Abstract
Objectives
To compare iron status in breastfed infants randomized to complementary feeding regimens that provided iron from fortified infant cereals or meats, and examined the development of the enteric microbiota among groups.
Study design
Forty-five exclusively breastfed 5 month old infants were randomized to commercially available pureed meats, iron- and zinc-fortified infant cereals, or iron-only fortified infant cereals as the first and primary complementary food through 9–10 months of age. Dietary iron was determined by monthly 3-d diet records. Iron status was assessed at end of the study by hemoglobin (Hb), serum ferritin (SF), and soluble transferrin receptor (STfR) measurements. In a subsample 14 infants, enteric microbiota were profiled in monthly stool samples (5–9 mo) by 16S rRNA gene pyrosequencing.
Results
Infants in cereal groups had 2–3 fold greater daily iron intakes vs the meat group (P < 0.0001). 27% of participants had low SF, and 36% were mildly anemic, without significant differences by feeding group; more infants in meat group had high STfR (p=0.03). Sequence analysis identified differences by time and feeding group in the abundances of several bacterial groups, including significantly more abundant butyrate producing Clostridium Group XIVa in the meat group (P=0.01)
Conclusions
A high percentage of healthy infants who were breastfed-only were iron deficient, and complementary feeding, including iron exposure, influenced the development of the enteric microbiota. If these findings are confirmed, reconsideration of strategies to both meet infants’ iron requirements and optimize the developing microbiome may be warranted.
Keywords: iron, complementary feeding, breastfeeding, phylogeny, microbiome
After approximately 6 months of age, term breastfed infants are increasingly dependent on other sources of iron to avoid iron deficiency, due to the depletion of the iron stores present at birth and to the low concentration of iron in human milk. Most commonly in the US, iron is provided to older infants by iron-fortified cereals. The absorption of the electrolytic iron used in these cereals is less than 5%.1 Although meats have been recognized as potentially good sources of more readily absorbable heme iron,1–4 with absorption up to 35%,5 only a small percentage of infants in the US consume meats during the first year of life.6, 7 Reliance on the poorly bioavailable electrolytic iron in commercial infant cereals, along with infants’ relatively high iron requirements, has led to an “Estimated Average Requirement” (EAR) of 6.9 mg of iron per day,8 and to a Recommended Dietary Allowance (RDA) of 11 mg per day to be applied to individuals’ estimated intake needs. Consumption of foods with a more favorable iron bioavailability might be sufficient to meet physiologic requirements at a lower dietary intake.
Beyond considerations about dietary strategies to meet iron requirements, studies in animals and older children have implicated iron exposure as a modulator of the enteric microbial profile, including promotion of pro-inflammatory organisms.9 With growing recognition of the importance of the enteric microbiome to immunity, these observations provide a link between infants’ diets and modulation of the developing immune system. After birth, the gastrointestinal tract undergoes a transformation from ostensible sterility to robust and adult-like colonization.10 Many factors influence the composition of the microbiome, including mode of delivery at birth, antibiotic use, and breast vs. formula feeding. Given the proclivity of many bacteria for iron, the poorly absorbed electrolytic iron in infant cereals could theoretically influence the enteric microbiome profile.
To date few studies have examined the potential for meats as a complementary food to meet iron requirements in breastfed infants, and to our knowledge, none have specifically considered the effect of complementary foods with different forms and amounts of iron on development of the gut microbiome. The objectives of this study were to compare iron status in infants who were breastfed only (no formula) and randomized to complementary feeding regimens which provided iron primarily from fortified infant cereals or from meats. In a subset of the infants, we also examined the bacterial profiles in the gastrointestinal tract.
METHODS
The iron status and microbiome outcomes were secondary outcomes in a randomized controlled intervention trial investigating zinc absorption from different complementary feeding regimens11. Briefly, healthy, term infants were randomly assigned to receive commercially available pureed meats, iron- and zinc-fortified infant cereals, or organic, whole grain iron-only fortified infant cereals. These foods served as the first complementary food and as a consistent component of the infants’ diets throughout the study from approximately 6 months through 9 months. Fruits, vegetables, teething biscuits, and unfortified cereals and other finger foods were allowed ad lib. The study foods were provided at monthly visits and monthly 3-day diet records were obtained. Between 9 and 10 months of age, serologic and hematologic biomarkers of iron status were obtained.
The last consecutive 14 infants recruited into the primary study were enlisted for the analysis of the enteric microbiome. Each mother-infant pair was randomized to a complementary feeding regimen according to overall study randomization procedures. All infants were from the same geographic location and were delivered vaginally; antibiotic use was monitored monthly and recorded. Fecal samples were obtained at monthly intervals from 5 through 9 months to characterize the longitudinal changes and the effects of different dietary patterns on the composition and diversity of the enteric microbiome.
Sample size per group for the iron status study was determined by power analysis for the zinc absorption studies previously reported 11, and the subjects for the microbiome analyses represented the final third of subjects enrolled. The entire study, including the microbiome component, was approved by the Colorado Multiple Institutional Review Board and written and informed consent was obtained from the parents of the infants.
Diets
Details of the nutritional composition of the three intervention foods are included in Table I, and have also been described elsewhere.11 The intervention foods (cereals or meats) were provided at monthly visits to encourage compliance. Mothers were encouraged to follow responsive feeding practices, and were provided monthly guidelines on approximate amounts of the assigned complementary foods to offer the infants. Recommendations included gradually increasing from 1 serving (15 g dry cereal or one 71 g jar of meat) per day by 7 months to 2 servings per day by 9 months.1 Monthly 3-day diet records were analyzed by a registered dietitian at the Clinical Translational Research Center (CTRC) Bio-nutrition Unit, using the Nutrient Data System for Research (NDSR, University of Minnesota, Minneapolis, MN, 55454) dietary analyses program. Iron intake was calculated only from complementary foods, and results do not include estimated intake from breast milk, which contributes a very small amount to daily iron intake. Duplicate diet records were collected at 9 months to determine total dietary iron intake for five days, in conjunction with 4 days of test-weighing to determine intake of human milk and metabolic collections for zinc stable isotope studies.11
Table I.
Nutritional content of study foods
| Cereal + Fe/Zn (dry) (IZFC) |
Cereal + Fe (dry) (IFC) |
Pureed Meat1 (M) |
|
|---|---|---|---|
| Serving size, g | 15 14 (1/4 c) |
14 (1/4 c) |
71 (1 jar) |
| Energy, Kcal2 | 60 | 60 | 70 |
| Protein, g2 | 1 | 1 | 8 |
| Fe, mg3 | 7.8 | 6.2 | 1.0 |
| Zn, mg3 | 1.2 | 0.3 | 2.1 |
| Phytate, mg3 | 23 | 107 | 0 |
| Phytate:Fe molar ratio | 0.25 | 1.5 | - |
Values for pureed beef and gravy;
from label;
from lab analyses.
Anthropometric measurements
Length and weight were measured at enrollment (6 months) and at each subsequent monthly (± 1 wk) visit (7, 8 and 9 months). All measurements were performed in duplicate by trained research personnel (DC). Length was assessed in the recumbent position using an infant stadiometer, which is accurate to 0.1cm (Holtain Ltd, Crosswell, Crymych, Pembs, UK). An electronic digital balance, which integrates 100 rapid serial measurements to provide a mean weight to the nearest gram (Sartorious Corp, Bohemia, NY) was used to obtain naked weights.
Sample Collections, Laboratory Analyses, and Microbiome Analyses
Iron content of 5-day duplicate diets was measured by atomic absorption spectrophotometry after digestion and quantitative reconstitution, as described previously for determination of zinc concentrations.11 Blood samples were drawn at approximately 0900 in the Pediatric CTRC. Serum ferritin, C-reactive protein and soluble transferrin receptor concentrations and hematologic indices were determined in the CTRC Core Lab by immunonephelomtry using the BNII Nephelometer (Siemens Healthcare Diagnostics Inc, Tarrytown, NY 10591) and manufacturer suggested protocols.
Monthly stool samples for microbiome analysis were obtained for infants in the iron-fortified cereals (n = 4), iron- and zinc-fortified cereals (n = 6), and meat (n = 4) groups. Baseline specimens were obtained at 5 months, before the initiation of complementary feeding. Approximately 1 g of fecal sample was collected by sterile swab from stool in trace mineral free cloth diapers (provided by the research team). The swabs were placed in test tubes with 3 mL of 70% ethanol and stored at −20°C. Mothers were given sterile gloves to wear when collecting the samples to minimize bacterial contamination.
DNA was extracted using the UltraClean fecal DNA kit (MoBio, Inc). Amplicons of the V1V3 variable region of the bacterial 16S rRNA gene were generated via broad-range PCR (30– 36 cycles) using the primers 27FYM+3 and 5’-barcoded 515R.12–14 We previously reported that amplification of the V1V3 region produced microbiome profiles that were highly correlated with full-length 16S rRNA sequences.15 However, because the forward primer, 27F, may be biased against amplification of bifidobacterial genes, we used a degenerate variant of this primer.12 PCR yields were normalized using a SequalPrep™ kit (Invitrogen, Carlsbad, CA), pooled, lyophilized, and gel purified, as previously described.16 Pooled amplicons were provided to the Center for Applied Genomics at the University of Toronto for pyrosequencing on a 454/Roche Life Sciences GS-FLX instrument using Titanium chemistry (Roche Life Sciences, Indianapolis, IN).
Pyrosequences were sorted into libraries by barcode and quality filtered using bartab.14 All pyrosequences were screened for nucleotide quality (bases at 5’ and 3’ ends with mean Q <20 over a 10 n.t. window were discarded), ambiguous bases (sequences with >1 N were discarded), and minimum length (sequences <200 n.t. were discarded). The mean trimmed sequence length was ~340 b.p. The Infernal RNA alignment tool17 was used to screen all sequences in terms of their fidelity to a Covariance Model (CM) derived from SSU rRNA secondary structure models.18, 19 Chimera screening was performed by the tool ChimeraSlayer, which required prior alignment with NAST-iEr.20 Putative chimeras and unalignable sequences were removed from subsequent analyses.
Genus-level and higher-level taxonomic calls were produced by the RDP Classifier, which performs naïve Bayesian taxonomic classification versus a training set.21 Pyrosequences were clustered in operational taxonomic units (OTUs) on the basis of taxonomic assignments. Ecological indices22 of richness (e.g., Sobs, Schao), diversity (e.g., Shannon’s diversity [Ho] and evenness [Ho/Hmax]), and coverage (e.g., Good’s index), were conducted with the software tool biodiv,23 through bootstrap resampling (1000 replicates) and rarefaction of the OTU distributions obtained from each specimen. The Good’s index of each sequence library, which measures sequence coverage, was >97.5%, indicating that most biodiversity was captured in each library (Figure 1 available at www.jpeds.com).
Data Management and Statistical Analyses
Results of iron status biomarkers were analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com). Iron deficiency was defined as a serum ferritin < 15 µg/ L 1, or soluble transferrin receptor > 1.8 µg/dL; iron deficiency anemia was defined as hemoglobin < 11.5 g/dL (adjusted for Denver altitude 2) in conjunction with low ferritin or high transferrin receptor. Group data sets were first assessed for normality. If data were normally distributed, 1 way ANOVA with Tukey Multiple Comparisons post-test were used to compare group means. Correlations were determined by Pearson correlation, and frequency of abnormal iron biomarkers by group were compared by chi square test. Data are presented as mean ± SEM unless otherwise noted.
The R-statistical package was used for statistical analyses of microbiome data (http://www.R-project.org, R Development Core Team, Vienna, Austria, 2011). Demographic characteristics were compared using ANOVA for continuous outcomes and Fisher exact tests for categorical outcomes. Outcome variables measuring the changes in OTU abundances through time (calculated as percent abundances at age 9 months minus those at age 5 months) were modeled by multivariable ANOVA with feeding group (FG), total dietary iron (TDI), and the interaction term FG*TDI evaluated as predictor variables.
RESULTS
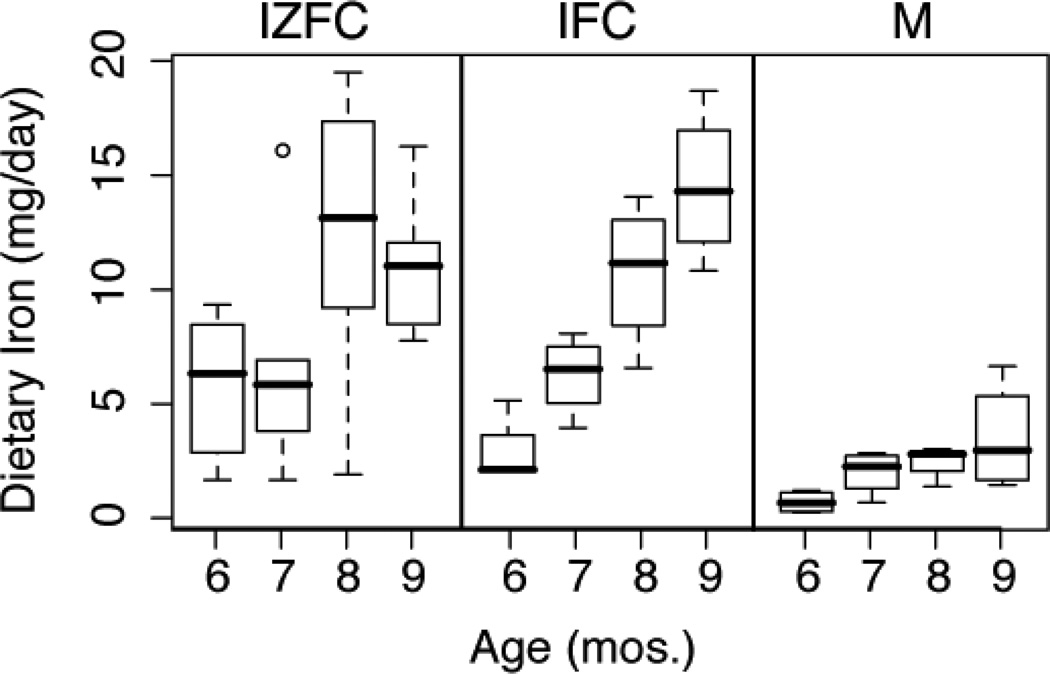
Monthly diet records indicated iron intakes reflective of the assigned feeding regimens. At every month, the mean iron intakes of the two cereal groups did not differ from each other and were significantly greater than that of the meat group (Figure 2). At 9 months, the average number of daily servings of the assigned intervention food was not different among the groups: 1.6, 1.3, and 2 for the iron- and zinc-fortified cereals, iron-fortified cereals, and meat, respectively. Likewise, neither mean total caloric intake nor intake of human milk differed significantly among groups. Protein intake was significantly higher in the meat group compared with that of the combined cereal groups: 2.9 ± 0.6 vs 1.6 ± 0.5 g/kg/d, respectively (P < 0.001).
Figure 2.
Longitudinal iron intakes (mg/day) by group. TDI for cereal groups significantly higher than meat group at each time point. P= 0.01, 0.002, 0.0003, and 0.0001 at 6, 7, 8, and 9 months, respectively.
Mean dietary iron intakes (mg/d) at 9 months, determined from duplicate diets were 11.8 ± 1.3, 7.5 ± 1.3, and 3.3 ± 0.4 for the iron- and zinc-fortified cereals, iron-fortified cereals, and meat groups, respectively. Intakes relative to body weight (mg/kg/day) were 1.0 ± 0.12, 1.5 ± 0.61, and 0.39 ± 0.16, for the iron- and zinc-fortified cereals, iron-fortified cereals, and meat groups, respectively. Iron intake was significantly higher for the two cereal groups compared with the meat group (P = 0.0001). No significant differences in linear growth or weight gain were observed among groups over the course of the study (data not shown).
Biomarkers of iron status were successfully obtained from 41 infants; mean results are presented in Table II and Figure 3 (Figure 3 available at www.jpeds.com). None of the means for biomarkers differed by feeding group, or by sex. Twenty-seven percent of all infants had low ferritin (< 15 ug/L), and 36% of all infants were mildly anemic (Hb < 11.5 g/dL), with no difference by group. Overall, 15 infants (37%) had elevated sTfR, including twenty two percent of infants in cereal groups, and 64% of infants in the meat group (P = 0.03). Dietary iron intake was not correlated with serum ferritin, either within or among dietary groups (r = −0.13, −0.28, and 0.16 for iron- and zinc-fortified cereals, iron-fortified cereals, and meat groups, respectively; P >0.3 for all). Infants with ferritin < 15 ug/L had significantly greater daily weight gain over the course of the study (P = 0.03).
Table II.
Summary of biomarker data1 by feeding group
| Biomarker | IZFC | IFC | M |
|---|---|---|---|
| Serum Ferritin (µg/L) | 41.2 ± 7.1 (14) | 37.2 ± 10.0 (13) | 27.5 ± 5.8 (14) |
| Without high CRP | 43.0 ± 7.6 (12) | 38.6 ± 10.3 (12) | 23.6 ± 4.7 (12) |
| sTransferrin Receptor (µg/dL) | 1.7 ± 0.1 (14) | 1.7 ± 0.1 (13) | 2.0 ± 0.1 (14) |
| Hemoglobin (g/dL) | 11.8 ± 0.2 (14) | 12.1 ± 0.2 (13) | 12.4 ± 0.3 (12) |
Mean ± SEM (n)
The 14 infants who participated in the microbiome component of this study were breastfed-only (no formula), and all had high adherence to the assigned feeding pattern, based on diet records, calculated dietary iron intake, and consumption of study foods provided.
The results of pyrosequencing 16S rRNA genes indicated a median of 2036 (IQR 1597–2486) high-quality pyrosequences per sample. Species-level sequence coverage values were uniformly high (median of 99.1%, IQR 98.6–99.4), indicating that the depth of pyrosequencing was sufficient to represent the biodiversity in the specimens.
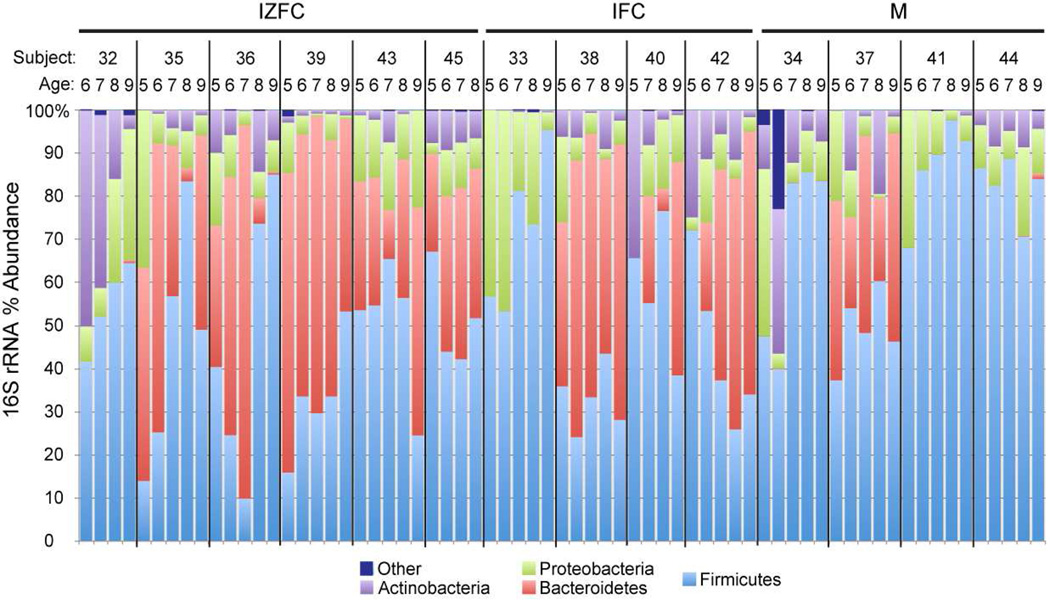
In agreement with other analyses of the infant fecal microbiome,10 members of the bacterial phyla Firmicutes (low G+C Gram positives) and Bacteroidetes were the most abundant microorganisms in the infant stool samples (Figure 4). However, the relative proportions of these phyla differed both within individuals over time and among individuals. For example, few Bacteroidetes species were observed in some participants: one in each of the cereal groups, and 3 in the meat group. In these individuals, members of the phyla Actinobacteria (high G+C Gram positives), Proteobacteria, or Firmicutes compensated for the relatively low abundance of Bacteroidetes.
Figure 4.
Longitudinal variation in abundances of bacterial phyla in infant fecal specimens.
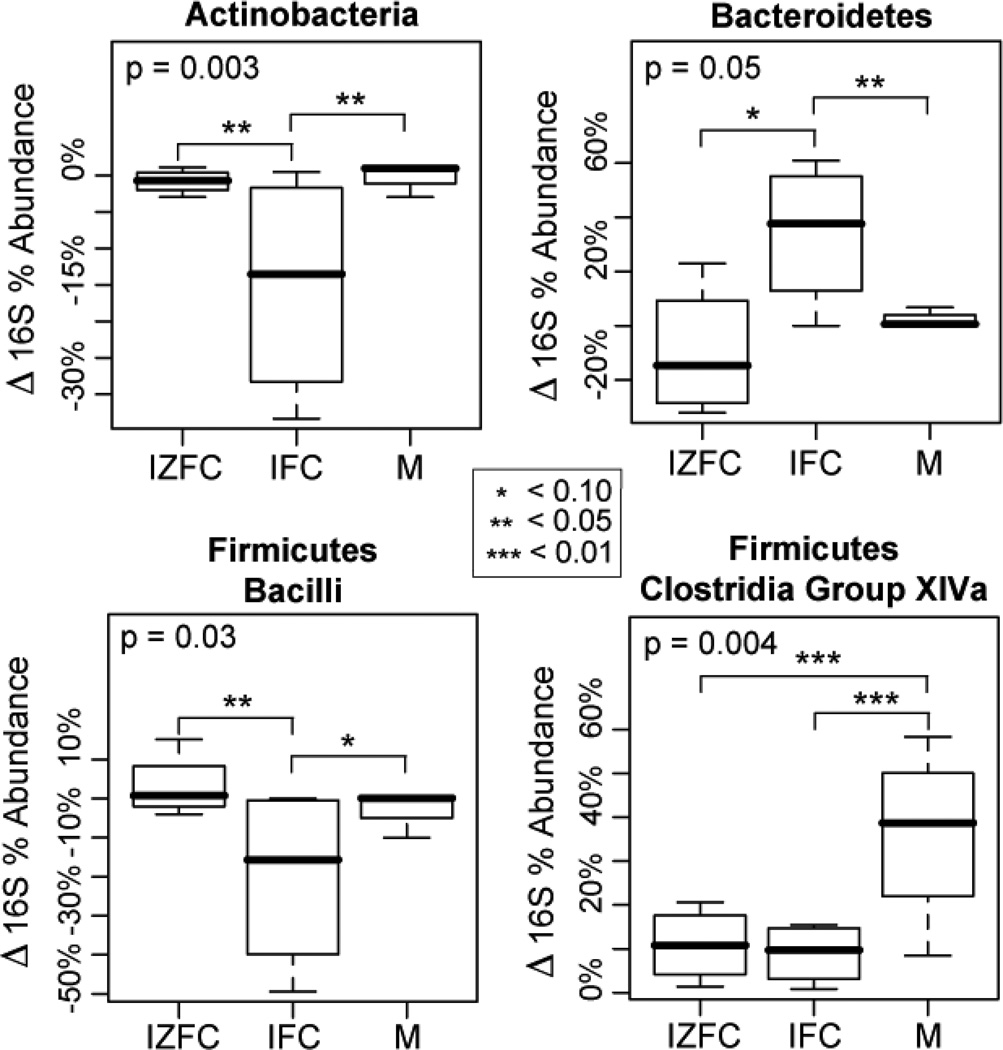
The longitudinal development of the infants’ enteric microbiomes differed significantly among complementary feeding groups. To control for differences in baseline microbiomes, we compared the change in percent abundances of microbial taxa from age 5 to 9 months in each feeding group, adjusting for dietary iron (Figure 5). Both feeding group and dietary iron were significantly associated with age dependent changes in several bacterial groups (Table III and Figures 5-8; Figures 6–8 available at www.jpeds.com). Most of the statistically significant differences that were observed contrasted iron-fortified cereals from iron- and zinc-fortified cereals and meat groups. For instance the phylum Actinobacteria (P = 0.003), specifically the genera Bifidobacterium (P = 0.004) and Rothia (P = 0.0007), decreased in abundance with time in the iron-fortified cereals group, but were relatively unchanged in iron- and zinc-fortified cereals and meat groups. Similarly, the relative abundance of the order Lactobacillales (phylum Firmicutes) also declined in iron-fortified cereals, compared with iron- and zinc-fortified cereals and meat (P = 0.04). In contrast, members of the order Bacteroidales were significantly more abundant in the iron-fortified cereals group compared with iron- and zinc-fortified cereals and meat (P = 0.02). Dietary iron also was a significant covariate in many of these analyses (Table III).
Figure 5.
Age-dependent changes in relative abundances of select phyla/sub-phyla between treatment groups. X-axis: three feeding groups. Y-axis: differences in percent abundances of bacterial groups from 5 to 9 months of age (%9mo - %5mo). Statistical significance was tested by ANOVA and adjusted for the potentially confounding variable for total dietary iron (TDI). Overall P-values are indicated in upper left corners of each boxplot, and P-values less than 0.1 are noted by asterisks. The analysis of all phylum/sub-phylum categories is presented in Figure 6, online.
Table III.
Effects of feeding group and dietary iron on gastrointestinal bacteria
| Taxonomic Level | Feeding Group1 | P-value2 | ||||
|---|---|---|---|---|---|---|
| IZFC | IFC | M | FG | TDI | FG*TDI | |
| Actinobacteria | ↔ | ↓ | ↔ | 0.003 | 0.004 | 0.009 |
| Actinobacteriales | ↔ | ↔ | ↔ | 0.025 | 0.24 | 0.089 |
| Rothia | ↔ | ↔ | ↔ | <0.001 | 0.010 | 0.002 |
| Bifidobacteriales | ↔ | ↓ | ↔ | 0.003 | 0.005 | 0.010 |
| Bifidobacterium | ↔ | ↓ | ↔ | 0.004 | 0.005 | 0.010 |
| Bacteroidetes | ↓ | ↑↑ | ↔ | 0.047 | 0.34 | 0.26 |
| Bacteroidales | ↓ | ↑ | ↔ | 0.022 | 0.55 | 0.13 |
| Firmicutes | ↑↑ | ↓ | ↑ | 0.30 | 0.24 | 0.29 |
| Bacilli | ↔ | ↓ | ↔ | 0.035 | 0.092 | 0.44 |
| Bacillales | ↔ | ↔ | ↔ | 0.35 | 0.17 | 0.19 |
| Lactobacillales | ↔ | ↓ | ↔ | 0.035 | 0.096 | 0.045 |
| Clostridia | ↑↑ | ↔ | ↑↑ | 0.44 | 0.72 | 0.13 |
| Clos. Group IV | ↔ | ↔ | ↔ | 0.19 | 0.15 | 0.049 |
| Clos. Group XIVa | ↑ | ↑ | ↑↑ | 0.005 | 0.21 | 0.013 |
| Clos. Others | ↑ | ↓ | ↔ | 0.41 | 0.49 | 0.75 |
| Proteobacteria | ↔ | ↔ | ↓ | 0.54 | 0.016 | 0.057 |
| Enterobacteriaceae | ↔ | ↓ | ↓ | 0.45 | 0.029 | 0.11 |
Δ% abundance of 16S pyrosequences from age 5 to 9 mo stratified by FG; ↑↑↑: >50%; ↑↑: >25%; ↑: >10%; ↔ -10%-10%; ↓: >-10%; ↓↓: >-20%; ↓↓↓: >-50%
Results of ANOVA tests of Δ16S abundance from age 5 to 9 mo. P-values are from MANOVA of FG, TDI, and the interaction term FG*TDI.
Among the phylum Firmicutes, the median abundance of the Clostridium Group XIVa clade increased by 40% in the meat feeding group, but only 10% in the iron-fortified cereals (P = 0.007) and iron- and zinc-fortified cereals (P = 0.01) groups (Figures 5 and >7). This cluster of clostridial species includes a variety of butyrate producing24 and immunomodulatory25 species. The other predominant clostridial clade, Clostridium Group IV, as well as other clostridia not belonging to groups XIVa or IV, did not show comparable associations with feeding group (Figure 7), indicating that the complementary foods had specific effects on select groups of clostridia.
Neither the phylum Proteobacteria nor any sub-groups of this phylum, including the genera Escherichia, Klebsiella, Haemophilus, and Shigella, were significantly associated with feeding group. However, the relative abundance of the family Enterobacteriaceae, which includes the enteric pathogen Escherichia coli, was significantly correlated with dietary iron (P = 0.03; Table III). This study was not sufficiently powered to detect changes in a group of relatively low abundance (e.g. median relative abundance of E. coli was1% for all samples).
The data suggested a trend toward greater biodiversity (i.e., species- and genus-richness) with age (Figure 1 and data not shown). The meat feeding group increased from a median of 62.1 species (IQR 46.7, 86.5) to 108.8 species (IQR 83.6, 131.4) at 5 and 9 mo, respectively. The iron- and zinc-fortified cereals group increased in species from 82.6 (IQR 52.8, 104.4) to 102.6 (IQR 94.9, 110.3), and iron-fortified cereals increased from 74.5 (IQR 57.5, 78.0) to 83.3 (IQR 59.4, 92.2) species at 5 and 9 mo, respectively.
DISCUSSION
Systematic comparisons of the effects of different complementary feeding patterns on nutritional outcomes in breastfed infants are very limited. Several important findings emerge. First, iron deficiency and iron deficiency anemia were surprisingly common in this group of healthy breastfed infants, regardless of feeding group. Second, the lack of correlation between dietary iron intake and biomarkers of iron status emphasizes the importance of other factors, such as bioavailability, growth rates, and iron endowment at birth. Third, different complementary feeding regimens in conjunction with breastfeeding are associated with significant differences in the development of the enteric microbiota, which may have implications for the developing immune system.
National data on rates of iron deficiency in infants under 12 months of age are not available for the U.S., but the rate of approximately one third with low serum ferritin, mild anemia, and elevated STfR observed in the present study is similar to previous observations in Denver breastfed infants.26 The transferrin receptor results indicated iron insufficiency was more common in the meat group, and a larger study may have detected other significant differences among the groups’ iron status. Nevertheless, the frequency of marginal iron status in all groups, despite the 2 to 3-fold higher dietary iron intake of the cereal groups compared with those of the meat group, highlights the differences in bioavailability from non-heme vs heme sources.
Current dietary data specifically for older breastfed infants in the US are limited, but results from the Infant Feeding Practices Study II indicated that the majority of 6 month old solely breastfed infants received less than recommended amounts of fortified infant cereals or meats.7 The mean intakes of iron for both of the cereal groups in the present study exceeded the EAR for infants between 6 and 12 months of age, but this may not be the case for those on “self-selected” diets for whom use of iron fortified infant cereal has reportedly declined substantially in recent years.6
Dietary factors are well known to affect the composition, diversity, and function of the gastrointestinal microbiome of infants10 and adults.27 Most studies of the dietary determinants of microbial community structure have focused on macronutrient balance.28–32 For example, in one study, consumption of animal protein and saturated fat was associated with Bacteroides enterotype,30 a finding not replicated in the meat group in our study. Human milk oligosaccharides represent a beneficial component in young breastfed infants, and foster growth of Bifidobacterium sp,33 but the impact of these carbohydrates in the context of breastfeeding with diverse complementary foods is less clear. The iron-fortified cereals group had a significantly lower abundance of bifidobactera, which may have been due to the higher phytate and fiber content of the cereal or other uncontrolled dietary factors; to the absence of micronutrient fortification beyond iron; to differences in the mothers’ milk oligosaccharide composition; or simply to chance. The results of this pilot trial indicate that complementary foods that differ substantially in micronutrient composition, including especially for iron and zinc,11 also influence development of the infant enteric microbiota.
As reported in other studies,10 we observed significant inter-infant variability in enteric microbiomes even at baseline before addition of complementary foods. Although all of the infants had been exclusively breastfed prior to the onset of the study, differences in breastmilk composition and/or other environmental or genetic factors are likely to influence the early development of the infant microbiome. Phylum-level changes were comparable with the data of Palmer et al,10 as exemplified by decreases in the relative abundances of Proteobacteria and Actinobacteria and the concomitant increase in Firmicutes.
We found differences in the phylum and sub-phylum abundances according to high (cereal) vs low (meat) iron intakes, and presumably to amounts of unabsorbed iron. Data from a small subset of the participating subjects (2/group) who ingested a non-absorbable fecal marker34 supported an approximately 10-fold difference in iron excretion between the cereal and meat groups (Krebs, unpublished data). Both feeding group and iron intake were significantly associated with longitudinal changes in the enteric microbiome (Table III). The Clostridium Group XIVa of the phylum Firmicutes, which rose significantly in abundance between ages 5 and 9 months in the meat arm relative to the two cereal arms, is notable for comprising a number of beneficial species capable of producing butyrate, a short chain fatty acid that is metabolized by enterocytes.24, 35 Because a reduced abundance of these microorganisms has been associated with inflammatory bowel disease in humans36, 37 and mice,25 these differential effects of the complementary foods could potentially affect the infants’ immunological development. These findings justify larger scale, randomized controlled trials to evaluate diet-induced effects on the microbiome and its modulation of infant health, metabolism and immune development.38
We found no evidence that iron fortification resulted in a greater abundance or diversity of potentially pathogenic microorganisms (e.g. Eschericia, Pseudomonas) among our study participants. In contrast to the report from Zimmerman et al for African children consuming iron fortified biscuits,9 we did not observe significant increases in enterobacterial abundance over time in any of the feeding groups. In fact, median abundances of Enterobacteriaceae decreased non-significantly by 5.5%, 10.3%, and 23.1% in iron- and zinc-fortified cereals, iron-fortified cereals, and meat groups, respectively from 5 to 9 months. Furthermore, we noted a significant decrease in Lactobacillales members only in iron-fortified cereals, whereas this bacterial group was unchanged over time in iron- and zinc-fortified cereals and meat groups. These two studies differed substantially, however, in the age of study populations (infants vs. 6–14 year old children), mode of feeding (human milk and complementary foods vs. iron fortified biscuits), and analytic methods (pyrosequencing vs. denaturing gradient gel electrophoresis). Determination of effects, both positive and potentially adverse, of specific micronutrient interventions on the progressive development of the microbiome of older infants will require targeted interventions, more extensive functional markers, and longer follow-up to characterize disease risk.
Limitations of the current study include reduced statistical power due to a relatively small sample size. Because of the pilot scale of this project, we did not adjust P-values for multiple comparisons, and therefore the results may include some false-positives. Furthermore, infants were allowed ad lib feeding for foods other than those assigned (eg, fruits and vegetables), which may have increased inter- and intra-subject variability in intestinal microbiomes, apart from treatment group assignment. Besides iron, differences in the assigned diets also included other micronutrient and macronutrient intakes, especially between the meat verses the two cereal groups. For example, zinc intake was similar between the iron- and zinc-fortified cereals and meat groups,11 and zinc has been associated with reduced virulence of enteropathogens.39 The small sample size also precluded examination of differences in the microbiome profiles according to iron status, which has recently been s uggested to alter the gut microbiota in an animal model.40
In summary, this study illustrates the striking difference in iron intake for older breastfed infants that results from complementary feeding patterns which emphasize iron fortified cereals vs meats. Independent of feeding group, approximately one third of these healthy breastfed infants had evidence of iron deficiency. The results of this pilot examination support the conclusion that dietary components, potentially including specific micronutrients, influence the character of the developing microbiome. Whether benefits or detriments are associated with a given pattern will require larger, controlled studies.
We conclude by noting that the results of this study have implications for recommendation for universal screening for anemia at 12 months, and the assessment of risk for iron deficiency by diet history.41 Many breastfed infants are at risk for iron deficiency well before 12 months of age, but screening by diet history seems to have low sensitivity for identifying risk. Measurement of a biomarker such as serum ferritin (concurrently with a marker of inflammation) would be more informative to identify iron deficiency before progression to anemia. In view of this complexity, broad-scale iron supplementation has been recommended for breastfed infants.41 The effectiveness and safety of this approach have not been tested, however, and potentially untoward effects have been associated with iron exposure for iron replete infants and young children, even in developed countries.42 Unfavorable impact on the enteric microbiome and on systemic and intestinal inflammation are other potential side-effects of untargeted iron fortification.9 We conclude that if an interaction between iron bioavailability from complementary foods and host microbiota is confirmed, reconsideration of strategies to meet iron requirements that also minimize adverse effects on the enteric microbiota will be warranted.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr SA Santorico (University of Colorado Denver) for statistical advice, as well as the parents and infants who participated in the study.
Supported by the National Institutes of Health (NIH; T32DK007658-20S, K24DK083772, and HG005964), NIH/National Center for Research Resources (UL1 RR025780 to Colorado County Technical Services, Inc), and National Cattlemen’s Beef Association (Beef Checkoff).
ABBREVIATIONS
- CTRC
Clinical Translational Research Center
- CF
complementary food
- EAR
Estimated Average Requirement
- FG
feeding group
- Hb
hemoglobin
- IQR
interquartile range
- RDA
Recommended Dietary Allowance
- SF
serum ferritin
- STfR
soluble transferrin receptor
- TDI
total dietary iron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of the study have been presented as abstracts as the Pediatric Academic Societies’ Meeting 2011 and the Experimental Biology Meeting 2011.
No Reprints are available from the author.
REFERENCES
- 1.American Academy of Pediatrics. Pediatric Nutrition Handbook. 6th ed. Elk Grove, IL: American Academy of Pediatrics; 2009. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Recommendations for preventing and controlling iron deficiency in the United States. MMWR Morb Mortal Wkly Rep. 1998;47(RR 3):1–36. [PubMed] [Google Scholar]
- 3.PAHO/WHO. Guiding Principles for Complementary Feeding of the Breastfed Child. Washington, DC: PAHO, WHO; 2003. [Google Scholar]
- 4.Hambidge KM, Sheng X, Mazariegos M, Jiang T, Garces A, Li D, et al. Evaluation of meat as a first complementary food for breastfed infants: impact on iron intake. Nutr Rev. 2011;69(Suppl 1):S57–S63. doi: 10.1111/j.1753-4887.2011.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461S–1467S. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- 6.Siega-Riz AM, Deming DM, Reidy KC, Fox MK, Condon E, Briefel RR. Food consumption patterns of infants and toddlers: where are we now? J Am Diet Assoc. 2010;110:S38–S51. doi: 10.1016/j.jada.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Dee DL, Sharma AJ, Cogswell ME, Grummer-Strawn LM, Fein SB, Scanlon KS. Sources of supplemental iron among breastfed infants during the first year of life. Pediatrics. 2008;122(Suppl 2):S98–S104. doi: 10.1542/peds.2008-1315m. [DOI] [PubMed] [Google Scholar]
- 8.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 9.Zimmermann MB, Chassard C, Rohner F, N'Goran EK, Nindjin C, Dostal A, et al. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d'Ivoire. Am J Clin Nutr. 2010;92:1406–1415. doi: 10.3945/ajcn.110.004564. [DOI] [PubMed] [Google Scholar]
- 10.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krebs NF, Westcott JE, Culbertson DL, Sian L, Miller LV, Hambidge KM. Comparison of complementary feeding strategies to meet zinc requirements of older breastfed infants. Am J Clin Nutr. 2012;96:30–35. doi: 10.3945/ajcn.112.036046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol. 2008;74:2461–2470. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. New York: Wiley; 1991. pp. 115–175. [Google Scholar]
- 14.Frank DN. BARCRAWL and BARTAB: Software tools for the design and implementation of barcoded primers for highly multiplexed DNA sequencing. BMC Bioinformatics. 2009;10:362. doi: 10.1186/1471-2105-10-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li E, Hamm CM, Gulati AS, Sartor RB, Chen H, Wu X, et al. Inflammatory bowel diseases phenotype, C. difficile and NOD2 genotype are associated with shifts in human ileum associated microbial composition. PLoS One. 2012;7:e26284. doi: 10.1371/journal.pone.0026284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank DN, Feazel LM, Bessesen MT, Price CS, Janoff EN, Pace NR. The human nasal microbiota and Staphylococcus aureus carriage. PLoS One. 2010;5:e10598. doi: 10.1371/journal.pone.0010598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nawrocki EP, Kolbe DL, Eddy SR. Infernal 1.0: inference of RNA alignments. Bioinformatics. 2009;25:1335–1337. doi: 10.1093/bioinformatics/btp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannone JJ, Subramanian S, Schnare MN, Collett JR, D'Souza LM, Du Y, et al. The comparative RNA web (CRW) site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics. 2002;3:2. doi: 10.1186/1471-2105-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutell RR, Lee JC, Cannone JJ. The accuracy of ribosomal RNA comparative structure models. Curr Opin Struct Biol. 2002;12:301–310. doi: 10.1016/s0959-440x(02)00339-1. [DOI] [PubMed] [Google Scholar]
- 20.Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magurran AE. Measuring Biological Diversity. Malden, USA: Blackwell Publishing; 2003. [Google Scholar]
- 23.Frank DN. XplorSeq: a software environment for integrated management and phylogenetic analysis of metagenomic sequence data. BMC Bioinformatics. 2008;9:420. doi: 10.1186/1471-2105-9-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66:1654–1661. doi: 10.1128/aem.66.4.1654-1661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krebs NF, Westcott JE, Butler N, Robinson C, Bell M, Hambidge KM. Meat as a first complementary food for breastfed infants: feasibility and impact on zinc intake and status. J Pediatr Gastroenterol Nutr. 2006;42:207–214. doi: 10.1097/01.mpg.0000189346.25172.fd. [DOI] [PubMed] [Google Scholar]
- 27.Kelly CJ, Colgan SP, Frank DN. Of microbes and meals: the health consequences of dietary endotoxemia. Nutr Clin Pract. 2012;27:215–225. doi: 10.1177/0884533611434934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metzler-Zebeli BU, Hooda S, Pieper R, Zijlstra RT, van Kessel AG, Mosenthin R, et al. Nonstarch polysaccharides modulate bacterial microbiota, pathways for butyrate production, and abundance of pathogenic Escherichia coli in the pig gastrointestinal tract. Appl Environ Microbiol. 2010;76:3692–3701. doi: 10.1128/AEM.00257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. Isme J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. e1–e2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson CL, Versalovic J. The human microbiome and its potential importance to pediatrics. Pediatrics. 2012;129:950–960. doi: 10.1542/peds.2011-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fairweather-Tait SJ, Minihane AM, Eagles J, Owen L, Crews HM. Rare earth elements as nonabsorbable fecal markers in studies of iron absorption. Am J Clin Nutr. 1997;65:970–976. doi: 10.1093/ajcn/65.4.970. [DOI] [PubMed] [Google Scholar]
- 35.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 36.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokol H, Lay C, Seksik P, Tannock GW. Analysis of bacterial bowel communities of IBD patients: What has it revealed? Inflamm Bowel Dis. 2008;14:858–867. doi: 10.1002/ibd.20392. [DOI] [PubMed] [Google Scholar]
- 38.Frank DN, Zhu W, Sartor RB, Li E. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol. 2011;19:427–434. doi: 10.1016/j.tim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crane JK, Byrd IW, Boedeker EC. Virulence inhibition by zinc in shiga-toxigenic Escherichia coli. Infect Immun. 2011;79:1696–1705. doi: 10.1128/IAI.01099-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dostal A, Chassard C, Hilty FM, Zimmermann MB, Jaeggi T, Rossi S, et al. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J Nutr. 2012;142:271–277. doi: 10.3945/jn.111.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age) Pediatrics. 2010;126:1040–1050. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- 42.Dewey KG, Domellof M, Cohen RJ, Landa Rivera L, Hernell O, Lonnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr. 2002;132:3249–3255. doi: 10.1093/jn/132.11.3249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.