Abstract
There has been extensive research investigating self-control in humans and nonhuman animals, yet we know surprisingly little about how one’s social environment influences self-control. The present study examined the self-control of chimpanzees in a task that required active engagement with conspecifics. The task consisted of transferring a token back and forth with a partner animal in order to accumulate food rewards, one item per token transfer. Self-control was required because at any point in the trial, either chimpanzee could obtain their accumulated rewards, but doing so discontinued the food accumulation and ended the trial for both individuals. Chimpanzees readily engaged the task and accumulated the majority of available rewards before ending each trial, and they did so across a number of conditions that varied the identity of the partner, the presence/absence of the experimenter, and the means by which they could obtain rewards. A second experiment examined chimpanzees’ self-control when given the choice between immediately available food items and a potentially larger amount of rewards that could be obtained by engaging the token transfer task with a partner. Chimpanzees were flexible in their decision-making in this test, typically choosing the option representing the largest amount of food, even if it involved delayed accumulation of the rewards via the token transfer task. These results demonstrate that chimpanzees can exhibit self-control in situations involving social interactions, and they encourage further research into this important aspect of the self-control scenario.
Keywords: self-control, chimpanzees, token transfer, delay of gratification, accumulation
Self-regulation is a critical aspect of goal directed behavior with at least a minimal future-oriented perspective, and it consists of a suite of self-regulatory processes that includes modulation of thought, affect, behavior, or attention via deliberate or automated use of specific mechanisms and supportive meta-skills (Karoly, 1993). Self-control is one of these processes in which an organism inhibits a response to an immediate desire in order to obtain a bigger or better reward in the future, and impulsivity is defined as the opposite of self-control. There has been extensive research investigating self-control and impulsivity in humans (e.g., Logue 1988; Mischel 1974, 1981; Rachlin 2000). The ability of young children to show self-control in situations in which they must delay gratification relates to a wide variety of abilities later in life, including higher levels of academic performance, social competence, verbal ability, attentiveness, planning and foresight, and tolerance of frustration (e.g., Mischel et al. 1988; Moffitt et al. 2011). Impulsivity, on the other hand, is related to lower IQ scores and higher delinquency rates in children (e.g., White et al. 1994) as well as other problems such as binge eating and alcohol abuse (e.g., Tangney et al. 2004). Comparative research suggests a continuum between humans and nonhuman animals in their capacity for self-control, demonstrating its relevance to many species (e.g., Beran et al. 1999; Evans and Westergaard 2006; Logue 1988; Logue et al. 1996; Rachlin 2000; Stevens et al. 2005; Tobin et al. 1996).
The self-control process by which an individual continuously foregoes an immediately available reward for a delayed, but larger reward is commonly referred to as delaying gratification (Mischel et al. 1989). Tasks that assess delay of gratification allow a participant to change from waiting for the later reward to taking the immediate reward at any time, sometimes using an accumulation procedure in which rewards collect over time until the individual interrupts this accumulation process to take the rewards and end the trial. A large proportion of the research on self-control in nonhuman animals has focused on delay of gratification, with many of these tests given to nonhuman primates, although other species have been tested also (e.g., Anderson et al. 2010; Beran 2002; Beran and Evans 2006; Bramlett et al. 2012; Evans and Westergaard 2006; Stevens et al. 2005; Vick et al. 2010). For example, chimpanzees have shown a marked ability to delay gratification, with some individuals inhibiting taking food items for multiple minutes (Beran 2002; Beran and Evans 2006; Beran et al. 1999; Dufour et al. 2007). Like children (Mischel et al. 1972; Miller and Karniol 1976; Toner and Smith 1977), chimpanzees have shown a propensity to use behavioral strategies (e.g., self-distraction) to cope with impulsivity in accumulation tests (Evans and Beran 2007). Also, chimpanzees will continue to delay gratification even if food items are not visible and even when an experimenter is absent, suggesting that these results are not likely an artifact of any specific experimental procedure (Beran and Evans 2006). These previous studies provide evidence that self-control manifests in the chimpanzee in a similar manner to that seen in human children. However, these studies have focused on individual chimpanzees that are isolated from their larger social network. Because an individual rarely uses its physical cognition devoid of its social environment, we were interested in studying self-control as it manifests in a social task.
In a recent paper, we demonstrated that chimpanzees were mostly successful in delay of gratification tasks across a range of relatively passive social situations (Evans et al. 2012). When chimpanzees worked independently on accumulation tasks in adjacent enclosures, some chimpanzees demonstrated impairments in performance compared to when they were tested alone, though there was not a direct relationship between the time at which each chimpanzee ended its own trial and when the partner ended its trial. In addition, their self-control was relatively unaffected when a conspecific was allowed to freely consume rewards in an adjacent enclosure and even when they worked together on the same accumulation task so that each individual’s rewards were contingent upon both chimpanzees continued ability to inhibit eating the items. Overall, chimpanzee self-control was modestly influenced by the social setting and only some chimpanzees exhibited these effects.
In the current study, we were interested in the role of a more active social interaction between chimpanzees and how performance on an accumulation task was affected when both individuals were required to jointly participate in the task. In addition to the novelty of active engagement with a conspecific, the present study introduced an additional element of risk that is a novel manipulation in this kind of self-control task. The chimpanzees not only had to actively engage in a social task with their partner while inhibiting ending an accumulation task early, but their own outcomes were also subject to their partner’s inhibitory control (or lack thereof) and willingness to participate. We investigated whether chimpanzees would actively engage in a token transfer task, passing a token back and forth with a partner in order to increase their rewards across a variety of conditions (e.g., prepotent foods, new partnerships, and in the absence of an experimenter). Furthermore, we investigated their preference behavior in the current task by giving them the option to take an immediately available food reward or to work with a partner to accumulate a different amount of food reward.
Primates are known to readily engage in token transfer, exchanging tokens with human experimenters (e.g., Brosnan and de Waal 2004, 2005; Chalmeau and Piegnot 1998; Chen et al. 2006). They also have demonstrated the ability to exchange lower preference foods for better foods, displaying self-control in their inhibition of consuming the immediately available food item to obtain a more preferred item in the future (Evans and Westergaard 2006; Lefebvre 1982; Lefebvre and Hewitt 1986). Monkeys and apes will engage in food and tool transfer and exchange directly with conspecifics (Beck, 1973; Brosnan and Beran 2009; Savage-Rumbaugh et al. 1978a; Westergaard and Suomi 1997). We utilized a token transfer and exchange procedure in the current study in which success in obtaining a better outcome was directly contingent on each individual’s self-control, tolerance of delayed reward, and willingness to engage another chimpanzee directly and repeatedly by transferring a token back and forth with that individual.
We predicted that chimpanzees would engage in the token transfer procedure to accumulate food rewards given their success in previous tasks utilizing an accumulation procedure (e.g., Beran 2002, Beran and Evans 2009). We predicted a potential decrease in self-control performance following the introduction of the phase in which the food rewards were then more immediately accessible to the chimpanzees (c.f., Boysen and Bernston 1995). We did not anticipate any difference in performance between testing phases that utilized a human experimenter and one that used an automated food delivery system given their previous success with both procedures (Beran and Evans 2006). Additionally, we anticipated that this transfer and exchange behavior would continue even when chimpanzees were moved into novel partnerships. Finally, given these chimpanzees’ success in several previous investigations of self-control and delay of gratification behavior, we expected them to be successful in the final phase by exhibiting flexibility in response patterns that optimized their access to the higher valued reward when given the option to take an immediately available food reward or to work with a partner to accumulate a different amount of food reward.
Experiment 1
Methods
Participants
We tested four chimpanzees (Pan troglodytes) from the Language Research Center at Georgia State University, including two females (Panzee: age 26, Lana: age 41) and two males (Sherman: age 39, Mercury: age 25). Apes were group housed but separated voluntarily into adjacent testing enclosures as necessary for testing. All chimpanzees worked for preferred foods, but received their normal diet of primate chow, fruits and vegetables and were never food or water deprived.
Three of four chimpanzees (excluding Mercury) have had extensive exposure to language-training using a lexigram system in which symbols represent words including food items, people, places, and activities (see Rumbaugh and Washburn 2003). The three language-trained chimpanzees (Sherman, Lana, and Panzee) were included in a token trading task in which they flexibly used lexigram tokens to obtain foods from an experimenter but did not spontaneously trade with their partner unless an experimenter required a trade interaction, and even then only after extensive training (Brosnan and Beran 2009). In an earlier series of studies, one chimpanzee (Sherman) had experience requesting and exchanging tools and food items with a conspecific using the lexigram system (see Savage-Rumbaugh et al. 1978a, 1978b). All four chimpanzees also have been tested previously in tasks involving tokens representing different quantities of foods as well as one task involving the choice between immediately consumable rewards and lexigram tokens exchangeable later for better rewards (Beran and Evans 2012; Beran et al. 2011; Evans et al. 2010).
Apparatus
Trials were presented using a board that displayed the potential food rewards available for each chimpanzee at opposite ends (see Figure 1). The token that the chimpanzees were required to transfer and exchange was a red or white plastic block that easily slid on the floor between the testing enclosures.
Figure 1.
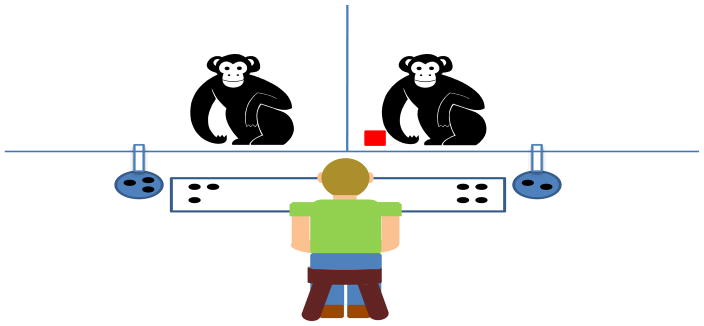
Experimental setup for Experiment 1. Chimpanzees engaged in token transfer by passing a token (shown to the left of the chimpanzee at right) back and forth, which led to the accumulation of delayed food rewards that were delivered into each animal’s respective bowl that was outside of their enclosure, but that could be obtained by either passing the token to the experimenter or simply pulling in the bowl (as depicted here), depending on the testing phase.
Design and Procedure
The task
Partner chimpanzees were separated into adjacent testing enclosures with full visual and auditory access to one another through the cage mesh that separated them. Enclosures were separated by approximately 25 centimeters, and tokens could be easily transferred between the cages by pushing them on the floor. The display board on the floor outside of the testing areas contained a predetermined number of food items available for accumulation following successful transfers. The experimenter first gave the token to one of the two chimpanzees, alternating which chimpanzee started with the token across trials and sessions. If a chimpanzee transferred the token to the partner, food would accumulate in its respective bowl. For example, if chimpanzee A transferred the token to its partner, the experimenter would move a single reward from chimpanzee A’s end of the display board into chimpanzee A’s bowl, and if chimpanzee B transferred the token to its partner, the experimenter would move a reward from chimpanzee B’s end of the display board into chimpanzee B’s bowl. This continued for each transfer of the token.
Depending on the testing phase, at any point in time, either chimpanzee could end the trial by (1) passing the token out of the enclosure in the direction of the experimenter after which the experimenter would give the chimpanzee their bowl or (2) by directly taking the food items instead of completing a transfer with the partner. This subsequently terminated the trial by ending the accumulation phase for both participants. The number of possible candies per trial, the number of trials per session, and the number of sessions conducted varied depending upon the testing phase. Partnerships consisted of Lana/Mercury and Panzee/Sherman unless indicated otherwise. Experimenters recorded the number of token transfers and accumulated food items per trial, along with trial duration. Trial duration included the total accumulation duration plus the time the chimpanzees required to take their accumulated rewards before they began eating them.
Training
To familiarize the chimpanzees with the testing procedure, all participants underwent a training phase in which they demonstrated proficiency at transferring the token to their partner to accumulate food items. This simply consisted of being prompted to first pass the token back and forth to each other, and then the chimpanzees were given the opportunity to either transfer the token (in this case the white plastic block) to their partner to accumulate up to ten food items per trial, or exchange the token for their accumulated rewards by passing the token to the experimenter. Each chimpanzee was prompted to transfer the token to the partner on their first attempt per trial by a combination of verbal prompts (“pass it to Sherman”) and gestures by the experimenter. After that, they were simply asked “what do you want to do?” These chimpanzees do respond within experimental contexts to such prompting from experimenters, although this is due to contextual effects that include prompting and pointing along with speaking rather than comprehension of the verbal statement itself. For the remainder of trials, there was no instruction at all from the experimenter. Sessions consisted of four trials with four-minute inter-trial intervals, in which the display board was re-baited to maintain ten food items per chimpanzee for each trial. A criterion was set so that each chimpanzee had to transfer the token to their partner at least five times each in two consecutive trials for three consecutive sessions before beginning the testing phase. Sherman and Panzee reached criterion in three sessions and Mercury and Lana reached criterion in four sessions.
Testing Phase 1: Token-Return Phase
Testing consisted of six two-trial sessions in which each chimpanzee could accumulate up to twenty food items per trial by passing back and forth the same token used in training. Inter-trial intervals were six minutes long during which the display board was re-baited to maintain twenty food items per chimpanzee per trial. At any point in time, either chimpanzee could end the trial by passing the token to the experimenter rather than its partner, and the experimenter then gave the out-of-reach bowls to the two chimpanzees so that they could eat the contents.
Testing Phase 2: Bowl-Retrieval Phase
Testing procedures were identical to those for Testing Phase 1 except for the method by which the chimpanzees ended the accumulation and the type of token used. We introduced the red token in this phase to facilitate ease of transfer across the testing enclosure floor as it was made of a lighter plastic material than the previous white plastic block. Also in this phase, the bowl in which the experimenter placed each chimpanzee’s accumulated rewards had an attached handle that was within reach of the chimpanzee. Therefore, instead of exchanging the token with the experimenter for the food bowl, either chimpanzee could end the trial in this phase by simply pulling in its bowl with the handle (and eating the food). This change was necessary because we wanted to ensure that the chimpanzees had the most intuitive option for ending a trial possible, and this was likely more intuitive given the added control with which they could take the proximal food through a simple action. This also was in closer accord with the past experiences of these chimpanzees with accumulation tasks such as this one (e.g., Beran 2002; Beran and Evans 2006; Evans and Beran 2007). We ran one pretest trial for each chimpanzee with 20 pieces of food before formal testing to confirm that they would engage this new experimental setup.
Testing Phase 3: New Partner Phase
Testing procedures were identical to those for Testing Phase 2 except for two features. Testing consisted of four two-trial sessions instead of six two-trial sessions. In addition, we repaired the chimpanzees to create novel pairings. Now, partnerships consisted of Sherman/Lana and Panzee/Mercury. Because of the new partnerships and restrictions on where individual chimpanzees could be housed during testing, Sherman was now tested in a different (but highly familiar) enclosure to facilitate transfers with Lana, and Panzee and Lana were tested in their original enclosures but had to pass the token in the opposite direction to facilitate token transfers with their new partners. This phase was designed to assess immediate and proficient token transfers and accumulation performance with new partners.
Testing Phase 4: Automated Phase
Testing procedures were identical to those for Testing Phase 2 except for the automated delivery of food items. Instead of the experimenter physically delivering the food rewards, a computer was set up so that rewards were delivered via an automated dispenser (see Beran and Evans 2006; Evans et al. 2012). This apparatus consisted of two Med Associates dispensers controlled by a Dell Optiplex personal computer via a Keithley Instruments digital I/O board. The dispensers released individual M&M’s candies into a vertically-hanging, transparent rubber tube that fed into the testing enclosure so that each chimpanzee had access to their own tubes. Each tube was covered by a transparent capped section that prevented the candies from falling out. The chimpanzees had continuous access to their respective tubes and could easily release the capped section at any given time during the testing session. This effectively ended a trial, prohibiting the release of any additional candies to either individual. The computer program controlling the automated dispensers was written in Microsoft Visual Basic. An experimenter controlled the release of candies and termination of trials from an area outside of the testing room (and out of sight from the chimpanzees) using a keyboard while watching the subjects via a closed circuit monitor. The accumulation procedure was automatically terminated if all candies were accumulated, effectively ending the trial and preventing any extra token passes. All chimpanzees had experience using the automated food dispenser in previous accumulation tasks (e.g., Beran and Evans 2006; Evans et al. 2012). We ran one pretest trial for each chimpanzee with 20 pieces of food before testing to ensure they would engage this new experimental setup.
Data Analysis
Data violated the assumption of normality. Therefore, for each chimpanzee, a Kruskal-Wallis test was used to compare the amount of food accumulated across the four testing phases – token, bowl, new partner, and automated. Analyses were conducted separately for each subject. In addition, we included descriptive statistics on the average number of candies accumulated, average trial durations, and average number of extra token transfers (occurring after all food items had accumulated) per individual for each testing phase.
Results
As predicted, we found no statistically significant difference in the amount of food accumulated across the four separate testing phases for Sherman, H(3) = 1.704, p = 0.636, Panzee, H(3) = 2.694, p = 0.441, Lana, H(3) = 2.644, p = 0.45, or Mercury, H(3) = 4.228, p = 0.238. As shown in Table 1, on average, chimpanzees accumulated 18.33 candies in the token phase, 18.94 candies in the bowl phase, 17.0 candies in the new partner phase, and 17.07 candies in the automated phase. Test trial durations were an average of 236 seconds for the token phase, 167 seconds for the bowl phase, 137 seconds for the new partner phase, and 163 seconds for the automated phase collapsed across all chimpanzees. Finally, we calculated the average number of extra token transfers for the first three test phases – chimpanzees averaged 1.23 extra passes for the token phase, 0.4 extra passes for the bowl phase, and 0.3 extra passes for the new partner phase. This meant that the chimpanzees, on average, passed the token one more time after all candies were accumulated, but rarely more than that one extra time.
Table 1.
Each chimpanzee’s performance in the test phases of Experiment 1.
| Testing Phase | Sherman | Panzee | Lana | Mercury | |
|---|---|---|---|---|---|
| Token-Return | Avg # of Candies Accumulated | 18.92 | 19.17 | 17.58 | 17.67 |
| Avg Trial Duration (sec) | 331 | 331 | 140 | 140 | |
| Avg # of Extra Transfers | 1.83 | 1.67 | 0.5 | 0.92 | |
| Bowl- Retrieval | Avg # of Candies Accumulated | 19.75 | 19.5 | 18.33 | 18.17 |
| Avg Trial Duration (sec) | 229 | 229 | 105 | 105 | |
| Avg # of Extra Transfers | 0 | 0 | 0.67 | 0.92 | |
| New Partner | Avg # of Candies Accumulated | 14.13 | 19.88 | 14 | 20 |
| Avg Trial Duration (sec) | 95 | 179 | 95 | 179 | |
| Avg # of Extra Transfers | 0.25 | 0.25 | 0.25 | 0.43 | |
| Automated | Avg # of Candies Accumulated | 17.17 | 17.17 | 17 | 16.92 |
| Avg Trial Duration (sec) | 164 | 164 | 161 | 161 |
Discussion
Our results indicated that all four chimpanzees were equally proficient at accumulating nearly all food items in all testing phases of Experiment 1. Performance remained consistent regardless of the method of trial termination, their partner’s identity, and whether a human or an automated device delivered food rewards. In addition, the chimpanzees actively engaged in token transfer with their partner allowing for the accumulation of delayed rewards for between one to four minutes depending upon the testing phase.
As is evident from a visual inspection of the data, the chimpanzees became more efficient, accumulating the food items over a shorter duration after the first testing phase. This increase in accumulation speed was likely driven by a combination of experience with the transfer task and the trial termination method which gave the chimpanzees more direct control over taking their own food items rather than having to engage the experimenter. Chimpanzees rarely engaged in additional, ineffective token transfers but instead disengaged the social task to obtain the accumulated food rewards when appropriate (i.e., when there were no more candies left to accumulate). Thus, delay of gratification was excellent, and chimpanzees were quite proficient in this new context that required actively performing a task with a partner rather than just waiting for more food to accumulate.
Experiment 2
Now we gave chimpanzees the choice between an available reward that could be immediately obtained without engaging in a social task and the token that signified transfer with a partner to accumulate rewards. This phase allowed us to determine whether they preferred an option that led to some amount of free food without the need for joint action with a partner or an option to alternate work with a partner to accumulate a different number of items as in previous test phases.
Methods
Design and Procedure
We used the same partnerships as in testing phases 1, 2, and 4 in Experiment 1. Also, we used the same token transfer and accumulation procedure, and the same termination procedure as outlined in testing phase 2 of Experiment 1 in which the chimpanzees pulled in their own respective bowl to end the trial and obtain any accumulated food items.
We introduced a two-option choice task at the outset of each trial in which one chimpanzee chose between a bowl that contained an immediately available food reward (or was empty) or the token that symbolized transfer with their partner for the accumulation of a different quantity of food items on the display board. Experimenter 1 (E1) was seated in front of the display board. Experimenter 2 (E2) presented the choice task via a testing bench with a sliding tray that could be pushed in towards the focal chimpanzee. To avoid cuing, E2 closed his eyes during the choice phase and E1 (who was out of direct view of the focal chimpanzee) announced the item that was chosen (token or bowl). If the chimpanzee chose the token, E2 gave the token to the focal chimpanzee and E1 carried out the transfer procedure as was done in Experiment 1. If the chimpanzee chose the bowl, E2 immediately gave the focal chimpanzee the food items in the bowl and also gave the partner an equal quantity of candies (from a bowl inside the bench). E1 removed the display board if the bowl was chosen instead of the token. Thus, in essence the focal chimpanzee chose either to take the immediate food reward, or take the token and engage in the task as it was outlined in Experiment 1.
Testing consisted of 12 seven-trial sessions in which each chimpanzee served as the subject (the chimpanzee making the choice between the bowl or the token) for six sessions, alternating subjects across sessions. We used the following distribution of trial types in which the optimality of selecting the token decreased across levels:
-
Level 1
Trials:
Bowl = 0 M&M’s; Token/Display board = 2 M&M’s
Bowl = 0 M&M’s; Token/Display board = 6 M&M’s
Bowl = 0 M&M’s; Token/Display board = 12 M&M’s
-
Level 2
Bowl = 2 M&M’s; Token/Display board = 12 M&M’s
-
Level 3
Bowl = 6 M&M’s; Token/Display board = 12 M&M’s
-
Level 4
Bowl = 6 M&M’s; Token/Display board = 2 M&M’s
Levels corresponded to the expected decrease in likelihood to choose the token over the bowl. For Level 1, selecting the token was the most optimal response given that the token could yield food, while the bowl contained nothing in all trial types. We included trials in which the bowl was empty to ensure that the chimpanzees would not simply stop using the token altogether, in case they always took visible food amounts that were offered as a choice. Selecting the token in Level 4 trials was the least optimal response as the token represented fewer candies than could be obtained immediately. We included two trials of the comparison between bowl-2 versus token-12 from Level 2 and one trial of all other comparisons per session. Trial types were randomized within and across sessions. Inter-trial intervals were four minutes.
Data Analysis
We were interested in whether chimpanzees adaptively shifted their proportion of responses to the token over the bowl as the token decreased in optimality. A chi-square test for trend was used to analyze the proportion of token selections across levels in which the token became increasingly less optimal. A two-tailed binomial sign test was used to test for a significant preference between token or bowl for each level for all individuals. In addition, we included descriptive statistics on the average number of candies accumulated and average trial durations per chimpanzee for each trial type if the token was chosen.
Results
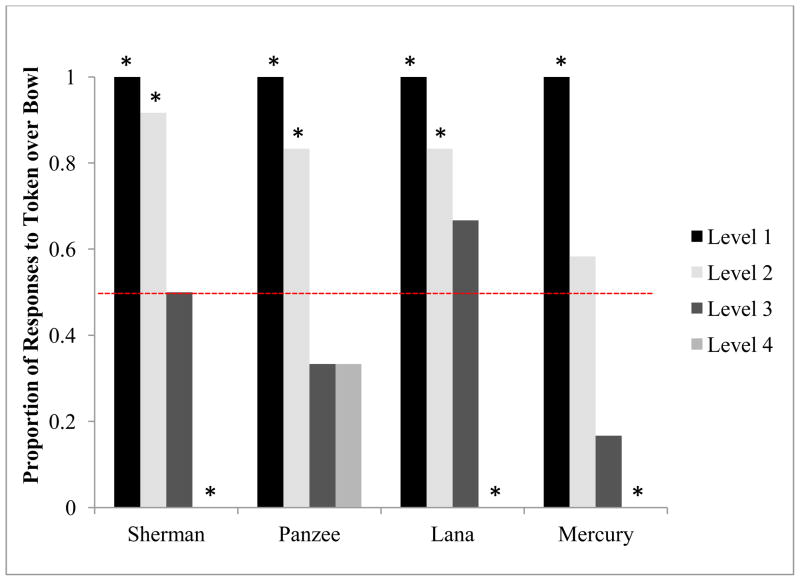
Preferences for the token or bowl for each level are shown in Figure 2. As predicted, the chi-square test for trend indicated that the proportion of token choices over bowl choices significantly decreased as the ratio between candies available for the bowl and token decreased. There was a significant decrease in the proportion of trials in which the token was selected across levels for Sherman, Χ2 (1, N=42) = 25.84, p < 0.001, Panzee, Χ2 (1, N=42) = 16.54, p < 0.001, Lana, Χ2 (1, N=42) = 22.51, p < 0.001, and Mercury, Χ2 (1, N=42) = 25.53, p < 0.001 (see Figure 2).
Figure 2.
A chi-square test for trend showed that the proportion of token choices significantly decreased as the token option became less optimal (i.e., as levels increased) in Experiment 2. This trend was statistically significant (p < .001) for all subjects. Levels corresponded to the expected decrease in likelihood to choose the token over the bowl. Asterisks indicate differences in preference for token or bowl that deviate from chance (shown by the dotted line) using a binomial distribution.
All individuals showed a significant preference for the token in Level 1, choosing the token in 100% of the trials, all p < 0.001. Three chimpanzees (excluding Mercury, p = 0.774) showed a significant preference for the token in Level 2: Sherman, p = 0.006, Panzee, p = 0.039, and Lana, p = 0.039. All chimpanzees were indifferent to the token and the bowl in Level 3, showing no significant preference for either item: Sherman, p = 1.0, Panzee, p = 0.688, and Lana, p = 0.688, Mercury, p = 0.219. Finally, three chimpanzees (excluding Panzee, p = 0.688) showed a significant preference for the bowl in Level 4, choosing the bowl in 100% of the trials, all p = 0.031.
Moreover, there was a high level of success in the accumulation phase when the token was selected. Performance levels indicated a success rate of above 98% on the accumulation task indicating that the chimpanzees almost always transferred the token until the maximum amount of candies available could be obtained when they chose to work with their partner (see Table 2). On average, chimpanzees required 54.14 seconds to receive all of the food items in the token transfer condition with a typical increase in time for the larger food sets, so there was a temporal cost associated with the token that was not present if the bowl was selected. Thus, even though subjects typically recovered all items using the token (minimizing that aspect of risk), there was the additional component of temporal cost to be considered when choosing between the token and bowl.
Table 2.
Chimpanzees’ performance across different trial types in Experiment 2, including the percentage of candies accumulated when the token was chosen and average trial durations.
| Sherman | Panzee | Lana | Mercury | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Trial Type | % Candies Accumulated | Avg Trial Duration (sec) | % Candies Accumulated | Avg Trial Duration (sec) | % Candies Accumulated | Avg Trial Duration (sec) | % Candies Accumulated | Avg Trial Duration (sec) |
| Bowl = 0 | ||||||||
| Token = 2 | 100% | 56.67 | 100% | 25.50 | 100% | 30.00 | 100% | 25.83 |
| Bowl = 0 | 100% | 43.17 | 100% | 47.00 | 100% | 35.33 | 100% | 45.00 |
| Token = 6 | ||||||||
| Bowl = 0 | ||||||||
| Token = 12 | 100% | 78.00 | 98.61% | 74.83 | 98.61% | 62.50 | 100% | 61.67 |
| Bowl = 2 | ||||||||
| Token = 12 | 100% | 83.82 | 100.00% | 71.10 | 100% | 60.50 | 100% | 61.29 |
| Bowl = 6 | ||||||||
| Token = 12 | 100% | 75.00 | 100.00% | 67.00 | 100% | 51.75 | 100% | 44.00 |
| Bowl = 6 | ||||||||
| Token = 2 | -- | -- | 100.00% | 36.50 | -- | -- | -- | -- |
Note. Data for two trial durations were not recorded because of equipment failure (Mercury: Level 1, bowl = 0, token = 6; Sherman: Level 3, bowl = 6, token = 12).
Discussion
When presented with the option between taking an immediately available food reward or working with a partner to accumulate a potentially larger amount of food, the chimpanzees’ preferences for the token shifted along with its corresponding optimality (i.e., as the difference between token rewards and bowl rewards became more discrepant, the chimpanzees typically opted for the item that led to the larger reward amount). As expected, the chimpanzees preferentially chose the token for transfer with their partner when it yielded the only reward set (Level 1; bowl = 0; token= 2, 6, or 12) or the larger reward set by a factor of six (Level 2; bowl = 2; token = 12), except for Mercury. However, the chimpanzees were not locked into this choice behavior as they preferentially chose the bowl when it instead yielded the larger reward set, except in one case for Panzee (Level 4; bowl = 6; token = 2). Interestingly, we saw more variation in choice behavior when the token represented the larger reward set, but only by a factor of two (Level 3; bowl = 6; token = 12). Sherman and Lana chose the token on 50–67 % of trials, whereas Panzee and Mercury chose the token in 16–33 % of trials. Given their highly sensitive discriminations of quantity in previous studies (e.g., Beran 2001, 2004), their choice of six immediate candies instead of 12 possible delayed candies indicates that tolerance for delayed reward for some chimpanzees decreases when they are required to work with a partner.
General Discussion
Chimpanzees readily engaged in a token transfer task with a conspecific that enabled the accumulation of food items. This test required the apes to actively engage a task and alternate actions with a partner, when either individual could terminate the trial by taking the accumulating food rewards at any given time. The novelty of continuous engagement with a social partner that directly led to the accumulation of food items introduced a new type of risk in that termination of the accumulation phase was no longer solely dependent upon the subject’s self-control, but instead also was dictated by the partner’s self-control (or lack thereof).
Chimpanzees accumulated nearly all available food items in Experiment 1, regardless of the method of trial termination, engaging in token transfer for up to four minutes, and rarely transferring the token more than necessary. Performance remained consistent with the introduction of more prepotent food items, perhaps due to the chimpanzees’ extensive experience with a similar paradigm using within-reach food rewards (Beran 2002; Beran and Evans 2006, 2009; Evans and Beran 2007). In addition, success was not likely contingent on an experimenter enforcing or exaggerating the transfer behavior as performance was consistent in the automated testing phase as well. Although a previous study with these same chimpanzees showed that human presence did not artificially inflate self-control (Beran and Evans, 2006), we felt that this phase was necessary so that we could rule out reliance on human intervention that has proven necessary in some previous token exchange experiments (e.g., Brosnan and Beran 2009). Finally, performance remained consistent across new-pairings of social partners, even among individuals that do not typically work or live together and otherwise are not highly affiliative (i.e., Mercury and Panzee). These results indicate robust and flexible self-control performance across a number of experimental manipulations.
When given the option to take an immediately available food reward or to work with a partner to accumulate a different amount of food reward, chimpanzees typically chose whichever option led to the greater amount of reward (except when the rewards differed only by a factor of two). When the greater reward amount had to be accumulated, foregoing the immediately available, guaranteed reward was risky in that the partner may not complete the token transfer process, potentially leading to fewer rewards than the immediately available option. Success in earlier phases of this experiment likely decreased this inherent risk as the chimpanzees had previous positive experience working with a partner who typically did not terminate the trial early in the accumulation phase. Furthermore, there was an inherent temporal cost to transferring the token rather than selecting the immediately available food.
The present results are in line with previous research on self-control with chimpanzees in which they have demonstrated a proficiency in delay of gratification paradigms, including tasks that required inhibiting taking accumulating food rewards for multiple minutes per trial (Beran 2002; Beran et al. 1999; Dufour et al. 2007). The full corpus of data from these studies now indicates that at least some chimpanzees show self-control abilities similar to those of human children (Mischel et al. 1972; Miller and Karniol 1976; Toner and Smith 1977). Specifically, chimpanzees can delay gratification for similar amounts of time as human children (Beran and Evans 2006), and like children, chimpanzees will divert their attention from task rewards resulting in greater delay of gratification (Evans and Beran 2007). Despite these results, we have an incomplete picture of self-control in chimpanzees as it manifests in a social setting (but see Boysen and Bernston 1995; Evans et al. 2012), and as it might manifest in other colonies of captive animals.
Beyond the aspect of self-control, the current task required that the chimpanzees repeatedly engaged in a coordinated interaction with their partner, which in turn facilitated the gradual accumulation of rewards for both individuals. Chimpanzees have demonstrated a similar capacity to exchange tokens and tools with conspecifics (Brosnan and Beran 2009; Savage-Rumbaugh et al. 1978a, 1978b), demonstrating the propensity to directly facilitate their own rewards via social engagement. Social tasks of this nature may be extended to investigate the level at which each individual understands its partner’s needs or motivation levels, and how those interact with their own similar or competing needs or motivations. Because a social animal rarely engages in cognitive processes or exhibits behavioral patterns in isolation from its social environment, we developed the paradigm described here so that we could combine the attributes of a self-control test with the need to engage in social behavior to get one step closer to explicitly testing the two in conjunction. Future research that more closely integrates an animal’s social world and its self-control abilities is needed to further address questions surrounding how the two interact and impact one another.
Acknowledgments
The research was supported by National Institute of Child Health and Development (grant HD-060563). Audrey Parrish was supported, in part, by the 2CI University Doctoral Fellowship from Georgia State University, and Bonnie Perdue was supported, in part, by the Duane M. Rumbaugh Fellowship from Georgia State University. The authors thank the animal care and enrichment team at the Language Research Center.
References
- Anderson JR, Kuroshima H, Fujita K. Delay of gratification in capuchin monkeys (Cebus apella) and squirrel monkeys (Saimiri sciureus) J Comp Psychol. 2010;124:205–210. doi: 10.1037/a0018240. [DOI] [PubMed] [Google Scholar]
- Beck BB. Cooperative tool use by captive hamadryas baboons. Science. 1973;182:594. doi: 10.1126/science.182.4112.594. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Summation and numerousness judgments of sequentially presented sets of items by chimpanzees (Pan troglodytes) J Comp Psychol. 2001;115:181–191. doi: 10.1037/0735-7036.115.2.181. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Maintenance of self-imposed delay of gratification by four chimpanzees (Pan troglodytes) and an orangutan (Pongo pygmaeus) J Gen Psychol. 2002;129:49–66. doi: 10.1080/00221300209602032. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Chimpanzees (Pan troglodytes) respond to nonvisible sets after one-by-one addition and removal of items. J Comp Psychol. 2004;118:25–36. doi: 10.1037/0735-7036.118.1.25. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA. Maintenance of delay of gratification by four chimpanzees (Pan troglodytes): The effects of delayed reward visibility, experimenter presence, and extended delay intervals. Behav Process. 2006;73:315–324. doi: 10.1016/j.beproc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA. Delay of gratification by chimpanzees (Pan troglodytes) in working and waiting situations. Behav Process. 2009;80:177–181. doi: 10.1016/j.beproc.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Evans TA. Language-trained chimpanzees (Pan troglodytes) delay gratification by choosing token exchange over immediate reward consumption. Am J Primatol. 2012;74:864–870. doi: 10.1002/ajp.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Evans TA, Hoyle D. Numerical judgments by chimpanzees (Pan troglodytes) in a token economy. J Exp Psychol Anim Behav Process. 2011;37:165–174. doi: 10.1037/a0021472. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Pate JL, Rumbaugh DM. Delay of gratification in chimpanzees (Pan troglodytes) Dev Psychobiol. 1999;34:119–127. doi: 10.1002/(SICI)1098-2302(199903)34:2<119::AID-DEV5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Boysen ST, Bernston GG. Responses to quantity: Perceptual versus cognitive mechanisms in chimpanzees. J Exp Psychol Anim Behav Process. 1995;21:82–86. doi: 10.1037/0097-7403.21.1.82. [DOI] [PubMed] [Google Scholar]
- Bramlett J, Perdue B, Evans T, Beran M. Capuchin monkeys (Cebus apella) let lesser rewards pass them by to get better rewards. Anim Cogn. 2012;15:963–969. doi: 10.1007/s10071-012-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, Beran MJ. Trading behavior between conspecifics in chimpanzees, Pan troglodytes. J Comp Psychol. 2009;123:181–194. doi: 10.1037/a0015092. [DOI] [PubMed] [Google Scholar]
- Brosnan SF, de Waal FBM. A concept of value during experimental exchange in brown capuchin monkeys. Folia Primatol. 2004;75:317–330. doi: 10.1159/000080209. [DOI] [PubMed] [Google Scholar]
- Brosnan SF, de Waal FBM. Responses to a simple barter task in chimpanzees, Pan troglodytes. Primates. 2005;46:173–182. doi: 10.1007/s10329-005-0125-0. [DOI] [PubMed] [Google Scholar]
- Chalmeau R, Peignot P. Exchange of objects between humans and captive western lowland gorillas. Primates. 1998;39:389–398. doi: 10.1007/BF02557563. [DOI] [Google Scholar]
- Chen MK, Lakshminarayanan V, Santos LR. How basic are behavioral biases? Evidence from capuchin monkey trading behavior. J Polit Econ. 2006;114:517–537. doi: 10.1086/503550. [DOI] [Google Scholar]
- Dufour V, Pelé M, Sterck EHM, Thierry B. Chimpanzee (Pan troglodytes) anticipation of food return: Coping with waiting time in an exchange task. J Comp Psychol. 2007;121:145–155. doi: 10.1037/0735-7036.121.2.145. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Chimpanzees use self-distraction to cope with impulsivity. Biol Lett. 2007;3:599–602. doi: 10.1098/rsbl.2007.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Addessi E. Can nonhuman primates use tokens to represent and sum quantities? J Comp Psychol. 2010;129:369–380. doi: 10.1037/a0019855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Perdue BM, Parrish AE, Menzel EC, Brosnan SF, Beran MJ. How is chimpanzee self-control influenced by social setting? Scientifica. 2012;2012:Article 9. doi: 10.6064/2012/654094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Westergaard GC. Self-control and tool-use in tufted capuchin monkeys (Cebus apella) J Comp Psychol. 2006;120:163–166. doi: 10.1037/0735-7036.120.2.163. [DOI] [PubMed] [Google Scholar]
- Karoly P. Mechanisms of self-regulation: a systems view. Ann Rev Psychol. 1993;44:23–52. doi: 10.1146/annurev.psych.44.1.23. [DOI] [Google Scholar]
- Lefebvre L. Food exchange strategies in an infant chimpanzee. J Hum Evol. 1982;11:195–204. doi: 10.1016/S0047-2484(82)80036-5. [DOI] [Google Scholar]
- Lefebvre L, Hewitt TA. Food exchange in captive chimpanzees. In: Taub DM, King FA, editors. Current perspectives in primate social dynamics. Van Nostrand Reinhold; New York, NY: 1986. pp. 476–486. [Google Scholar]
- Logue AW. Research on self-control: An integrating framework. Behav Brain Sci. 1988;11:665–709. doi: 10.1017/S0140525X00053978. [DOI] [Google Scholar]
- Logue AW, Forzano LB, Ackerman KT. Self-control in children: Age, preference for reinforcer amount and delay, and language ability. Learn Motiv. 1996;27:260–277. doi: 10.1006/lmot.1996.0014. [DOI] [Google Scholar]
- Miller DT, Karniol R. Coping strategies and attentional mechanisms in self-imposed and externally imposed delay situations. J Pers Soc Psychol. 1976;34:310–316. doi: 10.1037/0022-3514.34.2.310. [DOI] [Google Scholar]
- Mischel W. Processes in delay of gratification. Adv Exp Soc Psychol. 1974;7:249–292. doi: 10.1016/S0065-2601(08)60039-8. [DOI] [Google Scholar]
- Mischel W. Objective and subjective rules for delay of gratification. In: d’ Ydewalle G, Lens W, editors. Cognition in human motivation and learning. Psychology Press; New York, NY: 1981. pp. 33–58. [Google Scholar]
- Mischel W, Ebbesen EB, Zeiss AR. Cognitive and attentional mechanisms in delay of gratification. J Pers Soc Psychol. 1972;21:204–218. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Peake PK. The nature of adolescent competencies predicted by preschool delay of gratification. J Pers Soc Psychol. 1988;54:678–696. doi: 10.1037/0022-3514.54.4.687. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez ML. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Houts R, Poulton R, Roberts BW, Ross S, Sears MR, Thomson WM, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H. The science of self-control. Harvard University Press; Cambridge, MA: 2000. [Google Scholar]
- Rumbaugh DM, Washburn DA. Intelligence of apes and other rational beings. Yale University Press; New Haven, CT: 2003. [Google Scholar]
- Savage-Rumbaugh ES, Rumbaugh DM, Boysen S. Linguistically mediated tool use and exchange by chimpanzees (Pan troglodytes) Behav Brain Sci. 1978a;1:539–554. doi: 10.1017/S0140525X00076536. [DOI] [Google Scholar]
- Savage-Rumbaugh ES, Rumbaugh DM, Boysen S. Symbolic communication between two chimpanzees (Pan troglodytes) Science. 1978b;201:641–644. doi: 10.1126/science.675251. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Hallinan EV, Hauser MD. The ecology and evolution of patience in two New World monkeys. Biol Lett. 2005;1:223–226. doi: 10.1098/rsbl.2004.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. 2004;72:271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Tobin H, Logue AW, Chelonis JJ, Ackerman KT, May JGI. Self-control in the monkey Macaca Fascicularis. Anim Learn Behav. 1996;24:168–174. doi: 10.3758/BF03198964. [DOI] [Google Scholar]
- Toner IJ, Smith RA. Age and overt verbalization in delay-maintenance behavior in children. J Exp Child Psychol. 1977;24:123–128. doi: 10.1016/0022-0965(77)90025-X. [DOI] [Google Scholar]
- Vick SJ, Bovet D, Anderson J. How do African grey parrots (Psittacus erithacus) perform on a delay of gratification task? Anim Cogn. 2010;13:351–358. doi: 10.1007/s10071-009-0284-2. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ. Transfer of tools and food between groups of tufted capuchin (Cebus apella) Am J Primatol. 1997;43:33–41. doi: 10.1002/(SICI)1098-2345(1997)43:1<33::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- White JL, Moffitt TE, Caspi A, Bartusch DJ. Measuring impulsivity and examining its relationship to delinquency. J Abnorm Psychol. 1994;103:192–205. doi: 10.1037/0021-843X.103.2.192. [DOI] [PubMed] [Google Scholar]