Abstract
Objective
To report the retention rate of the Boston Keratoprosthesis Type 1 and to identify risk factors for keratoprosthesis loss.
Design
Cohort study.
Participants
300 eyes of 300 patients who underwent implantation of a Boston Keratoprosthesis Type I device between January 2003 and July 2008 by one of 19 surgeons at 18 medical centers.
Methods
Forms reporting preoperative, intraoperative, and postoperative parameters were prospectively collected and subsequently analyzed at a central data collection site.
Main Outcome Measures
Keratoprosthesis retention.
Results
A total cumulative number of 422 life years of device implantation are included in this analysis. The average duration of follow up was 17.1 ± 14.8 months with a range of one week to over 6.1 years. 93% of the 300 Boston Keratoprosthesis implanted were retained at their last follow up, corresponding to a retention time of 396 patient-years or 1.42 years / keratoprosthesis. The probability of retention after one year and two years was 94% and 89%, respectively. During the study period, 21 (7%) keratoprosthesis implants failed to retain the device; the reasons for keratoprosthesis loss include sterile keratolysis (9), fungal infections (8), dense retroprosthetic membranes (3), and bacterial endophthalmitis (1). Multivariate analysis demonstrated three independent risk factors for keratoprosthesis loss: autoimmune etiology (hazard ratio [HR] = 11.94; 95% confidence interval [CI] 3.31, 43.11), ocular surface exposure requiring a concomitant tarsorrhaphy (HR = 3.43; 95% CI 1.05, 11.22) and number of prior failed penetrating keratoplasties (HR = 1.64; 95% CI 1.18, 2.28).
Conclusions
The Boston Type 1 Keratoprosthesis appears to be a viable option for eyes that are not candidates for penetrating keratoplasty. Ocular surface disease due to an autoimmune etiology demonstrated the lowest retention rate.
Keratoprosthesis has emerged as a viable option when a traditional penetrating keratoplasty (PK) has a poor chance of success. Keratoprostheses have historically suffered from poor retention rates.1 In the 1850’s Nussbaum and Hussen both implanted an early keratoprosthesis design in human eyes, but none remained in place for more than 6 months.2,3 Over the next 25 years, other surgeons employed the use of keratoprostheses, but nearly all of these were extruded.1 Interest in artificial corneas subsequently decreased with the successful adoption of penetrating keratoplasty. The introduction of antibiotics and the discovery of inert plastics in the latter half of the 20th century prompted the development of various keratoprostheses with the goal of improving anatomical retention rates. Amongst these was the Boston Keratoprosthesis, which emerged as one of the most popular designs.4
The Boston Keratoprosthesis was initially developed at the Massachusetts Eye and Ear Infirmary and underwent a series of modifications to improve retention.5 By the year 2003, its use expanded to multiple surgeons and medical centers such that the Boston Keratoprosthesis Multicenter Study was initiated.6 Zerbe et al reported a 95% retention rate from 141 procedures, the largest sample of keratoprosthesis patients reported at the time.6 However, the study was limited by a relatively short follow-up time of 8.5 months (range: 0.03 – 24 months). Data collection continued for an additional 4 years after the initial publication and included preoperative, intra-operative, and post- operative data. The purpose of this paper is to update keratoprosthesis retention over a longer follow-up period and to identify risk factors for keratoprosthesis extrusion.
Methods
The Boston Keratoprosthesis is obtained from the Massachusetts Eye and Ear Infirmary. The technique for implanting the Boston Keratoprosthesis has been previously described and all surgeons reported using a similar technique.6
Data Collection
The Boston Keratoprosthesis Multicenter Study is a large prospective cohort study gathering data on Boston Keratoprosthesis Type I implanted since January 1, 2003. At the time the study was initiated, all surgeons known to be performing multiple procedures were contacted and encouraged to participate. Surgeons reported data using a mail-in report form evaluating approximately 70 perioperative variables. Submissions were voluntary, although all participants were encouraged to submit as complete data as was available, regardless of outcome. In compliance with Health Insurance Portability and Accountability Act of 1996 regulations, patients were assigned a unique study number. Forms were sent to a central collection site, under Institutional Review Board approval (Department of Ophthalmology, Albany Medical Center, Albany, NY). Institutional Review Board approval was also obtained by participating institutions. In general, follow-up is reported at one week, one month, six months, twelve months, and yearly thereafter by participating surgeons.
Some patients developed keratoprosthesis failure during the study period and then received a replacement keratoprosthesis; only data from the first implant was included in this study. Additionally, some patients underwent bilateral keratoprosthesis implantation; the first eye of these patients was included in the study.
Analysis
Based on previously published prognostic categories,7 the patients were categorized into the following groups: patients with severe autoimmune disease (ocular cicatricial pemphigoid [OCP] and Stevens Johnson Syndrome [SJS]), chemical injuries, herpes simplex (HSV) keratitis, Fuchs endothelial dystrophy, keratoconus, infectious keratitis, neurotrophic ulcers, limbal stem cell deficiency, pseudophakic bullous keratopathy (PBK), trauma, aniridia, miscellaneous, failed graft and unknown. Similar to previous keratoprosthesis outcomes reports, device survival is defined as anatomical retention at the last follow up date. 6, 8–10 Conversely, keratoprosthesis failure is defined as a lack of anatomical retention, which includes removal, extrusion, or loss of the eye. In some cases the keratoprosthesis was replaced with a new device and in others it was removed and replaced with a standard penetrating keratoplasty that did not contain a keratoprosthesis.
A Microsoft Excel spreadsheet was used to compile the data and SAS (version 9.2, SAS Institute Inc., Cary NC, USA) was used for all analyses. Because some surgeons sent data labeled as information for a particular follow-up time point, a follow-up date was imputed for these patients. Categorical variables were compared using the Fisher’s exact test or chi-square test when time was not a factor in the analysis. Similarly, continuous variables were analyzed using t-tests when time was not a factor. Retention (survival) was plotted using Kaplan-Meier curves; categorical variables were compared using the log-rank test. Multivariate analysis also utilized a Cox proportional hazards model to evaluate significant risk factors for failure. Covariates that demonstrated a significance level of p<0.05 on univariate analyses were included. Additionally, the number of prior failed PK were included because of prior work suggesting that the degree and chronicity of past inflammation may have prognostic significance.7 Similarly, because Aldave et al suggested that a primary keratoprosthesis is predictive of retention, this variable was also included a priori.9
Results
Between January 2003 and July 2008, information on 321 Boston Keratoprosthesis (Type I) implanted in 303 patients by 19 surgeons at 18 medical centers was received. Thirteen eyes were excluded because they represented re-implantation of a keratoprosthesis; only data from the first implant was included. Eight eyes of 8 patients that underwent sequential bilateral keratoprosthesis implantation were also excluded. The final analysis included 300 eyes of 300 patients.
The mean age at the time of implantation was 62.6 ± 18.9 years (range 10.5 – 96.7 years) and 48.1% were female; there was no difference between eyes that retained their prostheses versus those that failed in terms of age (p=0.278) or gender (p=1.000; Table 1). The procedure was performed in the right eye for 53.6% of the cases. Overall, 7.0% (n=21) keratoprosthesis failed to retain the device (Table 2, available at http://aaojournal.org). The average duration of follow up was 17.1 ± 14.8 months with a range of one week to over 6.1 years; 161 eyes (53.7%) had at least 1 year of follow-up and 91 eyes (30.3%) had at least 2 years of follow up. This analysis included 422.1 cumulative life years of device implantation.
Table 1.
Comparison of baseline demographics in patients with failed versus retained keratoprosthesis.
| Variable | Failure (n=21) | Retention (n=279) | Data Completeness | p-value |
|---|---|---|---|---|
| Age (years) | 58.4 ± 14.7 | 63.3 ± 19.4 | 50.0% | 0.278* |
| Female | 47.6% | 48.2% | 70.7% | 1.000† |
| Right Eye | 47.6% | 54.0% | 98.0% | 0.652† |
| Follow-up Time (months) | 14.7 ± 12.2 | 17.3 ± 15.0 | 93.0% | 0.441* |
two-tailed t-test
Fisher’s exact test.
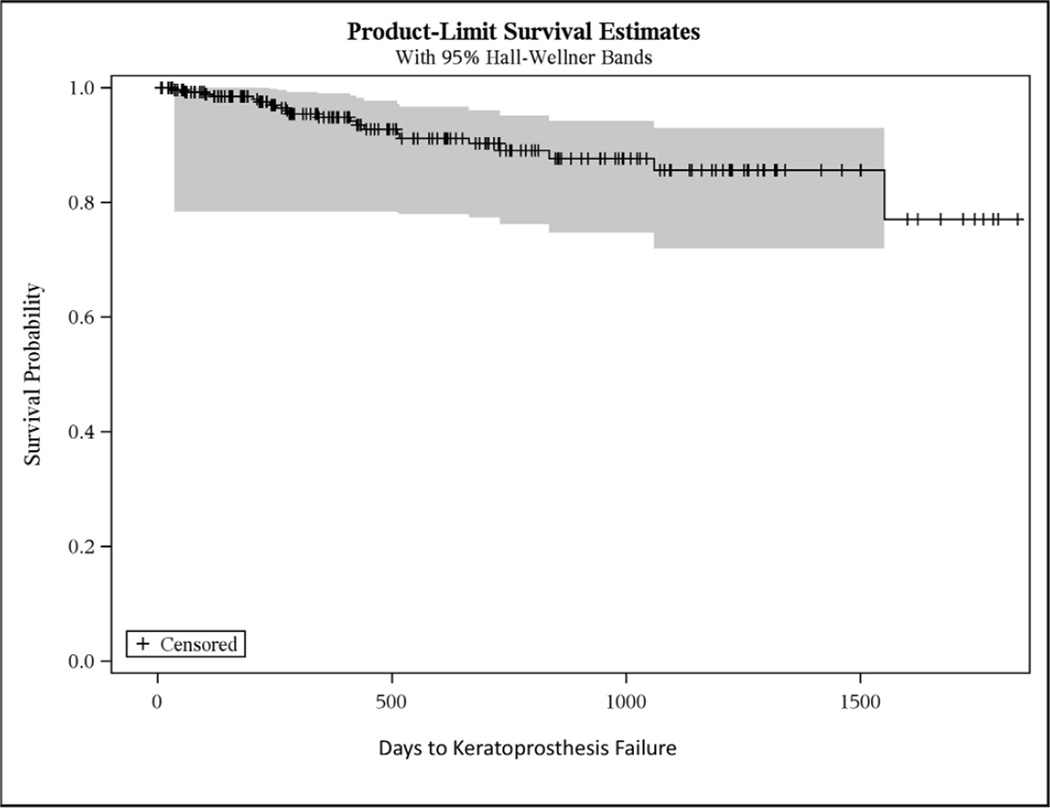
With 21 failures, the overall retention rate was 93% (Figure 1). The probability of retention after one year and two years of follow-up was 94% and 89%, respectively. The mean survival time was 3.8 years (standard error ± 0.09). Because fewer than 25% of eyes failed, the quartile survival estimate is undefined.
Figure 1.
Kaplan-Meier curve of keratoprosthesis failure.
The majority of eyes (86.2%) had experienced a failed cornea transplant prior to keratoprosthesis implantation; these eyes (n=244) had undergone an average of 2.3 ± 1.3 prior cornea transplants (range 1 – 8). Thirty-nine eyes (13.3%) underwent primary keratoprosthesis implantation because they were at high risk for graft failure, and 22 eyes (7.5%) underwent replacement of a prior keratoprosthesis. Primary procedures were more than three times as likely to fail as those undergoing de novo keratoprosthesis implantation (p=0.04) although there was no difference in the time to fail between these three strata (p=0.109). Furthermore, there was no relationship between primary procedures and failure on multivariate analysis (p=0.356). Of the primary keratoprostheses that failed (n=7), five were implanted for an autoimmune diagnosis, one for trauma, and one for carcinoma in-situ with associated exposure keratopathy due to Bell’s palsy.
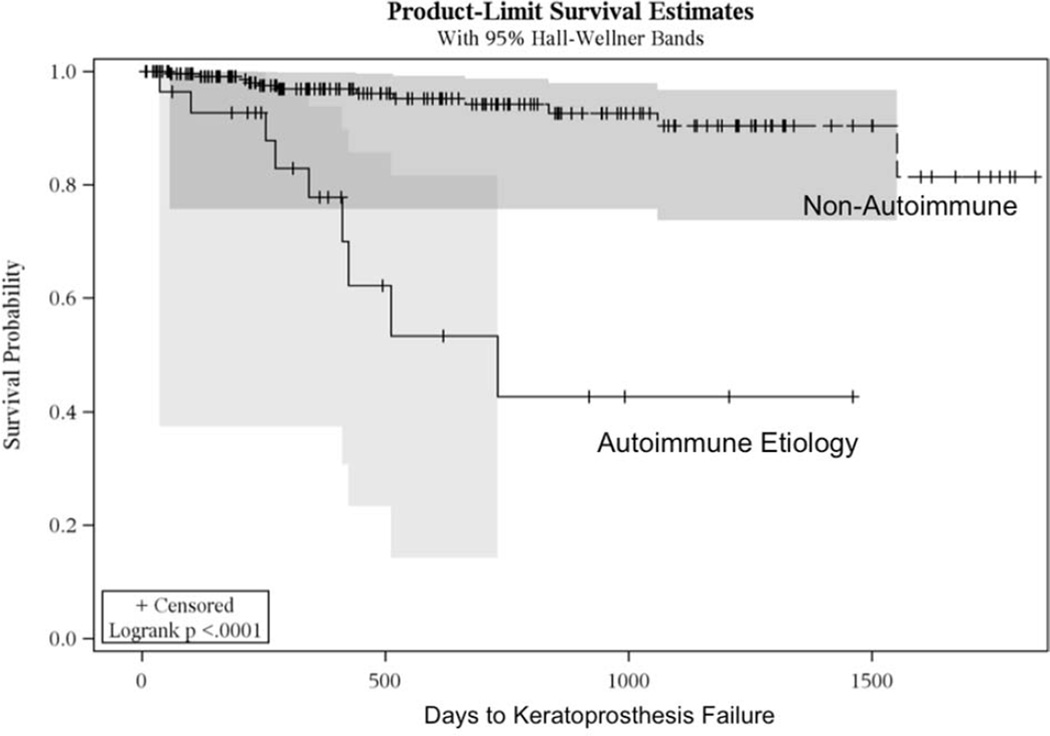
The Boston keratoprosthesis was utilized for a wide variety of ocular surface diseases (Table 3). The only etiology associated with failure was autoimmune disease (p<0.0001) for which the time to failure was significantly shorter. Autoimmune eyes had a quartile time to failure of 1.1 years (95% confidence interval 0.27, 1.4; Figure 2). No other diagnosis experienced a failure rate as high (25%) as autoimmune eyes during the study period. Patients in the autoimmune group had the lowest rate (71.0%) of retention and had an unadjusted odds of failure of 8.8 (95% confidence interval 3.33, 23.06) in comparison to the other etiologies assessed in this study (p<0.0001). If eyes with an autoimmune diagnosis are excluded, the overall retention rate is 95.5%; after one year of follow-up, the retention rate for patients without autoimmune disease is 95.9%, and after two years, 90.0%.
Table 3.
Failure rate of keratoprosthesis by surgical indication with comparison testing of time-to-failure data.
| Etiology | Failure (n=21) | Retention (n=279) | ||
|---|---|---|---|---|
| rate (%) |
n | n | p-value* | |
| Autoimmune | 29.0 | 9 | 22 | <0.0001 |
| Chemical Injury | 3.2 | 1 | 30 | 0.508 |
| Herpes Simplex Virus | 4.8 | 1 | 20 | 0.790 |
| Fuchs Dystrophy | 12.5 | 1 | 7 | 0.621 |
| Keratoconus | 9.1 | 1 | 10 | 0.969 |
| Infectious Keratitis | 5.3 | 1 | 18 | 0.643 |
| Neurotrophic Keratitis | 0 | 0 | 4 | 0.617 |
| Limbal Stem Cell Deficiency | 0 | 0 | 9 | 0.440 |
| Pseudophakic Bullous Keratopathy | 1.8 | 1 | 54 | 0.064 |
| Trauma | 15.4 | 2 | 11 | 0.520 |
| Aniridia | 14.3 | 1 | 6 | 0.566 |
| Miscellaneous | 8.6 | 3 | 32 | 0.889 |
| Failed Graft | 0 | 0 | 50 | 0.098 |
| Unknown | 0 | 0 | 6 | 0.390 |
log-rank test
For eyes classified as miscellaneous, underlying diagnoses leading to corneal replacement surgery included visually significant corneal scar (6), uveitis (3), thermal burn (2), Darier’s diseae (1), Alport’s syndrome (1), Salzmann’s nodular degeneration (2), Alpert’s syndrome (1) Wegener’s granulomatosis (1), atopic keratoconjuctivitis (1), gelatinous drop-like dystrophy (1), retinoblastoma (1), Herpes zoster virus (1), rheumatoid arthritis (1), ocular surface tumor (1), syphilitic keratitis (1), vernal keratoconjunctivitis (1), Goldenhaar’s syndrome (1), Mooren’s ulcer (1), congenital hereditary endothelial dystrophy (1), congenital rubella (1), graft versus host disease (1), rosacea (1), measles keratitis (1), trachoma (1), congenital alacrima (1), and anterior segment dysgenesis (1).
Figure 2.
Kaplan-Meier curves comparing eyes in the autoimmune category (solid line) to eyes that do not have an autoimmune disease (dashed line). Eyes receiving a keratoprosthesis for an autoimmune etiology fail faster in comparison to other etiologies (p<0.0001).
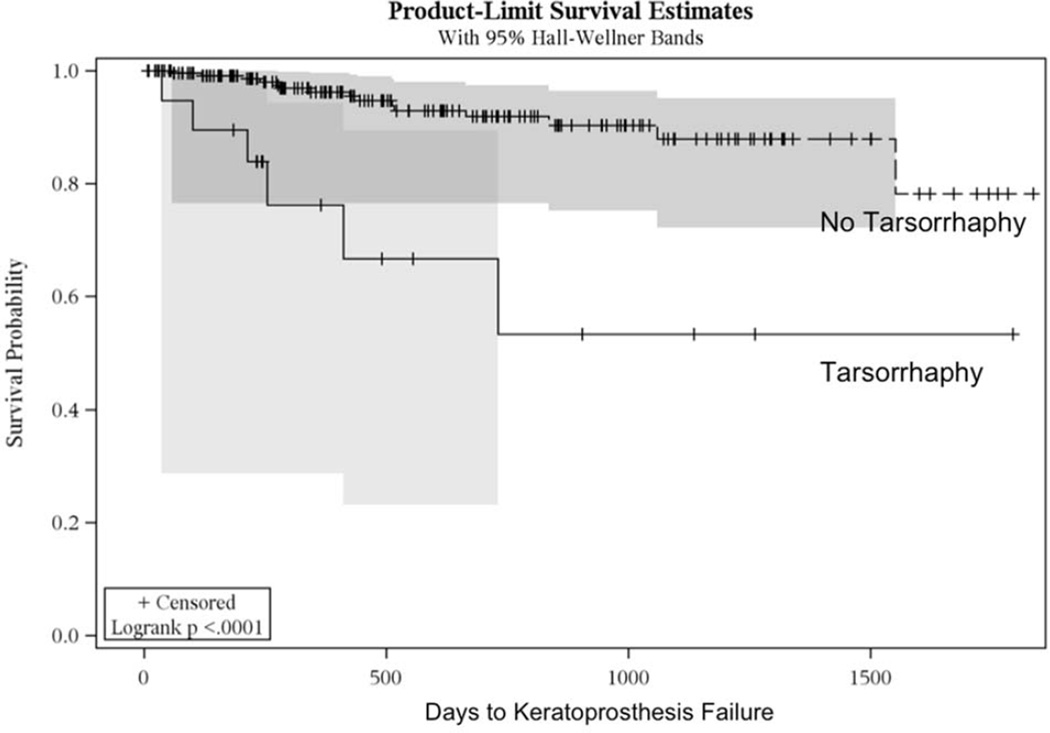
At the time of keratoprosthesis implantation, many patients underwent additional surgical procedures (Table 4). Of these, combined cataract extraction and tarsorrhaphy were each associated with a shorter time to failure; the mean time to failure for eyes undergoing combined cataract extraction was approximately one third of that in eyes not undergoing combined cataract surgery (p=0.009). However, this relationship was not significant on multivariate analysis, suggesting that other factors confound the association between combined cataract extraction and failure. Similarly, eyes undergoing tarsorrhaphy also failed three times sooner than those that did not receive a concurrent tarsorrhaphy (p<0.0001; Figure 3). Eyes that underwent a tarsorrhaphy, failed almost 6 times more frequently than eyes not undergoing tarsorrhaphy (p=0.002).
Table 4.
Time to failure of keratoprosthesis by additional surgical procedures performed at the same time as keratoprosthesis implantation.
| Procedure | Failure (n=21) | Retention (n=279) | |||
|---|---|---|---|---|---|
| rate (%) |
n | Mean Days to Failure (SE) |
n | p-value* | |
| Intracameral Steroid | 7.7 | 11 | 1431.9 ± 38.1 | 132 | 0.270 |
| Glaucoma Surgery | 4.8 | 1 | unstable | 20 | 0.563 |
| Pars Plana Vitrectomy | 8.6 | 6 | 979.3 ± 37.4 | 64 | 0.684 |
| Cataract Extraction | 16.4 | 9 | 589.1 ± 26.6 | 46 | 0.009 |
| IOL Removal | 3.1 | 1 | unstable | 31 | 0.549 |
| Iridectomy | 11.1 | 4 | 628.0 ± 23.6 | 32 | 0.414 |
| Iridoplasty | 12.5 | 2 | 929.7 ± 168.3 | 14 | 0.103 |
| Iridocorneal Synechiolysis | 0 | 0 | unstable | 15 | 0.373 |
| Tarsorrhaphy | 30.0 | 6 | 565.5 ± 68.6 | 14 | <0.0001 |
| Punctal Occlusion | 0 | 0 | unstable | 6 | 0.379 |
| Miscellaneous | 0 | 0 | unstable | 17 | 0.267 |
log-rank test
IOL = Intraocular Lens
SE = Standard Error
Mean days to failure is indicated because fewer than 25% (first quartile) of keratoprostheses failed for the majority of added procedures. Means indicated to be unstable were because the largest observation was censored and the estimation was restricted to the largest event time.
Figure 3.
Kaplan-Meier curves of keratoprosthesis failure in eyes with ocular surface exposure requiring a concomitant tarsorrhaphy (solid line) compared to those that did not (dashed line). Eyes that received a keratoprosthesis combined with tarrsorrhaphy failed faster than those that did not (p<0.0001).
Another perioperative factor associated with increased failure rates was the use of an aphakic keratoprosthesis, where the mean time to failure was less than half that of pseudophakic implants (p=0.015). This relationship was also not evident with multivariate analysis, again suggesting that it is confounded by other factors. Although there were only 3 instances in which the patient’s own cornea was used en lieu of a cadaveric donor cornea, these cases were associated with a shorter time to failure when compared to devices implanted in cadaveric donor carriers (p<0.0001).
There were no ocular (glaucoma, dry eye syndrome, age-related macular degeneration, cataract, diabetic retinopathy, aphakia, amblyopia, exposure keratopathy) or systemic (diabetes) comorbidities that were associated with an increased time to failure (p>0.05). With respect to prior ocular surgery (retinal detachment repair, prior cataract extractions, keratoprostheses, LASIK and lamellar keratectomy), eyes that had undergone glaucoma surgery prior to keratoprosthesis implantation had a lower failure fate (2.9%) in comparison to those who had not undergone prior glaucoma surgery (failure rate = 14.3%; p=0.013). However, there was no difference in the time to failure for these patients (p=0.169). One eye in the study underwent a prior LASIK and it had a faster time to failure (p=0.008).
Multivariate analysis (Table 5) demonstrated three independent risk factors for keratoprosthesis failure: autoimmune etiology disease (hazard ratio [HR] = 11.94; 95% confidence interval [CI] 3.31, 43.11), ocular surface exposure requiring a concomitant tarsorrhaphy (HR = 3.43; 95% CI 1.05, 11.22) and number of prior failed PKs (HR = 1.64; 95% CI 1.18, 2.28). It is important to note that even though the majority of tarsorrhaphies were performed for autoimmune eyes, concurrent tarsorrhaphy is a risk factor for failure independent of an autoimmune diagnosis. With respect to the number of failed PKs prior to keratoprosthesis implantation, the rates of failure were the highest in eyes with more failed PKs, although there were very few eyes with more than four prior failed grafts. For eyes with five failed PKs, 2/7 failed (22.2%). There were only two eyes with six or seven failed grafts, and neither of these eyes failed. Of the two eyes that had eight prior PKs, one failed (50%). As such, while increasing number of failed PK is an independent risk factor, it is unclear how many is too much.
Table 5.
Cox multivariate analysis.
| Variable | HR | 95% CI | Parameter Estimate |
SE | χ2 | p-value |
|---|---|---|---|---|---|---|
| Number of Failed PKs | 1.64 | 1.18 – 2.28 | 0.494 | 0.169 | 8.59 | 0.003 |
| Primary KPro | 2.23 | 0.41 – 12.28 | 0.803 | 0.870 | 0.85 | 0.356 |
| Aphakic KPro | 1.14 | 0.37 – 3.55 | 0.129 | 0.580 | 0.05 | 0.824 |
| Autoimmune Etiology | 11.94 | 3.31 – 43.11 | 2.480 | 0.655 | 14.34 | 0.0002 |
| Additional Procedures | ||||||
| Cataract Extraction | 2.10 | 0.64 – 6.89 | 0.741 | 0.607 | 1.49 | 0.222 |
| Tarsorrhaphy | 3.43 | 1.05 – 11.22 | 1.232 | 0.605 | 4.14 | 0.042 |
HR = Hazard Ratio
CI = Confidence Interval
SE = Standard Error
PK = Penetrating Keratoplasty
KPro = Keratoprosthesis
A total of 21 implants failed as a result of complications including: 9 cases of sterile keratolysis (five cases of sterile tissue necrosis with an intact prosthetic, 4 cases of keratoprosthesis extrusion), 8 fungal infections (7 cases of keratitis and one case of fungal endophthalmitis), 3 cases of replacement due to severe retro-prosthetic membranes, and 1 case of bacterial endophthalmitis. Of the 21 keratoprostheses that were not retained, 16 eyes received a replacement keratoprosthesis, 2 underwent penetrating keratoplasty without a keratoprosthesis, one developed a self-sealing membrane, and 2 eyes were enucleated.
Discussion
93.0% of the 300 Boston Keratoprostheses were retained at their last follow up, corresponding to a retention time of 396.0 patient-years or 1.42 years / implant. In this study, retention is defined as a binary outcome: the keratoprosthesis is retained or not. Complications leading to keratoprosthesis loss are typically urgent and often visually threatening. The first publication from the Boston Type 1 Keratoprosthesis Study Group reported a 95 % retention rate in 141 eyes that had an average follow-up time of 8.5 ± 6.1 months.10 Despite following a greater number of implanted devices (n=300) for a greater period of time (17.1 ± 14.8 months), the retention rate we report here is very similar. In addition, the overall retention rate in this study is comparable to previously published outcomes (Bradley: 83%, 2009; Aldave: 84%, 2009; Chew: 100%, 2009; Greiner: 80%, 2011).6, 8–11
Keratoprosthesis failure can be broadly attributed to three different pathological processes, which are not necessarily mutually exclusive and include: severe retroprosthetic membrane (RPM) formation, infection, and tissue necrosis. RPM’s are the most commonly encountered keratoprosthesis post-operative complication and occur in approximately 30% of eyes. 12 Most RPMs are typically treated with neodymium:yttrium-aluminum-garnet (Nd:YAG) laser membranotomy or by surgical membranectomy. However, when an aperture cannot be created in the membrane with either method, the keratoprosthesis can be replaced. This occurred in three eyes within this study and accounts for 14.2% of the failures observed.
Infection led to keratoprosthesis loss in 9 eyes, 7 of which resulted from fungal keratitis (one of which was polymicrobial with bacterial co-infection), one case of a fungal endophthalmitis, and one case of bacterial endophthalmitis. It is not surprising that fungal infections developed in eyes utilizing a continuous-wear contact lens and treated with topical steroids, both of which are risk factors for fungal keratitis. Only one keratoprosthesis was lost to bacterial endophthalmitis; the incidence is smaller than expected given that the prosthesis is neither biointegrated nor has a complete epithelial barrier. The relatively low infection rate may be due to the prophylactic use of antibiotics, including vancomycin. Massachusetts Eye and Ear Infirmary adopted the use of prophylactic vancomycin in 1999 in response to the observation at the time that all the cases of endophthalmitis were due to gram-positive organisms. At that institution, the incidence of bacterial endophthalmitis fell from 12% in ten years prior to the use of vancomycin to no cases in the following six years.5 Because this study reports on 431 cumulative years of follow up, it is reasonable to conclude that bacterial endophthalmitis is relatively uncommon in Boston keratoprosthesis patients, particularly when compared to the historically high rates of endophthalmitis among eyes receiving an artificial cornea. However, infectious endophthalmitis remains a concern, particularly in patients who may not be compliant using prophylactic antibiotics or in areas of the world where access to medications is limited.
Within the context of a keratoprosthesis, tissue necrosis can result in cornea perforation, hypotony, and / or instability, leading to extrusion of the device. Because these conditions can lead to severe sight-threatening complications (choroidal effusions, retinal detachments, endophthalmitis, and others), the treating physician can replace both the donor tissue carrier and the prosthetic when tissue necrosis becomes clinically significant. In this study, 4 eyes experienced extrusion of the device and 5 eyes experienced tissue necrosis necessitating keratoprosthesis replacement. Historically, tissue necrosis had been one of the most commonly encountered complications with the early keratoprosthesis designs. Although the exact etiology of corneal tissue necrosis is not known, potential contributing factors include increased collagenase activity in eyes with increased immune activity, formation of dense retrocorneal membranes that act as a barrier to corneal nutrition, ocular surface disease that includes exposure keratitis, and dry eye. In this multicenter study, 5 of the 9 eyes that developed tissue necrosis had a history of autoimmune diseases.
Autoimmune etiologies, such as ocular cicatricial pemphigoid and Stevens Johnson Syndrome, were found to be an independent predictor of keratoprosthesis failure (HR=11.94; p=0.0002). These eyes accounted for only 10.3% of the total number of implants, but resulted in 42.9% of failures. This finding is also similar to other large case series; in Greiner’s series, 37.5 % (3 of 8) of failures had a history of autoimmune disease leading to tissue necrosis.11 In Aldave’s report, 50% of the eyes that required a keratoprosthesis replacement experienced immune-related tissue necrosis.9
Multivariate analysis also demonstrated that placement of a tarsorrhaphy at the time of surgery was an independent risk factor for keratoprosthesis failure (HR=3.55; p=0.035). It is unlikely that the tarsorrhaphy itself contributes to failure; rather, it is more likely that a concomitant tarsorrhaphy is a surrogate for an unmeasured pre-operative risk factor for exposure-related complications. Surgeons typically place a tarsorrhaphy if there is a concern for post-operative exposure and poor contact lens retention. Poor lid apposition and excessive ocular surface exposure can contribute to evaporative desiccation of the ocular surface and also poor contact lens retention, which are known risk factors for dellen formation, persistent epithelial defects, and tissue necrosis.9, 13 Additional research into this finding is warranted before a change in practice should be considered.
Increasing number of previously failed grafts was also an independent risk factor for keratoprosthesis failure (HR=1.65; p=0.002). It is possible that eyes experiencing multiple graft failures had more extensive corneal neovascularization and more active immunity, leading to tissue necrosis. This result suggests that surgeons may want to consider keratoprosthesis placement earlier in patients at high-risk for repeated PK failure. However, more research is needed to determine the maximum number of grafts that should be considered prior to keratoprosthesis implantation.
Although there was no statistically significant difference between the retention rates for primary, first-time, or repeat keratoprostheses, primary keratoprostheses demonstrated the lowest retention rate of the three. This may reflect selection bias and the fact that this patient cohort included a greater percentage of eyes with autoimmune corneal disease. Similarly, since this was a multicenter study, we cannot rule out selection bias for every participating investigator or that some subjects may have had more or less complete follow up. However, all investigators shared the same interest in studying the role of keratoprostheses in complex ocular surface disorders, and as such we believe the data to be as complete as possible.
From this large multicenter study, the Boston Keratoprosthesis had a retention rate of 93.0%. Non-autoimmune eyes experienced a retention rate of 95.9%. Three factors found to be independent predictors of failure were autoimmune etiology, exposure keratopathy requiring a concurrent tarsorrhaphy, and increased number of prior failed penetrating keratoplasties. The most common cause of keratoprosthesis failure was infection and tissue necrosis.
Supplementary Material
Acknowledgments
This research was supported by NEI 1K08EY019686-03 (JBC), New England Cornea Transplant Research Fund (JBC), Research to Prevent Blindness Career Development Award (JBC), and Lion’s Club of Massachusetts (JBC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
A full listing of the Boston Type 1 Keratoprosthesis Study Group is available at http://aaojournal.org.
This article contains online-only material. The following should appear online-only: Table 2 and the Study Group.
References
- 1.Gomaa A, Comyn O, Liu C. Keratoprostheses in clinical practice - a review. Clin Experiment Ophthalmol. 2010;38:211–224. doi: 10.1111/j.1442-9071.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- 2.Heusser I. Ein Fall von Cornea artificialis. Denkschr Med Chir Gesellsch. 1860:127. [Google Scholar]
- 3.Nussbaum JN. Cornea artificialis, ein Substitut fur die transplantatio cornea. Deutsch Klin. 1853;5:367–372. [Google Scholar]
- 4.Klufas MA, Colby KA. The Boston keratoprosthesis. Int Ophthalmol Clin. 2010;50(3):161–175. doi: 10.1097/IIO.0b013e3181e20cca. [DOI] [PubMed] [Google Scholar]
- 5.Khan B, Dudenhoefer EJ, Dohlman CH. Keratoprosthesis: an update. Curr Opin Ophthalmol. 2001;12:282–287. doi: 10.1097/00055735-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Zerbe BL, Belin MW, Ciolino JB Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 keratoprosthesis study. Ophthalmology. 2006;113:1779–1784. doi: 10.1016/j.ophtha.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Yaghouti F, Nouri M, Abad JC, et al. Keratoprosthesis: preoperative prognostic categories. Cornea. 2001;20:19–23. doi: 10.1097/00003226-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bradley JC, Hernandez EG, Schwab IR, Mannis MJ. Boston type-1 keratoprosthesis: the University of California Davis experience. Cornea. 2009;28:321–327. doi: 10.1097/ICO.0b013e31818b8bfa. [DOI] [PubMed] [Google Scholar]
- 9.Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009;116:640–651. doi: 10.1016/j.ophtha.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 10.Chew HF, Ayres BD, Hammersmith KM, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28:989–996. doi: 10.1097/ICO.0b013e3181a186dc. [DOI] [PubMed] [Google Scholar]
- 11.Greiner MA, Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California, Davis. Ophthalmology. 2011;118:1543–1550. doi: 10.1016/j.ophtha.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Rudnisky CJ, Belin MW, Todani A, et al. Boston Type 1 Keratoprosthesis Study Group. Risk factors for the development of retroprosthetic membranes with Boston keratoprosthesis type 1: multicenter study results. Ophthalmology. 2012;119:951–955. doi: 10.1016/j.ophtha.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harissi-Dagher M, Beyer J, Dohlman CH. The role of soft contact lenses as an adjunct to the Boston keratoprosthesis. Int Ophthalmol Clin. 2008;48(2):43–51. doi: 10.1097/IIO.0b013e318169511f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.