Abstract
Immunological memory has traditionally been regarded as a unique feature of the adaptive immune response, mediated in an antigen-specific manner by T and B lymphocytes. All other hematopoietic cells, including natural killer (NK) cells, are classified as innate immune cells, which have been considered short-lived but can respond rapidly against pathogens in a manner not thought to be driven by antigen. Interestingly, NK cells have recently been shown to survive long term after antigen exposure and subsequently mediate antigen-specific recall responses. In this review, we will address the similarities between, and the controversies surrounding, three major viewpoints of NK memory that have arisen from these recent studies: (i) MCMV (mouse cytomegalovirus)-induced memory; (ii) cytokine-induced memory; (iii) liver-restricted memory cells.
Keywords: NK cells, adaptive immunity, immunological memory
What defines an immune memory cell?
Immunological memory is the ability of certain immune cells to “remember” a previous encounter with a pathogen and provide an enhanced response upon secondary encounter with the same pathogen. Memory cells are long-lived, respond more robustly to secondary infection and are phenotypically and epigenetically distinct from their naïve counterparts. Memory T cell formation is well characterized, and can be divided into three stages [1]. In the first stage, the “expansion” phase, naïve T cells clonally expand and differentiate into effector T cells after encounter with cognate antigen. In the second stage, the “contraction” phase, the majority (>90%) of effector T cells undergo apoptosis and surviving T cells enter the third stage - the “memory” phase. These long-lived T cells reside throughout the body and maintain their numbers through self-renewal. Memory T cells undergo robust expansion and heightened effector function upon re-encounter with their cognate antigen, termed the recall response. Antigen-specificity, a hallmark of adaptive immunity, is considered a prerequisite for the development of immunological memory. Innate immune cells, such as Natural Killer (NK) cells, lack the ability to undergo somatic rearrangements of their receptors and are thus thought to be incapable of forming memory. This concept is being challenged now as evidence for NK cell memory emerges. Once considered purely short-lived, rapid responders, NK cells now straddle the line between innate and adaptive immunity, possessing limited antigen-specificity, an extended lifespan, and mediating enhanced recall responses. Notably, not all of the classic features of adaptive immune memory have been demonstrated for memory NK cells. Recent NK cell research is challenging the concept of immunological memory, and is providing evidence for: 1) Antigen-specific NK memory cells induced by MCMV (mouse cytomegalovirus) infection, 2) NK memory cells induced by exposure to cytokines alone, and 3) Liver-restricted NK memory cells with highly antigen-specific recall responses. Here we will discuss how these emerging three perspectives differ in their definition of NK memory, and how memory NK cells diverge from classic adaptive memory. These features will be critical for understanding how, and to what extent, NK cells might enhance vaccination strategies.
Memory NK cells after MCMV infection: mimicking their T cell cousins?
Although NK cells are classically referred to as innate cells, they share many properties with their adaptive lymphocyte cousins; in particular, CD8+ T cells [2]. NK cells and T cells share a common lymphoid progenitor [3], express many common cell surface markers [4], and most importantly, function in a similar way in response to stimulus, producing cytokines and mediating cytotoxicity through the release of perforin and granzymes [1,4,5]. Could these shared attributes with T cells extend to the recently described “memory-like” properties of NK cells? Work by our group and others has established a view of NK cell memory that is based on viral antigen-driven proliferation through specific ligand/receptor interaction; this in turn generates a self-renewing, long-lived memory population with enhanced ability to respond to a secondary challenge [6].
Infection of mice with mouse cytomegalovirus (MCMV) is a well-established model of NK cell-virus interaction and highlights the critical nature of NK cells in resistance to viral infection. C57BL/6 mice carry the activating Ly49H receptor on ~50% of NK cells, which specifically recognizes the m157 MCMV protein on infected cells; this ligand-receptor interaction drives Ly49H–dependent expansion of NK cells during the acute phase of infection [7–9]. Using a system in which Ly49H+ NK cells were adoptively transferred into mice lacking this receptor, we showed prolific antigen-driven expansion of these Ly49H+ cells after MCMV infection. Expansion is followed by a contraction phase and the establishment of a long-lived pool of “memory” Ly49H+ cells, detectable as late as 70 days post infection [10]. This memory NK cell pool undergoes secondary expansion, displays enhanced effector function ex vivo, and offers increased protection against MCMV challenge, compared to naïve NK cells [10]. In a manner similar to a CD8+ T cell response, the initial expansion is dependent on interaction with antigen (m157); MCMV lacking this protein does not induce Ly49H+ proliferation or the establishment of a memory pool [10].
This antigen-driven expansion is critically dependent on interleukin (IL)-12 signaling [11]. NK cells lacking the IL-12 receptor do not proliferate in response to MCMV and fail to generate NK memory cells that can protect mice against challenge [11]. In addition, STAT4, but surprisingly not IFN-γ, signaling is important for the NK cell response to MCMV and the generation of NK memory [11]. These findings highlight the importance of cytokine signaling together with the m157-Ly49H interaction in driving NK expansion. It is unclear if the deficiencies in cytokine signaling directly impede these NK cells from becoming “memory” cells, or if there is simply a requirement for NK cell proliferation prior to the establishment of memory. Moreover, the contraction phase may also be a critical determinant in the development of NK memory [1,8,10]. Effector T cell apoptosis and memory T cell formation after viral infection are dependent on Bcl-2 and Bcl-2-regulating proteins such as Bim [12–14]. Similarly, NK effector cell contraction and transition into memory relies on appropriate apoptotic signaling. Notably, the kinetics of the NK cell contraction phase after MCMV infection resemble the gradual and continuous contraction of CD4+ T cells, rather than the rapid decline and plateau of CD8+ T cells [15]. Which cues inhibit apoptosis and allow a subset of Ly49H+ NK cells to become long-lived memory NK cells? This question remains to be fully addressed, but it is likely that pro-survival cytokines, including IL-15, are involved [2].
The identification of memory NK cells using specific cell surface markers, as is the standard for memory T cells, is an important unmet need in the field. NK cells in different phases of the viral response show remarkably distinct transcriptional profiles [4]. Using data generated by the ImmGen Consortium [URL www.immgen.org], we recently interrogated the differences between NK cells isolated at various times following MCMV infection, including Ly49H+ memory NK cells [16]. Owing to the nature of the systematically generated ImmGen dataset, memory NK transcriptional profiles can be readily compared to naïve NK cells and effector NK cells isolated early (36 hrs) and late (7 days) after MCMV infection, as well as with memory CD8+ T cells isolated following infection by Listeria monocytogenes or vesicular stomatitis virus [16]. This analysis yielded a subset of genes that are specifically upregulated in the memory NK cell and CD8+ T cell compartments, compared to naïve or effector cells, including Ly6c and CD49a [16]. Whether these genes are representative of long-lived NK cells in general, or whether antigen-driven proliferation is required to generate this memory profile, remains to be addressed. Additionally, it is of interest to compare memory NK cells to memory T cells isolated from the same mouse following MCMV infection. Interestingly, expansion of NKG2C+ NK cells has also been observed during some types of virus infection in humans [Box 1]. Despite progress in identifying NK memory signature genes using transcriptional profiling, there is still a need for specific cell surface markers that would allow for the study of NK memory without the dependence on an adoptive transfer system that relies on known ligand-receptor interactions.
Box 1: The existence of human virus-induced NK memory.
Does infection by viral pathogens lead to the development of memory NK cells in humans? NK cells are critical in controlling viral infections in humans; this has been revealed through individuals with rare NK cell-specific deficiencies that present clinically with uncontrolled viral infections, particularly those of the herpesvirus family (cytomegalovirus, Epstein-Barr virus, herpes simplex virus, and varicella zoster virus), and human papillomavirus [54]. The human cytomegalovirus (HCMV) ligand-NK receptor pair has not been identified, but the activating CD94-NKG2C receptor appears to be important in CMV recognition. Human NK cells expressing NKG2C exist in high frequency in HCMV-seropositive healthy subjects, as compared with HCMV-seronegative individuals [55]. Similar to the response of Ly49H+ NK cells during MCMV infection in mice, human NKG2C+ NK cells expand greatly in allogeneic transplantation patients during acute HCMV viremia [55]. A unique subset of NK cells that expresses NKG2C at high levels and the maturation marker CD57 persists at an elevated frequency in HCMV-seropositive individuals and increases after HCMV reactivation [55–57].
Interestingly, expansion of NKG2C+ NK cells has also been observed in HCMV-seropositive patients with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) [58]. Furthermore, NKG2C+ NK cells rapidly proliferate and persist for more than 2 months following hantavirus infection [59]. The kinetics of these NK cell responses strikingly resemble the expansion of Ly49H+ NK cells after MCMV infection. In patients with chikungunya virus (CHIKV) infection, there is a transient expansion and persistent survival of NK cells co-expressing NKG2C and CD57 [60]. Of note, NKG2C+ NK cells only expand during other viral infections if the individual had previously been infected with HCMV. In contrast to infection with HCMV, infection with herpes simplex virus 2 (HSV-2) does not significantly expand a specific subset of NK cells [61]. There have been a limited number of studies in humans investigating pathogen-induced expansion of specific NK cell subsets. For example, the frequencies of 2B4bright and NKp46dull human NK cells increase following both inoculation of influenza virus vaccine and exposure of NK cells to influenza virus in vitro [62]. CD57bright NK cells, but not CD57- and CD57dim NK cells, are enriched in patients with chronic human immunodeficiency virus 1 (HIV-1) infection [63]. Finally, Wu and colleagues have described a population of NK cells from individuals infected with Mycobacterium tuberculosis that express the memory-associated marker CD45RO [64]. These CD45RO+ NK cells are found in the pleural fluid, but not the peripheral blood, of infected patients. When stimulated with IL-12 ex vivo, these cells respond more robustly than their CD45RO- counterparts: a higher frequency expresses IFN-y; they display greater cytotoxicity against K562 cells, and they express higher levels of both subunits of the IL-12R (p1 and p2). In theory, if a vaccine could be designed to induce preferential expansion of effector NK cells and subsequent differentiation into memory NK cells specific to pathogens, this could increase vaccine efficacy and provide novel therapeutic approaches to clinically relevant viral infections such as CMV, herpes, hepatitis, and HIV.
To date, the evidence supporting this particular CD8+ T cell-type view on NK memory has been limited to MCMV. Are similar NK memory cells generated in response to other pathogens? Such studies have been hampered by a lack of known pathogen ligand-NK receptor pairs in other infection models. Although it has been reported that the NKp46 receptor specifically recognizes influenza hemagglutinin (HA) [17,18], this interaction does not lead to NK cell proliferation and may only induce bystander NK cell activation due to cytokines induced during viral infection [19]. Local proliferation of NK cells in the lungs of mice infected intra-nasally with influenza has not been observed [19]. Using a mouse model of genital herpes simplex virus 2 (HSV-2) infection, Abdul-Kareem and colleagues have shown that previously exposed NK cells are able to respond more robustly to challenge, as measured by IFN-y production and protection against lethal challenge. This recall response is HSV-2 specific and is independent of B and T cells [20]. Although no specific NK receptor was identified as mediating this protection, this study supports an antigen-specific recall response of memory NK cells in a distinct model of herpes virus infection.
We have shown that NK cells can mimic the virus-specific CD8+ T cell response and undergo activation, expansion, and contraction and develop into long-lived memory cells (Figure 1, left panel). These memory NK cells can be recovered from a variety of tissues, including spleen, liver, and peripheral blood [10]. Furthermore, in this model, memory NK cells are generated only through viral antigen-driven expansion, and although they clearly have an enhanced recall response to challenge with the same pathogen [10], these MCMV studies have not addressed whether these cells can offer protection against heterologous challenge.
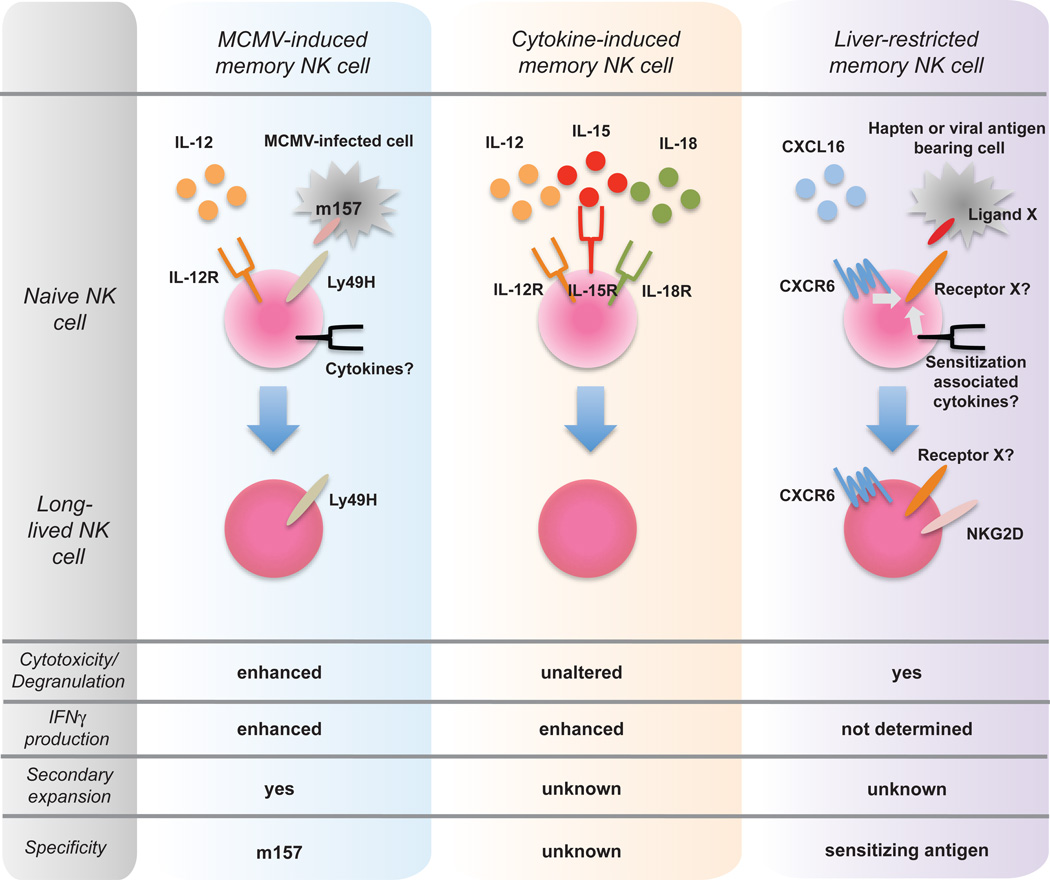
Figure 1. NK cells can take one of three paths towards becoming a memory cell.
Left panel: MCMV-induced NK memory cell is generated after the cognate recognition of the m157 MCMV protein on infected cells by the activating Ly49H receptor. Memory generation requires IL-12 and NK cell signaling through the IL-12R. Additional co-stimulatory signals by other cytokines and/or adhesion molecules might be required. Center panel: Cytokine-induced memory NK cells are generated after exposure to IL-12, IL-15, and IL-18. Sensitization with antigen is not required in this model. Right panel: Liver-restricted memory NK cell. Sensitization with haptens or specific antigens, in conjunction with CXCL16, is required to generate liver-restricted memory NK cells. The “precursors” might be selected by the cognate recognition of a hapten-modified self-proteins or foreign antigens, and develop into memory NK cells. Receptors responsible for the antigen specificity have not been identified.
Cytokine-induced “memory” NK cells
While some NK cell memory is antigen-driven, it is unlikely that NK cells can generate antigen-specific memory against a broad range of pathogens, given that they possess only germ line-encoded receptors. NK cells do, however, express receptors for and are exquisitely responsive to many cytokines, including IL-12, IL-15, and IL-18. Indeed, Yokoyama and colleagues found that activation by cytokines alone leads to the generation of NK cells with memory-like properties [21]. NK cells from Rag1 -deficient mice were activated overnight in vitro with IL-12, IL-18, and IL-15 and transferred into Rag 1-deficient recipient mice. Up to 3 weeks after transfer, a higher frequency of these pre-activated NK cells produced IFN-γ upon restimulation, compared to those NK cells that were not pre-activated. This robust response was elicited by restimulation with either cytokines (IL-12 and IL-15) or by engagement of the activating Ly49H or NK1.1 receptors. Interestingly, this enhanced response occurs both in cells that have not undergone division and those that are a product of division. Unlike viral antigen-driven memory NK cells [10], these memory-like NK cells do not possess a distinct cell surface phenotype nor do they respond with enhanced cytotoxicity upon restimulation [21].
While cytokine signaling is required for the generation of MCMV-induced memory NK cells [11], Yokoyama and colleagues showed that cytokine activation alone can elicit long-lived NK cells that provide an enhanced response upon restimulation [21]. The mechanism by which cytokine signaling generates memory-like function in NK cells is still unknown. No similar properties are found in B and T cell adaptive immune memory, as activation itself requires antigen-receptor signaling and co-stimulation [22]. However, it is possible that upon cytokine-induced activation, epigenetic changes occur at certain loci in NK cells, imprinting a memory-like phenotype. Indeed, a similar situation occurs during T helper 1 and T helper 2 differentiation at the Ifng and Il4 loci, and during memory T cell generation [23–25]. An epigenetic mechanism could account for why the progeny of the transferred cells – which have not been exposed to the activating cytokines in vitro - display this memory-like enhanced function [21]. However, it is important to note that, on a per cell basis, memory-like NK cells are not producing more IFN-γ. Rather, the frequency of these memory-like NK cells producing this cytokine is higher than it is in the control NK cell population. NK cells constitutively express IFN-γ mRNA transcripts [26], so it is likely that epigenetic modifications are not being made at the Ifng locus, but rather at other loci responsible for post-transcriptional or post-translational regulation of this cytokine.
While MCMV-induced memory NK cells are found in both lymphoid and non-lymphoid tissues [10], and hapten-induced memory NK cells are found in the liver [27], there is no defined reservoir for cytokine-induced memory-like NK cells. Yokoyama and colleagues did find a modestly higher frequency of memory-like than control NK cells in the lymph nodes at 7 days post-transfer [21]. This trafficking pattern is not explained by expression of CD62L, as both memory-like and control NK cells express similar levels. However, the authors did not look at later time points, so it is unclear whether these memory-like NK cells take up residence in a specific organ. A recent study by Sijts and colleagues provide some insight [19]. The authors demonstrated that influenza virus infection triggers migration of NK cells into the airways and proliferation of both immature and mature NK cells in the bone marrow (BM) but not spleen, lung, draining lymph node, and bronchoalveolar lavage. Adoptively transferred NK cells from influenza virus-infected mice survive longer than a few weeks in naïve recipient mice. These long-lived NK cells undergo homeostatic proliferation [Box 2] and secondary expansion in the BM in response to not only influenza virus infection, but also respiratory syncytial virus (RSV) infection. There is some evidence that the activating receptors NKp46 and NKp44 recognize HA of influenza virus [28–29]. However, Sijts and colleagues see a similar phenomenon during infection with the unrelated respiratory virus RSV, suggesting that these long-lived NK cells are generated not in response to a virus-specific antigen, but rather to inflammatory cytokines [19]. Respiratory illnesses often generate a “cytokine storm”, and a recent study measured cytokine levels in the plasma of patients with severe influenza and found elevated levels of IL-12, IFN-y, and IL-6 during infection [30]. Thus, it is possible that cytokine activation alone is generating these long-lived NK cells. These data provide evidence that the BM may be the long-term residence for these cytokine-induced memory-like NK cells.
Box 2: The special case of homeostatic proliferation.
Mature NK cells undergo homeostatic proliferation when transferred into recipient Rag × Il2rγ-deficient mice. These NK cells persist and are long-lived, similar to MCMV-induced memory NK cells [65] The kinetics of these NK cells undergoing homeostatic proliferation resemble those of Ly49H+ NK cells during MCMV infection. NK cells transferred into an immunodeficient recipient undergo expansion, peaking at day 5, and then go through a contraction phase over the next two weeks. Surprisingly, a small, but stable pool, of NK cells is maintained for up to 180 days, residing in both lymphoid and non-lymphoid tissues [65]. When compared to naïve NK cells from intact C57BL/6 mice, these homeostasis-driven NK cells have increased expression of IFN-y transcripts at day 7 and enhanced production of IFN-y and expression of CD107a, a marker of degranulation, in response to Ly49H and NKp46 stimulation. Not only are a higher percentage of these homeostasis-driven NK cells producing IFN-y, but also they produce more of this cytokine on a per cell basis. While these NK cells become activated and proliferate in response to MCMV infection even 60 days after transfer, whether they mediate enhanced effector function or protection from secondary challenge has not as yet been addressed.
What are the forces driving homeostatic proliferation and the generation of a long-lived population of NK cells? Homeostatic proliferation of T cells is driven not only by cytokines, such as IL-7, IL-15, and IL-21, but also by the interaction of TCR with self-MHC [66]. While IL-15 is required for NK survival during homeostatic proliferation, there is a dearth of evidence on the other factors necessary for this process [67–69]. One possibility is that NK cells require interaction with self-MHC, much in the same way that T cells do. During development in the BM, NK cells expressing inhibitory receptors that recognize self-MHC class I are “licensed” to become functional effector cells [70]. NK cells lacking these receptors become hyporesponsive, and are considered “unlicensed.” Thus, interaction with self-MHC could be another factor driving the formation of these long-lived NK cells during lymphopenia-induced proliferation. Raulet and colleagues, however, have shown that NK cells proliferate similarly when transferred into irradiated wild-type and p2-microglobulin-deficient hosts, suggesting that interaction with self-MHC is not a significant factor in this process [68]. And while naïve T cells require this interaction during homeostatic proliferation, memory T cells do not, suggesting that these long-lived NK cells may indeed be driven solely by cytokine signals. Further research is necessary to tease apart the requirements for generating these homeostatic-expanded NK cells.
Several questions about these cytokine-induced memory-like cells still remain. It is unclear whether this represents the in vivo situation during infection or other inflammation. Indeed, in the Yokoyama study, initial cytokine activation occurred in vitro, and cells were only again restimulated for cytokine production ex vivo [21]. Future experiments should interrogate the responses by these memory-like NK cells in an in vivo infection model. In addition, it is unclear whether activation by cytokines in vivo could generate and maintain such a population. In terms of host protection during infection, does it make sense to generate a population of non-specific memory-like NK cells?
Whether this phenomenon is physiological may not be the most important issue at hand. Adoptive immunotherapies utilizing purified NK cells are now being tested in patients with cancer and those undergoing hematopoietic stem cell transplant [31–32]. Cerwenka and colleagues have demonstrated that cytokine-induced memory-like NK cells cooperated with CD4+ T cells to mediate effective antitumor activity in vivo [33]. Moreover, Fehniger and colleagues asked whether cytokine activation could induce human NK cells to exhibit memory-like function [34]. Using their newly developed method for long-term culture of human NK cells in vitro, they found that a higher frequency of pre-activated human NK cells produces IFN-y upon restimulation with cytokines or K562 targets, even after cell division. The authors saw this enhanced function in both the CD56bright and CD56dim NK cell populations. This functional enhancement is not exhibited by NK cells that have been pre-activated by co-culture with K562 targets or by cross-linking of CD16 alone, suggesting that activation by cytokines uniquely imprints this memory-like enhancement of function. Similar to mouse memory-like NK cells, cytotoxicity is not enhanced. In contrast to mouse NK cells [21], human memory-like NK cells exhibit a distinct phenotype compared to their naïve counterparts, including higher expression of CD94, NKG2A, NKp46, and CD69 [34]. Expression of these markers, as well as NKG2C, correlates with the expression of IFN-y by pre-activated CD56dim NK cells. Although the authors do not describe a mechanism, they do rule out a contribution by IL-12R and phosphorylated STAT signaling. Additionally, as with mouse NK cells, they found no increase in IFN-y transcript levels between pre-activated and control NK cells. Thus, it remains to be determined what drives enhanced IFN-y production by memory-like NK cells in both mouse and human. Despite this, however, these studies provide insight into ways of enhancing NK cell function (Figure 1, center panel). It is a promising new approach that may lead to better NK cell immunotherapies.
Liver-restricted memory NK cells
Von Adrian and colleagues have demonstrated immunological memory in NK cells using a model of hapten-induced contact hypersensitivity (CH), which is a form of delayed-type hypersensitivity (DTH) induced by chemical haptens such as 2,4-dinitro-1-fluorobenzene (DNFB) and 4-ethoxymethylene-2-phenyloxazol-5-one (oxazolone) [27]. Mice are sensitized on the shaved abdomen or back with hapten, followed by challenge (referred to as “elicitation”) on the ear 4–7 days later with a non-irritant dose of hapten (no swelling without sensitization) and monitoring of ear-swelling thereafter. This ear-swelling can be detected only when the haptens used for sensitization and challenge are identical. CH was previously thought to be mediated only by T cells, but new findings suggest that NK cells in Rag-deficient mice can mount a CH response against haptens [35] (Figure 1, right panel). Interestingly, adoptive transfers of NK cells to naive Rag × Il2rγ-deficient mice revealed that CH-mediating NK cells can only be isolated from the liver of hapten-sensitized mice. Further studies demonstrated that Thy1+CD11b+CD27- and Thy1+Ly49C/I+ hepatic NK cells mediate CH [36]. Thy1+ NK cells are known to be immature cells recently derived from BM [37], but NK cells can acquire the expression of Thy1 after activation [38][39]. These data indicate that mature CD11b+CD27- and Ly49C/I+ NK cells can be activated after sensitization by haptens; however, unlike MCMV-induced expansion of Ly49H+ NK cells, stimulation by haptens does not induce preferential proliferation of this subset [27]. They also showed that hapten-modified B cells are preferentially killed by hepatic NK cells isolated from mice sensitized with the identical hapten [35]. These observations clearly indicate that hapten-specific NK cells are able to recognize hapten-modified molecules on the surface of B cells. Interestingly, hapten-specific NK cells are dependent upon the CXCR6 receptor for their function [35]. CXCL16, a unique ligand for CXCR6, is constitutively expressed on liver sinusoidal endothelium and known to promote the survival of iNKT cells [40]. CXCL16 itself inhibits cytotoxicity against hapten-modified B cells in vitro, and NK cells from Cxcr6-deficient mice or mice treated with anti-CXCR6 or anti-CXCL16 monoclonal antibody fail to mount CH responses and are unable to kill hapten-modified B cells [35]. Surprisingly, they showed similar DTH responses against not only haptens, but also virus-like particles of HIV, influenza, and UV-attenuated vesicular stomatitis virus [35]. How many diverse antigens can NK cells recognize? NKp46, an activating receptor expressed by all NK cells, has been reported to recognize the HA protein of influenza virus. However, these authors showed that virus-like particles of HA-deficient influenza are still able to vaccinate NK cells [35]. In their initial report, treatment with anti-NKG2D at challenge efficiently suppressed NK cell-mediated CH to haptens [27]; however, NKG2D does not generate diversity via gene recombination or alternative splicing. Curiously, CXCR6+ NK cells (or Thy1+CD11b+CD27- or Thy1+Ly49C/I+ NK cells) can be found in spleen or liver of unsensitized mice, but they are unable to mediate CH or kill hapten-modified B cells in vitro. These results indicate that dual stimulations – liver specific cytokines or chemokines (including CXCL16) and inflammatory stimuli associated with sensitization by haptens or immunization by virus-like particles – are prerequisite for the induction of antigen-specific NK cells or the expression of putative antigen-specific receptor(s) in hepatic NK cells.
Another question to be addressed is the mechanism of NK cell-mediated CH. Traditionally, T cell-mediated CH is classified into 3 steps. The first step involves the migration of dendritic cells (DCs) from the site of hapten application to the draining LN. Recent studies suggest that Langerin+ dermal DCs, which are distinct from Langerhans cells, are the most potent subset for the induction of CH [41–44]. In hapten-sensitized skin, proinflammatory cytokines produced by mast cells are crucial for the efficient migration of DCs [45–46]. The second step is the priming of hapten-specific T cells. In the draining LN, several co-stimulatory molecules, such as CD86 and OX40L, are responsible for the priming of hapten-specific T cells [47–48]. The third step is the re-activation of effector T cells. Previously primed effector T cells can induce edema at the skin upon secondary hapten exposure. This effect is dependent on cytotoxic granules and inflammatory cytokines such as IFN-γ and IL-17 [49–52]. These cytokines induce CXCL1 and CXCL2 and recruit neutrophils to hapten-challenged skin [49]. Although little is known about the mechanism of NK cell-mediated CH, Rouzaire and colleagues have reported that neither mononuclear cells, neutrophils, nor cytotoxic molecules such as granzyme B, TNF-α, FasL or TRAIL are detected in the inflammatory skin of NK cell-mediated CH [53]. Moreover, in T cell-mediated CH responses, the swelling of ear-skin increases upon repeated challenge with hapten, but curiously no enhancement is observed in NK-cell mediated CH.
Concluding remarks
Here we have reviewed the three major ‘paths’ leading to the generation of memory NK cells that have been recently described in the literature. The major underlying definition that links these diverse viewpoints is that memory NK cells are long-lived and exhibit a recall response. Beyond these points, each model has shown distinct requirements for tissue distribution, effector function, and antigen-specificity (Figure 2). Thus far, it has not been clearly elucidated what drives the formation or maintenance of the NK memory pool; there are likely a variety of factors (cytokines, chemokines, and co-stimulatory molecules) that are required. It is also important to address whether there is overlap between these three distinct types of memory NK cells. For example, can MCMV-induced memory NK cells residing in liver mediate DTH against MCMV? Are MCMV-induced memory NK cells protective against heterologous infections or highly antigen-specific as in the CH model? Is IL-12 also important for liver-restricted memory NK cells? Can the addition of IL-12 (and IL-15 plus IL18) boost MCMV-induced memory or DTH responses? In addition, it is of interest to assess whether certain subsets of NK cells (memory precursor) preferentially generate memory NK cells and whether they can be classified into central memory and effector memory, as is the case for CD8+ T cells. Future studies are also required to more clearly define the markers of a memory NK cell subset, to investigate other types of receptor-ligand-driven NK cell memory generation, and to elucidate the molecular mechanisms and specificity underlying the generation of memory NK cells.
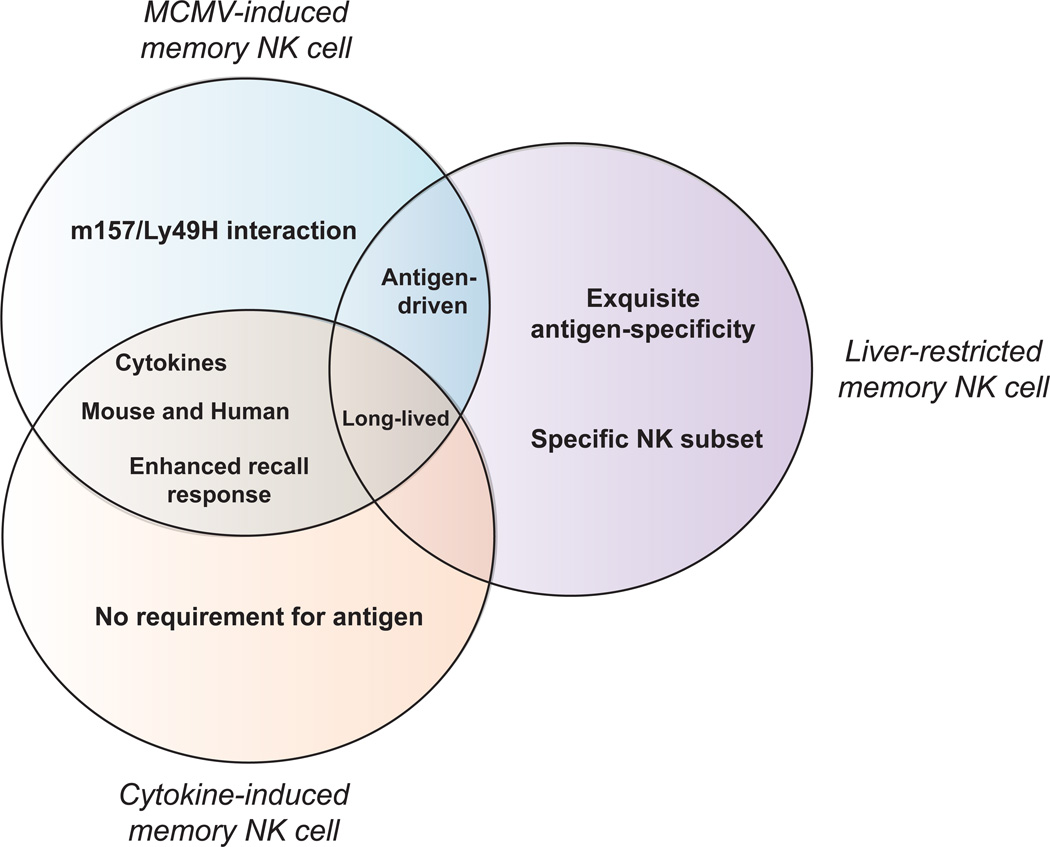
Figure 2. Differences and similarities between the three paths to memory.
The Venn diagram illustrates the three major perspectives of NK cell memory that have recently emerged. It is clear that the current definitions of NK cell memory are quite distinct and show little overlap; this underlines the need to more clearly delineate the mechanisms underlying the formation of memory by NK cells.
Highlights.
Mature NK cells can be long-lived and generate progeny with self-renewing capability
NK cells are altered after their initial activation, responding more robustly on subsequent stimulation
“Memory” NK cells can be generated after infection with cytomegalovirus, in vitro cytokine stimulation, or sensitization with haptens or viral particles, with the latter cells predominantly residing in the liver
Acknowledgements
LLL is an American Cancer Society Professor and supported by NIH grants AI066897, AI068129, and CA095137. GM is a Canadian Institutes of Health Research Bisby Fellow; TN is supported by the Uehara Memorial Foundation, the Natio Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and Japan Society for the Promotion of Science; and DWH is supported by an American Lung Association Senior Research Training Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu. Rev. Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat. Rev Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo M, et al. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 4.Sun JC, et al. NK cells and immune “memory”. J. Immunol. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama WM, et al. The dynamic life of natural killer cells. Annu. Rev. Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 6.Sun JC, et al. Immune memory redefined: characterizing the longevity of natural killer cells. Immunol Rev. 2010;236:83–94. doi: 10.1111/j.1600-065X.2010.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arase H, et al. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 8.Dokun AO, et al. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 9.Smith HRC, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci U.S.A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun JC, et al. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun JC, et al. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J. Exp. Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grayson JM, et al. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J. Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 13.Hildeman DA, et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrini M, et al. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homann D, et al. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 16.Bezman NA, et al. Molecular definition of the identity and activation of natural killer cells. Nat. Immunol. 2012;13:1000–1009. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazit R, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 18.Draghi M, et al. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J. Immunol. 2007;178:2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 19.Van Helden MJG, et al. The bone marrow functions as the central site of proliferation for long-lived NK cells. J. Immunol. 2012;189:2333–2337. doi: 10.4049/jimmunol.1200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdul-Careem MF, et al. Genital HSV-2 Infection Induces Short-Term NK Cell Memory. PLoS ONE. 2012;7:e32821. doi: 10.1371/journal.pone.0032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zehn D, et al. TCR signaling requirements for activating T cells and for generating memory. Cell. Mol. Life Sci. 2012;69:1565–1575. doi: 10.1007/s00018-012-0965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanno Y, et al. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu. Rev. Immunol. 2012;30:707–731. doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zediak VP, et al. The contribution of epigenetic memory to immunologic memory. Curr. Opin. Genet. Dev. 2011;21:154–159. doi: 10.1016/j.gde.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Weng N, et al. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat. Rev. Immunol. 2012;12:306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Leary JG, et al. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 28.Mandelboim O, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 29.Arnon TI, et al. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur. J. Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Heltzer ML, et al. Immune dysregulation in severe influenza. J. Leukoc. Biol. 2009;85:1036–1043. doi: 10.1189/jlb.1108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy EM, et al. Natural killer cells in human cancer: from biological functions to clinical applications. J. Biomed. Biotechnol. 2011;2011:676198. doi: 10.1155/2011/676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passweg JR, et al. Natural-killer-cell-based treatment in haematopoietic stem-cell transplantation. Best Pract. Res. Clin. Haematol. 2006;19:811–824. doi: 10.1016/j.beha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Ni J, et al. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J. Exp. Med. 2012 doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romee R, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012 doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paust S, et al. Adaptive immune responses mediated by natural killer cells. Immunol. Rev. 2010;235:286–296. doi: 10.1111/j.0105-2896.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurme M, Sihvola M. High expression of the Thy-1 antigen on natural killer cells recently derived from bone marrow. Cell. Immunol. 1984;84:276–284. doi: 10.1016/0008-8749(84)90099-6. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, et al. NK cell tolerance in mixed allogeneic chimeras. J. Immunol. 2003;170:5398–5405. doi: 10.4049/jimmunol.170.11.5398. [DOI] [PubMed] [Google Scholar]
- 39.Gillard GO, et al. Thy1+ NK [corrected] cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes. PLoS Pathog. 2011;7:e1002141. doi: 10.1371/journal.ppat.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geissmann F, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bursch LS, et al. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan DH, et al. Immunity. 2005) Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Bennett CL, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Biedermann T, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J. Exp. Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dudeck A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 47.Nuriya S, et al. The differential role of CD86 and CD80 co-stimulatory molecules in the induction and the effector phases of contact hypersensitivity. Int. Immunol. 1996;8:917–926. doi: 10.1093/intimm/8.6.917. [DOI] [PubMed] [Google Scholar]
- 48.Chen AI, et al. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 49.Kish DD, et al. CD8 T cells producing IL-17 and IFN-gamma initiate the innate immune response required for responses to antigen skin challenge. J. Immunol. 2009;182:5949–5959. doi: 10.4049/jimmunol.0802830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He D, et al. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J. Immunol. 2009;183:1463–1470. doi: 10.4049/jimmunol.0804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He D, et al. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J. Immunol. 2006;177:6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 53.Rouzaire P, et al. Natural killer cells and T cells induce different types of skin reactions during recall responses to haptens. Eur. J. Immunol. 2012;42:80–88. doi: 10.1002/eji.201141820. [DOI] [PubMed] [Google Scholar]
- 54.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–1558. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Vergès S, et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. U.S.A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez-Vergès S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foley B, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Béziat V, et al. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur. J. Immunol. 2012;42:447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- 59.Björkström NK, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petitdemange C, et al. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 2011;7:e1002268. doi: 10.1371/journal.ppat.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Björkström NK, et al. Characterization of natural killer cell phenotype and function during recurrent human HSV-2 infection. PLoS ONE. 2011;6:e27664. doi: 10.1371/journal.pone.0027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jost S, et al. Expansion of 2B4+ natural killer (NK) cells and decrease in NKp46+ NK cells in response to influenza. Immunology. 2011;132:516–526. doi: 10.1111/j.1365-2567.2010.03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong HS, et al. HIV infection is associated with a preferential decline in less-differentiated CD56dim CD16+ NK cells. J. Virol. 2010;84:1183–1188. doi: 10.1128/JVI.01675-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu X, et al. Human natural killer cells expressing the memory-associated marker CD45RO from tuberculous pleurisy respond more strongly and rapidly than CD45RO- natural killer cells following stimulation with interleukin-12. Immunology. 2011;134:41–49. doi: 10.1111/j.1365-2567.2011.03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun JC, et al. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J. Exp. Med. 2011;208:357–368. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyman O, et al. Homeostatic maintenance of T cells and natural killer cells. Cell. Mol. Life. Sci. 2012;69:1597–1608. doi: 10.1007/s00018-012-0968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prlic M, et al. In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jamieson AM, et al. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J. Immunol. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 69.Ranson T, et al. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–4893. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 70.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32:364–372. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]