Abstract
Background
Prior studies suggest individuals with body mass index (BMI) above vs. below 60 kg/m2 show lower percentage of excess body weight loss (%EBWL) following bariatric surgery.
Objectives
1. Test whether conclusions drawn about the effect of preoperative BMI on postoperative weight loss depend on the outcome measure; 2. Test for evidence of a threshold effect at BMI=60 kg/m2 and; 3. Test the effect from surgery to 12-month, relative to 12- to 36-month, follow-up.
Setting
Large University-affiliated hospital in New York.
Methods
Retrospective analyses of participants grouped according to preoperative BMI: 35–39.9 (n=232); 40–49.9 (n=1166); 50–59.9 (n=429); ≥60 (n=166).
Results
As anticipated, individuals with higher vs. lower preoperative BMI showed greater total body weight loss but lower %EBWL at all postoperative time points (all p’s<0.0005). However, these individuals also showed lower percentage of initial body weight loss (%IBWL) at all time points beyond 1 mo post-surgery (all p’s<0.0005). From 12- to 36-months, individuals with BMI: 35–39.9 showed 3.2±14.3 %IBWL (p<0.0001); 40–49.9 showed 1.0±8.9 %IBWL (p<0.0005); 50–59.9 showed −2.4±10.0 %IBWL (p<0.0005) and; ≥60 showed −3.6±11.5 %IBWL (p<0.0005). Overall F3,1989=20.2, p<0.0005.
Conclusions
Conclusions drawn about the effect of preoperative BMI may depend on the outcome measure. A dosage effect of preoperative BMI was apparent, with heavier individuals showing lower percentages of initial and excess weight loss, regardless of BMI above or below 60kg/m2. Finally, this effect was particularly apparent following the initial 12-month rapid weight loss phase, when less obese (BMI<50) individuals continued losing weight, while heavier individuals (BMI≥50) regained significant weight.
Keywords: gastric bypass, gastric banding, obesity, RYGB, preoperative weight
Obesity is increasing in prevalence (1) and is associated with a number of medical co-morbidities. (2) Currently, bariatric surgery represents the only treatment for obesity that demonstrates long-term effectiveness. (3, 4) Roux-en-Y gastric bypass (RYGB) is the most common bariatric procedure, leading to losses of up to 35% initial body weight sustained for over 15 years. (5) Despite the overall success of bariatric surgery, however, there is extreme variation in the amount of postoperative weight loss experienced by different individuals. For example, in a recent report on the Swedish Obese Subjects, (6) postoperative weight change ranged from 61 kg weight gain to a 106 kg weight loss at 10 years post-surgery. Reliable predictors of the variance in postoperative weight loss may help improve the effectiveness of bariatric surgery, but have not yet been established.
Prior studies report that super-super obese (SSO; body mass index [BMI] ≥ 60 kg/m2) vs. non super-super obese (non-SSO; BMI < 60 kg/m2) individuals show a lower percent excess body weight loss (%EBWL) following bariatric surgery. (8, 9) These data likely contribute to the general perception that SSO vs. non-SSO patients experience less benefit from bariatric surgery. However, an important factor that is frequently overlooked in the literature is that conclusions drawn about the impact of preoperative body weight on postoperative weight loss may depend on the particular [weight loss] outcome measure examined. (10) Super super obese vs. non-SSO individuals necessarily start with a greater proportion of total body weight comprised of excess body weight; thus, SSO vs. non-SSO patients and are less likely to achieve a high %EBWL following bariatric surgery. (11) Therefore, investigators examining only percent excess body weight loss (%EBWL) may be more apt to conclude that SSO vs. non-SSO individuals experience less positive weight loss outcome, even if they lose more absolute weight. (9, 10) Conversely, examining total body weight loss (TBWL) would likely lead to the conclusion that SSO vs. non-SSO individuals experience better weight loss outcome, even if they were to lose a lower percent of excess body weight, given that heavier individuals do tend to lose more absolute (total) weight with weight loss treatment. (10, 12) Only one relevant article (8) has examined percent of initial body weight loss (%IBWL [= %TBWL]), which may be less likely to yield a particular conclusion and has been suggested as an alternative to reporting postoperative %EBWL. (13, 14) Interestingly, this study found that SSO vs. non-SSO individuals showed a lower %EBWL at both 6 and 12 mo post-surgery but did not differ in %IBWL at either time point. (8) Thus, it remains unclear whether preoperative BMI has an effect on postoperative %IBWL.
An additional question that is inadequately addressed in the literature is whether weight change during the initial period (~12 months) of rapid weight loss differs from weight loss after the initial rapid weight loss period. From surgery to 12 months postsurgery, the range of weight change is relatively restricted, as the vast majority of patients appear to lose weight in a relatively linear fashion (i.e., steady weight loss with relatively little variation). (5) However, from 12 to 36 months post-surgery, very large individual differences in weight loss appear, with some individuals continuing to lose weight, while other individuals regain significant amounts of weight. (5) Only one relevant study (15) had adequate power for between group comparisons beyond 12 months, but did not evaluate %IBWL. The goal of this study was to compare postoperative weight loss using three different outcome variables (%EBWL, TBWL, %IBWL) in order to test the hypotheses that individuals with higher vs. lower preoperative BMI would show less %EBWL and more TBWL, but equivalent %IBWL to 36 months post-surgery.
Methods
Participants
The sample consisted of 1,993 (1708F, 285M) patients undergoing either laparoscopic gastric bypass (n = 1501) or laparoscopic gastric banding (n = 492) surgery from May 1, 2001 to May 1, 2011 at a large university-affiliated hospital in NYC. Participants ranged in BMI from 35 to 96 kg/m2 and age from 18 to 70 years. Participants were retrospectively grouped according to preoperative BMI: 35–39.9 (n=232); 40–49.9 (n=1166); 50–59.9 (n=429); ≥60 (n=166). 41.3% of the sample was Hispanic, 25.3% white, 28% African American, 0.6% Asian and 2.8% other. All participants met the criteria proposed by the National Institutes of Health Consensus Panel in 1991. (16) and permission to conduct this study was granted from the Institutional Review Board. Participant demographics are displayed in Table 1.
TABLE 1.
Participant Demographics
| Gastric Banding | Gastric Bypass | Combined | |
|---|---|---|---|
| Participants | 492 | 1501 | 1993 |
| Sex (F/M) | 428/64 | 1295/206 | 1712/281 |
| Age (years) | 38.92 ± 10.46 | 40.86 ± 10.98 | 39.43 ± 10.64 |
| Preop BMI (kg/m2) | 45.48 ± 6.48 | 48.42 ± 8.24 | 47.69 ± 7.95 |
| Preop EBW (kg) | 63.24 ± 18.72 | 71.96 ± 25.02 | 69.81 ± 23.94 |
| PreopTBW (kg) | 122.46 ± 10.98 | 131.65 ± 28.31 | 129.39 ± 27.09 |
Age and weight presented as mean ± SD.
Design
A retrospective analysis was conducted using the surgical database established and maintained at the Center for Bariatric Surgery at a large University-affiliated hospital in New York, a level 1A center for excellence in bariatric surgery. Data was retrieved for ethnicity, height (to calculate BMI), sex and age at 1 wk prior to surgery. Data was retrieved for body weight at 1 wk pre-, 1 month post-, 3 months post-, 6 months post-, 12 months post-, 18 months post-, 24 months post- and 36 months post- surgery. Data was included from patients who underwent surgery in the past 10 years, had 1-month and 3-month follow up data, and had data from at least two other time points. This inclusion criterion was utilized in order to increase the rates of available data and reduce the amount of data that would need to be imputed at later time points (i.e., 24- and 36- month follow-up). (18, 19) See Table 2 for body weight and the number of patients with available data at each time point. Participants were grouped according to preoperative BMI and between-group differences in postoperative weight loss were tested.
TABLE 2.
Body Weight, BMI and Weight Loss at Each Follow-Up Time Point
| 1mo | 3mo | 6mo | 12mo | 18mo | 24mo | 36mo | |
|---|---|---|---|---|---|---|---|
| N1 | 1993 | 1993 | 1814 | 1694 | 1535 | 1335 | 1116 |
| Weight (kg) | 116.8 ± 24.7 | 107.9 ± 25.7 | 97.6 ± 24.3 | 89.3 ± 24.5 | 88.6 ± 26.0 | 88.5 ± 26.0 | 89.7± 26.8 |
| BMI (kg/m2) | 43.1 ± 7.3 | 39.7 ± 7.7 | 35.9 ± 7.5 | 32.8 ± 7.6 | 32.5 ± 8.0 | 32.5 ± 8.1 | 32.9 ± 8.3 |
| %EBWL | 18.8 ± 6.6 | 33.0 ± 11.8 | 48.2 ± 17.2 | 60.9 ± 21.2 | 62.3 ± 21.7 | 63.1 ± 21.8 | 61.7± 21.3 |
| TBWL (kg) | 12.6 ± 4.9 | 21.5 ± 6.6 | 31.8 ± 10.9 | 40.1 ± 13.5 | 40.8 ± 11.6 | 40.9 ± 11.4 | 39.6 ± 10.0 |
| %IBWL | 9.7 ± 3.1 | 16.9 ± 5.1 | 24.9 ± 7.7 | 31.4 ± 9.4 | 32.2 ± 9.2 | 32.3 ± 9.0 | 31.5± 8.5 |
Data presented as Mean ± Standard Deviation
N1 = number of patients with available data at each time point prior to multiple imputation
BMI = body mass index
%EBWL = percent excess body weight loss
TBWL = total body weight loss
%IBWL = percent initial body weight loss
Statistical Analysis
Percent excess body weight loss was calculated using the middle of the 1983 Metropolitan Life Insurance tables for median frame + % weight lost + % excess BMI lost, with excess > 25 kg/m2. Mixed-model analyses of covariance (ANCOVAs) were used to assess postoperative weight loss, with follow-up time point (1, 3, 6, 12, 18, 24 & 36 months post-surgery) as the within subjects factor and BMI category (35–39.9, 40–49.9, 50–59.9, ≥60 kg/m2) as the between subjects factor. Pairwise comparisons were Bonferroni corrected. Age, sex, procedure type (banding vs. bypass) and ethnicity were controlled. At 1, 3, 6, 12, 18, 24 & 36 months post-surgery, data was available for 100, 100, 91, 85, 77, 67 and 56 percent of participants, respectively. Multiple imputation was used for missing values in accordance with established guidelines. (18, 19) Note: imputed results did not significantly differ from non-imputed results. SPSS version 19 (IBM, Armonk, NY) was used for all analyses. All tests were two-tailed and results were considered significant at p < 0.05. Analyses were repeated for RYGB and banding patient individually.
Outcomes and Follow-up
Body weight was assessed using a standard physician scale, with patients in light street clothing without shoes. Height (in order to calculate BMI) was assessed using a standard physician stadiometer. Percent excess body weight loss (%EBWL), total body weight loss (TBWL) and percent initial body weight loss (%IBWL) were the different weight loss outcomes tested. Excess body weight was calculated as actual weight – ideal weight. (11) Follow-up assessments took place at 1, 3, 6, 12, 18, 24 & 36 months post-surgery.
Results
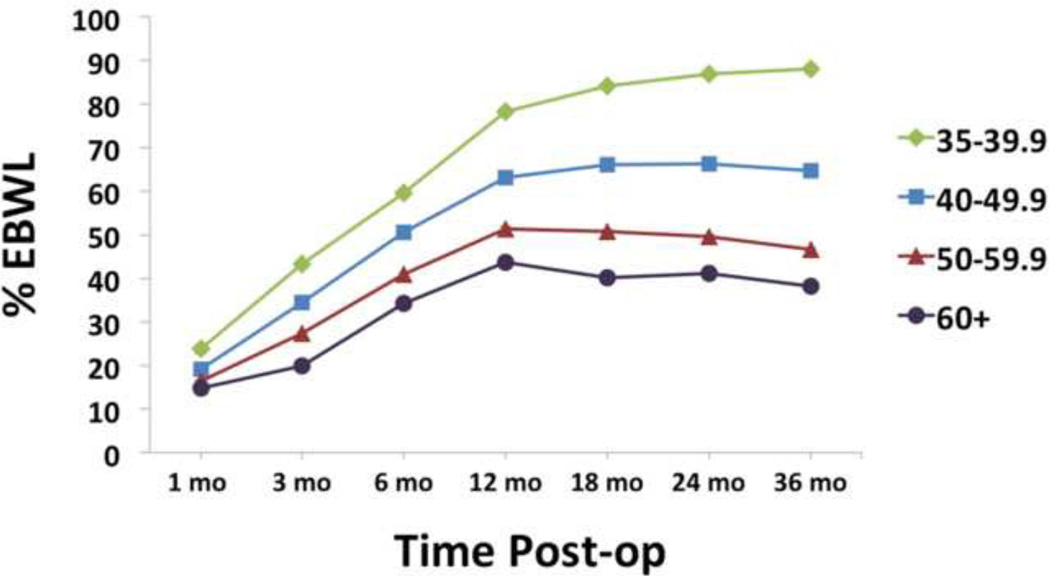
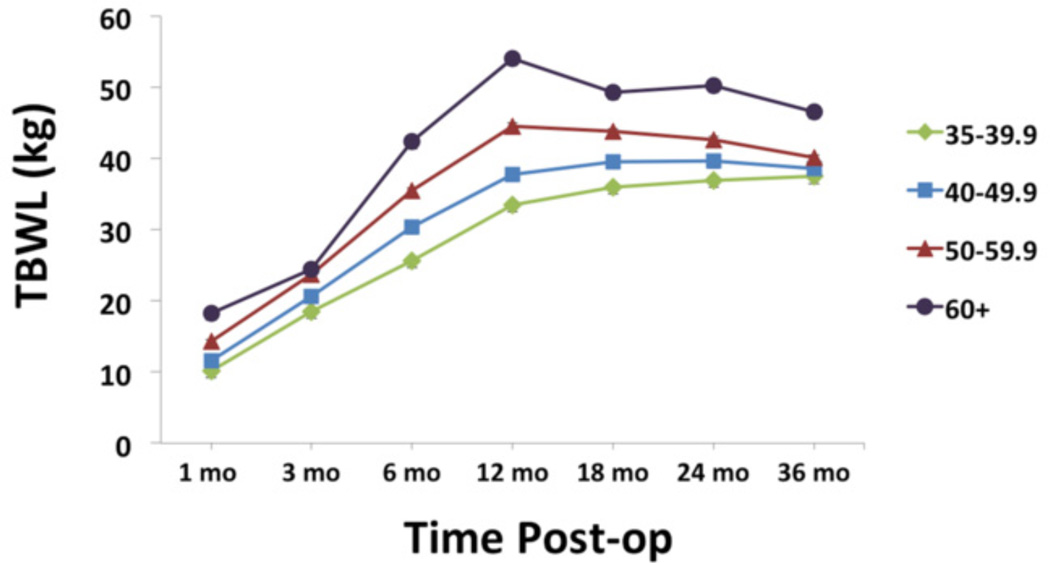
Individuals with higher vs. lower preoperative BMI showed lower %EBWL, overall F3,1989 = 46.6, p < 0.0005. A dosage effect was seen, with each BMI category showing significantly lower %EBWL than the next lower preoperative BMI category at all time points (all p’s < 0.0005). See Figure 1. Individuals with higher vs. lower preoperative BMI showed greater TBWL, overall F3,1989 = 11.8, p < 0.0005. A dosage effect was seen at most but not all time points, with each BMI category showing greater TBWL than the next lower BMI category between 6 and 24 months post-surgery (all p’s < 0.0005). See Figure 2.
FIGURE 1.
Percent excess body weight loss (%EBWL). Lighter vs. heavier individuals showed greater %EBWL at all time points (all p’s < 0.0005). Note: error bars (standard error of the mean) are included in all figures, but may not visible due to the small standard error relative to the size of the icons denoting different BMI categories.
FIGURE 2.
Total body weight loss (TBWL), expressed in kg. Lighter vs. heavier individuals showed lower TBWL at all time points (all p’s < 0.0005).
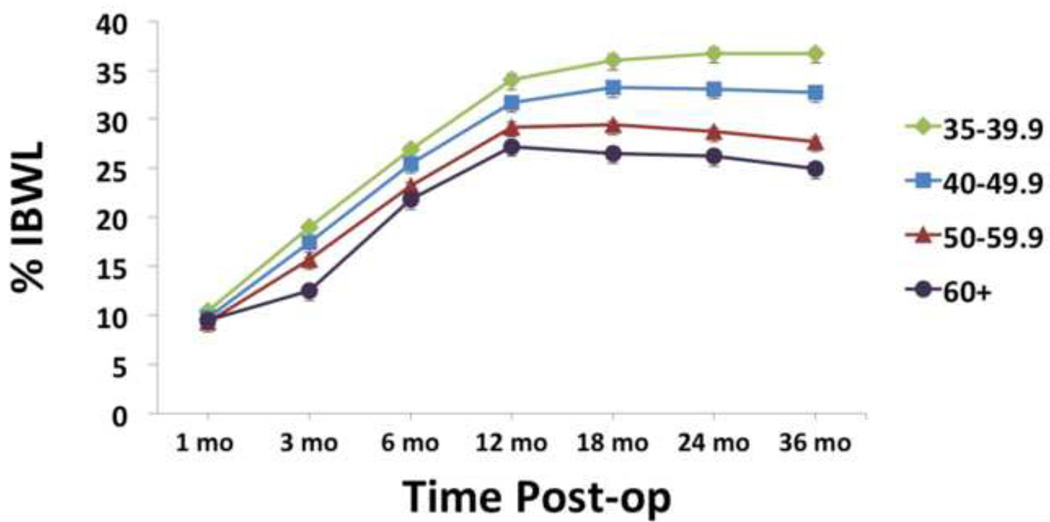
Individuals with higher vs. lower preoperative BMI showed lower %IBWL, overall F3,1989 = 20.2, p < 0.0005. No between-group difference was seen in %IBWL at 1 mo post-surgery. Beyond 1 mo post, however, a significant dosage effect was seen at all time points, with each BMI category showing lower %IBWL than the next lower BMI category (all p’s < 0.0005). Although significant, this effect was relatively small up to 12 months post-surgery. From 12 to 36 months post-surgery, however, this effect becomes more dramatic. From 12 to 36 months, individuals with BMI 35–39.9 lost 3.2 ± 14.3 %IBW (p < 0.0005), individuals with BMI 40–49.9 lost 1.0 (8.9) %IBW (p < 0.0005), individuals with BMI 50–59.9 gained 2.4 (10.0) %IBW (p < 0.0005) and individuals with BMI ≥60 gained 3.6 (11.5) %IBW (p < 0.0005). See Figure 3. Analyses repeated for RYGB and banding patients individually showed no significant difference in results.
FIGURE 3.
Percent initial body weight loss (%IBWL). Lighter vs. heavier individuals showed greater %IBWL at all time points after 3 mo post-surgery (all p’s < 0.0005).
Discussion
Ideally, preoperative weight would be entered as a covariate in between-subjects analyses comparing postoperative weight loss. However, as in the current analyses testing the effect of preoperative weight on postoperative weight loss, it is not possible to control for a predictor variable. Thus, results generated by such analyses are necessarily influenced by preoperative weight. Analyses in this study demonstrate how these influences may vary depending upon the outcome measure. In calculating %EBWL, patients who were more vs. less obese preoperatively fared less well after bariatric surgery. However it is important to bear in mind that more vs. less obese individuals typically start with more [total] body weight and a greater proportion of their body weight as excess. These individuals are, therefore, less likely to achieve a high %EBWL. (11) For example, if two individuals with an ideal body weight of 50kg both lose 50kg following RYGB, an individual weighing 100kg preoperatively will have lost 100% of their EBW, while an individual weighing 150kg preoperatively will have lost only 50% of their EBW (50% less weight loss).
Contrary to analyses using %EBWL, analyses using TBWL indicate that individuals who are more vs. less obese preoperatively fare better after bariatric surgery. However, more vs. less obese individuals typically lose more absolute weight when undergoing weight loss treatment. (10, 12) Thus, the opposing conclusions drawn by the use of these two different outcome measures are predictably influenced by preoperative body weight. Although %IBWL is still affected by baseline weight, it may be affected to a lesser extent than %EBWL. In the example above, the individual weighing 100kg preoperatively lost 50% of their IBW, while the individual weighing 150kg preoperatively lost 33% of their IBW (17% less weight loss). Given that heavier individuals tend to lose more weight after surgery, (10) let us consider a more representative scenario where the individual weighing 100kg preoperatively loses 50kg, while the individual weighing 150kg preoperatively loses 75kg, after surgery. Here, both patients have lost 50% of their IBW; however, there is still a large disparity in %EBWL (100% vs. 75% respectively). This may explain the seemingly perplexing findings of Farkas et al., (8) who demonstrated significant SSO vs. non-SSO differences in %EBWL, but no differences in %IBWL, at 6 and 12 mo post RYGB. Thus, despite the convention of reporting %EBWL in postoperative reports, (13) %IBWL may be a better weight loss outcome measure in analyses of the effect of preoperative weight on postoperative weight loss.
Contrary to our a priori hypothesis that preoperative BMI would not be related to postoperative %IBWL, heavier vs. lighter individuals at the time of surgery showed significantly lower %IBWL from 3 to 36 mo after surgery. Although differing from the Farkas et al. (8) report, this finding is consistent with other prior literature. (7, 9) However, it is important to note that we found no evidence of any specific separation between SSO and non-SSO, as has been previously suggested. (8, 9) These data indicate a dosage effect of preoperative BMI, with %IBWL diminishing successively as BMI category increases (Figure 3). Thus, our findings suggest a linear inverse relationship between pre- and post- operative BMI. It is likely that prior studies of SSO vs. non-SSO patients picked up this linear relation but, because of the a priori dichotomous grouping of more vs. less heavy participants into SSO vs. non-SSO patients, reported an effect of SSO vs. non-SSO.
Data from this sample also suggest that individuals experience weight loss at a relatively uniform rate out to 12 months post-surgery, irrespective of preoperative BMI or weight loss outcome measure assessed. Our findings indicate that the effect of preoperative BMI on post-operative weight loss becomes particularly pertinent past the first 12 months, when the range of weight loss increases (from −2 to 58 %IBWL at 12 months to −14 to 68 %IBWL at 36 months). There were significant between-group differences in weight loss from surgery 12 months post, as well as from 12 to 36 months post. However, between group differences in weight loss appear much larger from 12 to 36 months vs. surgery to 12 months (Figures 1, 2 & 3). Patients with a preoperative BMI from 35–49.9 kg/m2 continued to lose weight, while individuals with a BMI ≥ 50 kg/m2 regained significant weight, from 12 to 36 months post-surgery. As alluded to above, this is not necessarily suggestive of a threshold effect at BMI = 50, but rather a consequence of how groups were categorized. This finding illustrates the need for more longitudinal data as the period of rapid weight loss may mask the effects of significant predictors of post-operative weight loss outcome.
Finally, these findings may be relevant to the current debate about whether preoperative weight loss should always be recommended or required of bariatric patients. Although speculative, as preoperative weight change was not assessed in this study, these data may suggest that entering into surgery with a lower BMI could be beneficial. This may be particularly true for SO and SSO individuals beyond the initial rapid weight loss period, as these individuals showed weight regain beyond 12 mo post-surgery. However, it is also important to consider that a preoperative weight loss mandate would carry a risk of denying surgery to patients who were unable to meet this requirement but would nonetheless benefit from the procedure.
Limitations and Conclusion
Participant grouping and analyses in this study were retrospective in nature, limiting the inferences that can be drawn from the results. A large amount (up to 44%) of data was imputed at 36-month follow up; however, imputed results did not significantly differ from non-imputed results. Further, only patients undergoing laparoscopic gastric bypass or banding were included, limiting generalizability of findings to patients undergoing these procedures. In addition, only patients reporting for 1- and 3- month follow-up as well as at least two other follow-up visits were included in order to increase the percentage of available data at each time point. This, however, may also limit the generalizability of findings as these individuals were more likely to show up for more follow up visits. Due to incomplete or nonexistent data, we were unable to test the effect of, or control for, other variables that may have influenced the relation between preoperative BMI and postoperative weight loss, such as mobility and medications. Nonetheless, this study illustrates how prior reports may be biased towards a particular conclusion about the effect of preoperative BMI on postoperative weight loss, depending upon the weight loss measure chosen. For these analyses, we suggest that %IBWL may be less influenced by preoperative weight than %EBWL and TBWL. Further, findings in this study challenge the notion that there is a threshold effect at the level of super super obesity (BMI = 60) and, rather, suggest a (negative) dosage effect of preoperative BMI on postoperative weight loss when calculated as a percent (excess or initial). Finally, findings also show great dispersion in weight loss by BMI category after 12-month follow-up, with heavier individuals regaining weight, while less heavy individuals continue to lose weight.
Acknowledgments
Source of Funding: This research was supported by NIH grants KL2RR024157 and P30DK26687.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119:655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 3.Still CD, Benotti PWG, Gerhard GS, Petrick A, Reed M, Strodel W. Outcomes of preoperative weight loss in high-risk patients undergoing gastric bypass surgery. Arch Surg. 2007;142:994–998. doi: 10.1001/archsurg.142.10.994. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 5.Sjöström L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes. 2008;32:S93–S97. doi: 10.1038/ijo.2008.244. [DOI] [PubMed] [Google Scholar]
- 6.Sjöström CD, Lystig T, Lindroos AK. Impact of weight change, secular trends and ageing on cardiovascular risk factors: 10-year experiences from the SOS study. Int J Obes. 2011;35:1413–1420. doi: 10.1038/ijo.2010.282. [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Pagoto SL, Olendzki BC, et al. Predictors of weight status following laparoscopic gastric bypass. Obes Surg. 2006;16:1227–1231. doi: 10.1381/096089206778392284. [DOI] [PubMed] [Google Scholar]
- 8.Farkas DT, Vemulapalli P, Haider A, Lopes JM, Gibbs KE, Teixeira JA. Laparoscopic Roux-en-Y gastric bypass is safe and effective in patients with a BMI > or =60. Obes Surg. 2005;15:486–493. doi: 10.1381/0960892053723466. [DOI] [PubMed] [Google Scholar]
- 9.Tichansky DS, DeMaria EJ, Fernandez AZ, et al. Postoperative complications are not increased in super-super obese patients who undergo laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2005;19:939–941. doi: 10.1007/s00464-004-8929-3. [DOI] [PubMed] [Google Scholar]
- 10.Bloomston M, Zervos EE, Camps MA, Goode SE, Rosemurgy AS. Outcome following bariatric surgery in super versus morbidly obese patients: does weight matter? Obes Surg. 1997;7:414–419. doi: 10.1381/096089297765555395. [DOI] [PubMed] [Google Scholar]
- 11.Bray GA, Bouchard C, Church TS, et al. Is it time to change the way we report and discuss weight loss? Obesity. 2009;17:619–621. doi: 10.1038/oby.2008.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein LH, Wing RR. Aerobic exercise and weight. Addict Behav. 1980;5:371–388. doi: 10.1016/0306-4603(80)90011-8. [DOI] [PubMed] [Google Scholar]
- 13.Karmali S, Birch DW, Sharma AM. Is it time to abandon excess weight loss in reporting surgical weight loss? Surg Obes Relat Dis. 2009;5:503–506. doi: 10.1016/j.soard.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Sharma AM, Karmali S, Birch DW. Reporting weight loss: is simple better? Obesity. 2010;18:219. doi: 10.1038/oby.2009.289. [DOI] [PubMed] [Google Scholar]
- 15.Gould JC, Garren MJ, Boll V, Starling JR. Laparoscopic gastric bypass: risks vs. benefits up to two years following surgery in super-super obese patients. Surgery. 2006;140:524–529. doi: 10.1016/j.surg.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 16.NIH. Gastrointestinal surgery for severe obesity consensus statement. 1991;9:1–20. [PubMed] [Google Scholar]
- 17.Deitel M, Greenstein RJ. Recommendations for reporting weight loss. Obes Surg. 2003;13:159–160. doi: 10.1381/096089203764467117. [DOI] [PubMed] [Google Scholar]
- 18.Schafer JL. Analysis of Incomplete Multivariate Data. London: Chapman & Hall; 1997. pp. 1–444. [Google Scholar]
- 19.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]