Abstract
The identification of a drug that stimulates endogenous myelination and spares axon degeneration during multiple sclerosis (MS) could potentially reduce the rate of disease progression. Using experimental autoimmune encephalomyelitis (EAE), a mouse model of MS, we have previously shown that prophylactic administration of the estrogen receptor (ER) β ligand 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN) decreases clinical disease, is neuroprotective, stimulates endogenous myelination, and improves axon conduction without altering peripheral cytokine production or reducing central nervous system (CNS) inflammation. Here, we assessed the effects of therapeutic DPN treatment during peak EAE disease, which represents a more clinically relevant treatment paradigm. In addition, we investigated the mechanism of action of DPN treatment-induced recovery during EAE. Given that prophylactic and therapeutic treatment with DPN during EAE improved remyelination-induced axon conduction, and that ER (α and β) and membrane (m)ERs are present on oligodendrocyte lineage cells, a direct effect of treatment on oligodendrocytes is likely. DPN treatment of EAE animals resulted in phosphorylated ERβ and activated the phosphatidylinositol 3-kinase (PI3K)/ serine–threonine-specific protein kinase (Akt)/ mammalian target of rapamycin (mTOR) signaling pathway, a pathway required for oligodendrocyte survival and axon myelination. These results, along with our previous studies of prophylactic DPN treatment, make DPN and similar ERβ ligands immediate and favorable therapeutic candidates for demyelinating disease.
Keywords: Multiple sclerosis, experimental autoimmune encephalomyelitis, neuroprotective drug, remyelination, demyelination, estrogen receptor ligands, oligodendrocytes, axon conduction, second messenger signaling
Introduction
The development of directly neuroprotective treatments that prevent the loss of oligodendrocyte (OL) progenitors (OLP) and promote the proliferation and differentiation of OL is a treatment goal for multiple sclerosis (MS). Using experimental autoimmune encephalomyelitis (EAE), a mouse model of MS, a significant reduction in clinical symptoms has been shown with estradiol, estriol, and other estrogen receptor (ER) ligand treatments (Crawford et al., 2009a, Tiwari-Woodruff et al., 2007, Crawford et al., 2010, Matejuk et al., 2004, Strigard et al., 1990). The success of estrogens in ameliorating EAE clinical disease within the laboratory setting prompted clinical trials in Europe and the United States using estrogen therapy in human MS patients. However, synthetic estrogen supplementation is associated with increased risk of breast and uterine cancer, heart disease, and stroke (Prentice et al., 2009). Most of these side effects are thought to be mediated through ERα, not ERβ (Caringella et al., 2011). As a result, interest in ERβ as a target for neuroprotective therapy has become desirable.
To this end, we have shown that prophylactic administration of the ERβ ligand 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN) decreases clinical features of EAE, is neuroprotective, stimulates endogenous myelination, and improves axon conduction without altering peripheral cytokine production or reducing central nervous system (CNS) inflammation (Tiwari-Woodruff et al., 2007, Crawford et al., 2010). However, DPN treatments were administered prior to disease induction (Crawford et al., 2010, Tiwari-Woodruff et al., 2009). Thus, these experiments tested the potential for ERβ ligands to prevent or protect against future axonal damage. Theoretically, estrogen treatment in human relapse-remitting MS (RRMS) patients could protect against a future MS immune attack (relapse). But because treatment would be initiated after initial diagnosis, such treatment would not be considered prophylactic in the strictest sense. Given such limitations, it is important to investigate the therapeutic effects and mechanisms of potential treatments when administered following the onset of MS/EAE symptoms.
Though neuroprotection and remyelination were observed in DPN-treated EAE mice, the mechanism of action of DPN remains unknown. Recent reports have demonstrated an involvement of the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway in OL differentiation and CNS myelination during development (Flores et al., 2008, Narayanan et al., 2009). mTOR activation has also been demonstrated to promote OL differentiation (Narayanan et al., 2009). For successful remyelination to occur, activation/recapitulation of developmental myelination pathways, including PI3K/Akt/mTOR, may be required. The observation that DPN treatment during EAE increases the number of OL and axon myelination (Crawford et al., 2010) led us to hypothesize that therapeutic DPN treatment during EAE will be as effective as prophylactic treatment, and that ERβ ligand-induced remyelination/neuroprotection involves the activation of the PI3K/Akt/mTOR pathway. In this report, we verify the involvement of the ERβ ligand DPN in activating these signaling pathways to promote remyelination and repair during EAE. In addition, we show that therapeutic DPN treatment-activated second messenger signaling, which induces remyelination/neuroprotection, is not a rapid response and requires sustained treatment with DPN during EAE.
Materials and Methods
Animals
The generation, characterization, and genotyping of PLP_EGFP (proteolipid protein-enhanced green fluorescent protein) transgenic mice have been reported (Mallonet al., 2002). Mice were bred in house at the UCLA animal facility. All procedures were conducted in accordance with the NIH and were approved by the Animal Care and Use Committee of the Institutional Guide for the Care and Use of Laboratory Animals at UCLA.
Number of Mice
Three different treatment groups (normal, vehicle+EAE, and ERβ ligand+EAE) were used per experiment, with 10-12 animals per treatment group [(3 animals for electrophysiology recording + 3 animals for immunohistochemistry + 3-4 animals for Western blotting + 2 animals for electron microscopy (EM)]. Each experiment was repeated three times.
Reagents
DPN (Tocris Bioscience, Ellisville, MO), Miglyol 812 N liquid oil (gift from Sasol, Houston, TX), and myelin oligodendrocyte glycoprotein (MOG) peptide (amino acids 35–55; Mimotopes, Minneapolis, MN) were used. See Supplementary Material 1 for other chemicals and antibody details.
EAE Induction
Active EAE was induced in 8 week old male and female PLP_EGFP C57BL/6 mice (Tiwari-Woodruff et al., 2007, Crawford et al., 2010, Mangiardi et al., 2011). Specifically, active EAE was induced via immunization with 200 μg of MOG peptide, amino acids 35-55, in combination with Mycobacterium tuberculosis in complete Freund's adjuvant (CFA) on post-immunization Day 0 and 7. Additionally, mice were injected with Pertussis toxin (500 ng/mouse) on Day 0 and 2. Mice were monitored and scored daily for clinical disease severity according to the standard EAE grading scale: 0, unaffected; 1, tail limpness; 2, failure to right upon an attempt to roll over; 3, partial hind limb paralysis; 4, complete hind limb paralysis; and 5, moribund. Within each treatment group, the mean clinical score was determined daily, thereby yielding the mean clinical score for that treatment group. Mice were sacrificed at either Day 36 or 42 after disease induction.
DPN Treatment
Treatment with DPN (8 mg/kg) every other day starting on post-immunization Day 21 or 32 (therapeutic), or on Day 0 (prophylactic), continued for 4 days, 24 days, or throughout the entire duration of disease. The dose of DPN was chosen based on uterine weight measurements for biological response and previous EAE experiments using this compound (Tiwari-Woodruff et al., 2007, Crawford et al., 2010).
Rotorod Testing
Mice were tested for motor performance up to two times per week using a rotorod apparatus (Med Associates, Inc., St. Albans, VT), as previously described (Tiwari-Woodruff et al., 2007). Animals were placed on a rotating horizontal cylinder for a maximum of 200 seconds. Each mouse was tested on a speed of 3-30 rpm and given three trials for any given test day. The amount of time the mouse remained walking on the cylinder without falling was recorded. The three trials were averaged to report a single value for each mouse. Averages were then calculated for all animals within a given treatment group. All mice underwent two practice sessions prior to immunization (before Day 0).
Histopathology, Immunohistochemistry, Fluorescent, and Electron Microscopy (EM)
Paraformaldehyde (PFA)-fixed brain sections containing the corpus callosum (CC) were examined by immunohistochemistry using various series of cell type-specific antibodies, as previously described (Tiwari-Woodruff et al., 2007). In parallel, the CC was dissected and subjected to electron microscopy (EM) as previously described (Crawford et al., 2010). The following were used for immunohistochemistry to detect: axons: anti-NF200 (1:500, Millipore and 1:1000, Sigma), anti-SMI-32, anti-APP (1:1000 Abcam); astrocytes: anti-GFAP (1:1000, Millipore); OLPs: anti-PDGFRα/anti-olig2 + anti-Ki67 (1:500, Millipore); mature OLs: anti-CC1 (1:1000, Gene Tex); PLP_EGFP fluorescence; myelin: anti-MBP (1:1000, Millipore); T cells: anti-CD3 (1:1000, Abcam); and microglia/macrophage/monocyte: anti-CD45 (1:1000; PharMingen, La Jolla, CA). Second messenger antibodies are the same as those mentioned in the Western blot section. The second antibody step was performed by labeling with antibodies conjugated to Cy3 and Cy5 (1:1000, Vector Labs Burlingame, CA and Chemicon). IgG-control experiments were performed for all primary antibodies, and no staining was observed under these conditions. To assess the number of cells, a nuclear stain 4′,6-Diamidino-2-phenylindole, DAPI (2 ng/ml; Molecular Probes), was added to tissue sections for 15 minutes prior to final washes after secondary antibody addition. The sections were mounted on slides, allowed to dry, and coverslipped in fluoromount G (Fisher Scientific, Pittsburgh, PA). To detect apoptotic cells within the CC, the TUNEL TMR in situ cell death kit (Roche Applied Science, Mannheim, Germany) was used as previously described (Ziehn et al., 2010). For EM, PFA- and glutaraldehyde-perfused brains were cut sagittally. The genu area of CC was identified under a dissecting scope and 4 mm2 blocks [from the mid CC up to 1/3 splenium, corresponding to the CC area of plate 40-48 (Crawford et al., 2009b)] were carefully dissected. These blocks were further cut in 1 mm sections for Epon embedding.
Stereological and g-ratio Analysis
Immunostaining was quantified using unbiased stereology. All images (RGB) were converted to grayscale, split, and separated by color channel using ImageJ version 1.29 (Windows version of NIH Image; downloaded from http://rsb.info/nih/gov/ij). To avoid experimenter bias, auto-adjustment of brightness and contrast, as well as threshold of staining signal, was carried out by NIH software. A Grid Plug-in (Image J) was used for counting points per area of interest. CC1+ OLs, olig2+ and Ki67+ OLPs, CD3+ T cells and TUNEL+ labeled cells lying within the CC region were counted manually using X10 or X40 images and compared blindly between normal, EAE+vehicle, and EAE+ DPN treated groups. Pixel intensity of microglia/macrophage activation, myelin, and GFAP+ staining intensity within the dorsal column of the spinal cord or CC1 were also measured as percent area of immunoreactivity using ImageJ and were then recorded and compared statistically. Axon density was measured by the ratio of NF200+ pixel intensity and the total image pixel area. All pixel intensities were measured and compared using X10 and X40 magnified images.
For EM analysis, serial ultrathin sections of CC embedded in Epon were stained with uranyl acetate-lead citrate and analyzed as described in (Crawford et al., 2010). Images at 3,600X and 14,000X were analyzed using ImageJ. A grid was used to count axons per area of interest. The ratio of axon diameter to total fiber diameter (g-ratio) was measured by dividing the circumference of an axon without myelin by the circumference of the same axon including myelin (Crawford et al., 2010). For most axons, two encounters were measured. At least 500 axons were analyzed per treatment group.
Electrophysiological Recording Procedures
Brain slices corresponding approximately to plates 40-48 in the atlas of Paxinos and Franklin atlas (2001) were used for CAP recordings (Crawford et al., 2010). As described previously (Crawford et al., 2009a, Crawford et al., 2009b), mice were deeply anesthetized via inhalation of isoflurane and decapitated. The brain was removed and vibratome (Leica, Germany)-sectioned into 400 μm coronal sections while submersed in ice-cold carbogen (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF, in mM NaCl 124, KCl 5, NaH2PO4 1.25, NaHCO3 26, MgSO4 1.3, CaCl2 2, glucose 10; adjusted to pH 7). Slices were incubated for at least one hour in room-temperature ACSF. Two slices per animal containing the dorsal hippocampus (plates 35-48, in the atlas of Paxinos and Franklin 2001) were used for electrophysiology recordings of CAP and refractoriness (Crawford et al., 2010). Stimulation used for evoked CAPs was constant current stimulus-isolated square wave pulses. For analyses of the CAP amplitude, standardized input–output functions were generated for each slice by varying the intensity of stimulus pulses (200 μs duration, delivered at 0.2 Hz) in steps from approximately threshold level to an asymptotic maximum (0.3-4.0 mA) for the short latency negative CAP component. To enhance the signal-to-noise ratio, all quantitative electrophysiological analyses were conducted on waveforms which were the average of four successive sweeps. Evoked callosal CAP field potentials were amplified and filtered (bandpass=DC to 10 kHz) using an Axopatch 200A amplifier (Molecular Devices, Sunnyvale, CA), digitized at 200 kHz, and stored on disk for offline analysis.
Axon refractoriness is defined as the reduced excitability of an axon following an action potential. Axon damage can modify refractoriness and its measurement represents a diagnostic tool to measure axon health. To quantify refractoriness, the suppression of a second CAP response in paired stimulus trials is determined as previously described. Initially, a single stimulating pulse is given at a defined strength to establish a control response (C1). Following this response, two pulses of equal intensity and duration are generated which are separated by a variable time window, starting with an interpulse interval (IPI) of 8 ms and decreasing in 0.5 ms steps down to 1.5 ms. For analysis, the control response is subtracted from the paired stimulus responses at each IPI. This results in the response, which can be attributed to the second pulse (C2). The estimated N1 and N2 responses for C2 are then measured. Refractoriness is calculated for both N1 and N2 by dividing these C2 CAP component amplitudes by their respective C1 CAP amplitudes and multiplying by 100%. The results are graphed versus the IPI and analyzed using a non-linear regression analysis, with specific use of the Boltzmann sigmoid function. The IPI that results in a 50% reduction, in the CAP component, is used as a standard measure when making statistical comparisons between groups.
Western Analysis
The CC brain region, which contained some cortical tissue but no hippocampus, was dissected from individual animals, rapidly frozen in liquid nitrogen, and stored at -80°C. Polyacrylamide gel electrophoresis, electrophoretic transfer of protein bands to nitrocellulose, and immunoblotting was performed to detect cell-signaling proteins as described previously (Kumar et al., 2006). Briefly, Protein lysates were prepared from brain tissue in lysis buffer containing 50 mM HEPES pH 7.5, 150 mM NaCl, 1% TritonX-100, 1.5 mM MgCl2, 1 mM EDTA, 10 mM sodium pyrophosphate, 2 mM dithiothreotol (DTT), and protease inhibitor cocktail (Sigma, 1:100 dilution). Protein concentration was determined with protein assay reagent (BioRad, Hercules, CA) and protein aliquots were denatured immediately at 95°C in sodium dodecyl sulfate (SDS) -Laemmli sample buffer containing 2 mM DTT for 5 min and stored at -80°C. Prior to electrophoresis, protein lysate aliquots are thawed and reheated at 95°C for 3 min and 35 µg proteins were separated on 4-20% SDS-polyacrylamide gel (Invitrogen, Life Technology group, NY). Protein bands were blotted on Hybond nitrocellulose (Amersham, GE Health Care) overnight at 4°C at 30 Volts and blots were blocked with Tris buffered saline (50 mMTris-HCl pH 7.4 and 150 mM NaCl), 0.1% Tween (TBST) containing 10% powdered milk and 0.1% bovine serum albumin (BSA). The blots were incubated overnight at 4°C with the following primary antibodies in blocking buffer containing 5% milk: Rabbit anti-cleaved Caspase-3 (Chemicon; 1:200; 17 kDa); rabbit anti-Calpain S1 (Abcam 1:500; 27 kDa); rabbit anti-BDNF (Santa Cruz, 14 kDa) and mouse anti-IGF-1 (Millipore; 0.5 μg/ml, 7 kDa), mouse anti-IGF-1R (Millipore; 0.5 μg/ml, 90 kDa); rabbit anti-ERβ (Upstate; 1 μg/ml,53-65 kDa); rabbit anti-pERβ (Ser105, Abcam, 1:250, 66 kDa); mouse anti-GAPDH (Ambion, 1:20,000; 36 kDa); rabbit anti-Trk-B (Chemicon, 1:1000; 90 kDa) were used as primary antibodies. In addition, primary antibodies from Cell Signaling were also used at 1:1000 dilution: anti-AKT (60 kDa); anti-Phospho-AKT (Thr308, 60 kDa); anti-mTOR (289 kDa); anti-Phospho-mTOR (ser-2448; 290 kDa); anti-PI3K (60 kDa); anti-Phosphor-PI3K (Tyr199, 60 kDa); anti-PTEN (54 kDa); anti-Phospho-PTEN (ser380; 54 kDa); anti-P70S6 kinase (70 kDa); anti-Phospho-P70S6 kinase (Thr389, 70 kDa). Blots were washed three times in TritonX-100 containing Tris buffered saline. The washes were followed by incubation in horseradish peroxidase-labeled secondary antibodies (1:1000) against host of primary antibody for 1 h at room temperature and washed three times in TBST followed by chemiluminescence reaction that was carried out with reagent from Santa Cruz Biotech as per manufacturer's protocol. X-ray films were exposed for a period to capture protein bands. Band density was determined as scanning units and expression levels were quantified as compared to the levels of glyceraldehyde phosphate dehydrogenase (GAPDH).
Western analysis figures represent a gel with 6-7 lanes. Each lane represents an individual animal within the treatment group. Quantification graphs are from 4-8 animals/treatment group pooled over 2-3 different experiments.
Statistical Analysis
All the animal groups were coded and analyses were conducted blind to experimental condition by multiple researchers. Quantification of electrophysiology and immunostaining results was done similar to previous studies (Crawford et al., 2010, Tiwari-Woodruff et al., 2007). EAE clinical score differences and rotorod scores were determined by one-way ANOVA and Friedman Test (only for clinical scores) or Bonferroni's multiple comparison post-test. Differences were considered significant at the *p<0.05 level. Statistics were performed using Microcal Origin (Northampton, MA) or Prism 4 (GraphPad Prism Software Inc., La Jolla, CA). Western blot data are presented as means ± SEM (n=4-8) and analyzed by t-test for independent samples, or two-way ANOVA followed by Tukey posthoc test.
Results
Therapeutic treatment with DPN ameliorates EAE disease
To visualize and characterize the effects of therapeutic DPN treatment on demyelination and axon degeneration, active EAE was induced in PLP_EGFP transgenic C57BL/6 mice (Mallon et al., 2002, Mangiardi et al., 2011). PLP_EGFP mice show no fluorescence in neural cells or axons, but show high levels of green fluorescent protein in oligodendrocytes and myelin throughout the CNS.
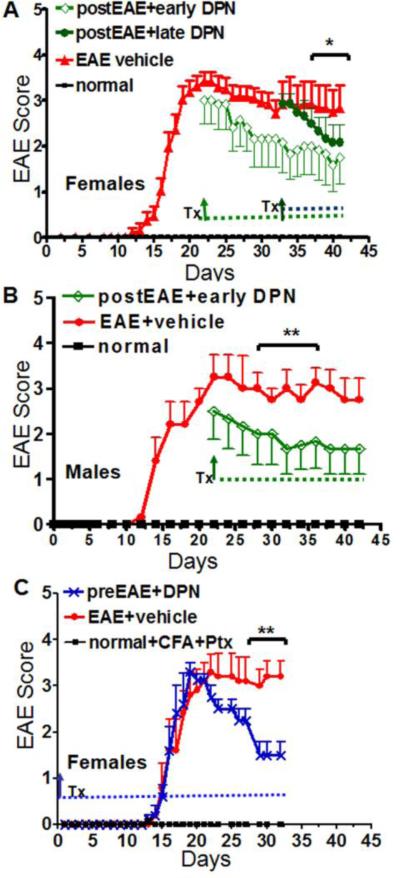
Active EAE was induced using MOG 35-55 peptide (Mangiardi et al., 2011) Figure 1). Groups of mice were treated with DPN (8mg/kg) or vehicle (ethanol+miglyol oil) via subcutaneous injection every other day starting at Day 21 (postEAE+earlyDPN) and Day 34 (postEAE+lateDPN) after onset of clinical disease symptoms. A group of mice receiving Mycobacterium tuberculosis in complete Freund's adjuvant (CFA) and pertussis toxin (PTX), but no MOG, did not develop clinical disease symptoms (normal group). Vehicle-treated EAE mice developed a persistent, chronic disease course. A significant reduction in EAE clinical disease severity, as measured using the standard EAE grading scale, was observed by the end of 4 weeks in the postEAE+earlyDPN treatment group (Figure 1A, B) and 5.5 weeks in the postEAE+lateDPN treatment group (Figure 1A, *p<0.05, **p<0.001). As a control, a group of mice was treated with DPN beginning at post-immunization Day 0 of EAE (preEAE+DPN; prophylactic treatment, Figure 1C), similar to published reports (Crawford et al., 2010). Prior to Day 20, prophylactic DPN treatment had no effect. Subsequently, prophylactic DPN treatment demonstrated a significant protective effect throughout the later stages of disease, consistent with previous findings (p<0.001, Figure 1C, n=10-12; (Crawford et al., 2010). Together, these results suggest that early therapeutic treatment with DPN, after the acute initial phase of EAE, is neuroprotective.
Figure 1. Therapeutic treatment with DPN significantly improves active EAE.
(A) C57BL/6 female mice (transgenic PLP_EGFP) were given DPN treatment (Tx) on Day 21 (green diamonds) and Day 34 (green circles) after initiation of active EAE. (B) Male mice were given DPN beginning on Day 21 (green diamonds) and continued until Day 42. (C) Another group of female mice was started on prophylactic DPN treatment on post-immunization Day 0 (green star) and continued treatment through the entire period of 32 days. Each experiment also contained a group that was given a mixture of ethanol+miglyol oil as vehicle (red). Animals were scored using the standard EAE grading scale. EAE scores of DPN-treated mice were significantly improved as compared to vehicle-treated mice (**p<0.001, *p<0.05, ANOVA Friedman test). Normal mice (A-B) or normal mice that were given only CFA and Ptx injections (C) did not show any disease and their clinical scores remained zero throughout the experiment. Number of mice in each group was as follows: normal, n=6-10; EAE+vehicle, n=10; preEAE+DPN, n=10; postEAE+DPN, n=10. Data are representative of experiments repeated three times
Therapeutic treatment with DPN does not decrease CNS inflammation but restores myelin density in spinal cord and corpus callosum in EAE animals
Final EAE clinical scores were assessed between Day 40-42 after initiation of disease. Groups of mice were fixed by cardiac perfusion with PFA for immunohistochemical analysis. The dorsal column of spinal cord sections from vehicle-treated, active EAE PLP_EGFP mice contained multiple areas with significantly decreased “green fluorescence”, indicative of oligodendrocyte and myelin pathology (Figures 2A i). Consecutive thoracic (T1-T5) spinal cord sections were immunostained and imaged to show the dorsal column, where areas of low green fluorescence were accompanied by inflammatory lesions with typical perivascular infiltration and accumulation of mononuclear cells (CD45+ cells co-stained with DAPI+ nuclei), as previously seen (Mangiardi et al., 2011).
Figure 2. Therapeutic treatment with DPN enhances myelin protein and oligodendrocyte numbers without affecting inflammation in the spinal cord and corpus callosum of EAE mice.
Representative images are shown from spinal cord and corpus callosum (CC)-containing brain sections of vehicle-treated EAE, DPN-treated EAE, and normal (experiment from Figure 1A) animals sacrificed at Day 42 post-EAE induction. The stained sections were imaged at either 10X or 40 X magnifications. The staining intensities or number of cells/0.4 mm2 were quantified and normalized to normal controls. (A) PLP_EGFP (green) spinal cord (i-dorsal column X10 magnification) and brain slices (ii-CC X40 magnification) were stained with DAPI (blue) to label nuclei and co-immunostained for common leukocyte antigen (CD45), T cells (CD3), and astrocytes (GFAP). A significant increase in CD45+, CD3+, and GFAP+ cells was observed in vehicle-treated and DPN-treated EAE groups. (B) Myelin basic protein (MBP-red) immunostaining in vehicle-treated EAE groups was significantly decreased as compared to other groups in the dorsal column (i-X10, iii) and CC (ii-X10, iv). (C) PLP_EGFP oligodendrocytes (OLs) were co-stained with DAPI (blue), mature OLs were labeled with adenomatous polyposis coli APC (CC1 antibody, red), proliferating OLPs were co-immunostained with Ki67 (green) and olig2 (red), and apoptotic cells were co-stained with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL; images not shown). Vehicle-treated EAE sections showed a significant decrease in numbers of PLP_EGFP+ cells (iv), CC1+ cells (v), olig2+Ki67+ cells, and an increase in PLP_EGFP cells that were also TUNEL+ (vii). A reversal of these numbers was observed in DPN-treated EAE sections. Scale bar is 100 μm (Ai, Bi-ii), 20 μm (Aii, Cii) and **p<0.001 ANOVAs; Bonferroni's multiple comparison post-test; n=8-10 mice per treatment group.
We have recently shown that CNS structures other than the spinal cord are negatively affected during EAE, leading to sensory, motor, and cognitive impairments similar to those seen in MS patients (Wensky et al., 2005, Hobom et al., 2004, Brown et al., 2007, MacKenzie-Graham et al., 2009, Rasmussen et al., 2007, Ziehn et al.). Specifically, integrity of the corpus callosum (CC) in MS reflects demyelinating lesions, diffuse tissue damage, and abnormalities in neural connectivity, making it a potentially useful surrogate marker of clinically significant brain abnormalities (Boroojerdi et al., 1998, Warlop et al., 2008, Ozturk et al., 2010). In addition to spinal cord, CC integrity was assessed using immunohistochemistry. Vehicle-treated EAE group CNS inflammation during EAE includes activation of microglia/macrophages and an increase in T cell numbers and cells of the monocyte lineage (Mangiardi et al., 2011, Tiwari-Woodruff et al., 2007). Similar to previous observations, vehicle-treated EAE mice had numerous multifocal to coalescing inflammatory cell infiltrates that were positive for CD45, a pan-leukocyte marker which labels all infiltrating leukocytes, including T cells (Figure 2A ii). In normal CC, CD45+ cells are mostly filamentous, denoting labeling of mostly resident CNS microglia population (data not shown), whereas vehicle-treated and DPN-treated EAE spinal cord and brain sections contained mostly globoid cells indicative of macrophages and T cells. (Figure 2A ii-iii). Astrogliosis is also a prominent feature of the chronic and widespread adaptive CNS immune response in EAE and MS (Liedtke et al., 1998, Wu et al., 1992). A significant increase in GFAP+ immunoreactivity was observed throughout the grey and white matter of the spinal cord (as seen earlier (Voskuhl et al., 2009)) and the CC of the vehicle-treated EAE group (Figure 2A iv). DPN-treatment during preEAE and early-postEAE did not attenuate the inflammatory and reactive astrocyte response as compared to the vehicle-treated EAE group (Figure 2A ii-iv). Number of CD45+ and CD3+ inflammatory cells as well as GFAP+ astrocytes in vehicle-treated and DPN-treated EAE groups were significantly increased (***p<0.001, Figure 2A vii-ix).
Degree of myelin loss was then assessed by myelin basic protein (MBP) immunostaining in the CC and dorsal column of thoracic spinal cord (Figure 2B i). Extensive demyelination occurred at the sites of cell infiltrates in vehicle-treated EAE mice as compared to normal mice. Significantly less demyelination occurred in all DPN-treated spinal cords and CC-containing brain sections (Figure 2B i, ii). Quantification of demyelination in vehicle-treated EAE mice by analysis of MBP staining density in delineated dorsal columns and CC revealed a ~30-40% (***p<0.001) decrease in myelin density as compared with healthy controls. In contrast, myelin staining was preserved in DPN-treated EAE groups (Figure 2B iii-iv).
The increase in MBP fluorescence intensity in DPN-treated preEAE and postEAE groups could be due to an increase in survival and/or decrease in OL cells. PLP_EGFP+ and CC1+ OL immunostained spinal cord and CC were imaged and quantified (Figure 2). A significant increase in number of PLP_EGFP cells and CC1+ (adenomatus polyposis coli, a mature OL marker) OLs was observed in DPN-treated EAE groups (Figure 2C i, ii, iv, and v; *p<0.05). Co-immunostaining with the OLP marker platelet derived growth factor receptor-α (PDGFR-α) and a cellular marker of proliferation, Ki67, and was not successful. PDGFR-α is a cell surface marker and even after co-staining with DAPI to label nuclei (and/or with Ki67, which only labels newly divided nuclei), it was extremely difficult to assign staining to one or multiple cells. Therefore, we switched to the OL/OLP marker olig2 (OL transcription factor 2), which co-stained with Ki67 and allowed us to assess newly divided OLPs. A significant decrease in proliferating OLPs in vehicle-treated groups was observed, and this was reversed with prophylactic and therapeutic DPN treatment (Figure 2B iii, vii; *p<0.05). Lastly, we assessed the status of cell death in the presence of DPN. Apoptotic marker terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining revealed a significant decrease of apoptotic PLP_EGFP OLs in the DPN-treated EAE group compared to the vehicle-treated EAE group (Mangiardi et al., 2011) (Figure 2B vii; *p<0.05). Taken together, the increased MBP fluorescence intensity in DPN-treated EAE groups correlated with an increase in OL and OLP numbers. Significantly, therapeutic DPN treatment during EAE occurred in the presence of inflammation, as evidenced by the presence of inflammatory cells and reactive astrocytes.
Therapeutic treatment with DPN results in remyelination, decreased axon damage, and improved callosal axon conduction
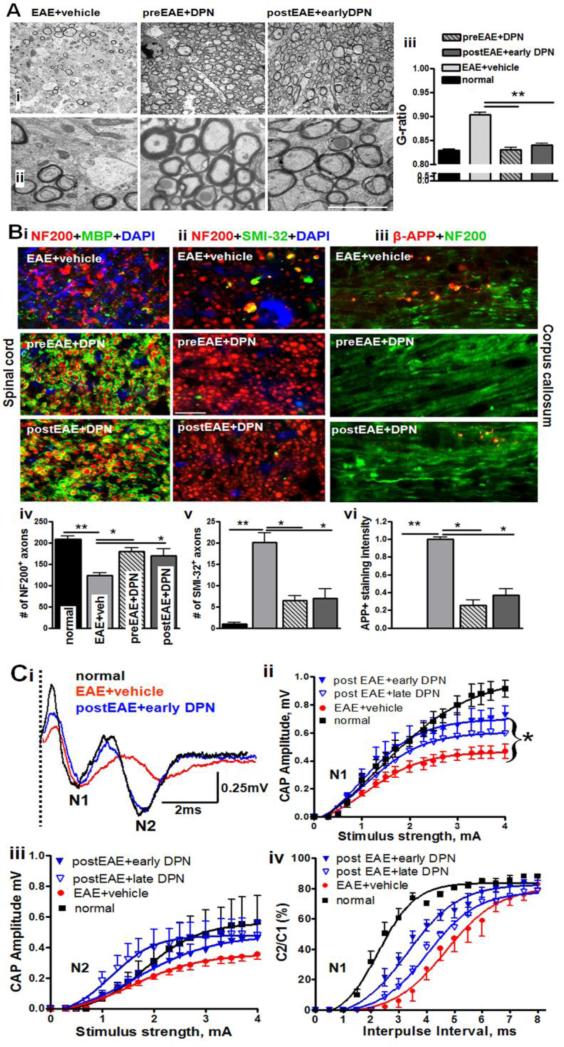
Detailed analysis of DPN-induced axon remyelination was performed by ultrastructure electron microscopic (EM) analysis of CC axons (Figure 3A-B). A DPN treatment-induced increase in myelinated axon numbers and a significant reduction in “g-ratio” (axon diameter/axon+myelin diameter) were observed. The g-ratio of EAE+vehicle CC (0.90±0.02) was significantly higher than normal (0.85±0.01) due to an increase in partially and completely demyelinating axons, and a few remyelinating axons with thin myelin (Figure 3A-B). The calculated g-ratios of postEAE+earlyDPN callosal axons decreased to 0.86±0.01, indicative of remyelination-induced recovery of demyelinated axons. The g-ratio for therapeutic DPN treatment group was similar to the prophylactic DPN treatment group's g-ratio of 0.83 ±0.02.
Figure 3. Therapeutic treatment with DPN enhances remyelination and restores corpus callosum axon conduction of EAE mice.
(A) Representative electron micrographs of CC from EAE+vehicle, preEAE+DPN, and postEAE+earlyDPN groups at a magnification of 3,600X (i) and 14,000X (ii) are shown. Measurement of myelin thickness showed significant reduction in vehicle-treated EAE mice as compared to normal and DPN-treated EAE mice. To quantify the extent of myelination, axon diameter and fiber diameter were measured (iii). Axon diameter/fiber diameter (g-ratio) showed a significant increase in vehicle-treated axons whereas a dramatic reduction in g-ratio was observed in DPN-treated EAE callosal axons. At least 4 mice from each group were analyzed and a minimum of 500 fibers were measured from each mouse. Scale bar is 1 μm. (**p<0.001, *p<0.05, ANOVAs; Bonferroni's multiple comparison post-test; n=8-14).
(B) Representative images of ventral funiculus of thoracic spinal cord sections immunostained with either NF200 (red), MBP (green), and DAPI (blue)-i; NF200 (red), SMI-32 (green), and DAPI (blue)-ii; or CC sections immunostained with β-APP (red), and NF200 (green)-iii from EAE+vehicle, preEAE+DPN, and postEAE+earlyDPN groups. Pre- and post-treatment with DPN during EAE significantly increased the number of myelinated axons and decreased the number of SMI-32+ axons in spinal cord (iv, v) and APP+ CC axons (vi). In the CC, APP staining intensity is normalized to vehicle-treated EAE animals. No APP+ axons were observed in normal group. Scale bar is 10 μm. (**p<0.001, *p<0.05, ANOVAs; Bonferroni's multiple comparison post-test; n=6-8).
(C) Compound action potential (CAP) responses were recorded from slices with midline-crossing segments of the CC overlying the mid-dorsal hippocampus. Typical CC CAPs from normal (black), vehicle-treated EAE (red), and postEAE+earlyDPN treated (blue) brain slices at Day 42 after disease induction are shown (i). There is a decrease in N1 and N2 amplitude in the vehicle-treated EAE group, as well as latency shift in N2 peak. Early DPN treatment during EAE induced an increase in N1 and N2 CAP amplitude compared to vehicle alone and normalization of N2 peak latency. Dashed vertical line represents CAPs beyond the stimulus artifact. Quantification of CAP amplitudes in the CC of vehicle-treated EAE mice showed a significant reduction in N1 and N2. DPN pre- and post-treatment showed a significant improvement in CAP response (ii, iii). Average C2/C1 ratios [obtained from plots of mean CAP amplitude elicited by the second pulse in each paired stimulation (C2) divided by the CAP amplitude to single pulse stimulation (C1)] were fitted to Boltzmann sigmoid curves. A rightward shift in curves for N1 shows decreased refractoriness in vehicle-treated and DPN-treated EAE groups (n=4). DPN-treated EAE callosal axons show a significant increase (a leftward shift in the curve compared to vehicle treatment alone) in refractoriness of N1 compared to those with vehicle treatment alone (iii). Number of mice = 4-6 per treatment group, number of CC sections per mouse = 3, total number of sections per treatment group = 12-15. Statistically significant compared with normal controls at 2-4 mA stimulus strength (*p<0.05; **p<0.001; ANOVAs; Bonferroni's multiple comparison post-test).
During chronic EAE, significant axon damage occurs along with inflammatory demyelination (Crawford et al., 2010). DPN-induced remyelination could potentially decrease demyelination-induced axon damage. Decreased axon damage in the presence of DPN (with both prophylactic and therapeutic treatment) was confirmed by performing immunohistochemistry using the monoclonal antibody SMI32, which recognizes a non-phosphoepitope on neurofilament proteins H and M, and an amyloid precursor protein (APP) monoclonal antibody, which detects accumulation of APP due to failed axonal transport. No sign of axon damage was observed in normal spinal cords and CC white matter (Figure 3B). Vehicle-treated EAE animals showed a significant decrease in number of myelinated axons and a significant increase in SMI-32+ immunoreactivity in spinal cord axons (Figure 3B i, iv, v). Pre- and post-treatment with DPN significantly increased the number of myelinated axons and decreased the number of SMI-32+ axons. Axons of vehicle-treated EAE mice showed swelling, beading, and areas of β-APP accumulation, similar to previous findings by our laboratory (Crawford et al., 2010). Further, pre- and post-treatment with DPN significantly decreased the number of APP+ axons in the CC as compared to the vehicle-treated EAE group indicative of treatment mediated preservation of axonal integrity (Figure 3B ii, vi).
Remyelination in response to DPN treatment in EAE animals should amount to improved axon conduction as compared to vehicle-treated EAE animals. Compound action potential (CAP) recordings of callosal axons were performed as previously described (Crawford et al., 2010). Coronal brain slices with midline-crossing segments of the CC, corresponding approximately to plates 40-48 in the atlas of Paxinos and Franklin (Franklin et al., 2001), were used for recording (Figure 3C i). Typical voltage traces showing two downward phases of the CAPs ‘N1’ and ‘N2’, likely representing fast depolarization from large, myelinated axons and slower depolarization from non-myelinated axons, respectively, are shown (Mangiardi et al., 2011). A distinct improvement in peak N1 and N2 CAP was observed in postEAE+earlyDPN CC as compared to vehicle-treated EAE CC (Figure 3C i-ii). This improvement in CAP amplitudes was similar to DPN pretreatment groups [not shown, please see (Crawford et al., 2010)] and significantly better than postEAE+lateDPN groups (Figure 3C ii, iii). To investigate the therapeutic treatment effect of DPN on EAE-induced axonal deficits, changes in axon refractoriness were estimated (Figure 3C iv). Rightward shifts in these curves correspond to increases in the refractory recovery cycle in axons and are indicative of functional deficit (Crawford et al., 2010, Mangiardi et al., 2011). In the normal group, the N1 component evoked by the second pair of pulses was 50% of the amplitude of a single pulse presentation at an interpulse interval (IPI) of 2.3 ±0.1ms. The IPI for the vehicle-treated EAE group had a slower response of 4.7 ±0.1ms. Therapeutic postEAE+earlyDPN callosal axons had a faster IPI of 3.3 ±0.2ms. With an IPI of 4.0±0.1ms postEAE+lateDPN myelinated axons were not different from that of the vehicle-treated EAE group. The IPIs for the N2 component of post-DPN-treated groups were not different from the vehicle-treated groups: 3.3 ±0.10ms (normal), 3.9 ±0.1ms (vehicle-treated EAE), 3.7 ±0.1ms (postEAE+earlyDPN), and 3.8 ±0.1ms (postEAE+lateDPN).
DPN treatment during EAE increases ERβ phosphorylation
We investigated the effect of DPN on ERβ expression of vehicle- and DPN-treated spinal cord and brain sections of EAE groups. We analyzed ERβ immunostaining in OLs present in the dorsal column of the spinal cord and CC of brain sections. Distinct ERβ immunoreactivity in OLs of normal, vehicle-treated EAE, and DPN-treated EAE group was observed (CC region, Figure 4A i). Phosphorylation of specific sites on ERs can occur as part of both ligand-induced and ligand-independent activities. ERβ phosphorylation (p-ERβ) emerges as an additional and significant determinant in regulating ERβ transcriptional competence (Sanchez et al., 2010). A significant increase in p-ERβ immunostaining in callosal OLs and other cells was observed in DPN-treated EAE group (Figure 4A i). For Western protein analysis, OL-rich CC was dissected out and homogenized from 3-5 animals/treatment group. CC homogenate from individual animals was run in a separate lane. Loading was further assessed by comparing levels of glyceraldehyde phosphate dehydrogenase (GAPDH). A significant increase in p-ERβ but not ERβ in DPN-treated EAE groups as compared to the vehicle-treated EAE group was observed (Figure 4A ii-iii; **p<0.001, t test).
Figure 4. DPN treatment during EAE induces ERβ, phosphorylation decreases apoptotic markers, and increases cell survival by increasing BDNF levels in the corpus callosum.
(A) DPN treatment during EAE caused an increase in the ERβ expression level (red) as well as the phosphorylated (p) ERβ (red) protein in PLP_EGFP+ callosal OLs (green). EAE animals treated with vehicle have significantly less immunostaining than the DPN-treated group (i). To quantify the potential increases in ERβ protein levels, Western analysis of dissected callosal protein lysates from normal, EAE+vehicle, and EAE+DPN groups was performed with antibody against ERβ, p-ERβ (p-ERβ; ser105), and GAPDH (ii). An increased level of p-ERβ was observed in protein lysates from the DPN-treated EAE group as compared to the vehicle-treated EAE group, which showed a significant decrease in p-ERβ (*p<0.05; **p<0.001; n=4-6). No significant difference in ERβ levels between groups was detected (iii). (B) During EAE, the inflammatory response induces demyelination and increases OL apoptosis. DPN treatment during EAE increases number of differentiated OLs, which can be attributed to the role of DPN in preventing OL apoptosis. To evaluate the effectiveness of DPN on EAE-induced OL apoptosis, immunohistochemistry and Western analysis of calpain and caspase-3 were performed. Vehicle-treated EAE brain sections exhibited an increase in calpain immunoreactivity in the CC along with reduced numbers of PLP_EGFP cells. DPN treatment during EAE significantly decreased calpain immunoreactivity and increased the number of healthy PLP_EGFP cells (i). Western analysis confirmed the increase in calpain and caspase-3 levels in callosal protein lysates of EAE+vehicle groups while DPN treatment during EAE decreased the levels of these proteins significantly, thereby confirming the role of DPN in prevention of apoptosis (**p<0.001; ANOVAs; n=5) (ii). (C) Analysis of BDNF and TrkB by immunohistochemistry and Western analysis revealed a significant increase in BDNF but not TrkB immunoreactivity in the CC of DPN-treated EAE animals (i-iii) as compared to vehicle-treated EAE animals (*p<0.001; ANOVAs; n=6). (D) Analysis of IGFR1 by immunohistochemistry revealed a significant increase in OLs of DPN-treated CC. IGF-1 and IGFR-1 Western analysis showed no significant differences among normal, EAE+vehicle or EAE+DPN groups, but more OLs were co-stained with IGF-1 and IGFR-1 (i-iii).
DPN treatment decreases EAE-induced calpain and caspase-3 activity
The effect of DPN on increased myelination and OL differentiation could be due to an increase in survival and/or a decrease in cell death of OL lineage cells. We further confirmed DPN-induced decrease in cell death by assessing expression of apoptotic cell markers. Calpains are Ca2+-dependent cysteine proteases characterized as critical mediators of both necrotic and apoptotic cell death following acute hypoxia, traumatic brain injury, and in mouse models of MS such as EAE (Das et al., 2008, Hesse et al.). Caspase-3 is a cysteine-aspartic protease that plays an essential role in apoptosis and is activated in dying OLs. An increased level of calpain (Figure 4B i) and caspase-3 (not shown) immunoreactivity was seen in the CC of the vehicle-treated EAE group compared to the DPN-treated EAE group. Analysis of calpain and caspase-3 by Western analysis showed a significant decrease in DPN-treated CC lysate as compared to vehicle-treated EAE CC lysate (Figure 4B ii-iii). Taken together, these results show an increase in apoptosis and cell death of OLs during EAE that is decreased significantly with DPN treatment.
DPN treatment increases BDNF levels during EAE
The survival of OLs with neurotrophic support can enhance axon myelination (Barres et al., 1993). DPN-induced OL survival and myelination may involve a direct or indirect effect on growth factors, brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), and related receptors tyrosine kinase receptor-type 2 (Trk-B) and insulin-like growth factor receptor 1 (IGFR-1). These growth factors and related receptors have been linked to OL survival and myelination. We found that DPN treatment during EAE caused a significant increase in BDNF immunoreactivity in the CC (Figure 4Ci), but no change was observed in Trk-B immunostaining (Figure 4C i) compared to vehicle-treated EAE animals (not shown). A significant reduction in the expression level of BDNF protein in the vehicle-treated EAE group compared to normal controls and DPN-treated EAE group was also confirmed by Western analysis (Figure 4C ii-iii). No difference in Trk-B protein level was observed between groups (Figure 4C ii-iii). Unlike increased BDNF immunoreactivity in CC, there was no change in IGF-1 immunostaining between groups. In contrast, increased levels of IGFR-1 immunostaining were observed in OLs of DPN-treated EAE groups (Figure 4D i). However, IGF-1 and IGFR-1 protein levels did not show a significant difference between groups, as assessed by Western analysis (Figure 4D ii-iii).
DPN treatment mediates phosphorylation of PI3K/Akt/mTOR proteins
Activation of signaling molecules via Trk-B/BDNF and IGF-1/Akt/mTOR pathways regulate transcription, translation, proliferation, and survival of OLs (Zeger et al., 2007, Van't Veer et al., 2009, Bibollet-Bahena et al., 2009). The abundant co-expression of estrogen, BDNF, IGF-1, and appropriate receptors in neurons and OLs indicates that crosstalk between the intracellular signaling pathways activated by growth factor receptor(s) and ERs is possible in many brain cells (Sohrabji et al., 2006). Given the significant effect of DPN on OLs and axon myelination, we aimed to identify cell signaling intermediate proteins in the OL-rich CC of the brain and dorsal column of spinal cords. We performed immunohistochemistry of brain slices and spinal cords and Western analysis of dissected CC protein lysates to investigate the involvement of PI3K/Akt/mTOR proteins.
Non-phosphorylated PI3K, Akt, or mTOR proteins were not different between normal, EAE+vehicle, and EAE+DPN groups as observed by Western analysis (Figure 5A-C). Phosphorylated (p)-PI3K was up regulated in mature OLs of EAE+DPN CC but not EAE+vehicle CC, as measured by immunohistochemistical analysis (Figure 5A). The p-PI3K levels measured by Western analysis showed a significant increase in EAE+DPN groups as compared to both EAE+vehicle and normal groups (Figure 5A ii-iii). Increased p-Akt protein levels were observed in vehicle-treated EAE protein lysates compared to the normal group. The p-Akt levels in EAE+DPN group were significantly higher than both the EAE+vehicle and normal groups (Figure 5B i-ii). The p-mTOR immunoreactivity was significantly increased in EAE+DPN myelinated fibers of CC (and spinal cord) and co-stained with myelin basic protein and differentiated OLs (Figure 5C i). In addition, this increase in p-mTOR protein in the EAE+DPN group was confirmed by Western analysis (Figure 5C ii-iii).
Figure 5. DPN treatment during EAE activates the PI3K/Akt/mTOR cell signaling pathway in the CC.
(A) Treatment with DPN during EAE has shown increased myelination. To address the potential involvement of PI3K/Akt/mTOR signaling pathway in DPN-treated EAE animals, we performed immunohistochemistry of CC-containing brain slices andWestern analysis of dissected callosal protein lysates. Using immunohistochemistry, robust p-PI3K immunostaining was seen co-labeled with CC1+ OLs (i). Likewise, by Western analysis, DPN-treated EAE mice revealed an elevated level of p-PI3K (**p<0.001) to support the role of DPN-mediated activation of PI3K (ii-iii). (B) Western analysis of Akt, p-Akt (Thr308), and GAPDH revealed no significant difference in the levels of Akt between, normal, EAE+vehicle, and EAE+DPN groups (i). However, a significant increase in p-Akt was detected in DPN-treated EAE mice to confirm the activation of p-Akt in response to upstream signaling events (*p<0.05; **p<0.001) (i-ii). (C) Activation of the mTOR pathway in DPN-treated EAE animals was assessed by immunohistochemistry, which revealed the presence of p-mTOR (Ser2448) in the CC and spinal cord dorsal column (i). Western analysis of mTOR, p-mTOR, and GAPDH presents a three-fold increase in p-mTOR levels in DPN-treated EAE mice relative to normal mice (ii-iii). (D) DPN treatment during EAE enhances P70S6K activation downstream of mTOR pathway to facilitate protein synthesis. Western analysis of P70S6K, p-P70S6K (Thr389), and GAPDH revealed undetectable levels of p-P70S6K in EAE+vehicle mice as compared to normal controls. DPN-treated EAE protein lysates showed a significant increase in p-P70S6K to confirm the activation of DPN-mediated mTOR signaling pathway (i-ii) (**p<0.001;ANOVA; n=6).
To confirm activation of p-mTOR, we investigated the status of critical downstream mTOR substrates, particularly P70 ribosomal protein S6 kinase (P70S6K), which regulates protein synthesis and phosphatase, and tensin homolog (PTEN), which potentially inhibits PI3K phosphorylation. We observed a significant reduction in phosphorylated (p)-P70S6K in the vehicle-treated EAE group (Figure 5D ii-iii) compared to DPN-treated EAE and normal groups. Surprisingly, an increase in p-PTEN was observed in EAE+DPN protein lysates compared to EAE+vehicle protein lysates (data not shown). Taken together, these results suggest that DPN activates the PI3K/Akt/mTOR pathway, a pathway necessary for OL survival and OL differentiation (Figure 6).
Figure 6. DPN-induced effect on callosal myelination during EAE is not a rapid effect, but requires sustained treatment.
(A) Active EAE was initiated in 8 week old C57BL/6 PLP_EGFP female mice. Animals were scored using the standard EAE grading scale and subjected to the Rotorod motor performance test (D). To address the potential timing of PI3K/Akt/mTOR pathway stimulation by DPN to enhance OL survival and axon myelination, DPN treatment was initiated post peak disease, at Day 21 in 2 groups of mice. A group of mice was injected with vehicle (red circles). Brains were collected after 4 (dark green circles) and 24 days (light green circles) of DPN treatment. (B, E) CC protein lysate were analyzed for phosphorylated and non-phosphorylated forms of Akt and mTOR proteins. (A, C, D) No significant difference in EAE clinical sores, rotorod performance, or protein expression was observed between EAE+vehicle and postEAE+DPN (DPN treatment for 4 days) groups. (A, D, F) In contrast, a significant improvement in EAE clinical scores, rotorod motor performance, and p-Akt and p-mTOR protein levels were observed in postEAE+DPN animals treated for 24 days (*p<0.05; **p<0.001; ANOVA; n=6-10 animals). Data are representative of experiments repeated twice.
DPN-induced increases in PI3K/Akt/mTOR pathway are not due to a rapid ligand-receptor binding response
ER ligands are known to affect different cell types immediately, potentially by binding to mERs and G protein-coupled receptors and downstream changes in calcium signaling (Thomas et al., 2005). During EAE, DPN treatment does not initially suppress clinical disease symptoms (Tiwari-Woodruff et al., 2007, Crawford et al., 2010). Instead, the DPN-mediated activation of the signaling pathway required for OL differentiation and remyelination appears to culminate over an extended period of continuous treatment with DPN.
We were interested in assessing potential immediate effects of DPN treatment. However, interpretation of these results would be difficult due to in vivo limitations of immediate drug access to the brain and the time required processing the tissue. Peak EAE disease symptoms are present by post-immunization Day 21 and, when it is started on this day, therapeutic DPN treatment ameliorates clinical disease significantly within two weeks of treatment (Figure 1 and 6A). Thus, the immediate effect of DPN treatment in EAE animals was assessed by starting DPN treatment on Day 21. DPN or vehicle injections were given to EAE mice for 4 or 24 days. Mice were assigned daily standard EAE clinical disease scores and subjected to a more rigorous Rotorod motor performance test (Figure 6A-B). Vehicle-treated EAE mice demonstrated an increase in EAE clinical scores beginning at Day 12 (Figure 6A). This increase in clinical scores corresponded with an abrupt and consistent decrease in the time for which the mice were able to remain on the rotorod, and this disability persisted throughout the observation period. DPN treatment started on Day 21 for 4 days did not result in any changes in the clinical scores or rotorod performance. Whereas, DPN treatment started on Day 21 and continued for 24 days resulted in significant recovery of EAE clinical scores and rotorod motor performance beginning after 7 days of DPN treatment, and this recovery persisted throughout. In contrast, the vehicle-treated EAE group did not show recovery of EAE clinical scores or rotorod performance.
On the fourth day and twenty fourth day of DPN treatment, the CC was dissected, collected, homogenized, and subjected to Western analysis of Akt, p-Akt, mTOR, and p-mTOR proteins. No significant difference in non-phosphorylated or phosphorylated Akt and mTOR were observed in the 4 day treated group, which correlated with the group's lack of improvement in EAE clinical score and rotorod motor performance (Figure 6A, B, C, E). Longer periods of DPN treatment (i.e., 24 days) were found to initiate significant changes in phosphorylated levels of Akt and mTOR signaling pathways as compared to vehicle-treated groups and short term DPN treatment (i.e., 4 day) (Figure 6C-E).
Discussion
This is the first study to show a direct protective effect of therapeutic treatment on OLs, ultimately leading to remyelination and functional axon conduction in an inflammatory demyelinating disease. The increased number of OLs results from a combination of DPN-induced cell protection and generation, as an overall decrease in calpain/caspase/TUNEL activity in combination with an increase in proliferating OLPs was observed. The effectiveness of therapeutic treatment with the ERβ ligand DPN was somewhat surprising, as significant demyelination and axon damage is visible by post-immunization Day 21 of EAE (Mangiardi et al., 2011, Tiwari-Woodruff et al., 2007). We do not believe that DPN reverses axon damage, but we do believe it induces recovery as a result of remyelination and attenuates ongoing axon damage. Nonetheless, DPN can be a source of cautious optimism for people diagnosed with MS. Patients are often diagnosed with MS only after the first few symptomatic episodes, and therapeutic treatment with DPN-like ERβ ligands could aid in recovery and/or decrease disease progression. Further, recent studies have shown that anxiolytic effects are mediated by ERβ (Oyola et al., 2012). As an anxiolytic agent, DPN could provide added benefit to MS patients, 30% of whom display anxiety-related symptoms. DPN will likely be well-tolerated in both males and females, as negative effects like breast and uterine endometrial cancer are thought to be mediated by general estrogen use (Caringella et al., 2011). Moreover, ERβ ligand treatment circumvents the feminizing effects of estrogens, which are a barrier for treatment of male MS patients.
Therapeutic treatment with DPN in EAE animals appeared to inhibit demyelination, inhibit axon damage, and stimulate remyelination similar to levels achieved with a prophylactic treatment regimen (Crawford et al., 2010). The mechanism of action by which estrogens and ER ligands influence OL function and myelination is not known. Here, we aimed to understand the mechanism of action of DPN by identifying various receptors and signaling molecules that may regulate ERβ-induced transcriptional activity in vivo (DonCarlos et al., 2009). DPN treatment during EAE elicited a significant increase in OL ERβ expression and increase in callosal ERβ phosphorylation. Increased ERβ expression is known to protect the CNS from a wide range of disorders (Hernandez-Fonseca et al., 2012, Aguirre et al., 2010, Meltser et al., 2008). DPN-induced increase in ERβ phosphorylation can regulate ERβ transcriptional activity and influence pathways involved in increasing OLP survival and axon myelination (Sanchez et al., 2010).
Estrogen and various growth factors such as BDNF and IGF-1 activate similar signaling pathways that lead to OL differentiation and myelination (Bibollet-Bahena et al., 2009, Tyler et al., 2009, Narayanan et al., 2009, Kumar et al., 2006, Halbreich et al., 1990, McEwen, 2002, Van't Veer et al., 2009, Varea et al., 2010, Garcia-Segura et al., 2010, Hirahara et al., 2009). Both estradiol and BDNF enhance differentiation of OLPs through the Trk-B receptor (Du et al., 2006, Van't Veer et al., 2009). Reduced levels of BDNF, reduced OLP numbers, and decreased myelin proteins were observed in transgenic mice heterozygous for BDNF (Vondran et al., 2010, VonDran et al., 2011). Similarly, our study points to the significance of BDNF for potential OLP proliferation and OL differentiation, as a significant reduction in BDNF is observed during EAE in conjunction with a decrease in OL numbers and demyelination. Conversely, a significant increase in BDNF expression levels within DPN-treated EAE mice is observed.
The essential role of IGF-1 in OL development and in promoting myelination is well-recognized (Bibollet-Bahena et al., 2009, Tyler et al., 2009, Chesik et al., 2008, Zeger et al., 2007). IGF-1 binding to IGF-1R initiates a protein tyrosine kinase and PI3K, leading to activation of Akt, which plays a role in cell survival. There is an abundant co-expression of estrogen and IGF-1/Trk-B receptors in neurons and OLs. Thus, interactions of the intracellular signaling pathways activated by growth factor receptors and ERs are possible in many brain cells. Studies have shown that Akt is phosphorylated by PI3K in response to growth factors (i.e., IGF-1) that promote myelination (Carson et al., 1993). p-Akt is involved in cell survival and exerts its anti-apoptotic response to prevent cell death. During EAE, OL death has been linked to increased calpain and caspase-3 (Guyton et al., 2010). In this study, we also observed an increase in calpain/caspase-3 activity and a concomitant decrease in Akt phosphorylation in EAE that was successfully reversed during prophylactic and therapeutic DPN treatment. It is noteworthy that activity of these signaling molecules is sustained in EAE brain tissue under continuous DPN treatment for several days. This observation presents a unique feature of ERβ that differs from classical short-term activation of Akt pathway. In essence, this controlled, continued activation of Akt pathway needed for repair of demyelinated regions resembles the genetically modified mouse model that permanently expresses Akt and is described as undergoing continued myelination (Flores et al., 2008). Akt has emerged as an integrator of various signals that increase myelination in the CNS and PNS.
PI3K/Akt signaling activates mTOR. Inhibition of mTOR in vivo limits myelination during development (Narayanan et al., 2009). mTOR activity includes phosphorylation and inactivation of a negative regulator of protein synthesis initiation, 4E-binding protein, and a positive regulator of protein synthesis, P70S6K (Bibollet-Bahena et al., 2009, Tyler et al., 2009, Hay et al., 2004). We observed a robust increase in phosphorylation of mTOR and P70S6K (Thr389). P70S6K facilitates assembly of the ribosome complex and activates translation by phosphorylating ribosomal protein S6. A similar increase in phosphorylation of P70S6K, along with its major substrate S6RP, was seen in callosal OLs of transgenic Akt-overexpressing mice (Narayanan et al., 2009, Jastrzebski et al., 2007). We explored the status of PTEN, a negative regulator of PI3K, in order to better understand ERβ signaling. Myelinating glial cells lacking PTEN are hyperstimulated by elevated PIP3 levels, which is sufficient to trigger membrane wrapping and hypermyelination in CNS and PNS (Goebbels et al., 2010). A similar effect is seen in conditional knockout of PTEN in OLs (Harrington et al., 2010). Against the predicted results, p-PTEN levels decreased in vehicle-treated EAE animals, whereas the levels were increased in DPN-treated EAE animals. CC lysate contains other cells (microglia and astrocytes) along with OLs, and proteins like p-PTEN may have dual roles. We are currently investigating the possibility that the PTEN levels primarily reflect changes in cells other than OL.
The association of estrogens with IGF-1R/Trk-B through the PI3K/Akt/mTOR signaling pathway may illustrate the point of convergence used by estrogen and IGF-1/BDNF to promote OLP proliferation, OL differentiation, and survival after demyelination (Tyler et al., 2009, Narayanan et al., 2009, Bibollet-Bahena et al., 2009, Vondran et al., 2010, VonDran et al., 2011). To further confirm the direct effect of DPN in OL cell lineage, more complex loss of function studies such as ERβ knock-out in OL cell lineage by cre-lox technology and use of rapamycin to block mTOR activity (Narayanan et al., 2009) prior to DPN-treatment in EAE mice will be required. Our study suggests a working model by which DPN treatment causes a potential increase in OLP proliferation, a decrease in OL apoptosis, and an increase in myelination by increasing phosphorylation of various proteins in the PI3K/Akt/mTOR pathway (Figure 7).
Figure 7. A working model of DPN-induced second messenger signaling that ultimately leads to increased myelination.
DPN binding to OL ERβ induces growth factor (BDNF/Trk-B and IGF-1/IGF-1R) activated phosphorylation of ERβ to pERβ. Such phosphorylation could facilitate the recruitment of unknown co-activators and various enzymes including PI3K. Ligand-mediated activation and autophosphorylation of Src family of tyrosine kinase initiate downstream signaling cascade by phosphorylation and activation. Downstream of PI3K, the pathway activates Akt and mTOR. Phosphorylated Akt activates a number of different pathways and plays an important role in decreasing apoptotic markers, such as calpain and caspase-3, to prevent cell death (as we have described in this report). A key target of p-Akt activation is mTOR, which controls protein synthesis by activating P70S6K. Phosphorylated P70S6K further phosphorylates S6, which is required for protein synthesis to induce cell survival, cell differentiation, and axon myelination.
The ERβ ligand, DPN, used in our previously published work and the present study, is a generic racemic mixture with a 70-fold higher relative binding affinity for ERβ over ERα and 170-fold higher relative potency in transcription assays (Meyers et al., 2001). Even with such low selectivity, DPN treatment at a dose of 8 mg/kg works specifically through ERβ. This dose does not activate ERα significantly, as ERβ-null mice with EAE are not affected by DPN (Tiwari-Woodruff et al., 2007). In addition, normal and EAE animals treated with 8 mg/kg and 10 mg/kg DPN daily for nearly 40 days did not show any toxic effects of treatment in various organs, nor did we observe toxicity-suggesting behavior. Along with DPN, other ERβ ligands with better affinity for ERβ could be developed as therapeutic alternatives for neuroprotective drugs capable of remyelination. In fact, there are several synthetic and natural ERβ-selective compounds (e.g., WAY-202041, WAY-200070, MF101, nyasol, liquiritigenin, Indazole-Cl, and R- and S-enantiomers of DPN) with better affinity and selectivity than DPN (>200-fold selectivity for ERβ over ERα) that illustrate various positive effects in the CNS (Weiser et al., 2009, Saijo et al., 2011, Minutolo et al., 2001, Paruthiyil et al., 2009). To address the inflammatory component of MS disease, therapeutic effectiveness of combination therapies (i.e., ERβ ligand plus immunomodulatory agent) should be further examined.
In conclusion, we have demonstrated that both prophylactic and therapeutic treatment with the ERβ ligand DPN ameliorate EAE clinical disease. DPN treatment has a direct protective effect on OLs, ultimately leading to remyelination and functional axon conduction. DPN treatment in EAE animals phosphorylated ERβ and activated the PI3K/Akt/mTOR signaling pathway, a pathway required for oligodendrocyte survival and axon myelination. Future studies will entail finding the most effective therapeutic ERβ ligand capable of prompting remyelination and neuroprotection.
Research highlights.
▶ Decrease in EAE clinical disease with therapeutic estrogen receptor β ligand, DPN.
▶ DPN improves axon conduction and rotorod motor performance in EAE mice.
▶ DPN increases ERβ phosphorylation and activates PI3K/Akt/mTOR signaling pathway
▶ Therapeutic DPN induces remyelination during EAE.
Acknowledgements
This work was generously supported by NMSS grant RG 4538-A-2 and NIH grant R21NS075198 to STW. We would like to thank Dr. Wendy Macklin and Dr. Terri Wood for helpful discussions and Ms. Anna Khalaj for manuscript editing. We would also like to thank the BRI core facility at UCLA for EM assistance and Sasol, North America for the generous gift of miglyol oil.
Abbreviations
- MS
multiple sclerosis
- EAE
experimental autoimmune encephalomyelitis
- CNS
central nervous system
- ER
estrogen receptor
- DPN
2,3-bis(4-hydroxyphenyl)-propionitrile
- OL
oligodendrocyte
- OLP
oligodendrocyte progenitor
- RRMS
relapsing-remitting MS
- PLP_EGFP
proteolipid protein-enhanced green fluorescent protein
- MOG
myelin oligodendrocyte glycoprotein
- CC
corpus callosum
- CAP
compound action potential
- IPI
interpulse interval
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling
- CD
cluster of differentiation
- GAPDH
glyceraldehyde phosphate dehydrogenase
- PI3K
phosphatidylinositol 3-kinase
- Akt
serine–threonine-specific protein kinase
- mTOR
mammalian target of rapamycin
- P70S6K
P70 ribosomal protein S6 kinase
- PTEN
phosphatase and tensin homolog
- GFAP
glial fibrillary acidic protein
- MBP
myelin basic protein
- PDGFRα
platelet-derived growth factor receptor-α
- olig2
OL transcription factor 2
- CC1
adenomatus polyposis coli
- DAPI
4’,6-diamidino-2-phenylindole
- BDNF
brain-derived neurotrophic factor
- IGF-1
insulin-like growth factor-1
- Trk-B
tyrosine kinase receptor-type 2
- IGFR-1
insulin-like growth factor receptor 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre C, Jayaraman A, Pike C, Baudry M. Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-beta. J Neurochem. 2010;115:1277–1287. doi: 10.1111/j.1471-4159.2010.07038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Jacobson MD, Schmid R, Sendtner M, Raff MC. Does oligodendrocyte survival depend on axons? Curr Biol. 1993;3:489–497. doi: 10.1016/0960-9822(93)90039-q. [DOI] [PubMed] [Google Scholar]
- Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109:1440–1451. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Hungs M, Mull M, Topper R, Noth J. Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalogr Clin Neurophysiol. 1998;109:230–237. doi: 10.1016/s0924-980x(98)00013-7. [DOI] [PubMed] [Google Scholar]
- Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502:236–260. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- Caringella AM, Di Naro E, Loverro G. [Clinical function of estrogen receptors in endometrial cancer]. Minerva ginecologica. 2011;63:495–504. [PubMed] [Google Scholar]
- Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–740. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- Chesik D, De Keyser J, Wilczak N. Insulin-like growth factor system regulates oligodendroglial cell behavior: therapeutic potential in CNS. J Mol Neurosci. 2008;35:81–90. doi: 10.1007/s12031-008-9041-2. [DOI] [PubMed] [Google Scholar]
- Crawford DK, Mangiardi M, Song B, Patel R, Du S, Sofroniew MV, Voskuhl RR, Tiwari-Woodruff SK. Oestrogen receptor {beta} ligand: a novel treatment to enhance endogenous functional remyelination. Brain. 2010 doi: 10.1093/brain/awq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DK, Mangiardi M, Tiwari-Woodruff SK. Assaying the functional effects of demyelination and remyelination: revisiting field potential recordings. J Neurosci Methods. 2009a;182:25–33. doi: 10.1016/j.jneumeth.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Crawford DK, Mangiardi M, Xia X, Lopez-Valdes HE, Tiwari-Woodruff SK. Functional recovery of callosal axons following demyelination: a critical window. Neuroscience. 2009b;164:1407–1421. doi: 10.1016/j.neuroscience.2009.09.069. [DOI] [PubMed] [Google Scholar]
- Das A, Guyton MK, Butler JT, Ray SK, Banik NL. Activation of calpain and caspase pathways in demyelination and neurodegeneration in animal model of multiple sclerosis. CNS Neurol Disord Drug Targets. 2008;7:313–320. doi: 10.2174/187152708784936699. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Azcoitia I, Garcia-Segura LM. Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology. 2009;34(Suppl 1):S113–122. doi: 10.1016/j.psyneuen.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Clinton-Luke P, Lercher LD, Dreyfus CF. Distinct effects of p75 in mediating actions of neurotrophins on basal forebrain oligodendrocytes. Mol Cell Neurosci. 2006;31:366–375. doi: 10.1016/j.mcn.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The Mouse Brain: in Stereotaxic Coordinates. Academic Press; 2001. [Google Scholar]
- Garcia-Segura LM, Arevalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res. 2010;181:251–272. doi: 10.1016/S0079-6123(08)81014-X. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30:8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton MK, Das A, Samantaray S, Wallace GCt, Butler JT, Ray SK, Banik NL. Calpeptin attenuated inflammation, cell death, and axonal damage in animal model of multiple sclerosis. J Neurosci Res. 2010;88:2398–2408. doi: 10.1002/jnr.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Lemus CZ, Lieberman JA, Parry B, Schiavi RC. Gonadal hormones, sex and behavior. Psychopharmacol Bull. 1990;26:297–301. [PubMed] [Google Scholar]
- Harrington EP, Zhao C, Fancy SP, Kaing S, Franklin RJ, Rowitch DH. Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Ann Neurol. 2010;68:703–716. doi: 10.1002/ana.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hernandez-Fonseca K, Massieu L, Garcia de la Cadena S, Guzman C, Camacho-Arroyo I. Neuroprotective role of estradiol against neuronal death induced by glucose deprivation in cultured rat hippocampal neurons. Neuroendocrinology. 2012;96:41–50. doi: 10.1159/000334229. [DOI] [PubMed] [Google Scholar]
- Hesse A, Wagner M, Held J, Bruck W, Salinas-Riester G, Hao Z, Waisman A, Kuhlmann T. In toxic demyelination oligodendroglial cell death occurs early and is FAS independent. Neurobiol Dis. 2010;37:362–369. doi: 10.1016/j.nbd.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara Y, Matsuda K, Gao W, Arvanitis DN, Kawata M, Boggs JM. The localization and non-genomic function of the membrane-associated estrogen receptor in oligodendrocytes. Glia. 2009;57:153–165. doi: 10.1002/glia.20742. [DOI] [PubMed] [Google Scholar]
- Hobom M, Storch MK, Weissert R, Maier K, Radhakrishnan A, Kramer B, Bahr M, Diem R. Mechanisms and time course of neuronal degeneration in experimental autoimmune encephalomyelitis. Brain Pathol. 2004;14:148–157. doi: 10.1111/j.1750-3639.2004.tb00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25:209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- Kumar S, Biancotti JC, Yamaguchi M, de Vellis J. Combination of Growth Factors Enhances Remyelination in a Cuprizone-induced Demyelination Mouse Model. Neurochem Res. 2006 doi: 10.1007/s11064-006-9208-6. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Cannella B, Mazzaccaro RJ, Clements JM, Miller KM, Wucherpfennig KW, Gearing AJ, Raine CS. Effective treatment of models of multiple sclerosis by matrix metalloproteinase inhibitors. Ann Neurol. 1998;44:35–46. doi: 10.1002/ana.410440110. [DOI] [PubMed] [Google Scholar]
- MacKenzie-Graham A, Tiwari-Woodruff SK, Sharma G, Aguilar C, Vo KT, Strickland LV, Morales L, Fubara B, Martin M, Jacobs RE, Johnson GA, Toga AW, Voskuhl RR. Purkinje cell loss in experimental autoimmune encephalomyelitis. NeuroImage. 2009;48:637–651. doi: 10.1016/j.neuroimage.2009.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon BS, Macklin WB. Overexpression of the 3'-untranslated region of myelin proteolipid protein mRNA leads to reduced expression of endogenous proteolipid mRNA. Neurochem Res. 2002;27:1349–1360. doi: 10.1023/a:1021623700009. [DOI] [PubMed] [Google Scholar]
- Mangiardi M, Crawford DK, Xia X, Du S, Simon-Freeman R, Voskuhl RR, Tiwari-Woodruff SK. An animal model of cortical and callosal pathology in multiple sclerosis. Brain Pathol. 2011;21:263–278. doi: 10.1111/j.1750-3639.2010.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejuk A, Bakke AC, Hopke C, Dwyer J, Vandenbark AA, Offner H. Estrogen treatment induces a novel population of regulatory cells, which suppresses experimental autoimmune encephalomyelitis. J Neurosci Res. 2004;77:119–126. doi: 10.1002/jnr.20145. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA, Canlon B. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118:1563–1570. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Minutolo F, Bertini S, Papi C, Carlson KE, Katzenellenbogen JA, Macchia M. Salicylaldoxime moiety as a phenolic “A-Ring” substitute in estrogen receptor ligands. J Med Chem. 2001;44:4288–4291. doi: 10.1021/jm010948j. [DOI] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29:6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyola MG, Portillo W, Reyna A, Foradori CD, Kudwa A, Hinds L, Handa RJ, Mani SK. Anxiolytic effects and neuroanatomical targets of estrogen receptor-beta (ERbeta) activation by a selective ERbeta agonist in female mice. Endocrinology. 2012;153:837–846. doi: 10.1210/en.2011-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk A, Smith SA, Gordon-Lipkin EM, Harrison DM, Shiee N, Pham DL, Caffo BS, Calabresi PA, Reich DS. MRI of the corpus callosum in multiple sclerosis: association with disability. Mult Scler. 2010;16:166–177. doi: 10.1177/1352458509353649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruthiyil S, Cvoro A, Zhao X, Wu Z, Sui Y, Staub RE, Baggett S, Herber CB, Griffin C, Tagliaferri M, Harris HA, Cohen I, Bjeldanes LF, Speed TP, Schaufele F, Leitman DC. Drug and cell type-specific regulation of genes with different classes of estrogen receptor beta-selective agonists. PLoS One. 2009;4:e6271. doi: 10.1371/journal.pone.0006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, Johnson KC, Kuller LH, Lane DS, Wactawski-Wende J, Brzyski R, Allison M, Ockene J, Sarto G, Rossouw JE. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170:12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S, Wang Y, Kivisakk P, Bronson RT, Meyer M, Imitola J, Khoury SJ. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing--remitting experimental autoimmune encephalomyelitis. Brain. 2007;130:2816–2829. doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERbeta-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145:584–595. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Picard N, Sauve K, Tremblay A. Challenging estrogen receptor beta with phosphorylation. Trends Endocrinol Metab. 2010;21:104–110. doi: 10.1016/j.tem.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27:404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigard K, Holmdahl R, Olsson T. Oestrogen treatment reduces duration of experimental allergic neuritis in rats and suppresses T cell responses to myelin. Acta Neurol Scand. 1990;81:436–442. doi: 10.1111/j.1600-0404.1990.tb00991.x. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci U S A. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Voskuhl RR. Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice. J Neurol Sci. 2009;286:81–85. doi: 10.1016/j.jns.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Gangoli N, Gokina P, Kim HA, Covey M, Levison SW, Wood TL. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J Neurosci. 2009;29:6367–6378. doi: 10.1523/JNEUROSCI.0234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Veer A, Du Y, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res. 2009;87:69–78. doi: 10.1002/jnr.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varea O, Arevalo MA, Garrido JJ, Garcia-Segura LM, Wandosell F, Mendez P. Interaction of estrogen receptors with insulin-like growth factor-I and Wnt signaling in the nervous system. Steroids. 2010;75:565–569. doi: 10.1016/j.steroids.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Vondran MW, Clinton-Luke P, Honeywell JZ, Dreyfus CF. BDNF+/- mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia. 2010;58:848–856. doi: 10.1002/glia.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VonDran MW, Singh H, Honeywell JZ, Dreyfus CF. Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion. J Neurosci. 2011;31:14182–14190. doi: 10.1523/JNEUROSCI.6595-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV. Reactive Astrocytes Form Scar-Like Perivascular Barriers to Leukocytes during Adaptive Immune Inflammation of the CNS. J Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warlop NP, Achten E, Debruyne J, Vingerhoets G. Diffusion weighted callosal integrity reflects interhemispheric communication efficiency in multiple sclerosis. Neuropsychologia. 2008;46:2258–2264. doi: 10.1016/j.neuropsychologia.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensky AK, Furtado GC, Marcondes MC, Chen S, Manfra D, Lira SA, Zagzag D, Lafaille JJ. IFN-gamma determines distinct clinical outcomes in autoimmune encephalomyelitis. J Immunol. 2005;174:1416–1423. doi: 10.4049/jimmunol.174.3.1416. [DOI] [PubMed] [Google Scholar]
- Wu E, Raine CS. Multiple sclerosis. Interactions between oligodendrocytes and hypertrophic astrocytes and their occurrence in other, nondemyelinating conditions. Lab Invest. 1992;67:88–99. [PubMed] [Google Scholar]
- Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, Nave KA, Rowitch D, D'Ercole AJ, Ye P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–411. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. 2010;90:774–786. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]