prescribed medication for comorbid psychiatric condition | 173 (65.0) |
ICD-9: International Classification of Diseases, 9th Revision
3.2 Healthcare utilization at the FQHC
Most (90.6%) patients attended at least one medical visit, averaging 1.7 medical visits per month of BMT. At least one behavioral health visit was utilized by 56.3% of subjects, averaging 1.6 visits per month while on BMT. Over half (53.0%) attended at least one on-site substance abuse counseling visit, averaging 1.2 sessions per month of treatment. Overall, subjects averaged 3.1 visits to the health center for any reason per month on BMT, ranging from 0.5 to 8.3 visits per month (Table 2).
Table 2.
Types and frequency of healthcare utilization at health center while receiving buprenorphine maintenance therapy (N=266)
| Medical visits* | |
| Number of patients who had at least 1 medical visit, (%) | 241 (90.6) |
| Mean number of visits per month of BMT for those who had at least 1 medical visit, (range) | 1.7 (0.07 to 6.9) |
| Mean number of visits per month of BMT for the total cohort, (range) | 1.6 (0.0 to 6.9) |
| Behavioral Health visits* | |
| Number of patients who had at least 1 behavioral health visit, (%) | 150 (56.3) |
| Mean number of visits per month of BMT for those who had at least 1 behavioral health visit, (range) | 1.6 (0.05 to 8.0) |
| Mean number of visits per month of BMT for the total cohort, (range) | 0.9 (0.0 to 8.0) |
| Substance Abuse Counseling visits* | |
| Number of patients who had at least 1 substance abuse counseling visit, (%) | 141 (53.0) |
| Mean number of visits per month of BMT for those who had at least 1 substance abuse counseling visit, (range) | 1.2 (0.05 to 4.3) |
| Mean number of visits per month of BMT for the total cohort, (range) | 0.6 (0.0 to 4.3) |
| All visits* | |
| Mean number of visits per month of BMT for the total cohort, (range) | 3.1 (0.5 to 8.3) |
If individuals stayed in treatment for less than a month, the total number of visits was set to one month
BMT: buprenorphine maintenance therapy
3.3 Buprenorphine dosing
In this cohort, the mean daily buprenorphine dose was 15.4mg, achieved 2–4 weeks post-induction, and 17.8 mg, based on the last prescription dispensed, with a range for both between 2–32mg. Less than a fifth (17.7%) of subjects were prescribed a daily dose ≤12mg and 56.4% received ≥16mg based on the last recorded prescription.
3.4 Substance Abuse Treatment Outcomes
3.4.1 Retention on Buprenorphine Treatment
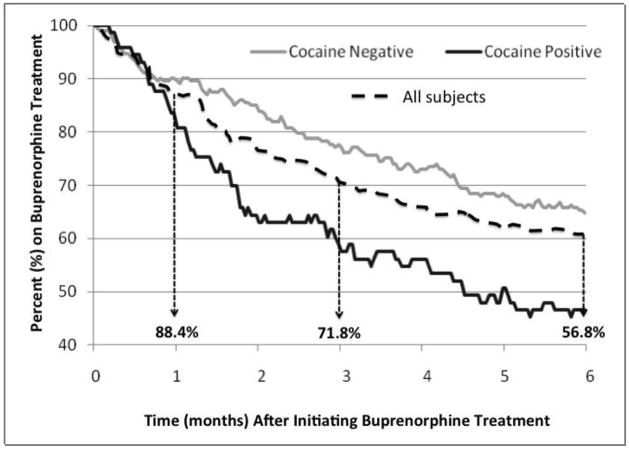
Of the 266 patients, 88.4% were maintained on BPN at 1 month, 71.8% at 3 months, and 56.8% at 6 months (Figure 1). Of the 136 patients who had at least 1 year of observation, 61.6% were still on BMT at 12 months (data not shown).
Figure 1.
Retention on buprenorphine maintenance therapy, overall and stratified by cocaine use (N=266)
In the adjusted analysis examining the likelihood of retention on BMT at 6 months (Table 3), the covariates found to be positively correlated were older age, female gender, HCV infection, receipt of psychiatric medication, and receipt of on-site substance abuse counseling; baseline cocaine use was negatively correlated with retention. In the adjusted analysis for 12-month retention on BMT, significant correlations persisted positively for receipt of psychiatric medication and receipt of on-site substance abuse counseling and negatively for baseline cocaine use.
Table 3.
Covariates associated with non-retention on buprenorphine maintenance therapy#
| Covariates | 6-Month Non-Retention | 12-Month Non-Retention | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=266 | N=136 | |||||||||||
| Unadjusted HR | Adjusted HR | Unadjusted HR | Adjusted HR | |||||||||
| H.R. | 95% CI | P value | H.R. | 95% CI | P value | H.R. | 95% CI | P value | H.R. | 95% CI | P value | |
| Age | 0.96 | (0.94, 0.98) | <0.01 | 0.96 | (0.94, 0.98) | <0.01 | 0.99 | (0.96, 1.02) | 0.62 | |||
| Gender | ||||||||||||
| Male | Ref | Ref | ||||||||||
| Female | 0.76 | (0.49, 1.1) | 0.19 | 0.59 | (0.37, 0.92) | 0.02 | 0.69 | (0.36, 1.3) | 0.26 | |||
| Site | ||||||||||||
| Site 1 | Ref | |||||||||||
| Site 2 | 0.80 | (0.54, 1.20) | 0.22 | 0.78 | (0.45, 1.4) | 0.39 | ||||||
| Prescriber Specialty | ||||||||||||
| Psychiatry | Ref | |||||||||||
| Primary Care | 0.85 | (0.79, 1.72) | 0.45 | 0.67 | (0.85, 2.59) | 0.16 | * | |||||
| Baseline Cocaine Screen | ||||||||||||
| Negative | Ref | Ref | Ref | Ref | ||||||||
| Positive | 1.73 | (1.18, 2.54) | 0.01 | 2.18 | (1.35, 3.50) | <0.01 | 2.06 | (1.20, 3.51) | <0.01 | 3.12 | (1.57, 6.16) | <0.01 |
| Any Cocaine Screen | ||||||||||||
| Negative | Ref | |||||||||||
| Positive | 1.36 | (0.94, 1.97) | 0.10 | * | 1.22 | (0.73, 2.04) | 0.44 | |||||
| Mood Disorder | ||||||||||||
| No | Ref | Ref | ||||||||||
| Yes | 1.06 | (0.81, 1.40) | 0.65 | 1.08 | (0.73, 1.59) | 0.68 | ||||||
| Prescribed psychiatric medication | ||||||||||||
| No | Ref | Ref | Ref | Ref | ||||||||
| Yes | 0.57 | (1.2, 2.6) | <0.01 | 0.69 | (0.47, 1.01) | 0.05 | 0.41 | (0.24, 0.69) | <0.01 | 0.36 | (0.20, 0.62) | <0.01 |
| HIV-infected | ||||||||||||
| No | Ref | Ref | ||||||||||
| Yes | 1.16 | (0.56, 1.8) | 0.98 | 1.39 | (0.66, 2.94) | 0.38 | ||||||
| HCV-infected | ||||||||||||
| No | Ref | Ref | Ref | |||||||||
| Yes | 0.74 | (0.52, 1.10) | 0.13 | 0.56 | (0.36, 0.86) | 0.01 | 0.95 | (0.56, 1.60) | 0.85 | |||
| Receipt of on-site substance abuse counseling | ||||||||||||
| No | Ref | Ref | Ref | Ref | ||||||||
| Yes | 0.55 | (0.37, 0.78) | <0.01 | 0.54 | (0.36, 0.79) | <0.01 | 0.39 | (0.22, 0.67) | <0.01 | 0.34 | (0.19, 0.59) | <0.01 |
| AIC Goodness of Fit | 1169.28 | 502.9 | ||||||||||
Estimates from a Cox Proportional Hazard model
Non-significant variables were excluded based on AIC in the multivariate model
AIC = Akaike Information Criterion; Ref = referent
Only about one third (32.7%) of the cohort persistently remained on BMT, receiving continuous BMT without any interruption in treatment. Therefore, about two thirds had nonpersistent treatment, experiencing at least one BMT gap of 2 or more weeks during their observation period. Overall, 39.9% of subjects experienced one gap while 17.7% had 2 gaps, 5.6% had 3 gaps, and 4.1% had 4 or 5 gaps. For the 67.3% who experienced treatment gaps, the mean gap length was 116.4 days, ranging from 14 to 482 days.
3.4.2 Opioid-free time
The 266 subjects enrolled in the BMT program underwent, on average, 1.6 monthly urine toxicology screens ranging from 0 to 4 tests per month. Just under a third (29.7%) of patients had all urine samples that were collected during observation test negative for opioids and 41.0% had all their urines test negative for cocaine (Table 4). About a third (33.5%) of patients had a baseline urine screen test positive for opioids and 26.7% tested positive for cocaine. Of the 134 patients who were still on BMT at the end of observation, 24.4% had their last urine test positive for opioids and 14.2% for cocaine. When examining all urines sampled in the last month of observation, 72.4% of these patients tested negative for opioids, 81.3% tested negative for cocaine, and 66.4% tested negative for both. Of the 132 patients who were not on BMT at the end of observation, 51.0% had their last urine test positive for opioids and 40.0% for cocaine. Only 34.9% had all urines in the last month test negative for opioids, 47.0% test negative for cocaine and 20.5% test negative for both.
Table 4.
Urine toxicology screening results of 266 opioid-dependent patients on buprenorphine maintenance therapy
| Number of urine screens done per person per month of BMT, mean (range) | 1.6 (0.0–4.1) | ||
| Percent of patients with all urines collected testing negative for opioids, (95% CI) | 29.7 (24.1–35.2) | ||
| Percent of patients with all urines collected testing negative for cocaine, (95% CI) | 41.0 (35.7–46.4) | ||
| Percent of baseline urine screens positive for opioids, (95% CI) | 33.5 (27.0–39.0) | ||
| Percent of baseline urine screens positive for cocaine, (95% CI) | 26.7 (21.0–32.0) | ||
| Patients still on BMT at end of observation period (N=134) | Patients not on BMT at end of observation period (N=132) | p value | |
| Last urine screen positive for opioids, % (95% CI) | 24.4 (17.0–32.0) | 51.0 (41.0–60.0) | <0.01 |
| Last urine screen positive for cocaine, % (n) | 14.2 (19) | 40.0 (53) | <0.01 |
| Urine screens negative for opioids in last month of treatment or observation, % (n) | 72.4 (97) | 34.9 (46) | <0.01 |
| Urine screens negative for cocaine in last month of treatment or observation, % (n) | 81.3 (109) | 47.0 (62) | <0.01 |
| Urine screens negative for both opioids and cocaine in last month of treatment or observation, % (n) | 66.4 (89) | 20.5 (27) | <0.01 |
BMT: buprenorphine maintenance therapy
In the multivariate analyses examining covariates of opioid-free time (Tables 5a–5c), each of the three definitions were analyzed separately. In examining opioid-free urines in the last month of treatment, receipt of psychiatric medication was positively correlated and any cocaine use was negatively correlated. Similarly, in examining the proportion of all collected opioid-free urine screens, the only covariate that was positively correlated was receipt of psychiatric medication. Last, in examining duration of opioid-free time, having a mood disorder diagnosis was positively correlated while baseline cocaine use was negatively correlated.
Table 5a.
Covariates associated with opioid-free time, defined as lack of opioids in the urine in the last month of observed treatment
| Covariates | Opioid negative urine samples in last month of BMT | |||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| Logistic Regression | Logistic Regression | |||||
| OR | 95% CI | P value | AOR | 95% CI | P value | |
| Age | 1.01 | (0.98, 1.03) | 0.46 | |||
| Gender | ||||||
| Male | Ref | |||||
| Female | 1.21 | (0.70, 2.07) | 0.49 | |||
| Site | ||||||
| Site 1 | Ref | |||||
| Site 2 | 0.98 | (0.59, 1.60) | 0.94 | |||
| Specialty | ||||||
| Psychiatry | Ref | |||||
| Primary Care Provider | 0.64 | (0.36, 1.10) | 0.11 | * | ||
| Baseline Cocaine Screen | ||||||
| Negative | Ref | |||||
| Positive | 0.52 | (0.29, 0.92) | 0.03 | |||
| Any Cocaine Screen | ||||||
| Negative | Ref | Ref | ||||
| Positive | 0.43 | (0.25, 0.72) | <0.01 | 0.43 | (0.25, 0.73) | <0.01 |
| Receipt of on-site substance abuse counseling | ||||||
| No | Ref | |||||
| Yes | 0.68 | (0.41, 1.11) | 0.13 | * | ||
| Mood disorder | ||||||
| No | Ref | |||||
| Yes | 1.56 | (0.88, 2.73) | 0.12 | * | ||
| Prescribed psychiatric medication | ||||||
| No | Ref | Ref | ||||
| Yes | 1.67 | (0.98, 2.83) | 0.06 | 1.66 | (1.03, 2.85) | <0.01 |
| HIV-infected | ||||||
| No | Ref | |||||
| Yes | 0.87 | (0.38, 1.98) | 0.75 | |||
| HCV-infected | ||||||
| No | Ref | |||||
| Yes | 0.87 | (0.52, 1.45) | 0.60 | |||
| AIC Goodness of Fit | 356 | |||||
Non-significant variables were excluded by AIC/criteria in the multivariate model
BMT = buprenorphine maintenance therapy; Ref = referent; AIC = Akaike Information Criterion; GLM=Generalized Linear Model; OLS= Ordinary Least Square Regression
Table 5c.
Covariates associated with opioid-free time, defined as the duration of opioid-free time while on prescribed buprenorphine
| Covariates | Proportion of all urine samples collected that were negative for opioids X [duration of BMT - gap times] (Duration of opioid-free time) | |||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| OLS | OLS | |||||
| Beta | 95% CI | P value | Beta | 95% CI | P value | |
| Age | 2.44 | (0.04, 4.82) | 0.05 | 2.08 | (−0.29, 4.44) | 0.09 |
| Gender | ||||||
| Male | Ref | |||||
| Female | 28.36 | (−19.0, 75.8) | 0.24 | |||
| Site | ||||||
| Site 1 | Ref | |||||
| Site 2 | 16.58 | (−28.1, 61.2) | 0.47 | |||
| Specialty | ||||||
| Psychiatry | Ref | |||||
| Primary Care Provider | 27.37 | (−20.0, 75.3) | 0.26 | |||
| Baseline Cocaine Screen | ||||||
| Negative | Ref | Ref | ||||
| Positive | −50.05 | (−102, 2.05) | 0.06 | −50.42 | (−99.1, −0.36) | <0.05 |
| Any Cocaine Screen | ||||||
| Negative | Ref | |||||
| Positive | −41.60 | (−86.1, 3.52) | 0.07 | * | ||
| Receipt of on-site substance abuse counseling | ||||||
| No | Ref | Ref | ||||
| Yes | 44.65 | (0.90, 88.3) | 0.05 | 39.87 | (−3.55, 83.3) | 0.07 |
| Mood disorder | ||||||
| No | Ref | Ref | ||||
| Yes | 72.00 | (23.8, 120.) | <0.01 | 65.9 | (18.23, 113.6) | <0.01 |
| Prescribed psychiatric medication | ||||||
| No | Ref | |||||
| Yes | 62.72 | (17.2, 108.) | 0.01 | * | ||
| HIV-infected | ||||||
| No | Ref | |||||
| Yes | −11.04 | (−39.0, 17.8) | 0.45 | |||
| HCV-infected | ||||||
| No | Ref | |||||
| Yes | −0.35 | (−18.2, 17.9) | 0.97 | |||
| AIC Goodness of Fit | 3512.2 | |||||
Non-significant variables were excluded by AIC/criteria in the multivariate model
BMT = buprenorphine maintenance therapy; Ref = referent; AIC = Akaike Information Criterion; GLM=Generalized Linear Model; OLS= Ordinary Least Square Regression
4. DISCUSSION
4.1 Substance Abuse Treatment Outcomes
The results confirm that BMT retention in a FQHC, a real-world clinical setting, is similar to results observed in other settings as varied as specialty substance abuse clinics, HIV clinics, academic centers, and hospitals and where patients with complex medical and psychiatric comorbidity were excluded. Of note, just above half of our cohort received daily buprenorphine dosing of ≤16mg. Given a recent meta-analysis which determined that daily doses of 16–32 mg predicted better retention than lower doses, the implication that even better retention rates could be achieved if higher dosages of buprenorphine are prescribed is intriguing and merits further investigation (Fareed et al., 2012).
Two factors were found to be significantly associated with improved retention on buprenorphine at both 6 and 12 months: receiving on-site substance abuse counseling and receiving psychiatric medication. Baseline cocaine use, however, was associated with poorer retention. The findings that on-site provision of counseling services improves retention are consistent with models that are either co-located or fully integrated (Basu et al., 2006; Sylla et al., 2007; Weiss et al., 2011). Furthermore, subjects prescribed psychiatric medications had improved retention also suggests that concomitant treatment of mental illness may be critical for BMT retention. Similar to other studies, cocaine use was associated with decreased retention (Sullivan et al., 2011). Indeed, non-cocaine users had higher BMT retention noted as early as 1 month after initiation (Figure 1). Early detection of cocaine should alert providers early into treatment and concentrate resources to help retain them in treatment, perhaps through counseling intensification (Copenhaver et al., 2007).
Last, being older, female, and HCV-infected were each associated with improved retention at 6 months though the association was not maintained at 12 months. A larger and longer study may be needed to better elucidate the association between these covariates and retention.
Opioid-free time, on the other hand, is not comparable across studies given the varied manner in which this variable has previously been defined and measured. Nevertheless, just under a third of patients were persistently opioid-free throughout the study and a similar proportion tested positive for opioids at baseline. As expected, however, opioid use decreased with improved BMT retention. Compared to those who discontinued BMT, those who were retained were significantly more likely to have negative terminal urine screens for both opioids and cocaine. Moreover, two factors associated with BMT retention were also associated with opioid-free time, including treatment for a mood disorder was associated with improved opioid-free time and not using cocaine.
These findings have broader implications globally. While countries in Western Europe, in particular France, has been prescribing BMT in primary care settings since 1996, other countries where BMT is more highly regulated (Bruce et al., 2007; Carrieri et al., 2006) can be assured that they can achieve good retention and reduce opioid use in real-world settings. Such findings are likely to have important health improvements where opioids contribute to negative health consequences (Altice et al, 2010).
In summary, improved treatment outcomes associated with substance abuse counseling and with screening for and treating mood disorders underscore the importance of integrating mental health screening and care into primary care-based BMT. The negative impact of cocaine use on study outcomes and lack of pharmacological therapy for treating cocaine addiction, alternative strategies that facilitate reductions or cessation in cocaine use among BMT patients is crucial, including cocaine-specific behavioral therapies (Penberthy et al., 2010; Petry et al., 2012). Given the constrained resources of FQHCs, however, these patients may need referrals to specialized community-based treatment programs. Such strategies may clinically improve BMT retention, increase opioid-free time, and continuously engage this vulnerable population in care at FQHCs. Moreover, the finding that two-thirds had at least one 2-week gap in treatment lasting on average 4 months underscores treatment non-persistence and re-entry into care in real-world settings. Such findings should inform clinical providers and researchers designing future clinical trials.
In addition, most (80.5%) patients initiated care at the FQHC specifically seeking BMT. This suggests that patients in communities where FQHCs provide BMT would enter care has great potential to also provide routine screening and treatment for a multitude of other comorbid conditions. Furthermore, no differences in treatment outcomes were noted between family practice and specialty psychiatry prescribers. All these findings underscore the value and need of integrating BMT programs into FQHCs to improve the likelihood that they can truly fulfill their role as patient-centered medical homes (PCMH) by addressing substance abuse treatment.
4.3 Limitations of study
This study was observational and retrospective with no controls and therefore lacks the rigor of a randomized controlled trial (RCT) in determining causal factors associated with the treatment outcomes examined. It does represent, however, a rigorous analysis using implementation science methodologies that provide important insight into expanding an evidence-based intervention, BMT, into a diverse clinical setting. Furthermore, given the sample size of 266 patients, associations could be identified between retention in BMT and opioid-free time, and the correlates examined for each. Prospective RCTs are needed to better differentiate the benefits of BMT in these settings, yet real-world implementation science studies are critical to examine how idealized RCTs are translated in community settings.
The retrospective chart review study limited the types and content of data collected to what providers recorded in charts during the course of real-world clinic visits. Therefore, additional data such as incarceration history, type of opioids used, route of drug use, attendance at off-site addiction counseling sessions, and reasons for treatment termination (e.g., relapse, transfer to methadone, or transfer to higher level of care), was not available. Given this limitation, we were unable to compare those subjects who were enrolled in counseling on-site with those who may have received counseling off-site. The strong association we found between on-site counseling and BMT retention, however, still indicates that integrating counseling on-site, as espoused by the PCMH model, is worthwhile to promote successful treatment. Moreover, the availability of EHR for chart review provided easy, systematic and complete extraction of real-world information such as visits, urine toxicology results, laboratory results, and problem lists. While data collection errors may still occur despite EHR access, we limited errors through double data collection techniques.
Though a statewide FQHC network was examined, data should be interpreted with caution due to the network being within one state and may not apply to FQHCs elsewhere. Nevertheless, the sites within the FQHC differed in number, training, specialty services and experience of staff involved directly and indirectly in the BMT program.
Furthermore, the FQHC BMT protocol is flexible and allows for differences in prescriber practice style and available resources. Frequency of patient visits, number of urine toxicology screens performed, thresholds for terminating BMT and for recommending higher levels of care, and insistence of patient participation in on-site or off-site substance abuse counseling are all dependent upon individual providers and thus variable. This variability, however, represents real-world practice and hence these results more accurately represent real-world outcomes. This study involved three primary care providers but only one psychiatrist, which may limit the value of the comparison of results between primary and specialty care services. The psychiatrist, however, was the most experienced BMT provider and thus could indeed potentially serve as the internal standard to which others could be compared.
We believe these study findings can greatly inform FQHCs across the nation to strive towards healthcare integration within PCMHs by providing BMT to their opioid-dependent patients. Moreover, identifying correlates of treatment success as defined by retention in care and opioid-free time could influence the design of other BMT programs. Factors proven to be associated with improved outcomes could determine what resources are needed to deliver effective quality care to opioid-dependent patients. Such factors could also help direct the allocation of resources within a program by identifying, for instance, the type of patient that may need extra resources at the outset of treatment.
The implications of expanding BMT to FQHCs could be significant not only to individuals but on a societal, economic, and public health level. Decreasing opioid dependence in communities and engaging these patients in primary care could result in increased health promotion and disease prevention and care, reducing emergency department visits (Schwarz et al., 2012), decreased opioid-related medical complications, including overdoses, abscesses, endocarditis, osteomyelitis, HIV and HCV transmission, and increased societal benefits including decreased healthcare costs, decreased crime, increased family cohesion, and increased employment. Expanding access to BMT for opioid-dependent persons has the great potential to provide considerable public health benefits (Krantz and Mehler, 2004).
Table 5b.
Covariates associated with opioid-free time, defined as the percentage of all collected urines free of opioids
| Covariates | Proportion of all urine samples collected that were negative for opioids | |||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| GLM Regression | GLM Regression | |||||
| OR | 95% CI | P value | AOR | 95% CI | P value | |
| Age | 1.00 | (0.96, 1.02) | 0.74 | |||
| Gender | ||||||
| Male | Ref | |||||
| Female | 1.72 | (0.94, 3.14) | 0.08 | * | ||
| Site | ||||||
| Site 1 | Ref | |||||
| Site 2 | 0.94 | (0.54, 1.60) | 0.82 | |||
| Specialty | ||||||
| Psychiatry | Ref | Ref | ||||
| Primary Care Provider | 0.57 | (0.30, 1.04) | 0.07 | 0.59 | (0.32, 1.10) | 0.10 |
| Baseline Cocaine Screen | ||||||
| Negative | Ref | |||||
| Positive | 0.73 | (0.40, 1.31) | 0.30 | |||
| Any Cocaine Screen | ||||||
| Negative | Ref | |||||
| Positive | 0.71 | (0.40, 1.22) | 0.22 | |||
| Receipt of on-site substance abuse counseling | ||||||
| No | Ref | |||||
| Yes | 0.94 | (0.55, 1.59) | 0.82 | |||
| Mood disorder | ||||||
| No | Ref | |||||
| Yes | 1.86 | (1.04, 3.31) | 0.03 | * | ||
| Prescribed psychiatric medication | ||||||
| No | Ref | Ref | ||||
| Yes | 1.89 | (1.08, 3.27) | 0.02 | 1.83 | (1.05, 3.18) | 0.03 |
| HIV-infected | ||||||
| No | Ref | |||||
| Yes | 1.11 | (0.46, 2.69) | 0.81 | |||
| HCV-infected | ||||||
| No | Ref | |||||
| Yes | 0.98 | (0.57, 1.69) | 0.96 | |||
| AIC Goodness of Fit | 1.02 | |||||
Non-significant variables were excluded by AIC/criteria in the multivariate model
BMT = buprenorphine maintenance therapy; Ref = referent; AIC = Akaike Information Criterion; GLM=Generalized Linear Model; OLS= Ordinary Least Square Regression
Acknowledgments
Funding: The National Institute on Drug Abuse provided career development funding for FLA (K24 DA017072).
Role of Funding Source: The National Institute on Drug Abuse had no role in the study design, data collection, analysis and interpretation of data, writing of the manuscript or decision to submit the manuscript for publication.
The authors would like to acknowledge Margaret Flinter, Mark Masselli and Nwando Olayiwola for their support at CHC, Inc. We would also like to thank Jamal Ahmed, Nora Gilbert, Erik Gonzalez, Hannah Jackson, and Benjamin Kuebrich for their valuable contributions in the data collection process.
Footnotes
Contributors: All authors participated in the research and manuscript preparation and have approved the final manuscript. The authors contributed to the other components of the paper:
- Literature Review: Haddad and Altice
- Statistical analysis: Zelenev and Altice
- First draft of manuscript: Haddad
- Data management: Haddad and Zelenev
- Study Design: Haddad, Zelenev and Altice
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, Samet JH. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171:425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford DP, LaBelle CT, Richardson JM, O’Connell JJ, Hohl CA, Cheng DM, Samet JH. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22:171–176. doi: 10.1007/s11606-006-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford DP, Saitz R, LaBelle C, Samet JH. Buprenorphine in the Primary HIV Care Setting. Forum for Collaborative HIV Research; Washington D.C: 2004. Buprenorphine Initiation and Maintenance in Primary Care: A Successful Interdisciplinary Approach. [Google Scholar]
- Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, Cunningham CO, Sullivan LE, Vergara-Rodriguez P, Fiellin DA, Cajina A, Botsko M, Nandi V, Gourevitch MN, Finkelstein R, Collaborative B. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S22–32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:59–79. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Sullivan LE, Smith-Rohrberg D, Basu S, Stancliff S, Eldred L. The potential role of buprenorphine in the treatment of opioid dependence in HIV-infected individuals and in HIV infection prevention. Clin Infect Dis. 2006;43(Suppl 4):S178–183. doi: 10.1086/508181. [DOI] [PubMed] [Google Scholar]
- Bae JW, Guyer W, Grimm K, Altice FL. Medication persistence in the treatment of HIV infection: a review of the literature and implications for future clinical care and research. AIDS. 2011;25:279–290. doi: 10.1097/QAD.0b013e328340feb0. [DOI] [PubMed] [Google Scholar]
- Barry DT, Irwin KS, Jones ES, Becker WC, Tetrault JM, Sullivan LE, Hansen H, O’Connor PG, Schottenfeld RS, Fiellin DA. Integrating buprenorphine treatment into office-based practice: a qualitative study. J Gen Intern Med. 2009;24:218–225. doi: 10.1007/s11606-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Smith-Rohrberg D, Bruce RD, Altice FL. Models for integrating buprenorphine therapy into the primary HIV care setting. Clin Infect Dis. 2006;42:716–721. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine--programme description and policy implications. Int J Drug Policy. 2007;18:326–328. doi: 10.1016/j.drugpo.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri MP, Amass L, Lucas GM, Vlahov D, Wodak A, Woody GE. Buprenorphine use: the international experience. Clin Infect Dis. 2006;43:S197–215. doi: 10.1086/508184. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Treatment Improvement Protocol (TIP) Series 40. Substance Abuse and Mental Health Services Administration (SAMHSA) (Ed.). Department of Health and Human Services; Rockville, MD: 2004. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Fry-Smith A, Day E, Lintzeris N, Roberts T, Burls A, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- Copenhaver MM, Bruce RD, Altice FL. Behavioral counseling content for optimizing the use of buprenorphine for treatment of opioid dependence in community-based settings: a review of the empirical evidence. Am J Drug Alcohol Abuse. 2007;33:643–654. doi: 10.1080/00952990701522674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Giovanniello A, Sacajiu G, Whitley S, Mund P, Beil R, Sohler N. Buprenorphine treatment in an urban community health center: what to expect. Fam Med. 2008;40:500–506. [PMC free article] [PubMed] [Google Scholar]
- Cunningham CO, Giovanniello A, Li X, Kunins HV, Roose RJ, Sohler NL. A comparison of buprenorphine induction strategies: patient-centered home-based inductions versus standard-of-care office-based inductions. J Subst Abuse Treat. 2011;40:349–356. doi: 10.1016/j.jsat.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK. Applied Missing Data Analysis. Guilford Press; New York: 2010. [Google Scholar]
- Fareed A, Vayalapalli S, Casarella J, Drexler K. Effect of buprenorphine dose on treatment outcome. J Addict Dis. 2012;31:8–18. doi: 10.1080/10550887.2011.642758. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Moore BA, Sullivan LE, Becker WC, Pantalon MV, Chawarski MC, Barry DT, O’Connor PG, Schottenfeld RS. Long-term treatment with buprenorphine/naloxone in primary care: results at 2–5 years. Am J Addict. 2008;17:116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- Friedman SR, Tempalski B, Brady JE, Friedman JJ, Cooper HL, Flom PL, McGrath MM, Gostnell K, Des Jarlais DC. Predictors of the degree of drug treatment coverage for injection drug users in 94 metropolitan areas in the United States of America. Int J Drug Policy. 2007;18:475–485. doi: 10.1016/j.drugpo.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Gunderson EW, Wang XQ, Fiellin DA, Bryan B, Levin FR. Unobserved versus observed office buprenorphine/naloxone induction: a pilot randomized clinical trial. Addict Behav. 2010;35:537–540. doi: 10.1016/j.addbeh.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AH, Gospodarevskaya E, Ritter AJ. A randomised trial of the cost effectiveness of buprenorphine as an alternative to methadone maintenance treatment for heroin dependence in a primary care setting. Pharmacoeconomics. 2005;23:77–91. doi: 10.2165/00019053-200523010-00007. [DOI] [PubMed] [Google Scholar]
- Ing EC, Bae JW, Maru DS, Altice FL. Medication persistence of HIV-infected drug users on directed administered antiretroviral therapy. AIDS Behav. 2011 doi: 10.1007/s10461-011-0082-0. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Jackman S. Estimation and inference via Bayesian simulation: an introduction to Markov chain Monte Carlo. Am J Poli Sci. 2000;44:375. [Google Scholar]
- Kakko J, Gronbladh L, Svanborg KD, von Wachenfeldt J, Ruck C, Rawlings B, Nilsson LH, Heilig M. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007;164:797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361:662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- Korthuis PT, Fiellin DA, Fu R, Lum PJ, Altice FL, Sohler N, Tozzi MJ, Asch SM, Botsko M, Fishl M, Flanigan TP, Boverman J, McCarty D, Collaborative B. Improving adherence to HIV quality of care indicators in persons with opioid dependence: the role of buprenorphine. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S83–90. doi: 10.1097/QAI.0b013e31820bc9a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz MJ, Mehler PS. Treating opioid dependence. Growing implications for primary care. Arch Intern Med. 2004;164:277–288. doi: 10.1001/archinte.164.3.277. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Ann Fam Med. 2007;5:146–150. doi: 10.1370/afm.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, Schottenfeld RS. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22:527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherland J, Botsko M, Egan JE, Saxon AJ, Cunningham CO, Finkelstein R, Gourevitch MN, Renner JA, Sohler N, Sullivan LE, Weiss L, Fiellin DA. Factors affecting willingness to provide buprenorphine treatment. J Subst Abuse Treat. 2009;36:244–251. doi: 10.1016/j.jsat.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor PG, Oliveto AH, Shi JM, Triffleman EG, Carroll KM, Kosten TR, Rounsaville BJ, Pakes JA, Schottenfeld RS. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. Am J Med. 1998;105:100–105. doi: 10.1016/s0002-9343(98)00194-6. [DOI] [PubMed] [Google Scholar]
- Parran TV, Adelman CA, Merkin B, Pagano ME, Defranco R, Ionescu RA, Mace AG. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend. 2010;106:56–60. doi: 10.1016/j.drugalcdep.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy JK, Ait-Daoud N, Vaughan M, Fanning T. Review of treatment for cocaine dependence. Curr Drug Abuse Rev. 2010;3:49–62. doi: 10.2174/1874473711003010049. [DOI] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol. 2012;80:276–285. doi: 10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA; Office of Applied Studies, editor. Results from the 2008 National Survey on Drug Use and Health: National Findings. Substance Abuse and Mental Health Services Agency; Rockville, MD: 2009. [Google Scholar]
- Schwarz R, Zelenev A, Bruce RD, Altice FL. Retention on buprenorphine treatment reduces emergency department utilization, but not hospitalization, among treatment-seeking patients with opioid dependence. J, Subst, Abuse Treat. 2012 doi: 10.1016/j.jsat.2012.03.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeffing JM, Martin LD, Fingerhood MI, Jasinski DR, Rastegar DA. Buprenorphine maintenance treatment in a primary care setting: outcomes at 1 year. J Subst Abuse Treat. 2009;37:426–430. doi: 10.1016/j.jsat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. J Gen Intern Med. 2005;20:1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Botsko M, Cunningham CO, O’Connor PG, Hersh D, Mitty J, Lum PJ, Schottenfeld RS, Fiellin DA. The impact of cocaine use on outcomes in HIV-infected patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S54–61. doi: 10.1097/QAI.0b013e3182097576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy. 2007;18:306–312. doi: 10.1016/j.drugpo.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L, Netherland J, Egan JE, Flanigan TP, Fiellin DA, Finkelstein R, Altice FL, Collaborative B. Integration of buprenorphine/naloxone treatment into HIV clinical care: lessons from the BHIVES collaborative. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S68–75. doi: 10.1097/QAI.0b013e31820a8226. [DOI] [PubMed] [Google Scholar]