Summary
Pulmonary thromboembolism associated with pancreatic endocrine neoplasia is extremely uncommon in humans and animals. Post mortem examination of an adult owl monkey revealed extensive pulmonary arterial thromboembolism and a well-demarcated mass attached to the pancreas. Histologically, the mass consisted of areas of interstitial fibrosis with loss of acini and islets and replaced by nests and sheets of polygonal cells with amphophilic cytoplasm, an eccentric rounded nucleus with stippled chromatin, and in some cells, with a single prominent eccentric nucleolus. Clusters of these cells were noted within vessels and adjacent lymph nodes. The cells did not stain with S-100 and insulin, stained rare individual cells with glucagon and somatostatin, but strongly stained with SP-1 Chromogranin. A few cells in pulmonary thrombi/emboli and adjacent lymph node stained also with SP-1 Chromogranin. Based on cell morphology, location, and immunohistochemical staining, the tumor was classified as pancreatic endocrine (islet cell) carcinoma with metastasis to regional lymph nodes and lung.
Keywords: immunohistochemistry, islet cell neoplasia, nonhuman primate
Pancreatic endocrine neoplasms (Islet cell tumors of the pancreas) are uncommon in humans (Batcher et al., 2011; Lloyd et al., 1984) and, with the exception of the ferret, (Weiss et al., 1998) much less common in domestic animals (Priester, 1974). In nonhuman primates, islet cell tumors of the pancreas appear to have a very low incidence with only a few cases reported in rhesus monkeys (McClure and Chandler 1982), and single reports in a pig-tailed macaque (McClure and Chandler, 1982), a marmoset (Dias et al., 1996) and a colobus monkey (Hobson and Turner, 2008). Compared to pancreatic adenocarcinomas, islet cell tumors grow at a much slower rate, can produce large amounts of specific hormones with associated dramatic clinical signs, and, in humans, can arise from familial genetic mutations (Batcher et al., 2011). Islet cell pancreatic tumors that do not produce large amounts of hormones are called nonfunctional and are characterized by being clinically silent but with poor prognosis (Batcher et al., 2011).
Few cases of pulmonary thromboembolism associated with islet cell tumors due to metastasis have been reported in humans (Fenkci et al., 2005; Sprogøe-Jakobsen & Karkov, 1991) and none, to the authors’ knowledge, in animals. Here we describe a case of pancreatic endocrine (islet cell) tumor with multiple pulmonary thromboembolism in an owl monkey.
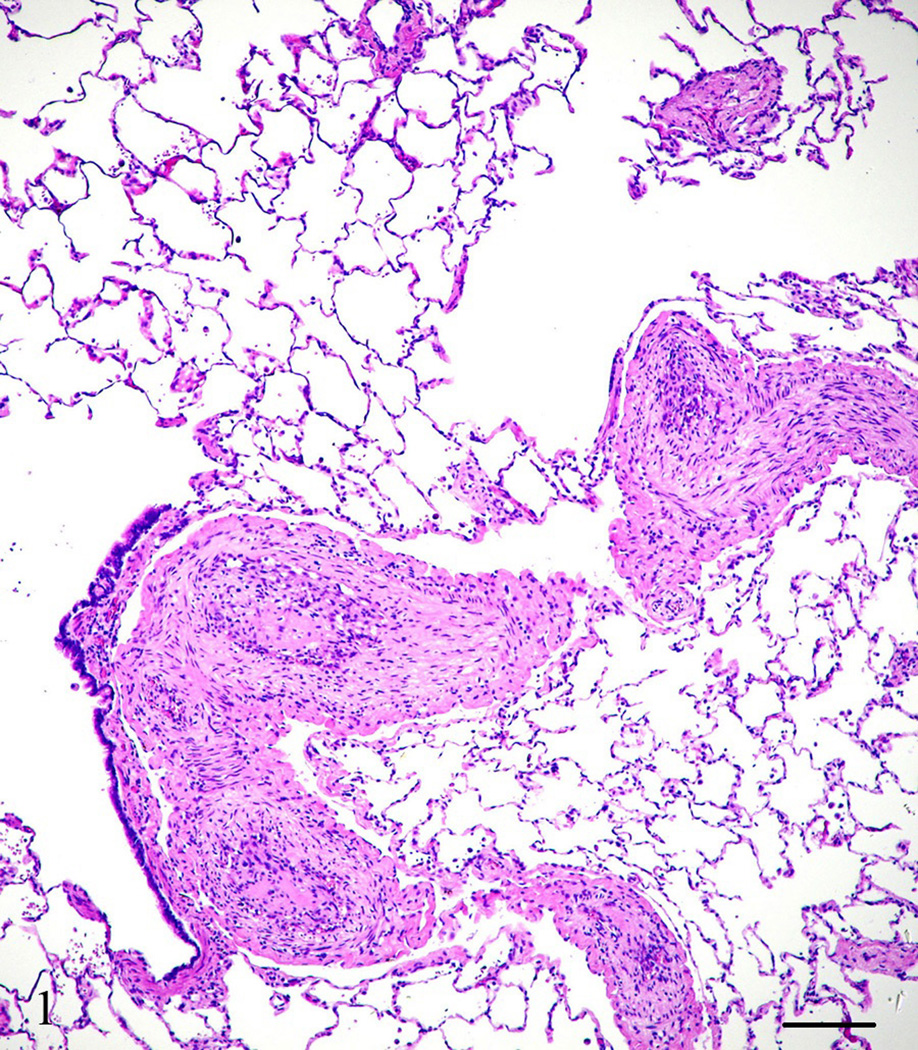
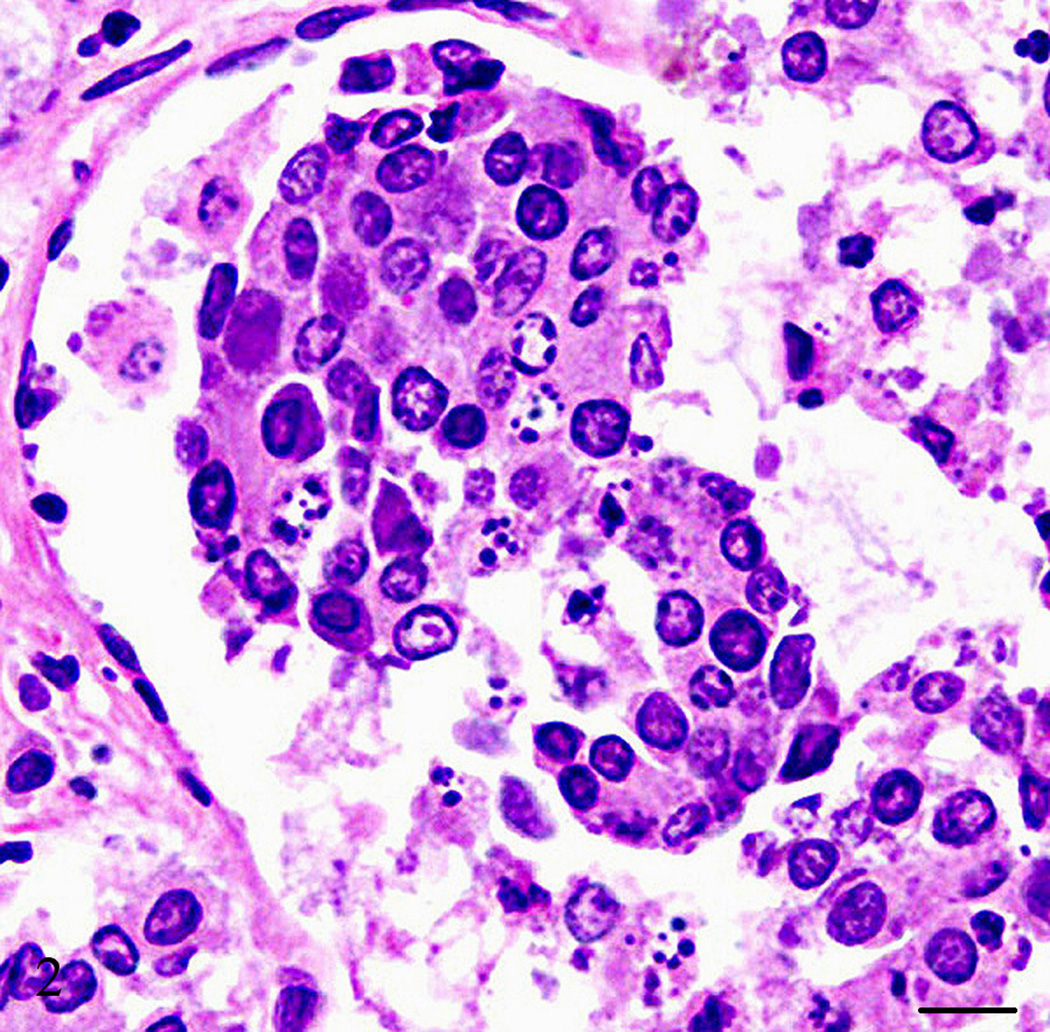
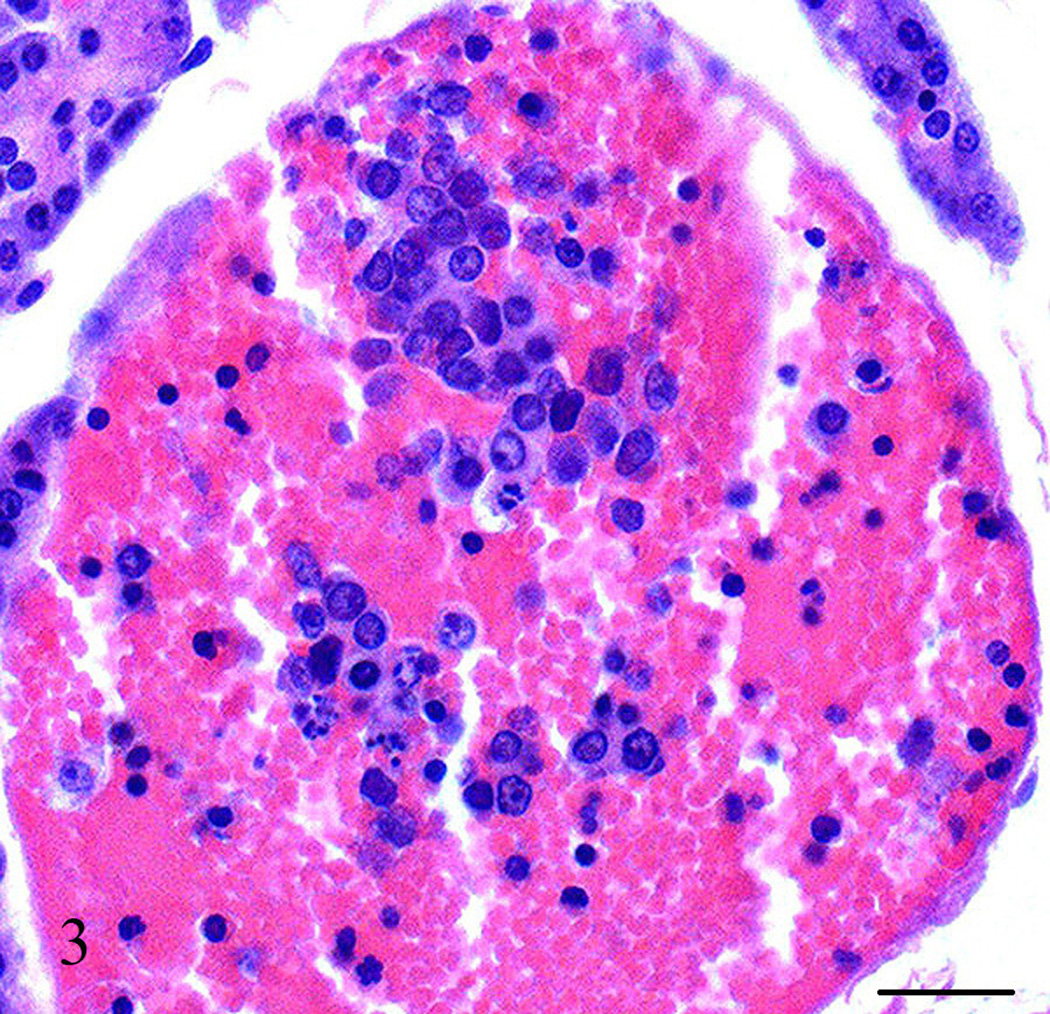
A captive-born, six-year old, male owl monkey (Aotus nancymae) was found dead. The animal was part of a malaria vaccine research study approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee and housed in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (ILAR, 1996). The animal had being immunized with a malaria candidate vaccine but was not infected at the time of death. At necropsy, the animal weighed 800 grams (within normal limits for the species) and appeared to be in good nutritional condition with adequate adipose stores. Mild subcutaneous edema was noted extending from the caudal half of the ventral abdomen to the prepuce and scrotum. Approximately, 30 ml of a brown clear fluid was found in the abdominal cavity and 15 ml of a red-tinged clear fluid in each hemithoracic cavity. The lungs were atelectatic. The heart left ventricular free wall was mild to moderately thicken. No other gross abnormalities were noted. Tissue samples from all major organs were collected, fixed in 10% neutral buffered formalin and processed for light microscopy. Microscopically, the heart left ventricular free wall was 3 times the thickness of the right ventricular free wall, however, there were more hypertrophied myocardial cells in the right ventricle than the left suggesting right ventricular wall hypertrophy. Multiple coronary arterioles had mild to moderate smooth muscle hypertrophy and multifocal minimal to mild myocardial interstitial fibrosis. The majority of the pulmonary arteries and arterioles had mild to moderate smooth muscle hypertrophy, mild to marked intimal thickening with increased matrix and mesenchymal cells, and partial to complete luminal occlusion with thrombi and organized and recanalized thrombi (Fig. 1). Diffuse mild alveolar lumina infiltrates of macrophages and red blood cells were observed along with a few areas of mild to moderate alveolar wall thickening and alveolar luminal fibrin accumulation. The liver had multifocal t o coalescing mild to moderate infiltrates of neutrophils admixed with necrotic hepatocytes and occasionally small colonies of short, plump cocco-bacilli organisms, diffuse mild biliary hyperplasia, and hepatocellular hemosiderosis. The kidneys showed multifocal mild perivascular and interstitial lymphocytic infiltrates with multiple randomly scattered cortical and cortico-medullary tubules containing intraluminal cellular debris and fragmented neutrophils. The pancreas had an approximately 3 by 2.5 mm well-demarcated mass attached that consisted of multifocal to coalescing areas of interstitial fibrosis with loss of acini and islets and replaced by nests and sheets of polygonal cells with amphophilic cytoplasm, an eccentric rounded nucleus with stippled chromatin, and in some cells, with a single prominent eccentric nucleolus (Fig. 2). Clusters of these cells were noted within vessels and many were necrotic and/or autolytic (Fig. 3). The adjacent lymph node appeared to contain some of these cells as well. No mitotic figures were observed. Immunohistochemistry was performed to further characterize the neoplasm. Paraffin-embedded tissues were cut to a thickness of 4 um, placed on ProbeOn Plus slides (Fisher Scientific, Pittsburgh, PA), and deparaffinized. A target retrieval solution (DAKO, Carpentaria, CA) was used for S-100 antibody and negative controls. A pressure cooker with citrate buffer pH 6.0 (Cell Marque, Rocklin, CA) was used for pretreatment with SP-1/Chromogranin and negative controls. Subsequently all slides were blocked two times with Dual Endogenous Block (DAKO) for five mins each and antibody was applied as follows: Glucagon (Zymed, San Francisco, CA) 1:400, rabbit polyclonal, 60 mins; Insulin (Zymed) 1:1,000, mouse monoclonal, 60 mins; Somatostatin (Zymed) 1:200, rabbit polyclonal, 60 mins; S-100 (DAKO) 1:10,000, rabbit polyclonal, 30 mins; SP-1/Chromogranin (Immunostar, Inc., Hudson, WI) 1:5,000, rabbit polyclonal, 60 mins. After two rinses with TBS, Envision rabbit or mouse (DAKO) was applied for 30 mins rinsed with TBS, DAB+ (DAKO) w as applied for 10 mins followed by two mins of CAT hematoxylin (Biocare, Concord, CA). As a negative control, a rabbit polyclonal or mouse monoclonal IgG was used in lieu of the antibody.
Figure 1.
Lung, Aotus nancymae. Multiple small-sized arteries showing complete luminal occlusion with organized thrombi. Hematoxylin and eosin stain. Bar = 250 µm
Figure 2.
Pancreas, Aotus nancymae. Pancreatic mass characterized by marked interstitial fibrosis with nests of polygonal cells with amphophilic cytoplasm and an eccentric rounded nucleus. Hematoxylin and eosin stain. Bar = 10 µm.
Figure 3.
Pancreas, Aotus nancymae. Clusters of neoplastic cells within a pancreatic blood vessel. Hematoxylin and eosin stain. Bar = 50 µm.
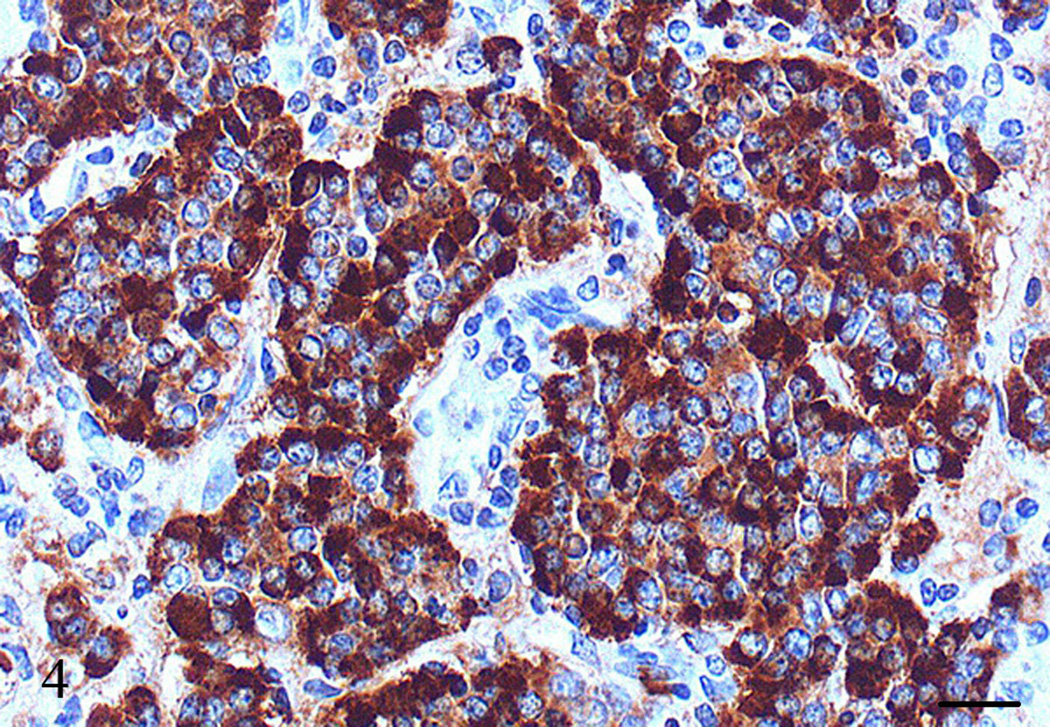
The neoplastic cells did not stain with S-100 and insulin, stained rare individual cells with glucagon and somatostatin, but strongly stained with SP-1/Chromogranin (Fig. 4). A few cells in pulmonary thrombi/emboli, blood vessels, and adjacent lymph node stained also with SP-1/Chromogranin (Figs. 5 and 6). Based on cell morphology, location, and immunohistochemical staining, the tumor was classified as pancreatic endocrine (islet cell) carcinoma with metastasis to regional lymph nodes and lung.
Figure 4.
Pancreas, Aotus nancymae. Nests and sheets of neoplastic cells in the pancreatic mass strongly stained with SP-1 Chromogranin. Immunohistochemistry, peroxidase staining, hematoxylin counter stain. Bar = 20 µm.
Figure 5.
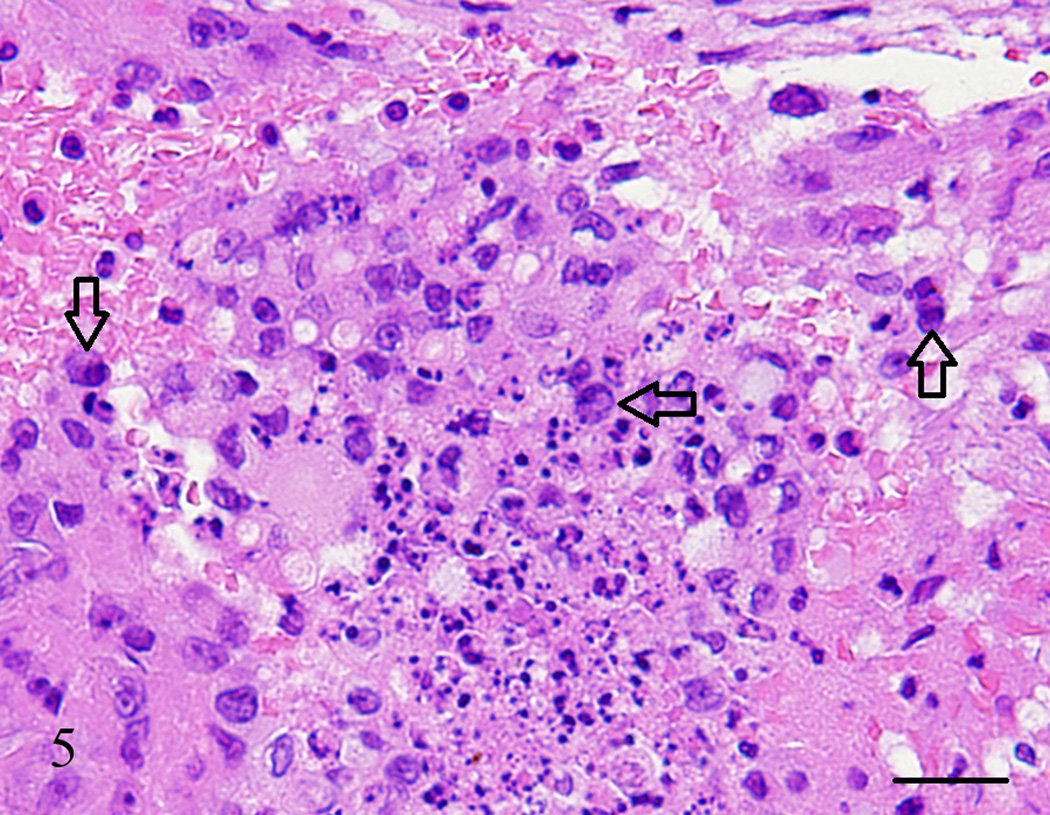
Lung, Aotus nancymae. Medium-sized artery lumen showing an organized thrombus with necrotic cells and a few suspect neoplastic cells (open arrows). Hematoxylin and eosin stain. Bar = 50 µm.
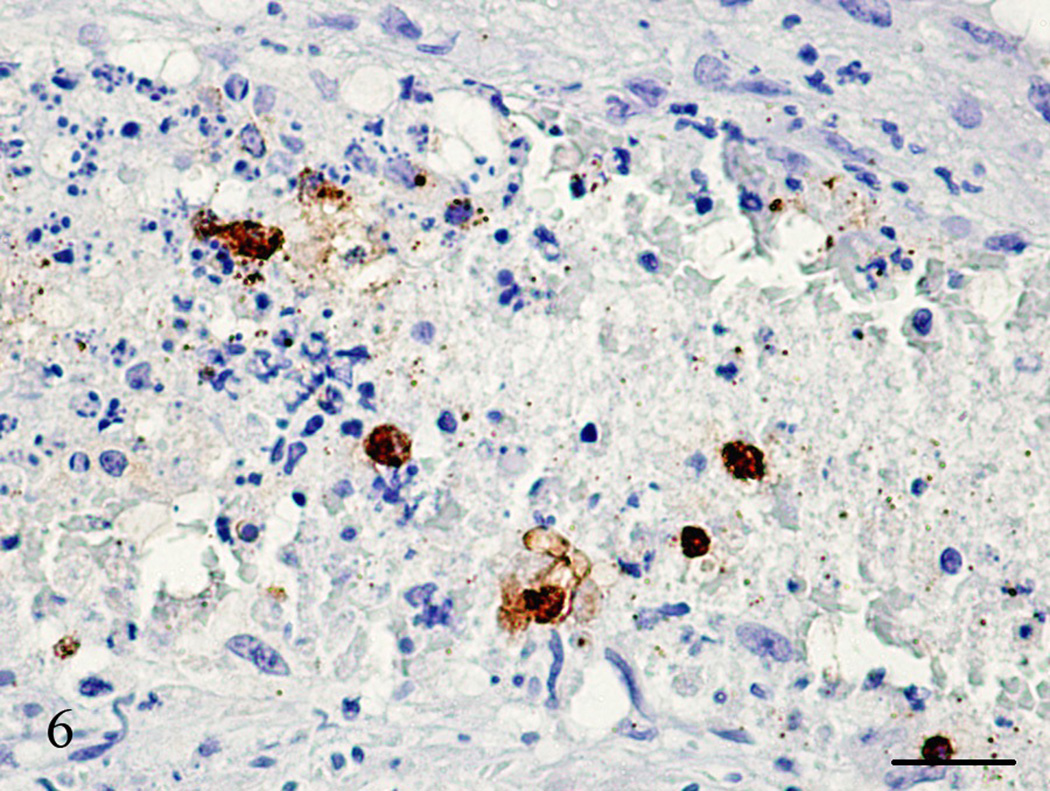
Figure 6.
Lung, Aotus nancymae. Same medium-sized artery lumen from Figure 5 showing an organized thrombus with necrotic cells and a few neoplastic cells demonstrated by being strongly stained with SP-1 Chromogranin. I mmunohistochemistry, peroxidase staining, hematoxylin counter stain. Bar = 50 µm.
Differential diagnoses for pancreatic neoplasms include acinar cell carcinomas which usually have a high mitotic rate and pancreatoblastomas which have distinct squamoid nests (Cavallini et al., 2009). No high mitotic rates or squamoid nests were observed in the present report. Pseudopapillary neoplasias can resemble islet cell tumors but are of non-endocrine origin and usually have foamy macrophage aggregates and large cytoplasmic hyaline globules (Meriden et al., 2011). Islet cell tumors are typically well demarcated in contrast to pancreatic adenocarcinomas which are much more poorly-defined (Asa, 2011). Islet cell tumors can have an invasive growth and tend to metastasize to lymph nodes, liver and other organs (Asa, 2011; Batcher et al., 2011). Microscopically, these tumors have round to polygonal cells with uniform nuclei and usually produce insulin and glucagon (Asa, 2011). Chromogranin immunolabeling is considered diagnostic for islet cell pancreatic tumors (Lloyd et al., 1984; Suh & Wang, 2008).
To our knowledge, pancreatoblastomas an d pancreatic pseudopapillary neoplasias have not been reported in animals. On the other hand, pancreatic acinar cell carcinomas have been reported in domestic animals (Skutelsky et al., 1987).
The cause of death in this animal was most likely due to disseminated pulmonary thrombosis. The lungs had diffuse, severe arterial/arteriolar narrowing and occlusion which lead to passive congestion of the liver and pulmonary hypertension with dilation of the right heart chamber and hypertrophy of individual myocardial cells (cor pulmonale). This would explain why the right ventricular wall myocytes were hypertrophied but grossly the wall appeared much thinner compared to the left ventricle wall. Agents or conditions known to induce disseminated intravascular thrombosis include: viral, bacterial and parasitic infections, neoplasias, immunologic endothelial injury, shock, vascular stasis, prolonged anesthesia, acidosis, tissue necrosis, gastric dilatation and volvulus, heatstroke, hyperosmolality, pancreatitis, snakebites, aflatoxicosis, incompatible blood transfusion, and purpura hemorrhagica (Caswell & Williams, 2007). The monkey was captive-born in an indoor closed colony subject to periodic health examinations. The colony was free of pathogenic viruses, bacteria, and parasites at the time of the animal’s death. Histologic examination of the animal did not reveal lesions suggestive of any of the other conditions except the pancreatic tumor. Chronic interstitial pneumonia and chronic bronchitis and bronchiolitis can result in proliferation of medial smooth cells and intima in medium and small sized pulmonary arteries, however, the monkey in this study did not showed pneumonia or bronchitis/bronchiolitis. Systemic arterial hypertension alone can cause arterial medial hypertrophy. In our case the monkey might have had systemic arterial hypertension since during histologic examination the heart showed multiple coronary arterioles with mild to moderate smooth muscle hypertrophy. How ever, similar vascular lesions were not observed in other organs except the lungs. The subcutaneous edema, hydrothorax, ascitis, and hepatomegaly found in this animal suggest right side heart failure. In addition, the hepatic, renal, and pancreatic lesions probably contributed to the deterioration of this animal but the main cause of death was attributed to the disseminated pulmonary thrombosis.
Only glucagon producing pancreatic islet cell tumors (glucagonomas) have been reported associated with pulmonary thromboembolism in humans (Fenkci et al., 2005; Sprogøe-Jakobsen & Karkov, 1991). Excessive production of glucagon can lead to pulmonary thrombi (Fenkci et al., 2005), however, the tumor cells in the owl monkey in the current report rarely stained with glucagon which suggest that another mechanism was responsible for the lung lesions. Pulmonary thromboembolism associated with neoplasia can result from large number of neoplastic cells detaching from the primary tumor migrating to the lung via the blood stream and consequently blocking small pulmonary arteries (Sancho-Chust et al., 2009). In addition, chemicals with direct procoagulant activity like tissue factor (TF) and cancer procoagulant (CP), or inflammatory cytokines like tumor necrosis factor (TNF), interleukine-1 (IL-1), and vascular endothelial growth factor (VEGF) can be released by neoplastic cells and activate the pulmonary vascular endothelium, attract inflammatory cells and predispose the formation of thrombi (De Cicco, 2004). Studies in humans suggest that elevated platelet and leukocyte counts in neoplastic processes are associated with vascular thrombosis (Shaib et al., 2010). Elevated D-dimer levels in patients with cancer and, specifically, high TF expression in patients with pancreatic cancer, have also been found to be predictive of vascular thrombosis (Shaib et al., 2010). In humans, pulmonary thromboembolism associated to neoplasia has a poor prognosis if not treated promptly.
Due to the finding of neoplastic cells in the owl monkey’s pulmonary thrombi/emboli, and since no pathogens or other lesions were found that might produce disseminated pulmonary thrombosis, we believe that the release of procoagulants by the neoplastic cells triggered the marked lung vasculopathy and consequently the death of the animal. This is, to our knowledge, the first report of a pancreatic endocrine tumor associated with multiple pulmonary thromboembolism in a nonhuman primate.
Acknowledgements
This study was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Comparative Medicine Branch, Laboratory of Malaria Immunology and Vaccinology, and the Office of Research Services. We thank Dr. Patrick Duffy for kindly letting us use the tissues in this case report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- 1.Asa SL. Pancreatic endocrine tumors. Modern Pathology. 2011;24(Suppl 2):S66–S77. doi: 10.1038/modpathol.2010.127. [DOI] [PubMed] [Google Scholar]
- 2.Batcher E, Madaj P, Gianoukakis AG. Pancreatic neuroendocrine tumors. Endocrine Research. 2011;36(1):35–43. doi: 10.3109/07435800.2010.525085. [DOI] [PubMed] [Google Scholar]
- 3.Cavallini A, Falconi M, Bortesi L, Crippa S, Barugola G, Butturini G. Pancreatoblastoma in Adults: A Review of the Literature. Pancreatology. 2009;9(1–2):73–80. doi: 10.1159/000178877. [DOI] [PubMed] [Google Scholar]
- 4.Caswell JL, Williams KJ. Respiratory System, Chapter 5. In: Grant Maxie M, editor. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 5th edition. Philadelphia, PA: Elsevier- Sanders; 2007. [Google Scholar]
- 5.De Cicco M. The prothrombotic state in cancer: pathogenic mechanisms. Critical Reviews in Oncology/Hematology. 2004;50(3):187–196. doi: 10.1016/j.critrevonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Dias JL, Montali RJ, Strandberg JD, Johnson LK, Wolff MJ. Endocrine neoplasia in New World primates. Journal of Medical Primatology. 1996;25(1):34–41. doi: 10.1111/j.1600-0684.1996.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 7.Fenkci SM, Fidan Yaylali G, Sermez Y, Akdam H, Sabir N, Kiraç S. Malign cystic glucagonoma presented with diabetic ketoacidosis: case report with an update. Endocrine Related Cancer. 2005;12(2):449–454. doi: 10.1677/erc.1.00957. [DOI] [PubMed] [Google Scholar]
- 8.Hobson DJ, Turner PV. Spontaneous pancreatic islet cell tumor in a black and white colobus monkey (Colobus guereza kikuyuensis) Journal of Medical Primatology. 2008;37(Suppl 1):11–15. doi: 10.1111/j.1600-0684.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academy Press; 1996. [Google Scholar]
- 10.Lloyd RV, Mervak T, Schmidt K, W arner TF, Wilson BS. Immunohistochemical detection of chromogranin and neuron-specific enolase in pancreatic endocrine neoplasms. American Journal of Surgical Pathology. 1984;8(8):607–614. doi: 10.1097/00000478-198408000-00004. [DOI] [PubMed] [Google Scholar]
- 11.McClure HM, Chandler FW. A survey of pancreatic lesions in nonhuman primates. Veterinary Pathology. 1982;19(Suppl 7):193–209. [PubMed] [Google Scholar]
- 12.Meriden Z, Shi C, Edil BH, Ellison T, Wolfgang CL, Cornish TC, Schulick RD, Hruban RH. Hyaline Globules in Neuroendocrine and Solid-pseudopapillary Neoplasms of the Pancreas: A Clue to the Diagnosis. American Journal of Surgical Pathology. 2011;35(7):981–988. doi: 10.1097/PAS.0b013e31821a9a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Priester WA. Data from eleven United States and Canadian colleges of veterinary medicine on pancreatic carcinoma in domestic animals. Cancer Research. 1974;34:1372–1375. [PubMed] [Google Scholar]
- 14.Sancho-Chust JN, Ferreres J, Pineda J, Molla MA, Giner F, Juan M, Blanquer J. Pulmonary tumor embolism as an initial manifestation of pancreatic adenocarcinoma. Respiratory Care. 2009;54(12):1732–1735. [PubMed] [Google Scholar]
- 15.Shaib W, Deng Y, Zilterman D, Lundberg B, Saif MW. Assessing risk and mortality of venous thromboembolism in pancreatic cancer patients. Anticancer Research. 2010;30(10):4261–4264. [PubMed] [Google Scholar]
- 16.Skutelsky E, Alroy J, Ucci AA, Carpenter JL, Moore FM. Modulation of carbohydrate residues in regenerative nodules and neoplasms of canine and feline pancreas. American Journal of Pathology. 1987;126(1):25–32. [PMC free article] [PubMed] [Google Scholar]
- 17.Sprogøe-Jakobsen U, Karkov JN. [Benign pancreatic g lucagonoma and recurrent pulmonary embolism with fatal course in a 36-year old man] Ugeskr Laeger. 1991;153(43):3015–3016. [PubMed] [Google Scholar]
- 18.Su N, Wang HL. The Pancreas. In: Humphrey PA, Dehner LP, Pfeifer JD, editors. The Washington Manual of Surgical Pathology. Philadelphia: Wolters Kluwer Health/Lippincott, Williams & Wilkins; 2008. pp. 232–241. [Google Scholar]
- 19.Weiss CA, Williams BH, Scott MV. Insulinoma in the ferret: clinical findings and treatment comparison of 66 cases. Journal of the American Animal Hospital Association. 1998;34(6):471–475. doi: 10.5326/15473317-34-6-471. [DOI] [PubMed] [Google Scholar]