Abstract
One major feature of the remarkable vocal repertoires of birds is the range of fundamental frequencies across species, but also within individual species. This review discusses four variables that determine the oscillation frequency of the vibrating structures within a bird's syrinx. These are (1) viscoelastic properties of the oscillating tissue, (2) air sac pressure, (3) neuromuscular control of movements and (4) source-filter interactions. Our current understanding of morphology, biomechanics and neural control suggests that a complex interplay of these parameters can lead to multiple combinations for generating a particular fundamental frequency. An increase in the complexity of syringeal morphology from non-passeriform birds to oscines also led to a different interplay for regulating oscillation frequency by enabling control of tension that is partially independent of regulation of airflow. In addition to reviewing the available data for all different contributing variables, we point out open questions and possible approaches.
Keywords: Vocal behavior, Sound frequency, Bird, Functional morphology, Signal evolution
1. Introduction
Vocal behavior in birds is characterized by remarkable temporal and acoustic diversity. Avian sounds are used in different functional contexts, suggesting that different vocal signals are the product of different selective pressures. Within the vocal repertoire of a species, the acoustic signals that are used in reproductive context are often the most complex and diverse, presumably because they are subject to strong sexual selection (e.g., Searcy and Nowicki, 2005). These signals are referred to as “song” and are distinct from simpler vocalizations, “calls”, with often distinct functional context. However, the acoustic parameters of vocal signals are subject to constraints that arise from the morphological, physiological and neural mechanisms underlying vocal production, which are also serving non-vocal functions (e.g., Podos and Nowicki, 2004).
Sound frequency is an important acoustic characteristic, and avian vocal sounds span a wide range of fundamental frequencies (F0) between approximately 100–12000 Hz. Evolutionary explanations for this range include ecological and behavioral hypotheses (e.g., Morton, 1975; Nowicki and Searcy, 2004) that all work within the framework of possible physical and physiological constraints. For example, the role of body size has been investigated as a possible determinant of the range of F0 (e.g., Wallschläger, 1980; Ryan and Brenowitz, 1985; Cardoso, 2012). Whereas body size explains a substantial amount of the variation in frequency range in a comparison of species across the full size range in birds (Fig. 1), the unexplained variation is of great interest in the context of signal evolution. Significant variation in the range of F0 in the vocal repertoire of individual species contrasts with much more limited ranges in others. Remarkable ranges of F0 are found within individual songs of species across different taxa. For example, the short song of the brown-headed cowbird (Molothrus ater) covers a frequency range of 450-12000 Hz, and the longer song sequences of European starlings (Sturnus vulgaris) contain syllables whose F0 ranges from 250 to 10,000 Hz. Broad frequency ranges are not restricted to songbirds, however. For example, the vocal repertoires of some Anseriformes (e.g., Tadorna tadorna) and Galliformes (e.g., Bonasa bonasia) span 1–8 kHz (e.g., Bergmann and Helb, 1982).
Fig. 1.
(A) Body size can explain some of the variation in fundamental frequency (F0) across bird species. The F0 range from a small selection of bird species from 7 orders was plotted against their average body mass. The black curve is a regression line calculated for the song center frequency of 90 songbird species (Wallschläger, 1980). (B) The frequency ranges are shown relative to the potential range calculated from Eq. (1), assuming labial length to scale with body mass as shown in the inset and assuming tissue stress of 10 kPa and 300 kPa for the low and high frequencies (stippled lines), respectively. The relationship between body mass and labial length (inset) is based on measurements of labial length in 7 songbird species (Riede and Goller, unpublished) and an estimate for the ostrich based on tracheal diameters (Yldiz et al., 2003).
These observations pose the question of how such remarkable F0 ranges are generated, and, at the same time, why higher and lower frequencies are not. A solid understanding of the proximate mechanisms of F0 control will be required for our interpretation of evolutionary patterns. Sound generating mechanisms in birds have been reviewed recently, including aspects of frequency control (e.g., Mindlin and Laje, 2005; Goller and Cooper, 2008; Suthers and Zollinger, 2008; Riede and Goller, 2010a). However, the main focus of earlier studies on vocal production was neural control of song characteristics. Neural control is, of course, an important aspect of frequency control, but it has to operate within the physical and morphological limits of the vocal organ. This point can be illustrated with the example of the human voice, where an individual's F0-range is largely determined by physical and biomechanical parameters (vocal fold histology, effective length and biomechanics of tension control) with minimal potential for expanding this range through vocal training (Titze, 2000). Consequently, F0-control can only be understood as a complex interplay between morphology of the oscillating tissue, biomechanics of the cartilaginous framework of the syrinx and neural control of active movements, and this review provides an update on our current knowledge of this interplay in birds.
Although morphological studies of the avian vocal organ date back more than 200 years (e.g., Hérissant, 1753; Cuvier, 1802; Fürbringer, 1888; Wunderlich, 1884; Setterwall, 1901), and neuromuscular control of song has received significant attention (reviewed by Goller and Cooper, 2008; Suthers and Zollinger, 2008; Riede and Goller, 2010a), the interplay between functional morphology and neural control has only recently become the focus of detailed studies in birds (e.g., Jensen et al., 2008; Riede and Goller, 2010b; Riede et al., 2010a; Prince et al., 2011). Here we review our current knowledge of the complex interaction between physical, physiological and neural mechanisms. First, we will review the basic anatomy of the vocal organ, followed by a discussion of the four variables that causally determine F0. Then we will review our current understanding in respect to three of these four variables, focusing on songbirds but including comparisons to other groups where data are available. Finally, we will discuss the broader implications of these complex relationships and point out gaps in our knowledge.
2. The avian vocal organ
The syrinx is a uniquely avian sound generating organ that is located at the tracheo-bronchial junction. Syrinx morphology differs substantially between different taxa (Fig. 2) and ranges from simple modifications of individual cartilages to elaborate drum-like structures that are composed of multiple fused cartilage rings. A varying number of muscles (1–6 pairs, although some authors subdivided some muscles and identified up to 9 pairs; Brackenbury, 1989; King, 1989) control the movements of cartilaginous components of the vocal organ. The basic set of two extrinsic muscles (i.e., one or both insertion sites are outside the syrinx) is complemented by intrinsic muscles (both insertion sites are in the syrinx area) in more complex syringes. The vibratory tissues of the syrinx are either called tympaniform membranes (e.g., doves and parrots) or labia (songbirds) (labium is used as a collective term for simplicity from here on; Fig. 2). In a number of avian taxa the vibrating tissues reside within the bronchial contributions to the syrinx, thus giving rise to two independently controlled sound sources, the left and right syrinx.
Fig. 2.
Schematic depiction of the syrinx morphology in the collard dove and in a songbird: (A) Collard dove syrinx ventral view. (B) Collard dove syrinx cross section (redrawn after Ballintijn, 1999). (C) External ventral view of a songbird syrinx. (D) Schematic of a cross section of the songbird syrinx (the section level is indicated by the vertical plane in C). (E and F) Schematic horizontal section through the syrinx of the dove (E) and the songbird (F). The respective levels are indicated by the horizontal line in A, and the horizontal plate in C, respectively. (G) Simulating the tympaniform membrane and labia oscillation assumes two major movement components: a latero-lateral component (top panel) and a superimposed cranio-caudal component (bottom panel). The cranio-caudal component induces an alternation between a convergent and divergent cross-sectional profile. This differential movement of the upper and lower part of the oscillating tissue produces larger asymmetries in the forces acting on the tissue in the opening and closing phase of the oscillation than the inward and outward movement alone, resulting in a positive pressure on the tissue during the opening phase and a net energy input to the tissue maintaining self-sustained oscillations. The oscillating tissue will be deformed during oscillation. Three main deformations are elongation (indicated by the arrow in F), shear (H) and collision (I). (A1, A2, A3, first, second and third bronchial ring; Ty, tympanum; T, tracheal ring; P, pessulus; ML, medial labium; LL, lateral labium; B, bronchial rings; TM, tympaniform membrane; TL, tracheolateral muscle; ST, sternotrachealis muscle; vS, ventral syringeal muscle; vTB, ventral tracheobronchial muscle, dTB, dorsal tracheobronchial muscle; dS, dorsal syringeal muscle).
Sound is produced in the syrinx by flow-induced tissue oscillations (Goller and Larsen, 1997a; Goller and Larsen, 2002; Jensen et al., 2007). Although direct evidence for the detailed vibratory behavior of the labia is not available for birds, it can be assumed that the basic mechanism for generation of self-sustained oscillations is similar to that in the mammalian larynx (e.g., Titze, 1988). Air flow passing between soft tissue masses causes these to respond. The labia are in a partially adducted position when increasing air sac pressure pushes them apart, expanding the intra-labial space. The labia are pushed into a convergent profile (Fig. 2G), and, as a consequence, flow velocity increases and intra-labial pressure decreases. The lateral movement is eventually stopped and reversed by increasing labial tension, Bernoulli forces and restrictions imposed by the cartilage framework. Now, as the labia move back medially, they assume a divergent cross sectional profile, and the pressure at the closest approximation of the labia increases to the point of flow separation. The more the labia approach each other, the more the subsyringeal pressure builds up again, thus restarting the cycle at some reversal point. Self-sustained oscillations require a dynamic alternation between the convergent profile of the oscillating tissue during opening movements and a divergent profile during closing movements (Fig. 2G). The change in profile establishes a change in pressure between the labia, which is essential for generating sustained oscillation of the vibrating tissues. This proposed mechanism of flow-induced tissue oscillation for the syrinx of birds is based on the theoretical foundation for vibratory behavior of the human vocal fold (Titze, 1988), which was successfully adapted for theoretical work on the sound generating mechanism in songbirds (e.g., Fee et al., 1998; Mindlin and Laje, 2005).
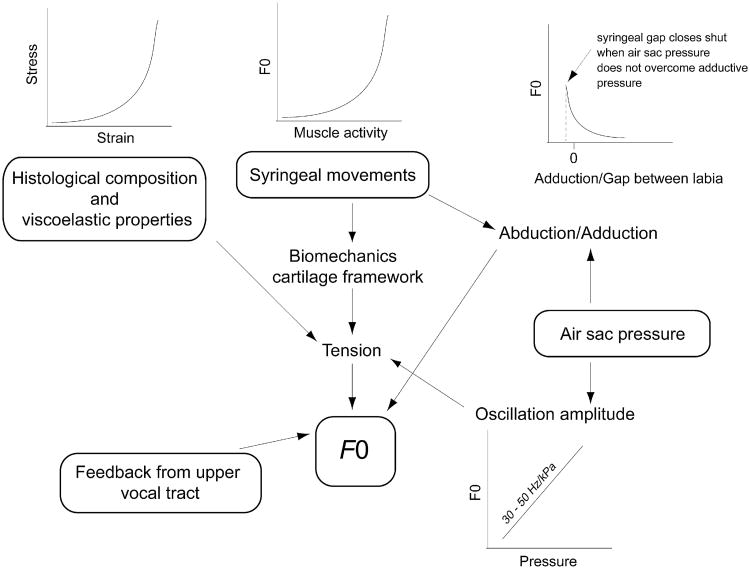
The rate of tissue oscillations determines F0 and is affected by four parameters and their variable interaction: (1) viscoelastic properties of labia, (2) air sac pressure, (3) active movements facilitated by syringeal muscles, and (4) nonlinear source-filter interactions (Fig. 3). Each of the first three parameters demonstrates a nonlinear characteristic. Furthermore, the interactions between these variables are nonlinear. These complex relationships make it particularly difficult to dissect their respective contributions to F0 regulation. Additionally, there is the possibility for nonlinear source-filter interactions, which can have a dramatic effect on F0. In the next section we will review how the four variables (visco-elastic properties, pressure, muscle generated movements, nonlinear source-filter interactions) affect F0.
Fig. 3.
Schematic overview of the 4 main mechanisms for controlling fundamental frequency (F0) and their ways of action as discussed in detail in the main text. Arrows indicate the relationships of this complex interplay between morphology, aerodynamics and neural control. The inset graphs show the nature of relationships (linear and nonlinear) and the effects of different mechanisms on F0.
3. Four different parameters generate a multidimensional parameter space for control of fundamental frequency
3.1. Histological composition and viscoelastic properties of vibrating tissues
The vibratory tissues of the avian syrinx, labia and tympaniform membranes, consist of connective tissue that is surrounded by epithelium (Fig. 2a). One epithelial surface is exposed to the tracheal or bronchial lumen and the other faces the lumen of the interclavicular air sac. The thickness of the connective tissue layer is highly variable and tends to be greater in songbirds (Warner, 1972; King, 1989; Riede and Goller, 2010b).
The composition and morphology of the soft connective tissue plays an important role in determining the oscillation characteristics of the labia. In general, mechanical properties of connective tissue are determined by the composition of its extracellular matrix (Fung, 1993; Cowin and Doty, 2007). Differences in the morphology of the connective tissue of vocal folds in mammals (e.g., Gray et al., 2000; Chan et al., 2007; Riede et al., 2010b; Klemuk et al., 2011) or labia in the songbird syrinx (e.g., Fee, 2002; Riede and Goller, 2010b) contribute to vocal differences. The extracellular matrix of connective tissue consists of fibrous proteins (elastin, collagen) and amorphous substances, such as glucosaminoglycans and proteoglycans (hyaluronan). The relative quantities of these substances together with the spatial arrangement of the protein fibers influence the mechanical properties of the tissue and therefore its oscillatory behavior. The fibrous proteins account to a large part for the tensile strength of the tissue, while amorphous substances such as hyaluronan (e.g., Chan et al., 2001) contribute to its viscous properties. The composition and spatial arrangement of fibers in the connective tissue determine its response to deformation, like stretch (Fig. 2F), shear (Fig. 2H) or collision (Fig. 2I).
The dimensions and morphology of the vocal organ can be expected to scale to some degree with body size, leading to the prediction that smaller animals with smaller vocal organs produce sounds with a higher F0. However, size dependence is complicated by the fact that nonlinear characteristics of the mechanical/viscoelastic properties of the labia have a large effect on F0. The viscoelastic properties of the vibrating tissue can be described by its stress-strain relationship. For example, straining the tissue in one direction causes it to stiffen, which will increase its internal stress. In a first approximation, vocal fold or labial oscillation behavior can be described as that of a string (Titze, 2000), where the oscillation rate is calculated according to the following equation:
| (1) |
In this relationship, L is string (or labial) length, σ is tissue stress (force per unit area), and ρ is tissue density (1.04 g cm−3). Elongation of the tissue (i.e., increase of L) has two opposing effects on F0. Whereas an increase in L results in a decrease in F0, this same elongation causes tissue stress to increase; this latter effect causes the longer string to effectively vibrate at higher rates. This net effect of F0 increase with lengthening has been demonstrated to occur in human phonation (Hollien and Moore, 1960; Hirano et al., 1969) as well as in excised larynx experiments. At strains larger than 20–30%, tissue stress becomes highly nonlinear, and individual, sex and species differences in frequency ranges of vocal folds of similar length can be attributed to these nonlinear effects (Haji, 1990; Min et al., 1995; Chan et al., 2007; Hunter and Titze, 2007; Zhang et al., 2009; Riede et al., 2010a). When connective tissue is strained, some stress is released as heat (Fung, 1993), which causes a drop in tissue oscillation rate and F0, if no compensation occurs either through further stretching of the vibrating tissue or increasing driving pressure.
We suggest here that these same physical relationships can be applied to songbird labia at least as a first approximation. The labia are positioned between the two endpoints of the third bronchial half ring (arrow in Fig. 2F), resembling a string mounted between two anchor points. The small size makes it very difficult to extract labial tissue for estimating the stress response (e.g., Fee, 2002; Riede et al., 2011a). However based on labial morphology and previous estimates, a stress response of 10–300 kPa for strains between 0% and 20% is a good approximation for songbird labia. For example, the length of the soft part of a starling labium (arrow in Fig. 2F) is about 2 mm, which results in an F0 of 775 Hz (at 10 kPa and 1.04 g cm−3). If this labium is stretched to 2.4 mm (i.e. strained by 20%), and tension does not simultaneously change, F0 will decrease to 646 Hz. However, straining will also change tension, and if in this example the stretching increases tension from 10 to 300 kPa, the resulting frequency will be 3538 Hz. Labial length in songbirds ranges from 0.5 mm in the smallest to approximately 5 mm in the largest species (Fig. 1B; Riede and Goller, unpublished data). We estimate that the length change that can be imposed on this tissue is up to 20–30%. The nonlinear relationship between stress and strain of the soft connective tissue of the labia is an important property that allows labia to generate a broad F0 range. Such a broad range has been documented for particularly the right side of the dual sound generator of some songbird species (e.g., Suthers, 1990; Allan and Suthers, 1994; Suthers and Goller, 1997; Suthers et al., 2004; Jensen et al., 2007).
The mechanical properties of the tissue are therefore an important aspect of frequency control, but direct data are not available for birds. Theoretically, these properties of the labia must depend on the composition of their extracellular matrix. The stress response of soft connective tissue is nonlinear due to the differential contribution of different protein fibers. The ratio between collagen and elastin fibers and the cross-linking between collagen fibers are predominantly responsible for tissue stiffness and its response to loading. Typically, elastin fibers provide resistance to deformation in the lower strain regions, and collagen fibers bear most of the load in the higher strain regions (e.g., Roach and Burton, 1957). The labia of different songbird species vary in their collagen-elastin ratio and in the orientation of fibers. Whereas the labia are fairly homogeneous in the zebra finch, different layered structures are found in other species (Fig. 4).
Fig. 4.
Labial morphology determines their viscoelastic properties. Examples from three songbird species indicate that the arrangement and composition of fibers in the extracellular matrix varies between species. Two different stains were used to image the two major fibrous proteins collagen (Masson's trichrome) and elastin (Elastica van Giesson). The labia in white-crowned sparrows and the European starling demonstrate a layer structure, whereas the extracellular matrix is uniform in the zebra finch. An example of song is depicted spectrographically and oscillographically for each species. Data are from Riede and Goller (2010b) and unpublished. Abbreviations: c.s – cross-sectioned collagen fiber; l.s. length-wise sectioned collagen fiber.
Tissue tension can be altered in different ways depending on how force is exerted onto the tissue. The main procedure to alter stress in the labia is through stretching them. Although it has not been shown directly in birds that labial stretching is used in F0 control, the strong correlation between ventral syringeal muscle activation and F0 suggests such a mechanism (Goller and Suthers, 1996a,b). The precise deformation of the labial tissue is determined by the degree to which the syringeal skeleton constrains its geometry and, thus, the transfer of force from syringeal muscles and aerodynamic variables. The nonlinear stress response of the oscillating tissue is therefore dependent on a number of variable factors, which remain to be investigated in detail in birds. Nevertheless, these relationships are largely responsible for the lack of a tighter coupling between body mass and F0 (Fig. 1). At this point more detailed information on the mechanical properties (Table 1) of the labia is required, because they are not a simple, homogeneous string. Each layer can be considered a separate string with a characteristic stress-strain relationship, giving rise to different amplitudes of oscillation (Titze, 2011). Labial morphology and specific viscoelastic properties contribute to the way tissue is drawn into oscillation. For example, at low frequencies sound may be generated by a pulse tone mechanism (e.g., Jensen et al., 2007; Sitt et al., 2008), which is characterized by oscillatory cycles of the entire labium with long closure and brief opening periods. In contrast, the modal vibratory mode is characterized by more symmetrical opening and closing movements, and in this dynamic regime variable portions of tissue can be engaged into vibration. The variable recruitment of tissue is facilitated by the inhomogeneous morphology of the extracellular matrix, most prominently the layer structure (Fig. 4; Riede and Goller, 2010b).
Table 1.
List of unexplored main questions in regard to control of fundamental frequency (F0).
| Main unanswered questions | Possible approach strategies |
|---|---|
| Determination of the viscoelastic properties of labia | Small scale stress strain tests (rheometry; pipette aspiration technique; atomic force microscopy) |
| Biomechanics of the syringeal cartilage framework and how muscle contraction acts on labial position and tension | 3D imaging in combination with finite element modeling |
| Synergistic tension control by ventral and dorsal syringeal muscles | Simultaneous recording of emg activity in respective muscles |
| To what degree individuals use different combinations in parameter space to generate similar frequencies | Detailed analysis of air sac pressure and emg data for the entire vocal repertoire of a species |
| Source-filter interactions | Flow and pressure recordings closely above and below the syrinx while vocal tract movements (vocal tract impedances) are altered |
| Comparative aspects of morphological specializations – how changes in cartilage framework and increased number of muscles make tension control partially independent of airflow regulation | Only very few groups have been investigated, but syringeal morphological specializations occur in many other groups and may provide insight into the evolution of F0-control mechanisms |
| Comparative aspects of the oscillatory tissue (tympaniform membrane (Non-Passeriformes vs labia (Passeriformes) and the function of additional thin membranes (e.g., medial tympaniform membranes in oscines; thin ventral membranes in other groups (e.g., King, 1989) | Systematic histological analysis of the oscillatory tissue in a number of avian orders will reveal whether labia and membranes are similar or categorically different. The role of additional membranes could be investigated through surgical manipulation of their elasticity or their elimination (as for example in Goller and Larsen, 1997a,b, 2002) |
Songbirds possess two independently controlled sound sources. Morphological and biomechanical differences of the vibrating tissues of the two sound generators (Fig. 4) will contribute to the tuning of the sound sources to different frequency ranges. This mechanism can lead to a significant expansion of the frequency range of a syrinx. Whereas large differences in labial size have been described for some songbird species (Jensen et al., 2008; Prince et al., 2011), the predicted differences in labial properties have not yet been explored.
3.2. Air sac pressure
Airflow is critical for starting tissue oscillation. It is generated by a pressure differential across the syrinx, i.e. by expiratory or inspiratory movements. The range of subsyringeal pressures for sound generation is similar to that in other vertebrates ranging between 0.3 and 3 kPa for most vocalizations, with maxima up to 5 kPa in very loud and/or high-pitched vocalizations (e.g., Gaunt et al., 1973).
The minimum air sac pressure necessary for starting syringeal vibrations is considered the phonation threshold pressure (PTP). In zebra finches, PTP ranges from 0.5 to 1.5 kPa (Riede et al., 2010b; Elemans et al., 2010). Computational modeling and experimental measurements of the mammalian larynx demonstrate that PTP increases with the pressure differential across the vocal organ, the stiffness of the oscillating tissue (damping characteristics and tissue wave velocity), and the prephonatory gap (i.e. adduction) between vocal folds (Titze, 1992). The same parameters must play a role in determining PTP in birds. However, in birds PTP is difficult to measure in vivo, because the interclavicular air sac pressure affects the position of the labia (Fig. 5, see below). Even though it cannot be accurately determined, PTP estimates are useful for comparison. The values for the zebra finch place the syrinx within the range of PTPs for the laryngeal sound source (Gans, 1973; Holmberg et al., 1988; Riede, 2011; Riede et al., 2011b). PTP is an important descriptive parameter of a vocal organ, because it identifies the minimum energy required to initiate phonation and therefore provides a measure of efficiency. Similarly, the upper subsyringeal pressure limit is also specific for each vocal organ and depends onthe same variables asPTP. The upper limitofregular phonation in the mammalian larynx is referred to as the phonation instability pressure, i.e. the pressure beyond which vocal fold oscillations become irregular (e.g., Zhang et al., 2007). Although data are not available in birds, it is likely that the maximum stability pressure is well below the maximal pressure that can be generated by birds.
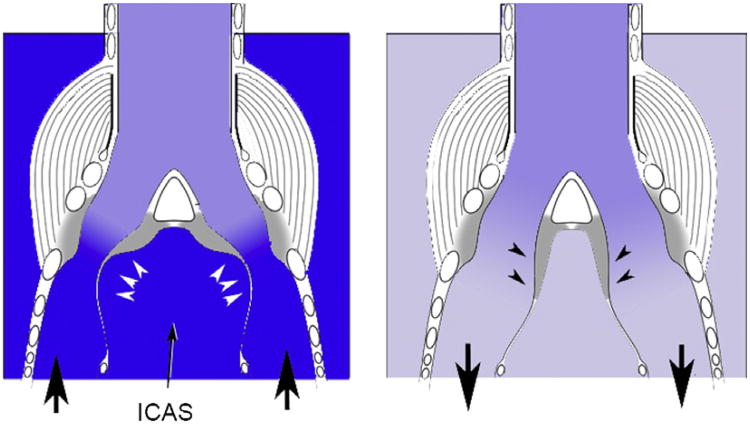
Fig. 5.
The pressure in the interclavicular air sac is similar to subsyringeal pressure and therefore contributes to adduction and abduction of the labia. Schematic views of a horizontal section of the syrinx for expiration (left) and inspiration (right) depict the pressure conditions in a blue scale (dark blue is higher pressure than lighter shades). The syrinx is situated in the interclavicular air sac (the attachment of the rostral end of the air sac membrane is on the trachea as depicted), exposing it to its pressure, whereas suprasyingeal tracheal pressure is close to atmospheric pressure. During expiration the elevated pressure in the interclavicular air sac (ICAS) pushes the labia into the bronchial lumen (white arrow heads) as the intralabial pressure gradient decreases toward atmospheric (suprasyringeal) pressure. During inspiration the subatmospheric pressure in the ICAS abducts the labia (black arrowheads). Arrows at the bronchi indicate the direction of airflow. See Fig. 2D for morphological details.
The effect of pressure on F0 can be explained as follows. If sub-syringeal air sac pressure increases while labial or membrane tension remains constant, oscillating tissue will be pushed increasingly more lateral during each oscillation cycle, decreasing the length/amplitude ratio. The wider excursion will strain labia more and therefore tension will be greater at maximum amplitude. This increased tension will cause a slightly faster closing movement of the oscillation cycle and therefore lead to a small increase in oscillation rate and, consequently, in F0. Simultaneously, the larger subsyringeal pressure will cause a faster build-up of the threshold pressure that opens the labial gap again. The effect of pressure on frequency is dependent on tissue tension. It will be higher at higher F0 simply because the tissue operates in the range where tension increases exponentially with strain. The pressure effect on F0 ranges between 5 and 70 Hz per 1 kPa subglottal pressure change in humans (Titze, 1989, 1988) and between 30–50 Hz per 1 kPa change in subsyringeal air sac pressure in zebra finches (Figs. 3, 6; Riede et al., 2010a).
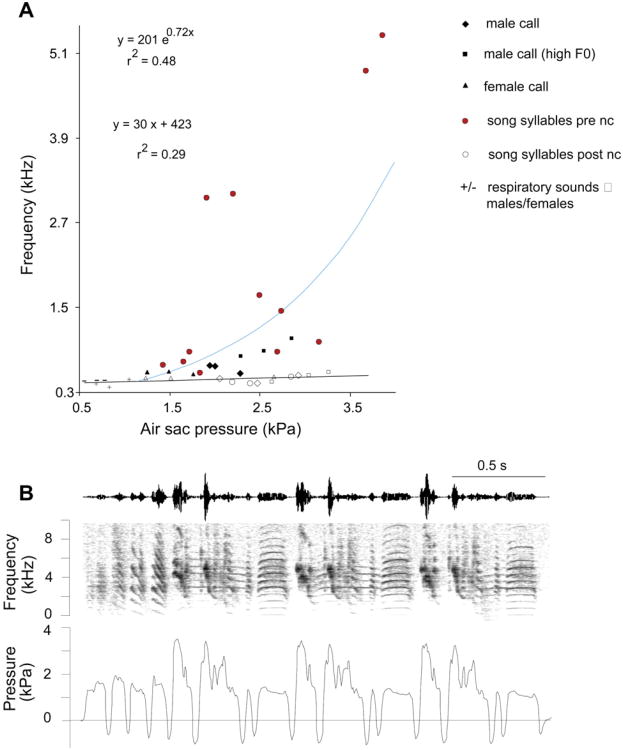
Fig. 6.
(A) Air sac pressure explains a small portion of F0 variation in zebra finches. In the intact birds (before bilateral denervation of the syringeal muscles), the relation between F0 and air sac pressure can be best described with an exponential model, while after denervation a linear model describes the relationship between the two variables. Call data had been presented in Riede et al. (2010a). Song data have been added. (B) Air sac pressure and spectrographic representation of a zebra finch song that contains several elements with high fundamental frequency (F0). High-F0 elements are generally produced with higher air sac pressure values, but substantial variation exists.
Respiratory pressure influences the syrinx via a second mechanism. In addition to generating airflow across the syrinx, it contributes to adducting the labia (Fig. 5). The syrinx is situated in the interclavicular air sac. Any difference in pressure between air sacs and/or the bronchial lumen will exert an additional adducting force on oscillating tissue, because the labia represent soft tissue walls separating the intra-bronchial lumen and the air sac lumen. In fact, the importance of a pressure balance between intra-bronchial lumen and air sac lumen has been shown by excised syrinx experiments, in which the simulation of a pressurized cavity (inter-clavicular air sac) around the syrinx is required for induction of vibrations (e.g., Rüppell, 1933). Increasing air sac pressure will therefore adduct the soft tissue into the bronchial lumen, which will increase the probability of collisions during oscillation. This adduction and resulting collisions will affect F0 as follows. Increased adduction of the opposing labia reduces the distance between them. At some point the two opposing labia touch each other, and further increase in adduction causes them to press against each other (tissue collisions). This continued adduction shortens the cycle time of the labia, thus producing a higher F0. In contrast, increased abduction causes the opposite effect. It slows down labial oscillations and causes F0 to decrease. The investigation of the effect of adduction is complicated because adduction is achieved actively by syrinx muscles (next section) as well as by the pressure balance between bronchial/tracheal lumen and air sac cavity.
3.3. Syringeal movements
Respiratory parameters provide a means for indirect F0 control. They determine the position of the vibrating tissues, and they facilitate oscillation of the labia. Muscles of the vocal organ allow direct neural control of F0, by affecting (a) the position of labia and (b) their tension.
The position of the labia is tied to their role as a valve. The most basic function of syringeal muscles is to adduct or abduct the labia. As explained above, adduction affects F0. In addition, some muscles change the length of the vibrating tissue directly, thus affecting tension. This direct control probably has the strongest effect on F0 because the tissue can be stretched to operate in its nonlinear stress range.
In the simplest morphology, the syrinx is controlled by two extrinsic syringeal muscles, the tracheolateral and sternotracheal muscles. In doves, these muscles can change the geometry of the tympanifom membrane by either abduction (contraction of the tracheolateral muscle) or adduction (contraction of the sternotracheal muscle). In addition, fibers of the tracheolateral muscle insert directly on the cranial end of the membrane, thus providing direct control of the stiffness and geometry of the membrane fold (e.g., Gaunt et al., 1982; Ballintijn et al., 1995; Elemans et al., 2006).
The larger number of muscles in more complex syringes may have evolved in part for facilitating a more efficient separation of the tasks of valve control and F0 control. In songbirds, these tasks are executed by different muscles. Yet, despite the greater independence between the two functions in songbirds, they remain coupled.
3.4. Feedback from upper vocal tract
Vocal tract geometry can affect F0 by supporting feedback mechanisms. A phenomenon called nonlinear source-filter coupling can influence the flow-induced labia or membrane oscillation. Vocal tract reactances can enhance the driving pressures of the oscillating tissue and the glottal flow, thereby increasing the energy level at the source. Interaction between the sound source and the vocal tract filter is controlled by cross-sectional areas of the suprasyringeal vocal tract. Although non-linear interactions have been investigated in theoretical work (e.g., Arneodo and Mindlin, 2009), experimental tests of the proposed mechanisms are very difficult.
4. Morphology and biomechanics of labia and lateral tympaniform membranes
The extracellular matrix of the labia and lateral tympaniform membrane contains collagen and elastin, the two main proteins that determine the viscoelastic properties of the tissue. In songbirds, the fiber composition and orientation has been studied in a few species, illustrating differences between these species in layer structure and overall fiber proportions. The labial composition is uniform in regard to fiber content and orientation in the zebra finch (Taeniopygia guttata) and shows a clear two-layer structure in the white crowned sparrow (Zonotrichia leucophrys) (Riede and Goller, 2010b) and a somewhat more complex but also layered structure in the European starling (Fig. 4). In white crowned sparrows there is a deep layer with a high concentration of elastic fibers while the superficial layer contains much more collagen fibers (Fig. 4). In starlings there are two central layers in which the collagen fibers are differently oriented. In addition there is a third superficial layer where collagen fibers appear more randomly oriented (Fig. 4). White crowned sparrow and starlings, in contrast to zebra finches, have vocal repertoires that encompass a greater range of F0. This evidence suggests that differences in labial histology may lay the foundation for different frequency ranges in the vocal repertoires of these species, but a larger comparative data set is needed to establish a clear link. The layer structure should allow recruitment of different portions of the labium into vibration, but direct evidence for such differential vibratory behavior of the labia is still missing (Riede and Goller, 2010b).
The size of the labia can give some insight into the lowest possible frequency range in a modal vibratory regime, but is not informative about the lowest possible oscillation frequency in the pulse tone mode. In the zebra finch, the left lateral labium is slightly smaller in males than in females, but the significance of this difference for frequency control is unclear (Riede et al., 2010a). In this species, most male and female vocalizations are harmonic stacks with low F0. Song is only produced by males and contains, in addition to low-frequency syllables, a few elements with high F0. However, these elements are generated on the right side of the syrinx (Goller and Cooper, 2004), and it is therefore unlikely that the smaller left lateral labium is the result of an expansion of the frequency range.
The presence of two sound generators in songbirds provides another possibility for comparison. Recordings of airflow through each sound source during song production allow determination of which sounds are contributed by each side of the syrinx. This evidence showed that in most species the left side of the syrinx contributes sound elements of a lower frequency range than does the right side (e.g. reviewed in Suthers and Goller, 1997; Suthers, 1999; Suthers and Zollinger, 2008). The respective frequency ranges of the two sides can overlap broadly (e.g., brown thrasher, Toxostoma rufum; Suthers, 1990; Suthers et al., 1994), but can also differ substantially with only partial overlap (e.g., northern cardinal, Cardinalis cardinalis; European starling, brown-headed cow-bird; Allan and Suthers, 1994; Suthers and Goller, 1997). In two cases this difference appears to be reversed (Suthers et al., 2011; Secora et al., 2012). Interestingly, even in species with a large difference between the frequency ranges of the left and right side, both sides can generate the same low frequency in the pulse-tone regime (e.g., European starling, zebra finch).
In the European starling the frequency range of the left-side generated sounds is 1–3.5 kHz, whereas that of the right-side generated syllables is 3–10 kHz (Uchida et al., 2010; Cooper and Goller, unpublished data). Both sides can generate pulse-tones between 250 and 800 Hz (Jensen et al., 2007). The size of the left and right labia is markedly different in this species. The lateral labium is slightly, but significantly, smaller on the right side, and the right medial labium is approximately only one fifth the size of the left medial labium (Prince et al., 2011). Another morphological difference was found in the Northern cardinal. In this species the left medial labium is 4.5 times thicker ventrally than the right medial labium. The left side of the syrinx generates sounds below 3.5 kHz, whereas F0 of right side generated sounds ranges between 3.5 and 7 kHz (Jensen et al., 2008). The small lateral difference in labial size in the zebra finch (Riede et al., 2010a) is associated with slightly higher lowest resonance frequency for the right labia (Fee, 2002).
5. Aerodynamic regulation of fundamental frequency
Eliminating neural control by denervating the syringeal muscles typically either prohibits phonation or results in markedly decreased F0 of all song syllables (early evidence reviewed in Suthers and Goller, 1997; subsequently e.g., Floody and Arnold, 1997; Suthers et al., 2004; Secora et al., 2012). Whereas this indicates the predominant role of direct muscular control of F0, it also allows investigation of the effect of driving air sac pressure on F0. In the intact zebra finch syrinx, F0 increases exponentially with driving pressure, but after denervation drops to a relatively small increase with pressure (a linear model suggests approx. 32 Hz per kPa; Fig. 4) (Riede et al., 2010a; Riede and Goller unpublished data). This role of air sac pressure in regulating F0 in the denervated syrinx gives a good estimate of the magnitude to which pressure changes can affect F0. It does not, however, account for the fact that in the intact syrinx elongation and geometry of the labia may be different as a result of the muscular control of labial adduction and tension. These active control mechanisms may generate a situation where nonlinear relationships are prominent, and the specific effect of air sac pressure on F0 may therefore be slightly different from the one in the denervated syrinx. In addition, the pressure differential between the bronchial lumen and the interclavicular air sac further influences this relationship and is not controlled for in the denervated syrinx. In the ring dove (Streptopelia risoria), fluctuations in the interclavicular air sac pressure are independent of modulations in subsyringeal air sac pressure and contribute to amplitude and frequency modulation of coos (Beckers et al., 2003). It is unknown how the interclavicular pressure is modulated. In songbirds no comparable data exist to our knowledge.
In a tyrannid suboscine, the great Kiskadee (Pitangus sulfuratus), air sac pressure is highly correlated with F0 of the 3 syllables (“kis-ka-dee”) of its territorial call sequence (Amador et al., 2008). Frequency modulation spans approximately 500–700 Hz. After a bilateral denervation of the syringeal muscles this relationship remained unchanged, suggesting that F0 is predominantly controlled by pressure. This relationship could be reproduced in a syrinx model, if a non-linear restitution force was assumed (Amador et al., 2008). This nonlinear relationship may determine to what degree pressure can drive changes in F0. Unfortunately, calibrated air sac pressure data are not available in this species for comparison. The syrinx of the Kiskadee features prominent intrinsic syringeal muscles, but these apparently are not involved in regulating F0 in this vocalization.
Most bird vocalizations are generated during expiration and self-sustained oscillations therefore arise from a pressure differential that tends to push the labia craniad. A few cases of inspiratory phonation have also been described (doves and zebra finch). In these cases, the labial geometry must be different in that the reversed pressure differential must push the labia downward. Differences in the geometry arising from these different movements must change the stress-strain relationship that applies during these vocalizations. In the zebra finch, inspiratory song elements are characterized by high fundamental frequency and are generated with lower absolute air sac pressure values than high-frequency expiratory syllables (Goller and Daley, 2001). However, in both cases, neural control of sound frequency was also present, making it difficult to assess the contribution of labial geometry in determining F0.
6. Muscular control of fundamental frequency
Both extrinsic syringeal muscles are activated during the coo in doves (Gaunt et al., 1982; Elemans et al., 2006). Whereas the sternotracheal muscle is tonically activated with little modulation throughout the coo sequence, the activity of the tracheolateral muscle shows more modulation, which is particularly pronounced during the trill portion of the coo. Assuming that the lateral tympaniform membranes are the main sound source (Goller and Larsen, 1997b; Elemans et al., 2006), both muscles can affect vibration frequency, by changing the position of the membrane folds and, in the case of the tracheolateral muscle, via fibers that directly insert on the membrane (Fig. 2). This expectation of a more direct involvement of the tracheolateral muscle is supported by the activation pattern, but the relationship between electromyographic (emg) activity and F0 is not a simple one (Elemans et al., 2006). Furthermore, at least during part of the trill, the amplitude and frequency modulation of the sound is paralleled by modulation of the subsyringeal air sac pressure, and, more importantly, the pressure of the interclavicular air sac is highly correlated with F0 throughout the coo (Beckers et al., 2003), creating a highly complex interplay of forces on the lateral tympaniform membranes. Because of this combination of control mechanisms it remains unclear to what degree the activity in both muscles contributes directly to frequency control. Although denervation experiments have not been done, the respective contributions of the activity in the tracheolateral muscle and of the pressure differential generated by modulating interclavicular air sac pressure have been explored in a model based on physiologically relevant parameters (Elemans et al., 2007). The results indicate that both control mechanisms are important with a stronger effect of muscle control. Reduction in tracheolateral muscle activation causes a lower F0 and reduces frequency modulation more than the change that occurs when the pressure differential is eliminated. The combined effect of both mechanisms results in a loss of frequency modulation, but in a smaller decline in F0 relative to the elimination of muscle control alone (Elemans et al., 2007).
In songbirds, intrinsic syringeal muscles are the main effectors of gating and tension control (e.g., Vicario, 1991; Goller and Suthers, 1995, 1996a,b; Larsen and Goller, 2002). The function of the intrinsic syringeal muscles has been inferred from emg recordings with simultaneous airflow data during spontaneous behavior and from direct stimulation of these muscles while the position of the labia was monitored with a fiberscope (Larsen and Goller, 2002). The lateral labium is withdrawn from the bronchial lumen by the ventral tracheobronchial muscle (vTB, abductor) and moved into the lumen by the dorsal tracheobronchial muscle (dTB, adductor). How the position of the medial labium is controlled is not clearly understood. Although it is likely that the ventral (vS) and dorsal (dS) syringeal muscles are involved, clear labial movements were not observed when these muscles were stimulated. As detailed below, emg data indicate a prominent role of the vS in the control of labial tension. Syringeal muscles display remarkably fast contraction kinetics, thus allowing very rapid labial gating movements and direct tension control (Elemans et al., 2004, 2008).
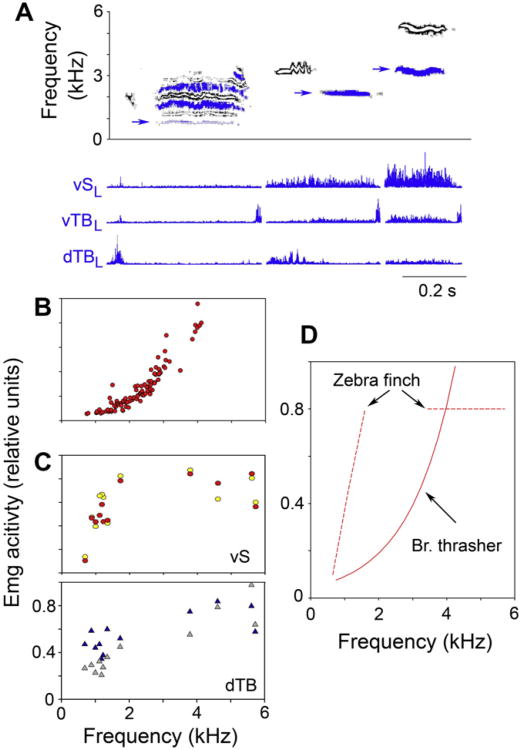
In the brown thrasher, activity in all measured syringeal muscles (vTB, dTB and vS) increases with increasing F0. Whereas the increase in activity associated with frequency control is relatively small in the main gating muscles (vTB and dTB) compared to their activation amplitude during gating tasks, vS activity increases exponentially with ipsilaterally generated F0 (Fig. 6a and b) (Goller and Suthers, 1996a,b). This tight relationship is also present on a short time scale, such that the amplitude pattern of the vS activation predicts the frequency modulation pattern of syllables (Goller and Suthers, 1996b).
In the zebra finch, the relationship between vS activation and F0 is less clear. For low-frequency sounds (0.5–1.5 kHz), emg activity of the vS and dTB increases with increasing F0, but for the high-frequency syllables neither the left nor the right vS or dTB show a strong correlation (Sitt et al., 2010; Fig. 6c). High-frequency syllables (2.5–7 kHz) are generated with the right side of the syrinx, whereas low-frequency syllables are generated with contributions from both sides (Goller and Cooper, 2004; Jensen et al., 2007). The two sets of frequencies most likely arise from different dynamical regimes of vibration. Low frequencies with rich upper harmonic content are generated by a mechanism similar to a pulse-tone register, whereas the high frequencies arise from a modal vibratory behavior that is also typical for most of the tonal sound production in birds (Jensen et al., 2007; Sitt et al., 2008, 2010). How the specific frequency is controlled during high-frequency sounds is not understood. Although vS and dTB are maximally activated, there is no directed change in emg amplitude within this frequency range. In addition, the same low frequencies may be generated by different mechanisms for different vocalizations of the repertoire, as suggested by dual flow recordings in the Australian magpie (Gymnorhina tibicen) (Suthers et al., 2011).
The different relationships between F0 and vS activity in thrashers and zebra finches is puzzling (Fig. 7d). A possible explanation could be that muscles that have not been monitored in the zebra finch, such as the dorsal syringeal muscle (dS), show differential activation during the high-frequency syllables. Preliminary results from emg recordings of dS do not support this explanation (Goller, Young and Mindlin, unpublished), but more work is needed to fully assess this possibility. It is also unlikely that modulation in the amplitude of driving pressure could effect changes in F0 within this range. Because the effect of air sac pressure on F0 is approximately 32 Hz per kPa after denervation, it is highly unlikely that the observed amplitude differences for high frequency syllables could account for changes in F0 that are in the kHz range (Fig. 6). More research is needed to elucidate F0-control in the zebra finch, but the complex interplay of dynamical behavior of the labia and biomechanical changes associated with unilateral phonation could play a role.
Fig. 7.
(A) In the brown thrasher vS activity is strongly correlated with fundamental frequency (F0). Spectrographic examples of 3 syllables (top panel) from the song of a brown thrasher whose left-side contributions (blue) vary in fundamental frequency (F0, indicated by arrows) are displayed with the accompanying emg activity (rectified) of three ipsilateral syringeal muscles (vS, ventral syringeal, vTB, ventral tracheobronchial, dTB, dorsal tracheobronchial). As the leftside generated frequency increases, so does emg activity in all three muscles. However, whereas the activity in dTB and vTB during the syllable is only a fraction of their maximal activation during adduction and abduction, respectively, vS activity is more tightly linked to F0. The left-side contributions were identified from simultaneously recorded bilateral airflow measurements (not shown). (Redrawn after Goller and Suthers, 1996b). (B) Emg activity in vS increases exponentially with increasing ipsilateral F0 in the brown thrasher. One representative example from the left side indicates a tight relationship, suggesting that vS contraction changes labial tension directly. Data points are measurements over 20 ms segments and are adjusted (by 8 ms) for delays between activation and acoustic effect induced by excitation-contraction coupling. (Redrawn after Goller and Suthers, 1996b). (C) In the zebra finch, emg activity in both vS muscles (top panel; left – red; right – yellow) and dTB (bottom panel; left – blue; right - grey) increases with F0 for the low-frequency (500–1600 Hz) elements of song syllables, but no longer shows a correlation with frequency for the right-side generated high-frequency elements. Each data point in this representative example for the song motif of one individual corresponds to the mean activation amplitude over a 20 ms segment with 8 ms rotation to account for excitation contraction coupling delays. Data were normalized to the peak amplitude and therefore the mean for the 20 ms period of highest activation can be less than 1. The distinct gap in F0 between low-frequency and high-frequency elements is typically present in zebra finches and likely reflects the switch between oscillatory regimes of the labia (Sitt et al., 2008). Data are from Goller and Cooper (unpublished); similar data for other individuals are also shown in Sitt et al., 2010). (D) The trendlines for the vS data sets of the two species illustrate the different relationships between vS activation and F0. Note that the extended frequency range of the zebra finch includes contributions from both syringeal sound generators, whereas only the left side is shown for the brown thrasher.
7. Conclusions
The exploration of how the complex interplay of functional morphological and dynamical parameters with central neural control gives rise to F0 is still in its infancy, but our current knowledge gives some general insight that is relevant to a number of research areas in bioacoustics. In particular, the question of how proximate mechanisms can inform us about evolutionary aspects of vocal behavior is of interest.
7.1. Neural control in the context of complex interplay
The different parameters (tissue properties, pressure, neuromuscular control of movements, potential source-tract interactions) generate a multidimensional parameter space for control of F0, which creates the possibility of generating the same F0 with different combinations of these parameters. This significant parameter space therefore creates the potential for individual specific regulation of F0. Inter-individual variation in labial histology, aerodynamic and biomechanical parameters presents an individual-specific target for neural control. Inter-individual variation in neural control patterns has not been investigated in much detail, but frequency control offers a tractable system for such investigation.
Because in most vocalizations, all aspects of neuromuscular control are highly stereotyped within individuals, we do not expect individual birds to generate F0 of a particular song syllable by different combinations of all involved parameters. However, as the variation around the general relationship of vS activation and F0 indicates (Fig. 7), different combinations may be used for the same frequency in different syllabic contexts. As a consequence, differential neural control of syringeal muscles will be required for generation of the same F0, if any of the other relevant parameters are different. For example, if air sac pressure is substantially different between two syllables, the position and tension of the labia will also be different, thus requiring different activation of gating and tension control muscles to generate the same F0. Although this effect will be small in most cases, it could combine with differential recruitment of labial tissue, generating a much different target for neural control.
This general point becomes specifically relevant if we consider variation of sound amplitude. Production of the same song syllables with increased sound amplitude typically requires an increase in subsyringeal air sac pressure (e.g., Plummer and Goller, 2008). This increase will cause a slight increase in F0 (Fig. 6, linear model), if muscle activation is held the same. Sound amplitude can vary substantially in some species, and ambient noise typically induces birds to sing more loudly (Lombard effect; for review Brumm and Zollinger, 2011). If birds hold F0 constant under variable driving pressures, some compensation in muscular control is required. Currently, it is not known whether birds compensate for the effects of pressure on F0. However, in the zebra finch fluctuations in F0 occur when the same song syllable is sung with different air sac pressure amplitudes (Goller et al., unpublished results). The observed changes are within the expected range for pressure-driven effects on F0 (see above), suggesting that neural compensation for the pressure effect does not occur. More research on this topic will be highly relevant to a number of current research areas.
Whether ambient noise changes the singing behavior of individual birds or populations has become an important focus of environmental research. Among the many noise-induced changes in singing behavior, it has been proposed that individual birds, in an effort to avoid low-frequency anthropogenic noise, dynamically sing their otherwise stereotyped songs at a slightly higher F0 (e.g., Verzijden et al., 2010). Because background noise reflexively induces the Lombard effect, generating song at higher sound amplitude may account for increased F0, if no or only incomplete compensation for the elevated pressure occurs. In other words, the observed increase in F0 may merely be a byproduct of singing with increased air sac pressure in order to achieve greater sound amplitude (see also discussion in Nemeth and Brumm, 2009, 2010). Consistent with this interpretation, the reported increases in F0 are small and in the order of magnitude that can be expected from pressure driven fluctuations (Fig. 6).
7.2. Fundamental frequency and the evolution of more complex muscular control of the syrinx
Vibrating tissues in airways perform two main functions, control of airflow and sound generation. In the “simple” syrinx, muscles influence F0 largely by changing the position of the vibrating tissues in the airstream and the resulting changes in tension. Additionally, as in the case of doves, some fibers of the tracheolateral muscle attach directly to the tympaniform membrane and therefore facilitate some more direct effect on its tension. However, a clear separation of abduction and adduction from tension control is not achieved. The evolution of intrinsic syringeal muscles in more “complex” syringes, such as the parrot and songbird syrinx, allows more separation of gating and tension control by increasing the degrees of freedom in the biomechanical control of the vibrating tissues. Whereas the two pairs of intrinsic muscles in the parrot syrinx are still mainly involved in the valving activity (Gaunt and Gaunt, 1985; Larsen and Goller, 2002), the songbird syrinx appears to have at least one muscle (vS) whose main function is control of F0 (Goller and Suthers, 1996b; Larsen and Goller, 2002). This direct control of labial tension enables birds to operate within the full range of frequencies that are physically possible, as well as a potential mechanism for very rapid frequency modulation (e.g., Elemans et al., 2004, 2008). The syrinx of oscine songbirds therefore appears to have gained an additional degree of freedom in F0 control compared to the vocal organs of non-passeriform groups and perhaps even suboscine groups. This added control mechanism is likely to have contributed to the spectral diversity found in oscine vocal repertoires (Table 1; see also Gaunt, 1983, 1987).
A second evolutionary step in increasing the frequency range of a vocal repertoire is the use of two independent sound generators that can be tuned to different F0-ranges. The syrinx of a number of bird taxa (e.g., penguins, owls, night-hawks, hummingbirds, cuckoos, swifts, songbirds; e.g., King, 1989) has two sound sources, but to what degree the dual sound source is used for expanding the range of F0 has not been investigated. In songbirds, the two sound generators tend to contribute differentially to the range of F0 of song, but this performance difference is not always accompanied by a strong morphological difference as in the case of the European starling. Other parameters involved in the determination of F0, such as layered composition of the labial extracellular matrix or differences in biomechanics, may explain the lack of strong size differences.
In the context of this discussion it is also interesting to ask to what degree vocal learning contributes to control of F0 and its range in vocal repertoires. Vocal learning, acquisition of a vocal template that shapes vocal behavior during ontogeny, appears to have evolved independently three times in birds (e.g., Jarvis et al., 2000). Of these three groups, parrots are limited by the presence of only one sound generator and less direct control of the tension of the vibrating tissues. Although parrots are renowned for their vocal ability, the range of F0 within individual repertoires is more limited than in oscine songbirds. We therefore postulate that neural control arising from vocal learning is not necessarily required for the emergence of a broad range of F0 in a bird's vocal repertoire. This notion may explain why birds that are not vocal learners can also generate broad ranges of F0. Vocal learning may, however, be a means for exploring the full potential of a vocal organ, including all dynamical regimes, large repertoires requiring a large number of rapid and precise motor gestures. Comparative work at the interface between functional morphology and neural control could shed some new light on the evolution of complexity in the vocal organ and the resulting behavior (Table 1).
The nonlinear characteristics of variables determining F0, most prominently viscoelastic properties of oscillating tissue, are important factors contributing to the uncoupling of body mass and F0 and why body size can explain only < 50% of the F0 variation across species (Fig. 1) (e.g., Cardoso, 2012). A better understanding of each of these factors cannot only explain why some species can produce sounds with very broad F0 ranges, but may also explain why F0 ranges vary between individuals (Table 1). For example, the expression of fibrous proteins depends on multiple factors, such as food and water availability, vocal training, immunity or pathologies, i.e. all factors shown to affect vocal behavior, yet without providing a causal link.
7.3. Importance of fundamental frequency in vocal signals
A thorough understanding of the mechanisms underlying control of F0 in birds does not only provide a foundation for our investigation of central motor control of vocal behavior, but also constitutes important information for other research areas, including signal evolution, ecological and sociobiological aspects of vocal communication. This importance is illustrated by evidence showing a perceptual and behavioral relevance of F0 in birds (e.g., Okanoya, 2000; Vignal and Mathevon, 2011). The above discussion of the interrelationship between F0 and sound intensity focused on the ecological relevance, but this relationship also opens avenues for communicative relevance. Fluctuations in F0 of otherwise stereotyped song may convey additional meaning, such as motivational aspects, as well as reflect internal physiological condition (e.g., endocrine status). If stereotypy of vocal signals including F0 is an important means for identifying the fitness of the signaling bird, variations in F0 induced by the complex interactions may play a significant role. Strong selection for an ability to compensate for fluctuations may occur in some species. Comparative work at all levels will be needed to elucidate further details in which F0 plays a role in the evolution of vocal communication.
Acknowledgments
The authors' research is supported by the National Institutes of Health (NIDCD 06876 and NIDCD 008612).
References
- Allan SE, Suthers RA. Lateralization and motor stereotypy of song production in the brown-headed cowbird. J Neurobiol. 1994;25:1154–1166. doi: 10.1002/neu.480250910. [DOI] [PubMed] [Google Scholar]
- Amador A, Goller F, Mindlin GB. Frequency modulation during song in a suboscine does not require vocal muscles. J Neurophysiol. 2008;99:2383–2389. doi: 10.1152/jn.01002.2007. [DOI] [PubMed] [Google Scholar]
- Arneodo EM, Mindlin GB. Source-tract coupling in birdsong production. Physical Rev E. 2009;79:061921. doi: 10.1103/PhysRevE.79.061921. [DOI] [PubMed] [Google Scholar]
- Ballintijn MR. PhD Thesis. Leiden Univ; The Netherlands: 1999. Vocal variation in the Collared dove. [Google Scholar]
- Ballintijn MR, ten Cate C, Nuijens FW, Berkhoudt H. The syrinx of the collared dove (Streptopelia decaocto): structure, inter-individual variation and development. Neth J Zool. 1995;45:455–479. [Google Scholar]
- Beckers GJL, Suthers RA, tenCate C. Mechanisms of frequency and amplitude modulation in ring dove song. J Exp Biol. 2003;206:1833–1843. doi: 10.1242/jeb.00364. [DOI] [PubMed] [Google Scholar]
- Bergmann HH, Helb HW. Stimmen der Vögel Europas. BLV Verlag; München: 1982. [Google Scholar]
- Brackenbury JH. Functions of the syrinx and the control of sound production. In: King AS, McLelland J, editors. Form and Function in Birds. Academic Press; London: 1989. pp. 193–220. [Google Scholar]
- Brumm H, Zollinger SA. The evolution of the Lombard effect: 100 years of psychoacoustic research. Behaviour. 2011;148:1173–1198. [Google Scholar]
- Cardoso GC. Paradoxical calls: the opposite signaling role of sound frequency across bird species. Behav Ecol Doi. 2012 http://dx.doi.org/10.1093/beheco/arr200.
- Chan RW, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg. 2001;124:607–614. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- Chan RW, Fu M, Young L, Tirunagari N. Relative contributions of collagen and elastin to elasticity of the vocal fold under tension. Anna Biomedi Eng. 2007;35:1471–1483. doi: 10.1007/s10439-007-9314-x. [DOI] [PubMed] [Google Scholar]
- Cowin SC, Doty SB. Tissue Mechanics. Springer; New York: 2007. [Google Scholar]
- Cuvier G. Über den unteren Larynx der Vögel. Reils Archiv Physiol. 1802;5:67–96. [Google Scholar]
- Elemans CP, Spierts IL, Müller UK, van Leeuwen JL, Goller F. Superfast muscles control dove's trill. Nature. 2004;431:146. doi: 10.1038/431146a. [DOI] [PubMed] [Google Scholar]
- Elemans CPH, Spierts IL, Hendriks M, Schipper H, Müller UK, van Leeuwen JL. Syringeal muscles fit the trill in ring doves (Streptopelia risoria L.) J Exp Biol. 2006;209:965–977. doi: 10.1242/jeb.02066. [DOI] [PubMed] [Google Scholar]
- Elemans CPH, Zaccarelli R, Herzel H. Biomechanics and control of vocalization in a non-songbird. J Royal Soc Interface. 2007;5:691–703. doi: 10.1098/rsif.2007.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemans CPH, Mead AF, Rome LC, Goller F. Superfast vocal muscles control song production in songbirds. PLoS ONE. 2008;3:e2581. doi: 10.1371/journal.pone.0002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemans CPH, Laje R, Mindlin G, Goller F. Smooth operator: avoidance of subharmonic bifurcations through mechanical mechanisms simplifies song motor control in adult zebra finches. J Neurosci. 2010;30:13246–13253. doi: 10.1523/JNEUROSCI.1130-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS. Measurement of the linear and nonlinear mechanical properties of the oscine syrinx: implications for function. J Comp Physiol A. 2002;188:829–839. doi: 10.1007/s00359-002-0349-z. [DOI] [PubMed] [Google Scholar]
- Fee MS, Shraiman B, Pesaran B, Mitra PP. The role of nonlinear dynamics of the syrinx in the vocalizations of a songbird. Nature. 1998;395:67–71. doi: 10.1038/25725. [DOI] [PubMed] [Google Scholar]
- Floody OR, Arnold AP. Song lateralization in the zebra finch. Horm Behav. 1997;31:25–34. doi: 10.1006/hbeh.1997.1368. [DOI] [PubMed] [Google Scholar]
- Fung YC. Biomechanics: Mechanical Properties of Living Tissues. Springer; New York: 1993. [Google Scholar]
- Fürbringer M. Specieller Theil. Holkema; Amsterdam: 1888. Untersuchungen zur Morphologie und Systematik der Vögel. [Google Scholar]
- Gans C. Sound production in the Silientia: mechanism and evolution of the emitter. Amer Zool. 1973;13:1179–1194. [Google Scholar]
- Gaunt AS. An hypothesis concerning the relationship of syringeal structure to vocal abilities. Auk. 1983;100:853–862. [Google Scholar]
- Gaunt AS. Phonation. In: Seller TJ, editor. Bird Respiration. CRC Press; Boca Raton: 1987. pp. 71–94. [Google Scholar]
- Gaunt AS, Gaunt SLL. Electromyographic studies of the avian syrinx in parrots (Aves:Psittacidae) Zoomorphology. 1985;105:1–11. [Google Scholar]
- Gaunt AS, Stein RC, Gaunt SLL. Pressure and air flow during distress calls of the starling, Sturnus vulgaris (Aves:Passeriformes) J Exp Zool. 1973;183:241–262. [Google Scholar]
- Gaunt AS, Gaunt SLL, Casey RM. Syringeal mechanics reassessed: evidence from Streptopelia. Auk. 1982;99:474–494. [Google Scholar]
- Goller F, Cooper BG. Peripheral motor dynamics of song production in the zebra finch. Ann N Y Acad Sci. 2004;1016:130–152. doi: 10.1196/annals.1298.009. [DOI] [PubMed] [Google Scholar]
- Goller F, Cooper BG. Peripheral mechanisms of sensorimotor integration during singing. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; Cambridge: 2008. pp. 99–114. [Google Scholar]
- Goller F, Daley MA. Novel motor gestures for phonation during inspiration enhance the acoustic complexity of birdsong. Proc Roy Soc Lond B. 2001;268:2301–2305. doi: 10.1098/rspb.2001.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Larsen ON. A new mechanism of sound generation in songbirds. Proc Natl Acad Sci USA. 1997a;94:14787–14791. doi: 10.1073/pnas.94.26.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Larsen ON. In situ biomechanics of the syrinx and sound production in pigeons. J Exp Biol. 1997b;200:2156–2167. doi: 10.1242/jeb.200.16.2165. [DOI] [PubMed] [Google Scholar]
- Goller F, Larsen ON. New perspectives on mechanisms of sound generation in songbirds. J Comp Physiol A. 2002;188:841–850. doi: 10.1007/s00359-002-0350-6. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Implications for lateralization of bird song from unilateral gating of bilateral motor patterns. Nature. 1995;373:63–66. [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles on gating airflow and sound production in singing brown thrashers. J Neurophysiol. 1996a;75:867–876. doi: 10.1152/jn.1996.75.2.867. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in controlling the phonology of bird song. J Neurophysiol. 1996b;76:287–300. doi: 10.1152/jn.1996.76.1.287. [DOI] [PubMed] [Google Scholar]
- Gray SD, Titze IR, Alipour F, Hammond TH. Biomechanical and histological observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109:77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- Haji T. Mechanical properties of the vocal fold. Practica Otologica Kyoto. 1990;83:793–808. [Google Scholar]
- Hérissant M. Recherches sur les organs de la voix des quadrupèds et de celle des oiseaux. Mém l'Acad Sci Paris. 1753:279–295. [Google Scholar]
- Hirano M, Ohala J, Vennard W. The function of the laryngeal muscles in regulating fundamental frequency and intensity of phonation. J Speech Lang Hear Res. 1969;12:616–628. doi: 10.1044/jshr.1203.616. [DOI] [PubMed] [Google Scholar]
- Hollien H, Moore GP. Measurements of the vocal folds during changes in pitch. J Speech Lang Hear Res. 1960;3:157–165. [Google Scholar]
- Holmberg E, Hillman R, Perkell J. Glottal airway and pressure measurement for soft, normal, and loud voice by male and female speakers. J Acoust Soc Am. 1988;84:511–529. doi: 10.1121/1.396829. [DOI] [PubMed] [Google Scholar]
- Hunter E, Titze IR. Refinements in modeling the passive properties oflaryngeal soft tissue. J Appl Physiol. 2007;103:206–219. doi: 10.1152/japplphysiol.00892.2006. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Ribeiro S, da Silva ML, Ventura D, Vielliard J, Mello CV. Behaviorally driven gene expression reveals song nuclei in hummingbird brain. Nature. 2000;406:628–632. doi: 10.1038/35020570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KK, Cooper BG, Larsen ON, Goller F. Songbirds use pulse tone register in two voices to generate low frequency sound. Proc R Soc Lond B. 2007;274:2703–2710. doi: 10.1098/rspb.2007.0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KK, Zollinger SA, Childress S, Suthers RA. Extended Abstracts. Corvallis, Oregon: 2008. Anatomy and vibration dynamics of the sound-producing medial labia in songbird syrinxes. Second International Conference on Acoustic Communication by Animals; pp. 106–107. [Google Scholar]
- King AS. Functional anatomy of the syrinx. In: King AS, McLelland J, editors. Form and Function in Birds. Academic Press; London: 1989. pp. 105–192. [Google Scholar]
- Klemuk SA, Riede T, Walsh EJ, Titze IR. Adapted to roar: functional morphology of tiger and lion vocal folds. PLoS ONE. 2011;6(11):e27029. doi: 10.1371/journal.pone.0027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen ON, Goller F. Direct observation of syringeal muscle function in songbirds and a parrot. J Exp Biol. 2002;295:25–35. doi: 10.1242/jeb.205.1.25. [DOI] [PubMed] [Google Scholar]
- Min YB, Titze IR, Alipour-Haghighi F. Stress–strain response of the human vocal ligament. Ann Otol Rhinol Laryngol. 1995;104:563–569. doi: 10.1177/000348949510400711. [DOI] [PubMed] [Google Scholar]
- Mindlin GB, Laje R. The Physics of Birdsong. Springer Verlag; Berlin: 2005. [Google Scholar]
- Morton ES. Ecological sources of selection on avian sounds. Am Nat. 1975;109:17–34. [Google Scholar]
- Nemeth E, Brumm H. Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization? Anim Behav. 2009;78:637–641. [Google Scholar]
- Nemeth E, Brumm H. Birds and anthropogenic noise: are urban songs adaptive? Am Nat. 2010;176:465–475. doi: 10.1086/656275. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Searcy WA. Song function and the evolution of female preferences: why birds sing and why brains matter. Ann N Y Acad Sci. 2004;1016:704–723. doi: 10.1196/annals.1298.012. [DOI] [PubMed] [Google Scholar]
- Okanoya K. Perception of missing fundamentals in zebra finches and Bengalese finches. J Acoust Soc Jpn (E) 2000;21:63–68. [Google Scholar]
- Plummer EM, Goller F. Singing with reduced air sac volume causes uniform decrease in airflow and sound amplitude in the zebra finch. J Exp Biol. 2008;211:66–78. doi: 10.1242/jeb.011908. [DOI] [PubMed] [Google Scholar]
- Podos J, Nowicki S. Performance limits on birdsong. In: Marler P, Slabbekoorn H, editors. Nature's Music: The Science of Birdsong. Elsevier Academic Press; New York: 2004. pp. 318–342. [Google Scholar]
- Prince B, Riede T, Goller F. Sexual dimorphism and bilateral asymmetry of syrinx and vocal tract in the European starling (Sturnus vulgaris) J Morphol. 2011;272:1527–1536. doi: 10.1002/jmor.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T. Subglottal pressure, tracheal airflow and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J Neurophysiol. 2011;106:2580–2592. doi: 10.1152/jn.00478.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Goller F. Peripheral mechanisms for vocal production in birds – Differences and similarities to human speech and singing. Brain & Language. 2010a;115:69–80. doi: 10.1016/j.bandl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Goller F. Functional morphology of the sound generating labia in the syrinx of two songbird species. J Anat. 2010b;216:23–36. doi: 10.1111/j.1469-7580.2009.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Fisher JH, Goller F. Sexual dimorphism of the zebra finch syrinx indicates adaptation for high fundamental frequencies in males. PLoS ONE. 2010a;5(6):e11368. doi: 10.1371/journal.pone.0011368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Lingle S, Hunter E, Titze IR. Cervids with different vocal behavior demonstrate different viscoelastic properties of their vocal folds. J Morphol. 2010b;271:1–11. doi: 10.1002/jmor.10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, York A, Furst S, Müller R, Seelecke S. Elasticity and stress relaxation of a very small vocal fold. J Biomechanics. 2011a;44:1936–1940. doi: 10.1016/j.jbiomech.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Tokuda IT, Farmer CG. Subglottal pressure and fundamental frequency control in contact calls of juvenile Alligator mississippiensis. J Exp Biol. 2011b;214:3082–3095. doi: 10.1242/jeb.051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach MR, Burton AC. The reason for the shape of the distensibility curves of arteries. Can J Biochem. 1957;35:681–690. [PubMed] [Google Scholar]
- Rüppell W. Physiologie und Akustik der Vogelstimme. J Orn. 1933;81:433–542. [Google Scholar]
- Ryan MT, Brenowitz EA. The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am Nat. 1985;126:87–100. [Google Scholar]
- Searcy WA, Nowicki S. Reliability and Deception in Signaling Systems. Princeton Univ. Press; Princeton: 2005. The Evolution of Animal Communication. [Google Scholar]
- Secora KR, Peterson JR, Urbano CM, Chung B, Okanoya K, Cooper BG. Syringeal specialization of frequency control during song production int eh Bengalese finch (Lonchura striata domestica) PLoS ONE. 2012;7:e34135. doi: 10.1371/journal.pone.0034135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setterwall CG. PhD dissertation. University of Lund; Sweden: 1901. Studier öfver syrinx hos polymyoda passeres; p. 133. [Google Scholar]
- Sitt JD, Amador A, Goller F, Mindlin GB. Dynamical origin of spectrally rich vocalizations in birdsong. Phys Rev E. 2008;78:011905. doi: 10.1103/PhysRevE.78.011905. [DOI] [PubMed] [Google Scholar]
- Sitt JD, Arneodo EM, Goller F, Mindlin GB. Physiologically driven avian vocal synthesizer. Phys Rev E. 2010;81:031927. doi: 10.1103/PhysRevE.81.031927. [DOI] [PubMed] [Google Scholar]
- Suthers RA. Contributions to birdsong from the left and right sides of the intact syrinx. Nature. 1990;347:473–477. [Google Scholar]
- Suthers RA. The motor basis of vocal performance in songbirds. In: Hauser MD, Konishi M, editors. The Design of Animal Communication. MIT Press; Cambridge, MA: 1999. pp. 37–62. [Google Scholar]
- Suthers RA, Goller F. Motor correlates of vocal diversity in songbirds. In: Nolan V, Ketterson ED, Thompson CF, editors. Current Ornithology. Vol. 14. 1997. pp. 235–288. [Google Scholar]
- Suthers RA, Zollinger SA. From brain to song: the vocal organ and vocal tract. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; Cambridge: 2008. pp. 78–98. [Google Scholar]
- Suthers RA, Goller F, Hartley RS. Motor dynamics of song production by mimic thrushes. J Neurobiol. 1994;25:917–936. doi: 10.1002/neu.480250803. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Vallet EM, Tanvez A, Kreutzer M. Bilateral song production in domestic canaries. J Neurobiol. 2004;60:381–393. doi: 10.1002/neu.20040. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Wild JM, Kaplan G. Mechanisms of song production in the Australian magpie. J Comp Physiol. 2011;197:45–59. doi: 10.1007/s00359-010-0585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze IR. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am. 1988;83:1536–1552. doi: 10.1121/1.395910. [DOI] [PubMed] [Google Scholar]
- Titze IR. On the relation between subglottal pressure and fundamental frequency in phonation. J Acoust Soc Am. 1989;85:901–906. doi: 10.1121/1.397562. [DOI] [PubMed] [Google Scholar]
- Titze IR. Phonation threshold pressure: a missing link in glottal aerodynamics. J Acoust Soc Am. 1992;91:2926–2935. doi: 10.1121/1.402928. [DOI] [PubMed] [Google Scholar]
- Titze IR. Principles of Voice Production. Published by the National Center for Voice and Speech; Denver, CO, USA: 2000. [Google Scholar]
- Titze IR. Vocal fold mass is not a useful quantity for describing F0 in vocalization. J Speech Lang Hear Res. 2011;54:520–522. doi: 10.1044/1092-4388(2010/09-0284). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida AM, Meyers RA, Cooper BG, Goller F. Fibre architecture and song activation rates of syringeal muscles are not lateralized in the European starling. J Exp Biol. 2010;213:1069–1078. doi: 10.1242/jeb.038885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijden MN, Ripmeester EAP, Ohms VR, Snelderwaard P, Slabbekoorn H. Immediate spectral flexibility in singing chiffchaffs during experimental exposure to highway noise. J Exp Biol. 2010;213:2575–2581. doi: 10.1242/jeb.038299. [DOI] [PubMed] [Google Scholar]
- Vicario DS. Contributions of syringeal muscles to respiration and vocalization in the zebra finch. J Neurobiol. 1991;22:63–73. doi: 10.1002/neu.480220107. [DOI] [PubMed] [Google Scholar]
- Vignal C, Mathevon N. Effect of acoustic cue modifications on evoked vocal response to calls in zebra finches (Taeniopygia guttata) J Comp Psychol. 2011;125:150–161. doi: 10.1037/a0020865. [DOI] [PubMed] [Google Scholar]
- Wallschläger D. Correlation of song frequency and body weight in passerine birds. Experientia. 1980;36:412. [Google Scholar]
- Warner RW. The anatomy of the syrinx in passerine birds. J Zool Lond. 1972;168:381–393. [Google Scholar]
- Wunderlich L. Beiträge zur vergleichenden Anatomie und Entwicklungsgeschichte des unteren Kehlkopfes der Vögel. Nova Acta Ksl Leop-Carol Dt Akad Naturf. 1884;48:1–80. [Google Scholar]
- Yldiz H, Bahadir A, Akkoç A. A study on the morphological structure of syrinx in ostriches (Struthio camelus) Anat Histol Embryol. 2003;32:187–191. doi: 10.1046/j.1439-0264.2003.00462.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Reynders WJ, Jiang JJ, Tateya I. Determination of phonation instability pressure and phonation pressure range in excised larynges. J Speech Lang Hear Res. 2007;50:611–620. doi: 10.1044/1092-4388(2007/043). [DOI] [PubMed] [Google Scholar]
- Zhang K, Siegmund T, Chan RW, Fu M. Predictions of fundamental frequency changes during phonation based on a biomechanical model of the vocal fold lamina propria. J Voice. 2009;23:277–282. doi: 10.1016/j.jvoice.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]