Abstract
Background
Older Americans are facing an epidemic of chronic diseases and are thus exposed to anticholinergics (AC) that might negatively affect their risk of developing mild cognitive impairment (MCI) or dementia.
Objective
Investigate the association between impairment in cognitive function and previous AC exposure.
Design
A retrospective cohort study.
Setting
Primary care clinics in Indianapolis, Indiana.
Participants
3690 older adults who have undergone cognitive assessment and had a one-year medication dispensing record.
Outcome
Cognitive function was measured in two sequential steps; a two-step screening process followed by a formal diagnostic process for participants with positive screening results.
Exposure
Three patterns of AC exposure were defined by the duration of AC exposure, the number of AC medications dispensed at the same time, and the severity of AC effects as determined by the Anticholinergic Cognitive Burden List.
Results
In comparison to older adults with no anticholinergic exposure and after adjusting for age, race, gender, and underlying comorbidity, the odds ratio (OR) for having a diagnosis of MCI was 2.73 (95% confidence interval, CI; 1.27, 5.87) among older adults who were exposed to at least three possible anticholinergic for at least 90 days; and the OR for having dementia was 0.43 (95% CI; 0.10, 1.81).
Conclusion
Exposure to medications with severe anticholinergic cognitive burden may be a risk factor for developing MCI.
Keywords: anticholinergics, cognitive impairment, dementia, mild cognitive impairment, elderly
INTRODUCTION
More than 7 million Americans are suffering from dementia or mild cognitive impairment (MCI) and half of them are coping with at least two additional chronic diseases that require treatment with more than five medications1-4. The elderly population is sensitive to experiencing drug-related adverse effects that negatively impact their cognitive function such as exposure to anticholinergics (AC)5-10. It is estimated that more than 9 million older Americans, including those with cognitive impairment, are prescribed at least one AC with negative cognitive effects5,7.
The negative cognitive effects of AC have been known for decades and were assumed to be reversible and transient5,8-10. More recently, a new hypothesis has been emerging that connects the effect of AC exposure to the pathogenesis of Alzheimer disease (AD)11-14. The basis for this connection between AC and AD pathology was primarily investigated in Parkinson’s disease11. Perry et al found that the continuous use of AC for at least two years doubled the prevalence of both amyloid plaque and neurofibrillary tangle densities in Parkinson’s disease patients11. This hypothesis was further supported by recent animal studies12,13. Caccamo and colleagues studied the effect of AC on the development of Aβ peptides in transgenic mice that express several features similar to the human AD brain and found that a long-term blockade of the M1 receptor with the use of AC increased the presence of Aβ peptides in the cortex, hippocampus, and amygdala12.
We recently completed a systematic evidence review (SER) of the literature, which confirmed that AC have an acute negative effect on cognition (delirium) but found only few longitudinal studies that evaluated the long-term exposure to AC as a risk factor for developing chronic cognitive impairment7,28. Our SER found several gaps in the literature. First, few studies evaluated long-term effects of anticholinergics on cognition in the elderly and their results are conflicting6-11,30. One recent study reported a potentially reversible association between AC use and cognitive decline30. Second, the measurement of drug exposure in the longitudinal studies was not based on actual medication dispensing records, including the recent study that found a reversible association between AC exposure and cognitive deficit7,28,30. Third, the only study that had access to dispensing data did not have access to a comprehensive cognitive assessment9, thus most likely not recognizing half of the cognitively impaired patients among their control group4-16.
As a first step in enhancing prescribing patterns for older adults with chronic diseases and reducing their risk of developing MCI or dementia, we are presenting the findings of a one year retrospective cohort study of primary care patients aged 65 and older to better understand the relationship between cognitive function, comorbidity and AC use. The data of the proposed study was generated by merging the cognitive assessment of more than 4000 older patients enrolled in the 2002-2004 Indianapolis Dementia Screening and Diagnosis study (IDSD)3,16-18 with their one-year drug dispensing data captured by the Regenstrief electronic medical record system15,19,20.
We hypothesized that after adjusting for potential confounders and in comparison to primary care patients who were not exposed to AC, those who were exposed to at least one severe anticholinergic or to three mild anticholinergics for at least two months would have a higher risk of cognitive impairment as defined by the presence of positive screening for dementia, having a diagnosis of MCI, or suffering from dementia.
METHODS
Data Source and Sample
Subjects were selected from the Indianapolis Dementia Screening and Diagnosis (IDSD) study, which has been described in detail in previous studies3,16-18. Briefly, the IDSD study targeted 4197 participants aged 65 and older who were receiving their primary care services within the Wishard Health Services (WHS) in Indianapolis from January 2002 until October 2003. A two-stage procedure was applied to screen eligible participants for dementia, based on both the six-item screener21 and an abbreviated version of the Community Screening Instrument for Dementia (CSI-D)16,22.
Subjects with cognitive impairment were invited to participate in formal diagnostic assessments which included a standardized neuropsychological testing, neurological examinations, medical record review, and a structured interview with an informal caregiver such as spouse, child, or other relative. Approximately half of these patients refused participation in the diagnostic assessment. In comparison to the decliners, those who accepted were younger (73.8 vs. 75.4; P = 0.01) and had poorer CSI-D performance (18.3 vs. 19.2; P = 0.07). There were no group’s differences in race, gender, comorbid conditions, psychotropics, or chart documentation of dementia or depression17.
Using the diagnostic assessment results, a team consisting of a psychologist, neuropsychologist, geriatrician, and geriatric psychiatrist made the final diagnosis of dementia or MCI16-18,23-25. For this study we merged the IDSD screening and diagnostic data with the Regenstrief Medical Record System (RMRS), an electronic system that has captured Indianapolis medical data since 1972, including drug dispensing data at pharmacies affiliated with the Wishard Memorial Hospital and the 39 health care clinics within the WHS15,19,20. RMRS captures more than 85% of the drug dispensing data of all participants receiving care within the WHS system15,19, 20. Patients with no RMRS-based drug dispensing information have private insurance and are more affluent than those with drug dispensing data captured by the RMRS15,19, 20. We had access to one year of drug dispensing data prior to the patients’ screening and final diagnosis. Five hundred and seven out of the total 4197 participants did not have any drug dispensing record during this study period, and were excluded. These excluded patients were slightly more likely to be female, non-white, and to have no cognitive impairment. Our analyses focused on the remaining 3690 participants.
Cognitive Outcomes
Based on the above screening and diagnosis process, a total of 562 participants (out of the 3690 eligible participants) were considered to have cognitive impairment, i.e., screened positive on the six-item screener and the CSI-D. The six-item screening instrument is a brief tool measuring temporal orientation and new learning ability21. The CSI-D evaluates multiple cognitive domains (language, memory, attention and calculation among others) and includes a standardized interview of physical and social function from a caregiver informant or relative if available22. Patients who made at least one mistake on the six-item screener and subsequently scored ≤24 on the CSI-D were considered to have cognitive impairment requiring further diagnostic evaluation.
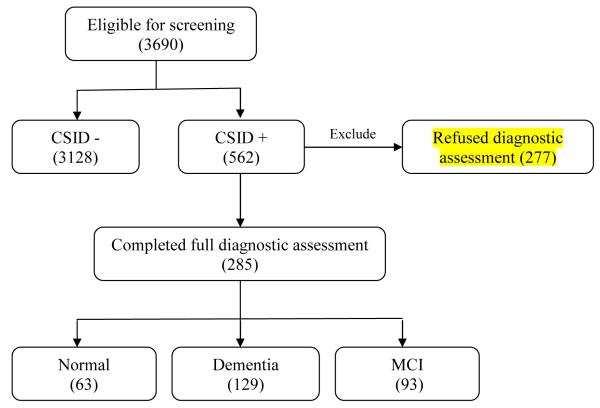
The second outcome of interest was the final diagnoses of participants, i.e., diagnosis of dementia (n=129) or MCI (n=93). Patients who screened negative on the six-item screener and the CSI-D (n=3128) or those who had normal cognition following their positive screening assessment (n=63) were considered to have no cognitive impairment (n=3191). In the analysis of the second outcome, we excluded 277 subjects who screened positive but refused to participate in subsequent diagnostic assessments. Figure 1 shows the tree-diagram for patient selections.
Figure 1.
Assessment Method
AC Exposure
We used the Anticholinergic Cognitive Burden (ACB) list to determine the anticholinergic activity of medications taken by our study cohort. The content validity of the ACB list was based on a systematic evidence review of 27 studies that measured the anticholinergic activities of a drug and evaluated the association between such activities and the cognitive function in older adults5,7. Based on this systematic review, a list of medications with anticholinergic activity was presented to an expert interdisciplinary team that included geriatricians, geriatric pharmacists, geriatric psychiatrists, general physicians, geriatric nurses, and aging brain researchers. This team categorized the above medications into mild (ACB score = 1) or severe anticholinergics (ACB score = 2 or 3). Drugs with mild anticholinergic effects were defined as those with serum anticholinergic activity or in-vitro affinity to muscarinic receptors but with no known clinically relevant negative cognitive effects. Drugs with established and clinically relevant cognitive anticholinergic effects were considered severe anticholinergics5,7. The ACB list has been shown to correlate well with a list developed from laboratory markers of anticholinergic activity26, and with cognitive performance among more than 13,000 British older adults27.
We structured participants’ exposure to AC based on three dimensions: the burden of AC, duration of AC exposure, and number of AC taken at the same time. Due to the complexity of drug dispensing records, we categorized the continuous or ordinal data for each of the above three dimensions into sub-groups so that an aggregated overall exposure to anticholinergics could be obtained for each patient. Anticholinergic burden was categorized as no burden (receiving no drug with an ACB score of 1, 2, or 3), mild burden (receiving at least one drug with an ACB score of 1), and severe burden (receiving at least one drug with an ACB score of 2 or 3). This categorization was based on our previous studies on clinically relevant negative cognitive effects of anticholinergics5,7. The duration of exposure was alternatively defined as the participant’s continuous use of AC (irrespective of the number of medications and their anticholinergic burden) for at least 30 days, 60 days, or 90 days. Finally, we tallied the number of mild and severe AC the participant took at the same time during the one-year period before cognitive assessment.
Covariates
Participant’s demographics (age, gender, and race) were included as covariates in multivariate analyses because they are likely associated with cognitive impairment3,14,16. We used the RMRS to identify the ten common chronic conditions that the participant had, including hypertension (HTN), arthritis, congestive heart failure (CHF), coronary artery disease (CAD), cancer, chronic obstructive pulmonary disease (COPD), diabetes, stroke, kidney disease, and liver disease. The RMRS use the international classification of diseases (ICD-9) codes documented by the physician during any ambulatory or hospital visit within Wishard Health Services since 1990. In addition, hypertension was defined based on three factors, an ICD-9 codes of hypertension, a blood pressure measurement (SBP ≥ 160 mmHg or DBP ≥ 95 mmHg), or use of antihypertensive medications. These co-morbidities, especially HTN, CHF, CAD, and stroke, may be important confounders since they tend to be regularly treated by mild AC and are considered risk factors for cognitive impairment. Finally, we calculated the annual Chronic Disease Score (CDS) to measure the severity of co-morbidity based on a participant’s medication profile28. The CDS ranges between 0 and 20, with higher score indicating greater chronic disease burden and utilization28.
Statistical Analyses
We first performed bivariate analyses to compare demographics and comorbidities across participant groups that were defined based on the screening (cognitive impairment versus no cognitive impairment) or the diagnostic assessment (MCI, and dementia). We further compared the rate of having cognitive impairment across participants with alternative exposure patterns. Group differences were compared using chi-square tests for categorical variables, and t-tests or analyses of variance (ANOVA) for continuous variables.
In multivariate analyses, we first estimated separate logistic regression models. The dependent variable in all models was whether the participant had cognitive impairment or not. The independent variable was three separate exposure patterns to AC. (1) Minimal mild anticholinergic burden (exposure pattern I): A binary variable was defined that equaled one if the participant had an ACB score =1 for less than three medications with a duration of exposure ≥90 days, and 0 otherwise. (2) Accumulative mild anticholinergic burden (exposure pattern II): A binary variable was defined that equaled one if the participant had an ACB score=1 for at least three medications with a duration of exposure ≥90 days, and 0 otherwise. The rational for this exposure structure was from our clinical expertise that taking three mild AC has a similar anticholinergic burden as taken one severe AC. (3) Severe anticholinergic burden (exposure pattern III): A binary variable was defined that equaled one if the participant had an ACB score = 2 or 3 for at least one medication with a duration of exposure ≥60 days, and 0 otherwise. All models controlled for the patient demographics and comorbidities.
We further estimated multivariate nominal regression models to determine the independent impact of AC exposure on dementia and MCI. The dependent variable was categorized as no cognitive impairment, diagnosis with MCI, and diagnosis with dementia, with no cognitive impairment being the control (omitted) group in each model. The key independent variable for exposure and covariates in the models was defined in the same way as described above.
RESULTS
Overall characteristics of the cohort
Compared with participants who screened negative, those with cognitive impairment were older and more likely to be non-white and male (Table 1). They also had a higher number of comorbidities and a higher rate of vascular burden as determined by the presence of CHF, CAD and stroke. Table 2 shows that compared with participants with no cognitive impairment, those diagnosed with dementia or MCI tended to be male, non-white, and older, and tended to have a higher rate of stroke.
Table 1.
Description of Participants, by screening results (N=3690)
| Screening negative (n=3128) |
Screening positive (n=562) |
P value | |
|---|---|---|---|
| Female (%) | 71.3% | 64.2% | 0.0008 |
| African Americans (%) | 59.3% | 68.1% | <0.0001 |
| Age (mean, SD) | 71.27 ± 5.56 | 74.90 ± 6.94 | <0.0001 |
| Number of chronic conditions (mean, SD) |
3.61 ± 1.71 | 3.91 ± 1.67 | 0.0001 |
| CDS (Median, Q1-Q3) | 5 (3-7) | 5 (3-7) | 0.87 |
| With HTN (%) | 99.1% | 99.8% | 0.08 |
| With CHF (%) | 35.1% | 42.2% | 0.001 |
| With CAD (%) | 36.9% | 40.3% | 0.12 |
| With Stroke (%) | 27.8% | 37.7% | <0.0001 |
CDS: Chronic Disease Score; HTN: Hypertension; CHF: Chronic Heart Failure; CAD: Coronary Artery Disease.
Table 2.
Description of Participants, by diagnostic groups (N=3413)
| Normal* (n=3191) |
MCI (n=93) |
Dementia (n=129) |
P value** | |
|---|---|---|---|---|
| Female (%) | 71.23% | 70.97% | 59.69% | 0.02 |
| African Americans (%) | 59.61% | 69.89% | 69.77% | 0.01 |
| Age (mean, SD) | 71.3±5.6 | 72.8±6.1 | 76.4±6.7 | <0.001 |
| Number of chronic conditions (mean, SD) |
3.6±1.7 | 3.9±1.7 | 3.9±1.6 | 0.05 |
| CDS (Median, Q1-Q3) | 5 (3-7) | 5 (4-8) | 5 (2-7) | 0.17 |
| With HTN (%) | 99.1% | 100.0% | 99.2% | 0.65 |
| With CHF (%) | 35.2% | 39.8% | 36.4% | 0.63 |
| With CAD (%) | 36.9% | 40.8% | 37.2% | 0.73 |
| With Stroke (%) | 27.8% | 40.9% | 48.8% | <0.001 |
Normal include subjects who screened negative and those who had no cognitive impairment at diagnostic assessment. CDS: Chronic Disease Score; HTN: Hypertension; CHF: Chronic Heart Failure; CAD: Coronary Artery Disease.
P-value is for the global comparison of the normal, MCI, and dementia groups.
Bivariate association between anticholinergic burden and cognition
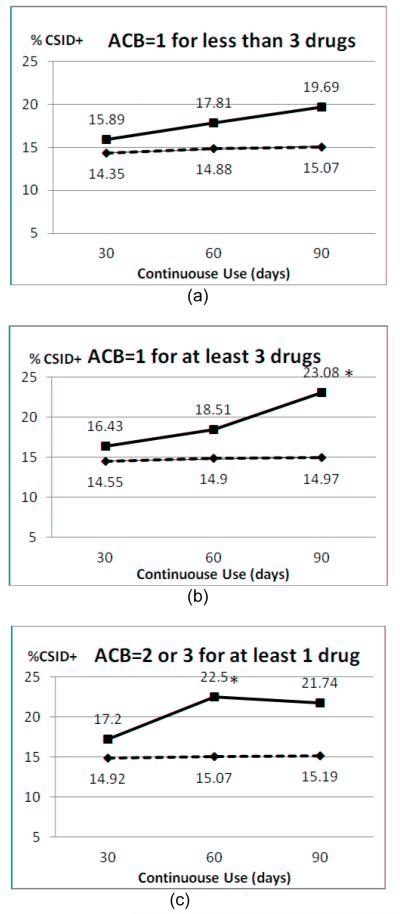
Prior to structuring our final AC exposure patterns and in order to determine the appropriate exposure duration, we conducted exploratory analyses on possible combinations of anticholinergic burden, duration of exposure, and number of medications used at the same time. Figure 2 shows the cognitive impairment rate across alternative exposure patterns defined along the three dimensions. In panel (a) of Figure 2, we held the anticholinergic burden at ACB=1 and the number of medications < 3, and found that the duration of exposure tended to be positively related to the rate of cognitive impairment (CSID+); compared to patients with exposure time <90 days, patients with exposure time ≥90 days had a higher rate of CSID+, although such difference was not statistically significant (i.e., 19.69% vs. 15.07%, p=0.16). In panel (b) of Figure 2, we found a similar trend of increased cognitive impairment as exposure time increased, when holding the anticholinergic burden at ACB=1 and the number of medications ≥ 3 (23.08% when exposure time ≥ 90 days, 14.97% when exposure time < 90 days, P=0.02). Marginally significant difference was found for patients with exposure time ≥ 60 days vs. < 60 days, holding the anticholinergic burden at ACB=2 or 3 and the number of medications ≥1 (panel (c), 22.5% vs. 15.07%, P=0.05). We used the results of these exploratory descriptive analyses to help structure the key independent variables in our multivariable analyses.
Figure 2.
Anticholinergic exposure and CSI-D status (*: P<0.05)  Longer than the days, ----Shorter than the days
Longer than the days, ----Shorter than the days
Multivariate analyses investigating the association between anticholinergic burden and cognitive impairment
Our multivariate analyses adjusted for two sets of potential confounders for the association between AC exposure and cognitive impairment; participant demographics and their underlying general comorbidity (see models A1, B1, and C1 in table 3), or their underlying vascular comorbidity (see models A2, B2, and C2 in table 3). Compared to the 777 non-AC users included in our study, patients with exposure pattern I (minimal exposure to mild anticholinergics, n=127) did not show a significantly increased likelihood of having cognitive impairment (OR=1.23, P=0.39 in model A1; and OR=1.20, P=0.40 in model A2); patients with exposure pattern II (accumulative exposure to mild anticholinergics, n=117) were 50% more likely than other patients to have cognitive impairment although such a difference was not statistically significant (OR=1.50, P=0.09 in model B1; and OR=1.46, P=0.11 in model B2); and participants with exposure pattern III (exposure to severe anticholinergic, n=80) were twice as likely as other patients to have cognitive impairment (OR=2.08, P=0.01 in model C1; and OR=2.13, P=0.01 in model C2).
Table 3.
Exposure to Anticholinergics and Positive Dementia Screening Result Controlling for Patient Covariates (n=3690)
| Parameter (Default) |
Exposure pattern I compared to no exposure |
Exposure pattern II compared to no exposure |
Exposure pattern III compared to no exposure |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model A1 |
Model A2 |
Model B1 |
Model B2 |
Model C1 |
Model C2 |
|||||||
| P value |
OR (95% CI) |
P value | OR (95% CI) |
P value |
OR (95% CI) |
P value | OR (95% CI) | P value |
OR (95% CI) |
P value | OR (95% CI) |
|
|
Defined exposure pattern |
0.39 | 1.23 (0.77, 1.96) |
0.46 | 1.20 (0.75, 1.91) |
0.09 |
1.50 (0.94, 2.37) |
0.11 |
1.46 (0.92, 2.32) |
0.01 |
2.08 (1.20, 3.62) |
0.01 |
2.13 (1.22, 3.71) |
| Sex (Male) |
<.001 | 0.61 (0.50, 0.75) |
<0.001 | 0.61 (0.50, 0.74) |
<.001 | 0.61 (0.50, 0.75) |
<.001 | 0.61 (0.50, 0.74) |
<0.001 | 0.60 (0.49, 0.74) |
<0.001 | 0.60 (0.49, 0.74) |
| RACE (Non-White) |
0.03 | 0.81 (0.66, 0.98) |
0.03 | 0.80 (0.66, 0.98) |
0.03 | 0.80 (0.66, 0.98) |
0.03 | 0.80 (0.66, 0.98) |
0.03 | 0.80 (0.65, 0.97) |
0.03 | 0.80 (0.65, 0.97) |
| Age (year) | <.001 | 1.10 (1.08, 1.11) |
<0.001 | 1.09 (1.08, 1.11) |
<.001 | 1.10 (1.08, 1.11) |
<.001 | 1.09 (1.08, 1.11) |
<.001 | 1.10 (1.08, 1.11) |
<.001 | 1.10 (1.08, 1.11) |
| CDS | 0.73 | 1.01 (0.98, 1.04) |
0.71 | 1.01 (0.98, 1.04) |
0.78 | 1.00 (0.97, 1.04) |
0.75 | 1.01 (0.98, 1.04) |
0.85 | 1.00 (0.97, 1.03) |
0.86 | 1.00 (0.97, 1.03) |
| NUMCHRON | 0.02 | 1.07 (1.01, 1.14) |
/ | / | 0.03 | 1.07 (1.01, 1.14) |
/ | / | 0.02 | 1.07 (1.01, 1.14) |
/ | / |
| HTN | / | / | 0.19 | 3.88 (0.52, 29.1) |
/ | / | 0.19 | 3.85 (0.51, 28.9) |
/ | / | 0.18 | 3.97 (0.53, 29.8) |
| CHF | / | / | 0.41 | 1.09 (0.89, 0.35) |
/ | / | 0.43 | 1.09 (0.88, 1.34) |
/ | / | 0.39 | 1.10 (0.89, 1.35) |
| CAD | / | / | 0.59 | 1.06 (0.86, 1.31) |
/ | / | 0.63 | 1.05 (0.86, 1.30) |
/ | / | 0.54 | 1.07 (0.87, 1.31) |
| Stroke | / | / | <0.01 | 1.38 (1.13, 1.68) |
/ | / | <0.01 | 1.38 (1.13, 1.68) |
/ | / | <0.01 | 1.39 (1.14, 1.69) |
Exposure pattern I: ACB=1 for less than 3 medications for more than 90 days; Exposure pattern II: ACB=1 for at least 3 medications for more than 90 days; Exposure pattern III: ACB=2 or 3 for at least 1 medication for more than 60 days. CDS: Chronic Disease Score; NUMCHRON: number of chronic conditions; HTN: Hypertension; CHF: Chronic Heart Failure; CAD: Coronary Artery Disease.
Models A1, B1, and C1 included gender, race, age, CDS, and number of chronic condition as confounders; Models A2, B2, and C2 included gender, race, age, CDS, HTN, CHF, CAD, and Stroke as confounders.
Multivariate analyses investigating the association between anticholinergic burden and MCI and dementia
Participants under exposure pattern II (n=94) were approximately 170% more likely than participants not under exposure pattern II to have MCI (OR=2.73, P=0.01 in model E1; and OR=2.63, P=0.01 in model E2). However, participants with other patterns of exposure were not independently associated with a diagnosis of MCI. In addition, patients with all defined exposure patterns were not statistically significantly associated with a diagnosis of dementia (see table 4).
Table 4.
Exposure to Anticholinergics and Diagnosis of Mild Cognition Impairment or Dementia Controlling for Patient Covariates (n=3413)
| Parameter | Exposure pattern I compared to no exposure |
Exposure pattern II compared to no exposure |
Exposure pattern III compared to no exposure |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model D1 |
Model D2 |
Model E1 |
Model E2 |
Model F1 |
Model F2 |
|||||||
| P value |
OR (95% CI) | P value | OR (95% CI) | P value |
OR (95% CI) | P value | OR (95% CI) | P value |
OR (95% CI) | P value | OR (95% CI) | |
| MCI (n=93) |
0.17 | 1.81 (0.77, 4.28) |
0.21 | 1.74 (0.74, 4.12) |
0.01 |
2.73 (1.27, 5.87) |
0.01 |
2.63 (1.22, 5.69) |
0.15 | 1.66 (0.50, 5.44) |
0.38 | 1.70 (0.52, 5.59) |
| Dementia (n=129) |
0.20 | 0.40 (0.09, 1.66) |
0.16 | 0.35 (0.08, 1.49) |
0.25 | 0.43 (0.10, 1.81) |
0.20 | 0.39 (0.09, 1.65) |
0.60 | 2.19 (0.76, 6.32) |
0.16 | 2.17 (0.74, 6.37) |
Model 1 included gender, race, age, CDS, and number of chronic condition as potential confounder; Model 2 included gender, race, age, CDS, HTN, CHF, CAD, and Stroke as potential confounders.
DISCUSSION
Our study found an association between anticholinergic burden and the risk of developing cognitive impairment. However, we found that such an association required both high anticholinergic burden and two to three months of continuous exposure to such a high burden. The crude risk of having cognitive impairment among older adults attending primary care clinics was increased by 50% (receiving at least three mild AC for more than 90 days) to 100% (receiving one or more severe AC for more than 60 days).
However, when we studied the association between high anticholinergic burden and having a diagnosis of dementia or MCI, the impact of high anticholinergic burden was less clear. Although receiving at least 90 days of three mild AC increased the odds of having a diagnosis of MCI by more than 170%, such an exposure did not increase the probability of dementia diagnosis. Furthermore, we found no association between severe AC use and either dementia or MCI. These conflicting results might be due to our small sample size with only 80 participants belonging to exposure pattern III and our detection of prevalent not incident cases of dementia or MCI. Regarding the AC effect on dementia, one may also consider that the one-year design of our study, with its small analytic window between outcome and exposure, may be a sufficient time frame for patients with dementia to be recognized by the their physicians who discontinue these medications. Another explanation might be that anticholinergics disturb the function of acetylcholine in the brain but may require a longer duration of receptor antagonism to develop neurodegenerative pathology and subsequent neuronal death. Such an explanation is supported by the Perry et al investigation that found a minimum exposure of two years to AC as the threshold for developing neurofibrillary tangles or amyloid plagues11.
Our study fills some gaps from the previous longitudinal studies that addressed the same question of chronic effects of AC on the aging brain6-11. Perry et al found that amyloid plaque densities were more than 2.5-fold higher in Parkinson’s disease patients treated with AC for at least two years compared with untreated patients or those treated for less than two years11. This large effect is similar to our finding of the presence of an association between high anticholinergic burden and MCI (OR of 2.53). However, the Perry definition of AC was very limited and included only Parkinson medications, which would have been categorized as severe AC by our ACB list.
Our findings were somewhat similar to a study that randomly recruited patients from general practices in southern France8. After adjustment for other possible causes of cognitive impairment, baseline use of AC was associated with one-year incidence of MCI (OR 5.12, 95% CI 1.94 to 13.51) but not with dementia8. This study used a detailed informant interview and a comprehensive neuropsychological assessment to make the diagnosis of MCI but used only the medical records of the general practitioners to identify the incidence of dementia over the subsequent eight years following the first-year cognitive assessment8. Furthermore, this study did not have access to drug dispensing data and used two home visits separated by 12 months to determine the presence of AC8.
In another longitudinal study that did not specifically evaluate the association between AC and dementia or MCI but focused on cognitive decline, Bottiggi et al used retrospective data from a longitudinal elderly cohort at an Alzheimer’s Disease Research Center6. This study found that AC use did not lead to an accelerated rate of decline in global cognitive status but it did lead to an accelerated rate of decline in scanning and visuomotor tracking and components of executive functioning6. This study used a very selective patient sample that was already attending memory care practices and therefore are not representative of the typical at-risk population. Furthermore, the study did not have access to continuous drug dispensing information to determine the continuous exposure to AC6. Finally, Roe et al conducted a retrospective cohort study of 836 community-dwelling older adults to compare the prevalence of AC use in older adults with probable dementia with that of a matched comparison group. They used the pharmacy claim data as the source for determining both the presence of dementia and AC exposure. Patients taking donepezil (n = 418) constituted the dementia group. Patients not taking donepezil (n = 418) constituted the comparison group. The prevalence of AC use was compared in the treatment and comparison groups over a 3- to 12-month follow-up period. This study found that older adults with dementia were more likely to use AC than matched comparison group patients (33.0% vs. 23.4%;P = .001)9. However this study design could not determine whether AC exposure led to the development of dementia, and a dementia diagnosis was based on the use of dementia medication and thus at least two thirds of dementia cases were most likely missed9. Finally, our current study had similar finding to our recently published paper that studied a community sample of only African American older adults aged 70 and older residing in Indianapolis and found that exposure to medication with severe anticholinergic activities had a higher probability of developing cognitive impairment over years of follow-up with an OR of 1.46 (95% CI 1.07 – 1.99) but there were no increased risk of developing dementia (OR 1.08 with 95% CI of 0.47 – 2.49)29. In our community study, we had no access to drug dispensing data and we enrolled only African Americans.
Our study has some limitations. First, the undetected demented or MCI cases in the cognitively normal group. The sensitivity of the six items screener for dementia detection is 98% and the CSI-D sensitivity is 87%. Using these sensitivity measures, we anticipate the presence of 179 patients with dementia among the 3128 patients who screened negative on the six-item screener and the CSI-D (n=3128). These false positive cases will underestimate the association between AC and cognitive impairment. Thus, our results are conservative.
Second, our study did not systematically measure medication adherence, we used drug dispensing as a surrogate for actual medication exposure. The accuracy of drug dispensing in capturing medication exposure is close to 95% for adherence for antihypertensive medications31,32. Third, study subjects who were exposed to AC and developed severe adverse cognitive events might have discontinue taking AC and thus diminish the strength of association between AC exposure and cognition. Fourth, potential biases in our exposure measure may come in the form of over-the-counter medications (OTC) AC not captured by RMRS or out-of-system prescription dispensing. However, all of our study subjects are older adults who received care within the Wishard Health Services system before 2006 (the launch of MEDICARE-part D that covers prescription drugs) and the majority of them are MEDICARE and MEDICAID beneficiaries who cannot afford out-of-pocket expenses or even the co-payment for prescription drugs. Thus, their prescription filling occurred at special pharmacies in Wishard that provide drugs for free to those who cannot afford it. Therefore, the RMRS has a very high probability of capturing the entire drug dispensing data for our proposed cohort. Regarding OTC medications, these are still available via the Wishard pharmacy department at very little cost ($2), and every office visit conducted within Wishard captures the entire prescribed and OTC medication regimen taken by every patient (affirmed by the medical assistant) and is automatically entered into the RMRS drug data. Fifth, our electronic medical record data did not capture other potentially important patient covariates that may confound the effect of AC on cognitive impairment, such as patients’ socio-economic status, education level, depressive symptoms, ApoE genotyping and alcohol and tobacco use. Future studies need to adjust for such important confounders. However, our model controlled for detailed demographics and comorbid conditions which should minimize the confounding effect of unobserved factors. Sixth, our study may suffer from the association by reverse causation. There is a remote possibility that individuals with unrecognized cognitive symptoms might be treated more with AC and since cognitive symptoms usually appear one to three years prior to MCI diagnosis, a causal link between AC exposure and MCI needs to take into account this potential source of bias. Finally, due to the limitation of our study design (retrospective cohort with one year follow-up) and sample size, we are unable to determine the reversibility of association between AC exposure and cognitive impairment, or the duration from exposure to diagnosis. A recent study30 found an increased risk of incident dementia and cognitive impairment for continuous AC users but not for discontinued users, suggesting potential reversibility of the association.
In conclusion, our data supports limiting the use of anticholinergics among older adults and at least having a sufficient conversation between prescribers and patients with regard to balancing the benefit and the harms of these medications, especially when the potential duration of their use is longer than 2 to 3 months.
Acknowledgments
Funding Source: This study was funded by the NIH/NIMH (R24 MH080827). Dr. Boustani was supported by Paul A. Beeson Career Development Award in Aging (K23 AG 26770-01) from the National Institute on Aging, the Hartford Foundation, The Atlantic Philanthropy, and the American Federation of Aging Research; Dr. Callahan was supported by funding from NIH (K24 AG24078).
Appendix: Anticholinergic Cognitive Burden Scoring of Drugs
| Score 1 | Score 2 | Score 3 |
|---|---|---|
| Alimemazine | Amantadine | Amitriptyline |
| Alverine | Belladone alkaloids | Amoxapine |
| Alprazolam | Carbamazepine | Atropine |
| Atenolol | Cyclobenzaprine | Benztropine |
| Brompheniramine maleate | Cyproheptadine | Brompheniramine |
| Bupropion hydrochloride | Empracet | Carbinoxamine |
| Captopril | Loxapine | Chlorpheniramine |
| Chlorthalidone | Meperidine | Chlorpromazine |
| Cimetidine hydrochloride, | Methotrimeprazine | Clemastine |
| Ranitidine | Molindone | Clomipramine |
| Clorazepate | Oxcarbazepine | Clozapine |
| Codeine | Pethidine hydrochloride | Darifenacin |
| Colchicine | Pimozide | Desipramine |
| Coumadin | Dicyclomine | |
| Diazepam | Dimenhydrinate | |
| Digoxin | Diphenhydramine | |
| Dipyridamole | Doxepin | |
| Disopyramide phosphate | Flavoxate | |
| Fentanyl | Hydroxyzine | |
| Furosemide | Hyoscyamine | |
| Fluvoxamine | Imipramine | |
| Haloperidol | Meclizine | |
| Hydralazine | Nortriptyline | |
| Hydrocortisone | Olanzapine | |
| Isosorbide | Orphenadrine | |
| Loperamide | Oxybutynin | |
| Metoprolol | Paroxetine | |
| Morphine | Perphenazine | |
| Nifedipine | Procyclidine | |
| Prednisone | Promazine | |
| Quinidine | Promethazine | |
| Risperidone | Propentheline | |
| Theophylline | Pyrilamine | |
| Trazodone | Quetiapine | |
| Triamterene | Scopolamine | |
| Thioridazine | ||
| Tolterodine | ||
| Trifluoperazine | ||
| Trihexyphenidyl | ||
| Trimipramine | ||
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United States Census Bureau Available at http://www.census.gov/Press-Release/www/releases/archives/population/006808.html.
- 2.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions of the elderly. Arch Intern Med. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 3.Schubert CC, Boustani M, Callahan CM, Perkins AJ, Carney CP, Fox C, Unverzagt F, Hui S, Hendrie HC. Comorbidity profile of dementia patients in primary care: Are they sicker? JAGS. 2006;54(1):104–109. doi: 10.1111/j.1532-5415.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- 4.Boustani M, Peterson B, Hanson L, et al. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138(11):927–937. doi: 10.7326/0003-4819-138-11-200306030-00015. [DOI] [PubMed] [Google Scholar]
- 5.Boustani M, Campbell, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4:311–320. [Google Scholar]
- 6.Bottigi KA, Salazar JC, Yu L, Caban-Holt AM, Ryan M, Mendiondo MS, Schmitt FA. Long-term cognitive impact of anticholinergic medications in older adults. Am J Geriatr Psychiatry. 2006;14(11):980–984. doi: 10.1097/01.JGP.0000224619.87681.71. [DOI] [PubMed] [Google Scholar]
- 7.Campbell N, Limbil T, Boustani M, Ott C, Fox C, Maidment I, Schubert C, Munger S, Fick D. The Cognitive Impact of Anticholinergics: A Clinical Review. Clin Interv Aging. 2009;4(1):225–233. doi: 10.2147/cia.s5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Rithie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006 Feb 1; doi: 10.1136/bmj.38740.439664.DE. doi:10.1136/bmj.38740.439664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roe C, Anderson M, Spivack B. Use of anticholinergic medications by older adults with dementia. J Am Geriatr Soc. 2002;50:836–842. doi: 10.1046/j.1532-5415.2002.50208.x. [DOI] [PubMed] [Google Scholar]
- 10.Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56:2203–2210. doi: 10.1111/j.1532-5415.2008.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry EK, Kilford L, Lees AJ, Burn DJ, Perry RH. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann Neurol. 2003;54:235–238. doi: 10.1002/ana.10639. [DOI] [PubMed] [Google Scholar]
- 12.Caccamo A, Oddo S, Billings LM, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Haring R, Fisher A, Marciano D, et al. Mitogen-activated protein kinase-dependent and protein kinase C-dependent pathways link the m1 muscarinic receptor to β-amyloid protein secretion. J Neurochem. 1998;71(5):2094–2103. doi: 10.1046/j.1471-4159.1998.71052094.x. [DOI] [PubMed] [Google Scholar]
- 14.Jedrziewski MK, Lee VM-Y, Trojanowski JQ. Lowering the risk of Alzheimer’s disease: Evidence-based practices emerge from new research. Alzheimer’s & Dementia: J Alz Assoc. 2005;1(2):152–160. doi: 10.1016/j.jalz.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.McDonald CJ, Overhage JM, Tierney WM, et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54(3):225–253. doi: 10.1016/s1386-5056(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 16.Boustani M, Callahan CM, Unverzagt FW, et al. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20(7):572–5771. doi: 10.1111/j.1525-1497.2005.0126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boustani M, Perkins A, Fox C, et al. Who refuses dementia assessment in primary care? Int J Geriatric Psychiatry. 2006;21:556–563. doi: 10.1002/gps.1524. [DOI] [PubMed] [Google Scholar]
- 18.Callahan CM, Boustani M, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care. A randomized controlled trial. JAMA. 2006;295:2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 19.Stroupe KT, Murray MD, Stump TE, Callahan CM. Association between medication supplies and healthcare costs in older adults from an urban healthcare system. J Am Geriatr Soc. 2000 Jul;48(7):760–768. doi: 10.1111/j.1532-5415.2000.tb04750.x. [DOI] [PubMed] [Google Scholar]
- 20.Fisch M, Callahan CM, Kesterson JG, Nichols C, Tierney WM. The use of an electronic patient record system to identify advanced cancer patients and antidepressant drug use. J Palliat Med. 1999;2(4):403–409. doi: 10.1089/jpm.1999.2.403. Winter. [DOI] [PubMed] [Google Scholar]
- 21.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Hall KS, Ogunniyi AO, Hendrie HC, et al. A cross-cultural community-based study of dementias: methods and performance of the survey instrument Indianapolis, USA and Ibadan, Nigeria. Int J Geriatr Psychiatr. 2000;15:521–531. doi: 10.1002/1099-1166(200006)15:6<521::aid-gps182>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull. 1988;24(4):641–652. [PubMed] [Google Scholar]
- 24.American Psychiatric Association Press . The International Statistical Classification of Diseases and Related Health Problems: 1 and 2. 1992. ICD-10. Report No.: V.3. [Google Scholar]
- 25.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. American Psychiatric Association; Washington, DC: 1987. Rev. [Google Scholar]
- 26.Sink KM, Loyato J, Williamson J, et al. Anticholinergic burden is associated with worse physical function: The ginkgo evaluation of memory study (GEMS) Journal of the American Geriatrics Society. 2008;56:S11–S11. [Google Scholar]
- 27.Fox C, Richardson K, Maidment I, Smithard D, Katona C, Livingston G, et al. MRC-CFAS Anticholinergic medication use and cognitive impairment in the older population. The Medical Research Council Cognitive Function and Ageing Study. JAGS. 2011 doi: 10.1111/j.1532-5415.2011.03491.x. DOI: 10.1111/j.1532-5415.2011.03491.x. [DOI] [PubMed] [Google Scholar]
- 28.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 29.Campbell NL, Boustani MA, Lane KA, Gao S, Hendrie H, Khan BA, Murrell JR, Unverzagt FW, Hake A, Smith-Gamble V, Hall K. Use of Anticholinergics and the Risk of Cognitive Impairment in an African-American Population. Neurology. 2010;75:152–159. doi: 10.1212/WNL.0b013e3181e7f2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrière I, Fourrier-Reglat A, Dartigues JF, Rouaud O, Pasquier F, Ritchie K, Ancelin ML. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009 Jul;169(14):1317–1324. doi: 10.1001/archinternmed.2009.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Medical Care. 1999 Sep;37(9):846–57. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006 Aug;15(8):565–74. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]