Abstract
The enzymatic activities of CD39 and CD73 play strategic roles in calibrating the duration, magnitude, and chemical nature of purinergic signals delivered to immune cells through the conversion of ADP/ATP to AMP and AMP to adenosine, respectively. This drives a shift from an ATP-driven proinflammatory environment to an anti-inflammatory milieu induced by adenosine. The CD39/CD73 pathway changes dynamically with the pathophysiological context in which it is embedded. It is becoming increasingly appreciated that altering this catabolic machinery can change the course or dictate the outcome of several pathophysiological events, such as AIDS, autoimmune diseases, infections, atherosclerosis, ischemia-reperfusion injury, and cancer, suggesting these ecto-enzymes are novel therapeutic targets for managing a variety of disorders.
Keywords: cytokine, Treg, macrophage, neutrophil, sepsis, CD39, CD73, ectonucleotidase
How the purinergic system shapes immune/inflammatory responses
The immune system is a tightly regulated and integrated cell network that functions to preserve and restore homeostasis. A task accomplished by patrolling organs for signs of microbial invasion or tissue injury and triggering defensive inflammatory and then restitutive responses following infection and injury [1]. However, inappropriate activation of the immune system can lead to unacceptable levels of collateral tissue damage and the development of various pathophysiological conditions, such as allergic and autoimmune diseases or ischemia/reperfusion injury [2].
The purinergic system is an evolutionarily selected system deputed to fine-tune immune cell functions, such as cell-to-cell interactions, cytokine and chemokine secretion, surface antigen shedding, intracellular pathogen removal, and generating reactive oxygen species (ROS) [3–5]. Purinergic mediators, such as ATP and adenosine, are released into the extracellular space in response to metabolic disturbances or other types of insults [6–8], and operate both as sensory and efferent signals to shape immune responses. ATP is released either by cell lysis or by non-lytic mechanisms including: (i) exocytosis of ATP-containing vesicles, (ii) through nucleotide-permeable channels (connexin/pannexin hemichannels, maxi-anion channels, volume-regulated anion channels or P2X7 receptor channels), (iii) via transport vesicles that deliver proteins to the cell membrane, and (iv) via lysosomes [9]. Following the release of ATP into the extracellular space, CD39 (ecto-nucleoside triphosphate diphosphohydrolase 1, E-NTPDase1) converts ATP into AMP, and then CD73 (ecto-5'-nucleotidase, Ecto5'NTase) dephosphorylates AMP into adenosine. In addition to CD39 and CD73, which are the major nucleotide metabolizing enzymes that regulate immunity and inflammation, there are other less well understood cell surface-associated enzyme activities involved in the catabolism of extracellular nucleotides, which include alkaline phosphatases, pyrophosphatases, and phosphodiesterases as well as the counteracting ATP-regenerating ectoenzymes adenylate kinase and nucleoside-diphosphate kinase [10]. The receptors P1 and P2 expressed on the surface of immune cells are activated by adenosine and ATP, respectively, and mediate the immunomodulatory effects of purines. Four G protein-coupled P1 or adenosine receptors have been cloned and designated as A1, A2A, A2B and A3, whereas the P2 receptors have been classified into two subfamilies, the ionotropic P2X (P2X1–7) and metabotropic P2Y (P2Y1,2,4,6,11–14) receptors [11].
CD39 and CD73 are important for calibrating the duration, magnitude, and composition of the “purinergic halo” surrounding the immune cells (Figure 1). The expression and activity of both CD39 and CD73 undergo dynamic changes in accordance with the pathophysiological context in which they are embedded [12], and it is increasingly appreciated that altering this catabolic machinery can modulate the course and dictate the outcome of several pathophysiological conditions, such as infections, AIDS, autoimmune diseases, atherosclerosis, ischemia-reperfusion injury, and cancer [13–17]. In this review, we provide a background on the molecular regulation of CD39 and CD73, outline the role of CD39 and CD73 in governing immune cell function, and detail the role of these enzymes in regulating the course of immune-mediated diseases.
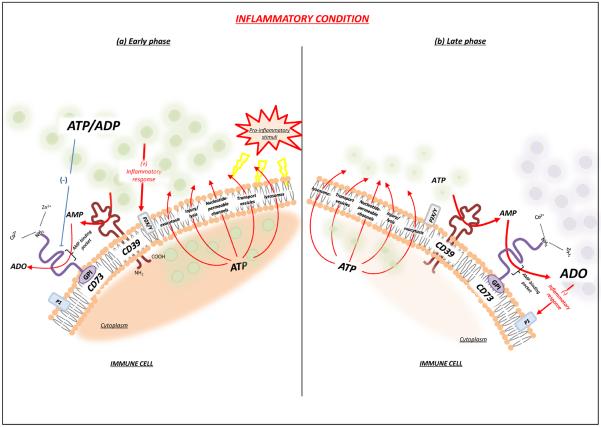
Figure 1.
CD39 and CD73 shape the “purinergic halo” surrounding immune cells. The occurrence of pathological events, such as inflammation, promotes a massive accumulation of ATP, which serves as a key “danger” signal, triggering a series of proinflammatory responses (a). However, negative feedback also takes part in this context because the increased ATP secretion, as observed in the early phase of inflammation, is followed by its sequential degradation to AMP by CD39, and to adenosine by CD73. Adenosine promotes a depressive action on the immune cell activity and exerts a potent anti-inflammatory effect (b). Abbreviations: ADO, adenosine; GPI, glycosylphosphatidyl inositol.
Molecular biology and regulation of CD39 and CD73
CD39 is an integral membrane protein that phosphohydrolyzes ATP, and less efficiently ADP, in a Ca2+- and Mg2+-dependent fashion, to yield AMP [18]. Human CD39 is a putative 510-amino acid protein with seven potential N-linked glycosylation sites, 11 Cys residues, and two transmembrane regions [19]. Structurally, it is characterized by two transmembrane domains, a small cytoplasmic domain comprising the NH2- and COOH-terminal segments, and a large extracellular hydrophobic domain consisting of five highly conserved domains, known as apyrase conserved regions (ACR) 1–5, which are pivotal for the catabolic activity of the enzyme [18]. The amino acid sequences of ACR 1 and ACR 5 contain a phosphate-binding motif (DXG), which has been shown to be critical for stabilizing the interaction between the enzyme and its nucleotide substrate during phosphate cleavage. In addition, two ACR residues, Glu 174 in ACR 3 and and Ser 218 of ACR 4 are also necessary for enzyme function [18,20]. CD39 becomes catalytically active upon its localization on the cell surface, and its glycosylation is crucial for correct protein folding, membrane targeting, and enzyme activity [20].
CD39 is constitutively expressed in spleen, thymus, lung, and placenta [21–24], and in these organs it is associated primarily with endothelial cells and immune cell populations, such as B cells, natural killer (NK) cells, dendritic cells, Langerhans cells, monocytes, macrophages, mesangial cells, neutrophils, and regulatory T cells (Tregs) [25]. CD39 expression is regulated by several pro-inflammatory cytokines, oxidative stress and hypoxia [13,26] through the transcription factors Sp1 [26], Stat3, and zinc finger protein growth factor independent-1 transcription factor [27]. In addition, the expression of CD39 is increased in several solid tumors (colorectal cancer, head and neck cancer, pancreatic cancer) as well as in chronic lymphocytic leukemia, suggesting this enzyme is also involved in the development and progression of malignancies [16]. Recently, a soluble catalytically active form of CD39 has been shown to circulate in human and murine blood [28].
The second step in the metabolism of purine nucleotides is accomplished by CD73, which dephosphorylates extracellular AMP to adenosine. Structurally, CD73 is a dimer of two identical 70 kD subunits, anchored to the plasma membrane via a C-terminal serine residue, Ser 523, linked to glycosylphosphatidyl inositol (GPI), with no membrane-embedded protein segments [29]. A soluble form of CD73 can be shed from the membrane through proteolytic cleavage or hydrolysis of the GPI anchor by phosphatidylinositol-specific phospholipase [10,30].
The mature form of CD73 consists of 548 amino acids with an estimated molecular mass of 61 kDa, comprising a 26-amino acid signal peptide at the N-terminus and a C-terminal sequence for the attachment to the pre-formed GPI anchor. The N-terminal domain coordinates the binding of two catalytic divalent metal ions (Zn2+ and Co2+), whereas the C-terminal domain provides the binding pocket for AMP [29]. Of note, ADP and ATP act as competitive inhibitors of CD73 [29,31].
CD73 is found in a variety of tissues, including colon, brain, kidney, liver, lung, and heart [32]; on leukocytes derived from peripheral blood, spleen, lymph nodes, thymus and bone marrow [32]; as well as on endothelium [33]. There is evidence that the expression and function of this enzyme are upregulated under hypoxic conditions [34,35], as well as by the presence of several pro-inflammatory mediators, such as transforming growth factor (TGF)-β, interferons (IFNs), tumor necrosis factor (TNF)-α, interleukin (IL)-1β and prostaglandin E2 [36, 37]. An increase in CD73 expression has also been reported in several neoplastic tissues [36], suggesting the involvement of this enzyme in the onset and progression of neoplasia.
The function of CD39 and CD73 in cells of the immune system
Given that the combination of CD39 and CD73 degrade ATP, ADP, and AMP to adenosine, they can be viewed as “immunological switches” that shift ATP-driven pro-inflammatory immune cell activity toward an anti-inflammatory state mediated by adenosine (Figure 1) [36]. In addition to host cells, several pathogens (i.e. Escherichia coli, Staphylococcus aureus, Toxoplasma gondii and Trichomonas vaginalis) are endowed with a streamlined CD39/CD73-like machinery, which fosters the establishment and propagation of infections [38,39]. The role of CD39 and CD73 in regulating the function of several immune cell types, including lymphocytes, neutrophils, monocytes/macrophages, dendritic cells, and endothelial cells is discussed in detail below.
Lymphocytes
Several regulatory mechanisms are used to maintain immune homeostasis, preventing autoimmunity and moderating the inflammation induced by pathogens and environmental insults. In this regard, Tregs are widely considered the primary mediators of peripheral tolerance, playing a pivotal role in preventing autoimmune diseases (i.e. type 1 diabetes), as well as in limiting chronic inflammatory disorders, such as asthma and inflammatory bowel diseases. However, they can also prevent sterilizing immunity to certain pathogens (i.e. viruses) and limit anti-tumour immunity [40]. CD39 and CD73 are highly expressed on the surface of Foxp3+ Tregs and have been increasingly used as markers of Tregs [41,42]. The catabolic activity of the CD39/CD73 axis is synchronized with the activation status of these cells. Indeed, murine Tregs display increased CD39 activity only upon activation of their T-cell receptor (TCR), and this enzyme was found to be inactive in non-stimulated cells [43] (Figure 2). This increased ATP-metabolizing activity appears to be critical for the immunosuppressive activity of Tregs [44]. In addition, it was speculated that the enhanced CD39 activity allows the entrance of these cells into inflamed regions, where it reduces the extracellular level of ATP, thereby decreasing P2 receptor-mediated Treg cell death [43] (Figure 2). Deaglio et al. [45] showed using CD39 deficient mice that CD39, in concert with CD73, facilitates the pericellular generation of adenosine, which mediates a substantial portion of the immune suppressive and anti-inflammatory activities of Tregs (Figure 2). This inhibitory control by Treg-derived adenosine is mediated by the engagement of A2A receptors on effector T (Teff) cells [45]. Of note, Romio et al. [46] observed that the CD73-derived adenosine produced by Tregs downregulates NF-κB activation in Teff cells through A2A receptors, thereby reducing the release of a broad spectrum of proinflammatory cytokines and chemokines. A recent paper by Ohta et al. [47] has highlighted the occurrence of a self-reinforcing loop in the immunosuppressive activity of Tregs, driven by adenosine generation. In particular, the activation of A2A receptors, expressed on Tregs, promoted the expansion of these cells, which in turn acquired an increased immunoregulatory activity (Figure 2) [47]. The importance of this autocrine signaling loop has been recently confirmed in the kidney, where it was shown to control ischemia and reperfusion-induced inflammation and injury [48].
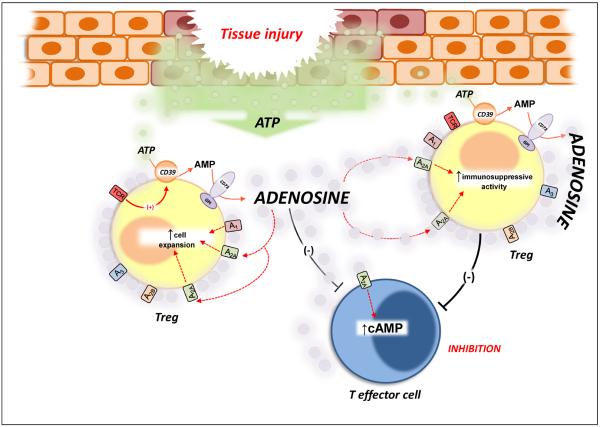
Figure 2.
The CD39/CD73 pathway modulates Treg activity. The activation of T cell receptor (TCR), expressed on Tregs, induces CD39 activity. This increment of ATP-metabolizing activity is critical for the immunosuppressive activity of Tregs because it facilitates the pericellular generation of adenosine, a substantial component of the immunosuppressive and anti-inflammatory functions of Treg cells. The inhibitory action of Treg-derived adenosine can be ascribed to the activation of A2A receptors expressed on T effector cells, which undergo reduced immune activity. In addition, adenosine generation triggers a self-reinforcing loop of Treg functions because the stimulation of A2A receptors, expressed on these cells, elicits their expansion and increases their immunoregulatory activity. Abbreviations: GPI, glycosylphosphatidyl inositol; TCR, T cell receptor.
In humans, it has been reported that 90% of Foxp3+ Tregs are CD39+ [41, 49]. Although surface expression of CD73 on Tregs is negligible [49; 50], CD73 is abundant in the cytoplasm of these cells [50]. Pharmacological studies have shown that human Tregs also degrade ATP to adenosine via CD39 and CD73 and that this adenosine is immunosuppressive [50]. We speculate that CD73 is secreted from human Tregs and that this secreted form is responsible for the conversion of AMP to adenosine. Alternatively, other AMP-metabolizing enzymes may also contribute to the degradation of AMP produced by CD39.
A recent study has shown that T helper (Th) 17 cells are also equipped with CD39 and CD73, which suppress immune responses by Th17 cells through the production of adenosine. [27]. The expression of both CD39 and CD73 on Th17 cells is tightly regulated by the factors that induce Th17 differentiation, namely IL-6 and TGF-β. In particular, IL-6, through stimulation of the transcription factor Stat3, and TGF-β, through downregulation of the growth factor independent-1 transcription factor, are both essential for increasing the expression of ectonucleotidases during Th17 cell differentiation [27].
Neutrophils
Neutrophils are crucial effectors of the innate defence against bacterial infections [51]. A key feature of these inflammatory cells is their ability to perceive adverse conditions, migrate toward compromised tissues, and trigger a series of antimicrobial effector mechanisms [52]. Several lines of evidence support the view that these events are subjected to control and coordination by ATP and adenosine that, once released from neutrophils, act in an autocrine fashion to regulate neutrophil functions, often with opposite outcomes [53]. Indeed, the released ATP can induce the activation of neutrophils by enhancing their oxidative burst and their adhesion to the vascular endothelium [54]. By contrast, an increase in adenosine concentration, consequent to the rapid degradation of ATP by ecto-enzymes, can lead to a substantial inhibitory effect, thus establishing a feedback loop to avoid potential tissue injury arising from a prolonged inflammatory response [54,55].
Neutrophils widely express CD39 [56] and, to some extent, CD73 [57], both of which appear to be critical players in the regulation of neutrophil activity by controlling extracellular purinergic gradients [58]. In particular, inadequate activity of the CD39/CD73 axis has been associated with amplified and uncontrolled activation of neutrophils [59,60], amplified chemotactic functions [61,62], and an increased adhesion to the vascular endothelium [59,63].
Monocytes and macrophages
Macrophages are components of the mononuclear phagocyte system, a group of immune cells produced in the bone marrow or yolk sac that includes blood monocytes and resident tissue macrophages [51]. These cells have key functions in coordinating the initiation, maintenance, and resolution of inflammation through the phagocytosis and clearance of pathogens and tissue debris, as well as the release of inflammatory, angiogenic, and tissue remodeling mediators (i.e. cytokines, free radicals, prostanoids, antimicrobial peptides, VEGF, and proteinases) [38].
By regulating purine concentrations in the extracellular space, the CD39/CD73 system is important for fine-tuning macrophage differentiation and activity. In particular, a lack of CD39 leads to ATP accumulation, which spurs macrophages to release a plethora of pro-inflammatory cytokines, such as IL-1β, IL-18, IL-6, and TNF-α [64–66]. In addition, a recent study by Zanin et al. [67] has provided evidence that proinflammatory M1 macrophages decrease both the expression and activity CD39 and CD73, leading to reduced ATP degradation. By contrast, M2 macrophages, which are characterized by the production of anti-inflammatory cytokines (IL-10 and IL-1 receptor antagonist) and tissue remodeling molecules, showed an increased expression and activity of both catabolic enzymes, followed quickly by the conversion of ATP into adenosine. Thus, M2 macrophages generate an adenosine-rich environment, which in turn can augment the anti-inflammatory and tissue remodeling activities of these cells [68].
Dendritic cells
Dendritic cells capture antigens in peripheral tissues, which they then deliver to the T cell-rich regions of secondary immune organs. They process the antigens and present them on their surface to T cells, thereby initiating an adaptive immune response against the antigens [51].
Ectonucleotidases on dendritic cells are involved in regulating the activation of lymphocytes. Mizumoto et al. [23] reported that CD39 on either bone-marrow derived dendritic cells or Langerhans cells, a type of dendritic cell in the skin, is required for the optimal stimulation of hapten-reactive T cells. Mechanistically, CD39 was proposed to be essential for preventing the desensitization of P2 receptors, which are required for the optimal function of dendritic cells.
In addition, it has been observed that CD73 expressed on follicular dendritic cells is important for the adhesive interaction between these cells and germinal center B cells, suggesting a regulatory role in B cell maturation [69,70]. It is, however, unclear whether CD73 facilitates dendritic cell adhesion to B cells through its enzymatic activity or by serving as a bona fide adhesion molecule.
Endothelial cells
The vascular endothelium, besides providing a physical barrier that separates circulating blood from the surrounding tissues, is highly sensitive to changes occurring in the vascular milieu. In particular, endothelial cells are actively involved in maintaining cardiovascular homeostasis by regulating hemostasis, immune cell diapedesis, and vascular functions, just to name a few [71]. In this regard, the endothelial CD39/CD73 axis regulates hemostasis by converting the local environment from a prothrombotic ATP/ADP-rich state, to an antithrombotic, adenosine-rich environment [72–74]. Accordingly, alterations in the expression and activity of CD39 or CD73 can give rise to disorders of thromboregulation [73,75].
CD39 and CD73 are also involved in orchestrating leukocyte trafficking in response to chemotactic stimuli [58,59,76]. By triggering a rapid and dynamic change in the pericellular concentration of purines in the proximity of both endothelial and immune/inflammatory cells, these enzymes can influence immune cell adhesion to the endothelial layer. This adhesion is generally stimulated by high ATP concentrations and reduced by increased adenosine levels [76]. This concept is supported by the observation that mice lacking CD39 or CD73 display an increased adhesion of leukocytes to the vascular endothelium [25,59,66,77]. In particular, the impaired adenosine generation occurring in these knockout animals was associated with increased endothelial activation, monocyte recruitment, and platelet aggregation, suggesting a critical role for these enzymes in the pathophysiology of vascular inflammation [75,78]. In contrast to its role in vascular endothelium, CD73 does not affect the permeability of lymphatic endothelium [79].
Regulation of immunity by ectonucleotidases in infections
There is increasing evidence that the ability of several microorganisms to evade the control of the immune system arises from their high nucleotide metabolic versatility, which favors their invasion of and dissemination in the host [80]. Pathogens can also exploit ectonucleotidases located on the outer surface of a cell or tissue to generate an adenosine-rich milieu, which allows them to escape immune surveillance [81–85]. By contrast, in certain scenarios, CD39 and CD73 can also curb infections and associated inflammation and mortality [65,86]. Increased expression and activity of the CD39/CD73 axis have been described during infections induced by several microorganisms, including protozoa (belonging to the genus Leishmania, Trypanosoma, Toxoplasma, Trichomonas, Giardia) [81,83,87–91], fungi (Candida parapsilosis) [84], and bacteria [62,65,82,85,86, 92–94].
Parasites
A direct correlation has been observed between the expression level of ectonucleotidases in Leishmania parasites and their virulence [81]. In particular, Leishmania amazonensis strains endowed with higher ectonucleotidase activity are more effective in establishing an infection because they increase the production of adenosine at the site of infection, which impairs immune functions [83]. In addition, both in vitro and in vivo studies have confirmed a direct relationship between the virulence of Trypanosoma cruzi, the causative agent of Chagas disease, and the activity of ectonucleotidases expressed by these protozoa [88,90,91].
An increase in CD73 expression has also been observed in the brain of mice infected with Toxoplasma gondii, which promotes bradyzoite differentiation and cyst formation by a mechanism dependent on the generation of adenosine, a molecule that is essential for the parasite life cycle [39]. Thus, the pharmacological blockade of CD73 might be a promising therapeutic approach to treat human toxoplasmosis.
Fungi
Ecto-5'-nucleotidase activity is also important in the pathophysiology of infection by Candida parapsilosis. This emerging fungal pathogen expresses an ecto-5'-nucleotidase on its surface that,– through the production of adenosine – can inhibit the phagocytosis of the fungus by immune cells, facilitating the survival and progression of the infectious agent [84].
Bacteria
Several bacteria exploit ectonucleotidases to promote infection in the host. Legionellapneumophila is endowed with an ecto-triphosphate diphosphohydrolase similar to human CD39 that contributes to virulence by facilitating the entry of the pathogen into host macrophages and epithelial cells [92]. Staphylococcus aureus and Bacillus anthracis deploy adenosine synthase A, a cell-wall anchored protein with 5'-nucleotidase activity, to synthesize adenosine as a mechanism for escaping from phagocytic clearance [82]. In addition, a recent paper by Fan et al. [85] showed that an ecto-5'-nucleotidase expressed on the cell surface of Streptococcus sanguinis contributes to the virulence of this bacterium, allowing the survival of the pathogen through the immunosuppressive activity of adenosine.
In contrast, there is also evidence that ectonucleotidases can protect against bacterial infection. Théâtre et al. [86] recently showed that upon intra-tracheal instillation of Pseudomonas aeruginosa, transgenic mice overexpressing human CD39 in airway epithelia developed an enhanced inflammatory response and displayed improved bacterial clearance [86]. The authors speculated that the increased CD39 activity, by reducing the extracellular ATP level, limited the desensitization of P2 purinergic receptors, which helped to promote airway inflammation in response to bacterial challenge [86]. Finally, Haskó et al. [65] have shown that CD73 is an important check-point for limiting bacterial spread during polymicrobial sepsis. In particular, CD73 increased the survival of mice with sepsis by decreasing bacterial growth, reducing inflammatory cytokine levels, and lessening organ injury (i.e. lung and kidney) [65].
Viruses
The enhanced expression and activity of CD39 and CD73 have been observed in endothelial cells infected with cytomegalovirus (CMV). The increase in local adenosine production, associated with the upregulation of ecto-nucleotidases, generates an immunosuppressive and antithrombotic microenvironment, which enables the virus to more easily enter the target cell [95].
Leal et al. [96] have found increased ectonucleotidase activity and enhanced CD39 expression on lymphocytes of individuals infected with human immunodeficiency virus (HIV), suggesting a possible role for ecto-nucleotidases in the immune dysfunction associated with this disease. Schulze et al. have corroborated this observation [97], demonstrating an increased proportion of Tregs expressing CD39 in different cohorts of HIV-infected patients, as well as a positive correlation between CD39 expression on Tregs and disease progression [97]. Nikolova et al. [14] found that HIV-positive patients had a higher number of CD39+ Treg, and that their Teff exhibited an increased sensitivity in vitro to the suppressive effect of adenosine, which was related to the elevated expression of immunosuppressive A2A receptors [14].
Regulation of immunity by CD39 and CD73 in autoimmune diseases
Alteration of the CD39/CD73 machinery can disrupt the complex mechanisms underlying immune tolerance to self-antigens, driven mainly by Treg, thus contributing to the development of several autoimmune diseases [25].
Experimental allergic encephalomyelitis (EAE) and multiple sclerosis (MS)
Evidence is accumulating for the involvement of the CD39/CD73 pathway in the onset of EAE, a murine model of MS. Early studies showed that CD73 knockout mice displayed resistance to the induction of allergic encephalomyelitis, and a lower number of inflammatory cells infiltrating the central nervous system (CNS), as compared with wild type mice [98]. The contribution of CD73 to the migration of pathogenic T cells into the CNS was then confirmed by Thompson et al. [99], who observed fewer lymphocytes in brain tissue from CD73 knockout mice with allergic encephalomyelitis compared to the number of lymphocytes in the CNS of wild type animals. The mechanism by which CD73 contributes to the migration of pathogenic T cells into the CNS involves an adenosine-mediated induction increment for CX3CL1 (chemokine C-X3-C motif ligand 1) in the epithelium of the choroid plexus [100].
It has been observed that the administration of interferon (IFN)-β, which is used to treat subjects with MS, increases CD73 expression, and thus the endogenous concentrations of adenosine, in patients at level of the endothelium of CNS microvasculature, in the blood brain barrier and astrocytes, as well as in serum [101,102]. These findings suggest that CD73-derived adenosine contributes to the beneficial effects of IFN-β, but this evidence is discordant with data obtained in the murine model of MS, in which CD73 contributes to neuroinflammation via adenosine generation. For this reason, further investigations are needed to better characterize the role of this enzyme in the pathophysiology of MS.
Altered CD39 functions have also been shown to contribute to the pathophysiology of MS. In patients affected by MS, a reduced number of CD39+ Treg cells was noted, in conjunction with defective control of Th17 cell proliferation, cells that are critically involved in the pathogenesis of this autoimmune disorder [43, 103]. During the remission phase of MS, patients could counterbalance this Th17 cell overactivity by triggering an expansion of CD39+ Treg [104].
Rheumatoid arthritis
Moncrieffe et al. [105] have reported high expression of CD39 on CD4+ T cells isolated from the synovial fluid of patients with juvenile arthritis. The authors found two distinct populations of these cells: one that expressed Foxp3 and other markers of Tregs, and another that was Foxp3− and displayed a memory phenotype. Although these Foxp3− cells could hydrolyze ATP, they were unable to suppress the proliferation of Teff cells. In addition, they released pro-inflammatory cytokines (IL-2, IL-17, and IFN-γ) and probably contributed to joint inflammation. Given that both the CD39+CD4+ T Foxp3+ and Foxp3−cells isolated from the joint had reduced CD73 expression and activity in comparison with circulating T cells, the authors posited that incomplete generation of adenosine may contribute to the inflammation of the joint [105].
It has long been known that the antiinflammatory effects of methotrexate, a drug widely used in the clinical practice to manage arthritis, are mediated, at least in part, by increased release of adenine nucleotides and their CD73–mediated conversion to adenosine [106]. Moreover, in a recent investigation of CD73 in the context of an experimental model of rheumatoid arthritis in mice, Flögel et al. [57] observed an upregulation of CD73 on neutrophils, monocytes, and macrophages in the synovial fluid, which was paralleled by the enhanced expression of A2A receptors in neutrophils and inflammatory monocytes in the arthritic knee joint. The authors demonstrated that this upregulated CD73 can be exploited to convert an inactive A2A receptor agonist precursor into its active anti-inflammatory analog selectively at the site of inflammation, where the CD73/A2A receptor axis was induced [57]. The advantage of converting this prodrug as a local effect in the joints was the reduction in side effects that are normally present when A2A agonists are administered systematically [107,108].
Glomerulonephritis
Careful observation of CD73 deficient mice revealed that the chronic lack of CD73 was associated with an autoimmune inflammatory phenotype, which at the renal level affected the glomerular endothelium, leading to glomerular inflammation, injury and interstitial cellular infiltrate, with consequent proteinuria and decreased kidney function [109].
Inflammatory bowel diseases
In the past few years, increasing attention has been paid to the involvement of CD39 and CD73 in regulating inflammatory bowel diseases. In this regard, it has been observed that in a murine model of dextran sodium sulphate (DSS)-induced colitis, the genetic ablation of CD39 is associated with increased disease severity [110]. In humans, a common single nucleotide polymorphism associated with decreased CD39 expression is enriched in patients with Crohn's disease as compared to healthy controls, confirming the protective role of CD39 against inflammatory bowel diseases [110].
CD73 is also protective, as the intrarectal administration of DSS to CD73 knockout mice augmented the severity of colitis, increased levels of tissue IL-1β and TNF-α, and decreased the expression of several tight-junction-associated proteins, leading to increased gastrointestinal permeability [111].
Regulation of immunity by CD39 and CD73 in allergic diseases
The growing awareness about the involvement of purinergic signaling in shaping immune and inflammatory responses during allergic reactions [112] has fostered scientific interest in the roles of CD39 and CD73 in this context. Recently, a key role for CD73 has been unraveled in the development of airway inflammation after allergen exposure, as mice lacking CD73 display reduced airway hyperresponsiveness to methacholine as well as decreased eosinophil and mast cell infiltration, as compared to control mice [113]. In addition to its role in pulmonary allergy, recent lines of evidence indicate the critical involvement of CD39 and CD73 in regulating contact hypersensitivity [114]. In particular, Tregs obtained from CD39 knockout mice challenged with hapten were unable to suppress the adherence of Teff to vascular endothelium, thus indicating a pivotal role for adenosine generated by the CD39 pathway in decreasing leukocyte tissue influx through endothelial cells, as well as in preventing a hypersensitivity reaction [114].
Regulation of immunity by CD39 and CD73 in ischemia-reperfusion injury
Multiple lines of evidence have demonstrated that adenosine, produced as a result of the coordinated function of CD39 and CD73, is pivotal in protecting tissue against hypoxic and ischemic insults. Early studies, based on both genetic knockout models and pharmacological inhibition of CD39 and CD73 in mice, showed that these enzymes are crucial for protecting against increased vascular permeability and neutrophil extravasation during local hypoxia [32,34,59]. The increased vascular permeability and neutrophil extravasation noted in CD39 and CD73 knockout mice could be reversed by stimulating adenosine receptors by exogenous administration of 5'-(N-ethylcarboxamido) adenosine (NECA), a general adenosine receptor agonist, or by exogenous reconstitution with soluble forms of CD39 and CD73. These results were later extended to models of organ-specific ischemia/reperfusion: in CD39 or CD73 knockout mice the organ injury and inflammation that followed cerebral [66], cardiac [15,115–117], renal [48,118–120], hepatic [121–124], intestinal [125,126], and hindlimb [127] ischemia were more severe than in the corresponding wild type mice. A common theme in these studies was that pharmacological adenosine receptor activation or the administration of soluble CD39 or CD73 before the induction of ischemic insult elicited a protective effect in CD39 or CD73 knockout mice as well as in wild type animals.
The protective role of CD39 in ischemia reperfusion injury has been confirmed using transgenic mice overexpressing the enzyme. In this regard, the overexpression of this enzyme conferred protection in a murine model of warm renal ischemia reperfusion injury [128]. Adoptive transfer experiments showed that the expression of CD39 on both bone marrow-derived cells and the vasculature contributed to protection [128]. Analogously, the overexpression of CD39 was protective in a murine model of liver transplantation [124]. This beneficial effect did not appear to be mediated by elevated levels of CD39 in the liver parenchyma itself, but rather by a CD39-mediated reduction in CD4+ cells in the donor liver [124].
Regulation of immunity by CD39 and CD73 in atherosclerosis and arterial calcification
Current knowledge about the complex mechanisms underlying the pathogenesis of atherosclerosis highlights the importance of several immune/inflammatory mediators in the initiation and progression of this disorder [129], and vascular CD39 and CD73 regulate several steps in the atherogenic process by governing purinergic signalling [130,131]. Apolipoprotein E (ApoE) knockout mice, an experimental model of atherosclerosis, show decreased CD39 expression and activity in thoracic aorta, which correlates with reduced vascular reactivity and the presence of atherosclerotic plaques in aortic roots and arches. The authors speculated that the accumulation of ATP and ADP in ApoE knockout mice may cause desensitization of P2 receptors, which in turn may contribute to decreased blood flow, which may predispose an individual to atheroma formation [132]. In a carotid artery wire injury model, mice lacking CD39 had a decrease in the migration of vascular smooth muscle cells and reduced neointimal formation, which indicated that CD39 contributes to harmful neointima formation [133]. Clearly, more research is needed to define the role of CD39 in regulating atherosclerosis.
Genetic ablation of CD73 in ApoE-knockout mice increased atheroma formation, likely resulting from a reduced inhibitory control exerted by adenosine on immune cells, which are actively involved in the atherogenic process [134]. In addition, severe endothelial dysfunction has been observed in CD73 knockout mice after wire injury of carotid arteries, which displayed an increased expression of vascular cell adhesion molecule (VCAM)-1 and enhanced nuclear factor-kappa B activity with an increase in neointimal plaque formation and sustained macrophage infiltration in comparison with wild type animals [78]. Thus, CD73 appears to protect against atherosclerosis.
There is recent evidence for the involvement of CD73 in regulating arterial calcification, a pathological process characterized by calcium deposition in the intimal or medial layers of the vessel wall, which is associated with an increased risk of cardiovascular events [135,136]. In particular, a correlation between mutations of the NT5E gene, resulting in non-functional CD73, and the presence of symptomatic arterial and distal joint calcifications has been reported [137,138]. The mechanism of disease appears to involve increased alkaline phosphatase levels and the accumulation of calcium phosphate crystals. The fact that CD73 knockout mice do not show arterial calcification suggests that caution should be exercised when extrapolating mouse data to humans regarding the role of CD73 in vascular diseases.
Regulation of immunity by CD39 and CD73 in diabetes
The term diabetes denotes a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both [139]. Diabetes can result from the autoimmune destruction of β cells in the pancreas with consequent insulin deficiency (type 1 diabetes) or abnormalities of adipose tissue, liver, and muscle that result in resistance to insulin action (type 2 diabetes) [139].
A recent study has demonstrated that the susceptibility of mice to streptozotocin-induced diabetes, an experimental model of type 1 diabetes, was influenced by the level of expression of CD39. CD39 knockout mice become diabetic faster and with a higher overall incidence than wild type animals [140]. The onset of diabetes in these animals was ascribed to a dysregulation of immunity rather than impaired glucose tolerance, as reconstitution of CD39 knockout mice with bone marrow from wild type animals reduced the incidence of diabetes [140]. By contrast, transgenic mice over-expressing CD39 were protected from streptozotocin-induced diabetes, displaying reduced islet T cell infiltration and inflammatory gene expression in comparison with wild type animals. This protective effect was reversed by the inhibition or deletion of the A2A or A2B receptors [140], which confirms the suppressive effect of A2 receptor activation on diabetes [141]. It is noteworthy that CD39 knockout mice have impaired glucose and lipid metabolism even in the absence of streptozotocin; however, the role of the immune system in mediating this effect is not clear [142].
In patients with type 2 diabetes, impaired glycemic control is associated with increased proportions of CD39+ cells and NTPDase activity in peripheral blood mononuclear cells. The authors speculated that CD39 may have a balancing regulatory role in the inflammatory processes occurring in patients with type 2 diabetes [143].
Recently, it has been observed that the deletion of CD73 is associated with increased serum levels of blood glucose, insulin, triglycerides, and free fatty acids. In addition, CD73 knockout mice display increased intramyocellular lipid levels and a reduction in insulin-induced Akt phosphorylation, thereby suggesting a role of CD73-derived adenosine in promoting muscle insulin action [144].
Regulation of immunity by CD39 and CD73 in cancer
Increasing evidence suggests that interactions between tumor cells and their microenvironment are essential for tumorigenesis [145]. Within the neoplastic milieu, cancer and immune cells closely interact to generate an immunosuppressive environment by releasing immunomodulatory factors, which supports neoplastic growth [146].
Several studies have pointed to the critical task carried out by CD39 and CD73 in generating this immunosuppressed environment, characterized by increased adenosine levels, which promotes the development and progression of cancer (Figure 3) [16]. Observations of high expression and activity of CD39 and CD73 in several blood or solid tumors (see Table 1) suggest roles for these enzymes in promoting tumor growth and infiltration [16,147].
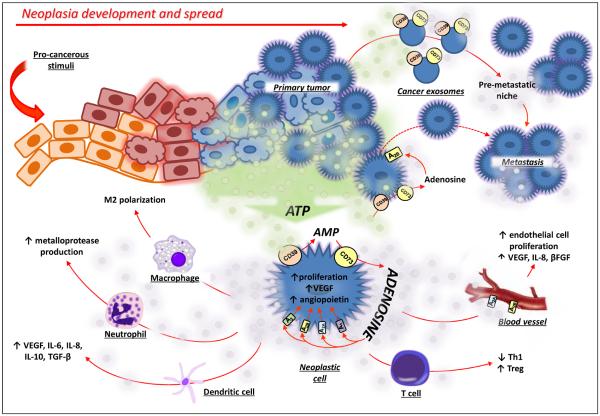
Figure 3.
The CD39/CD73 axis in neoplastic development and progression. Within the tumor environment, ATP is released and is converted into adenosine by upregulated CD39 and CD73. Adenosine promotes cancer growth by acting directly on neoplastic cells through A1, A2A, A2B, and/or A3 adenosine receptor activation and by subsequent enhancement of invasiveness and metastatic ability. The engagement of A2A and A2B receptors on endothelial cells enhances the production of pro-angiogenic factors (β-FGF, VEGF and IL-8). In addition, the adenosine generated by CD39/CD73 expressed either on primary neoplastic cells or on cancer exosomes generates an immunosuppressive environment by acting on macrophages, neutrophils, dendritic cells and T cells.
Abbreviations: IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; VEGF, vascular endothelial growth factor; β-FGF, fibroblast growth
Table 1.
CD39 and CD73 in solid and blood neoplasm.
| Cancer types | CD39/CD73 Expression and Activity | Refs |
|---|---|---|
|
| ||
| Solid Neoplasia | ||
|
| ||
| Glioma | Increased CD73 mRNA | [161] |
|
| ||
| Head Neck | Increased CD39 and CD73 expression and activity in Treg cells | [162] |
|
| ||
| Melanoma | Increased CD73 expression is associated with a highly invasive phenotype of melanoma | [156] |
| Increased CD39 expression is related to the differentiation of melanocytes into malignant cells | [163] | |
|
| ||
| Thyroid | Increased CD73 expression and activity in papillary carcinoma | [164] |
|
| ||
| Breast | Increased CD73 mRNA and protein level and increased enzyme activity | [151] |
| Involvement of CD73 in tumor growth and the suitability of this enzyme as a marker of breast cancer progression | [165] | |
| CD73 expression correlates positively with epidermal growth factor receptor expression/function | [166] | |
|
| ||
| Pancreas | Increased CD39 mRNA | [153] |
|
| ||
| Liver | CD39 expression on Tregs facilitates hepatic growth of metastatic melanoma | [152] |
|
| ||
| Colon | No changes in CD39 expression in human | [167] |
| Increased CD73 expression | [168] | |
|
| ||
| Bladder | Increased CD73 expression | [169] |
|
| ||
| Ovarian | Increased CD39 and CD73 expression | [170] |
|
| ||
| Prostate | CD73 activity promotes prostate cancer growth and metastasis | [171] |
|
| ||
| Blood Neoplasia | ||
|
| ||
| Leukemia | Increased CD39 expression and activity on chronic lymphocytic leukemia cells. | [172] |
| Increased CD39 expression on CD4+ T lymphocytes in patients with chronic lymphocytic leukemia. | [173] | |
|
| ||
| Lymphoma | Increased CD39 expression on CD4+ and CD8+ T cells in patients with follicular lymphoma | [174] |
The CD39/CD73 complex participates in the process of tumor immunoescape, by inhibiting the activation, clonal expansion, and homing of tumor-specific T cells (in particular, T helper and cytotoxic T cells), impairing tumor cell killing by cytolytic effector T lymphocytes, dictating, via pericellular generation of adenosine, a substantial component of the suppressive capabilities of Treg and Th17 cells, and enhancing the conversion of type 1 macrophages into tumor-promoting type 2 macrophages [16,17,147] (Figure 3). Myeloid-derived suppressor cells (MDSCs), which are a recently discovered tumor infiltrating immune cell type [148], also appear to promote tumor growth by a CD39-mediated mechanism. This is indicated by the observations that MDSCs obtained from patients with colorectal cancer expressed high levels of CD39 and displayed an increased inhibitory effect against anti-tumor T cells in comparison with MDSCs from healthy donors [149]. Recent mechanistic studies have shown that in addition to cell-associated CD39 and CD73, CD39- and CD73-expressing cancer exosomes can also raise adenosine levels within the tumor microenvironment, thereby inhibiting T cell responses through stimulating A2A and A2B receptors [150].
Beside its immunoregulatory roles, the ectonucleotidase pathway contributes directly to the modulation of cancer cell growth, differentiation, invasion, migration, and metastasis [151–157] (Figure 3). The CD39/CD73 system can also contribute, via adenosine generation, to tumor angiogenesis [158,159] (Figure 3). In particular, CD39, the dominant vascular cell ectonucleotidase, is important for both the initiation of angiogenesis and the progression of neovascularization [160], and CD39 on vasculature mediates the angiogenic process in mouse models of melanoma, lung [158] and liver malignancy [152].
Overall, the above lines of evidence suggest the evaluation of CD39 and/or CD73 expression and activity as potential prognostic markers in cancer patients, as well as the exploration of pharmacological modulation of these enzymes as innovative strategies to thwart neoplastic development and progression [36]. It is noteworthy that several small molecule inhibitors and monoclonal antibodies developed against these enzymes show anti-tumor efficacy and a favorable tolerability profile in several murine models of malignancy [36].
Concluding remarks
Over the past 30 years, increasing evidence has underscored the role of the purinergic system in coordinating tissue immune responses under both physiological conditions and in the presence of inflammation. In this context, the CD39/CD73 pathway is a critical checkpoint; through its coordinated activity, it regulates the types and level of purinergic receptor activation, by finely shaping the chemistry, magnitude, and duration of purinergic signalling, in accordance with the various extracellular conditions.
The use of pharmacological tools as well as the availability of transgenic mice has improved the characterization of the CD39/CD73 axis in fine-tuning immune cell responses under adverse conditions, revealing a close correlation between alterations in enzyme expression and activity and the onset of various immune-related diseases, (i.e. infections, AIDS, autoimmune diseases, atherosclerosis, ischemia-reperfusion injury and cancer). The studies briefly outlined in this review article support the notion that pharmacological modulation of CD39 and/or CD73 represents a viable approach in restoring immune homeostasis, and thus managing disorders that are driven by dysfunctional immune responses.
Highlights
CD39 and CD73 are important for calibrating the duration, magnitude, and composition of the “purinergic halo” surrounding immune cells
CD39 and CD73 degrade ATP, ADP and AMP to adenosine, they can be viewed as “immunological switches” that shift ATP-driven pro-inflammatory immune cell activity toward an anti-inflammatory state mediated by adenosine
CD39 and CD73 are highly expressed on the surface of Foxp3+ Tregs and have been increasingly used as markers of Tregs
CD39 and CD73 are important for the immunosuppressive activity of Tregs
CD39 and CD73 generate an immunosuppressed environment, characterized by increased adenosine levels, which promotes the development and progression of cancer
Acknowledgements
This work was supported by National Institutes of Health grant R01GM66189 and USAMRMC grant 09065004.
Glossary
- Arteriogenesis
describes the growth of functional collateral arteries from preexisting arterio-arteriolar anastomoses in response to ischemia.
- Atheroma
is an asymmetric focal thickening of the intima. The atheroma is composed of a lipid core, a residue of necrotic “foam cell-forming macrophages”, that migrate into the intima and ingest lipids, as well as by a connective tissue matrix derived from smooth muscle cells that migrate from the media into the intima, where they proliferate and change their phenotype to form a fibrous capsule around the lipid core.
- Biophase concentration
concentration reached by an endogenous or an exogenous ligand at its biological site of action.
- Bradyzoite
(brady = slow in Greek) is the slow growing and cyst-forming stage of Toxoplasma gondii. After oral ingestion, the bradyzoites invade the intestinal epithelium and differentiates into tachyzoites, the active form of the protozoa, which disseminates and replicates within the host.
- Chagas disease
also known as American trypanosomiasis, is a tropical chronic infection caused by Trypanosoma cruzi, a flagellate protozoan parasite. The disease affects an estimated 8–10 million people in Central (Mexico) and South America (Argentina, Bolivia, Brazil, Chile Colombia, Paraguay and Venezuela), putting them at risk of developing life-threatening cardiac (myocarditis, dilated cardiomyopathy) and gastrointestinal complications (megaesophagus, megacolon and achalasia).
- Diapedesis
is the movement of lymphocytes out of blood vessels into nearby tissues and is critical for immune system function and inflammation. It occurs by migration of blood leukocytes either directly through individual microvascular endothelial cells (the transcellular route) or between them (the paracellular route).
- Intra-tracheal instillation
direct injection of a compound into the tracheal lumen.
- Multiple sclerosis (MS)
is an immune-mediated disease of the central nervous system (CNS) that is heterogenous in its pathological substrate, clinical presentation and disease progression. The histopathology of MS classically comprises focal white matter demyelination in the brain and spinal cord, featuring inflammation and edema initially, followed by axonal damage, loss of oligodendrocytes, microglial activation, and astroglial scarring.
- Neointimal formation
is a thickened layer of arterial intima formed especially on a prosthesis or in the presence of atherosclerosis by migration and proliferation of cells from the media.
- T-cell receptor (TCR)
is a complex of integral membrane proteins that participates in the activation of T cells in response to the presentation of antigen.
- Treg cells
play an indispensable role in maintaining immunological unresponsiveness to self-antigens and in suppressing excessive immune responses deleterious to the host.
- Trypomastigotes
infective form of the parasite Trypanosoma cruzi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crimeen-Irwin B, et al. Failure of immune homeostasis the consequences of under and over reactivity. Curr. Drug. Targets. Immune. Endocr. Metabol. Disord. 2005;5:413–422. doi: 10.2174/156800805774912980. [DOI] [PubMed] [Google Scholar]
- 2.Sitkovsky MV, Ohta A. The `danger' sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Haskó G, et al. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci. Signal. 2009;2:pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 5.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperlágh B, et al. ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem. Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 7.Sperlágh B, et al. Potent effect of interleukin-1 beta to evoke ATP and adenosine release from rat hippocampal slices. J. Neuroimmunol. 2004;151:33–39. doi: 10.1016/j.jneuroim.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Sperlágh B, Vizi ES. The role of extracellular adenosine in chemical neurotransmission in the hippocampus and Basal Ganglia: pharmacological and clinical aspects. Curr. Top. Med. Chem. 2011;11:1034–1046. doi: 10.2174/156802611795347564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredholm BB, et al. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol. Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Kukulski F, et al. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv. Pharmacol. 2011;61:263–299. doi: 10.1016/B978-0-12-385526-8.00009-6. [DOI] [PubMed] [Google Scholar]
- 12.Schetinger MR, et al. NTPDase and 5'-nucleotidase activities in physiological and disease conditions: new perspectives for human health. Biofactors. 2007;31:77–98. doi: 10.1002/biof.5520310205. [DOI] [PubMed] [Google Scholar]
- 13.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv. Pharmacol. 2011;61:301–332. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolova M, et al. CD39/adenosine pathway is involved in AIDS progression. PLoS Pathog. 2011;7:e1002110. doi: 10.1371/journal.ppat.1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bönner F, et al. Resident cardiac immune cells and expression of the ectonucleotidase enzymes CD39 and CD73 after ischemic injury. PLoS One. 2012;7:e34730. doi: 10.1371/journal.pone.0034730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastid J, et al. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2012 doi: 10.1038/onc.2012.269. in press. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B. CD73 promotes tumor growth and metastasis. Oncoimmunology. 2012;1:67–70. doi: 10.4161/onci.1.1.18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heine P, et al. The C-terminal cysteine-rich region dictates specific catalytic properties in chimeras of the ectonucleotidases NTPDase1 and NTPDase2. Eur. J. Biochem. 2001;268:364–373. doi: 10.1046/j.1432-1033.2001.01896.x. [DOI] [PubMed] [Google Scholar]
- 19.Maliszewski CR, et al. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J. Immunol. 1994;153:3574–3583. [PubMed] [Google Scholar]
- 20.Smith TM, Kirley TL. Cloning, sequencing, and expression of a human brain ecto-apyrase related to both the ecto-ATPases and CD39 ecto-apyrases1. Biochim. Biophys. Acta. 1998;1386:65–78. doi: 10.1016/s0167-4838(98)00063-6. [DOI] [PubMed] [Google Scholar]
- 21.Enjyoji K, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann H. Two novel families of ectonucleotidases: molecular structures, catalytic properties and a search for function. Trends Pharmacol. Sci. 1999;20:231–236. doi: 10.1016/s0165-6147(99)01293-6. [DOI] [PubMed] [Google Scholar]
- 23.Mizumoto N, et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat. Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 24.Kapojos JJ, et al. Enhanced ecto-apyrase activity of stimulated endothelial or mesangial cells is downregulated by glucocorticoids in vitro. Eur. J. Pharmacol. 2004;501:191–198. doi: 10.1016/j.ejphar.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Dwyer KM, et al. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eltzschig HK, et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalmin F, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Yegutkin GG, et al. Metabolism of circulating ADP in the bloodstream is mediated via integrated actions of soluble adenylate kinase-1 and NTPDase1/CD39 activities. FASEB. J. 2012;26:3875–3883. doi: 10.1096/fj.12-205658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sträter N. Ecto-5'-nucleotidase: Structure function relationships. Purinergic. Signal. 2006;2:343–350. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuts DP. Crystal Structure of a Soluble Form of Human CD73 with Ecto-5' Nucleotidase Activity. Chembiochem. 2012;13:2384–2391. doi: 10.1002/cbic.201200426. [DOI] [PubMed] [Google Scholar]
- 31.Knapp K, et al. Crystal Structure of the Human Ecto-5'-Nucleotidase (CD73): Insights into the Regulation of Purinergic Signaling. Structure. 2012;20:2161–2173. doi: 10.1016/j.str.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Thompson LF, et al. Crucial role for ecto-5'-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennon PF, et al. Neutrophil-derived 5'-adenosine promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J. Exp. Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Synnestvedt K. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eltzschig HK, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beavis PA, et al. CD73: A potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Regateiro FS, et al. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur. J. Immunol. 2011;41:2955–2965. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 38.Haskó G, Pacher P. Regulation of macrophage function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012;32:865–869. doi: 10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahamed DA, et al. CD73-generated adenosine facilitates Toxoplasma gondii differentiation to long-lived tissue cysts in the central nervous system. Proc. Natl. Acad. Sci. USA. 2012;109:16312–16317. doi: 10.1073/pnas.1205589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vignali DA, et al. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandapathil M, et al. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J. Immunol. Methods. 2009;346:55–63. doi: 10.1016/j.jim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuler PJ, et al. Separation of human CD4+CD39+ T cells by magnetic beads reveals two phenotypically and functionally different subsets. J. Immunol. Methods. 2011;369:59–68. doi: 10.1016/j.jim.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borsellino G, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 44.Ernst PB, et al. Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J. Immunol. 2010;185:1993–1998. doi: 10.4049/jimmunol.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romio M, et al. Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells. Am. J. Physiol. Cell. Physiol. 2011;301:C530–539. doi: 10.1152/ajpcell.00385.2010. [DOI] [PubMed] [Google Scholar]
- 47.Ohta A, et al. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012;3:190. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinsey GR, et al. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J. Am. Soc. Nephrol. 2012;23:1528–1537. doi: 10.1681/ASN.2012010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dwyer KM, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am. J. Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mandapathil M, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J. Biol. Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antonioli L, et al. Adenosine deaminase in the modulation of immune system and its potential as a novel target for treatment of inflammatory disorders. Curr. Drug. Targets. 2012;13:842–862. doi: 10.2174/138945012800564095. [DOI] [PubMed] [Google Scholar]
- 52.Barletta KE, et al. Regulation of neutrophil function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012;32:856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 54.Eltzschig HK, et al. Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends. Cardiovasc. Med. 2008;18:103–107. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Pulte ED, et al. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb. Res. 2007;121:309–317. doi: 10.1016/j.thromres.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flögel U, et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl. Med. 2012;4:146ra108. doi: 10.1126/scitranslmed.3003717. [DOI] [PubMed] [Google Scholar]
- 58.Linden J. Cell biology. Purinergic chemotaxis. Science. 2006;314:1689–1690. doi: 10.1126/science.1137190. [DOI] [PubMed] [Google Scholar]
- 59.Eltzschig HK, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 60.Kukulski F, et al. NTPDase1 controls IL-8 production by human neutrophils. J. Immunol. 2011;187:644–653. doi: 10.4049/jimmunol.1002680. [DOI] [PubMed] [Google Scholar]
- 61.Corriden R, et al. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J. Biol. Chem. 2008;283:28480–28486. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reutershan J, et al. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 63.Eltzschig HK, et al. Nucleotide metabolism and cell-cell interactions. Methods Mol. Biol. 2006;341:73–87. doi: 10.1385/1-59745-113-4:73. [DOI] [PubMed] [Google Scholar]
- 64.Lévesque SA, et al. NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur. J. Immunol. 2010;40:1473–1485. doi: 10.1002/eji.200939741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haskó G, et al. Ecto-5'-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J. Immunol. 2011;187:4256–4267. doi: 10.4049/jimmunol.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrovic-Djergovic D, et al. Tissue-resident ecto-5' nucleotidase (CD73) regulates leukocyte trafficking in the ischemic brain. J. Immunol. 2012;188:2387–2398. doi: 10.4049/jimmunol.1003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zanin RF, et al. Differential macrophage activation alters the expression profile of NTPDase and ecto-5'-nucleotidase. PLoS One. 2012;7:e31205. doi: 10.1371/journal.pone.0031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Csóka B, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Airas L, Jalkanen S. CD73 mediates adhesion of B cells to follicular dendritic cells. Blood. 1996;88:1755–1764. [PubMed] [Google Scholar]
- 70.Airas L. CD73 and adhesion of B-cells to follicular dendritic cells. Leuk. Lymphoma. 1998;29:37–47. doi: 10.3109/10428199809058380. [DOI] [PubMed] [Google Scholar]
- 71.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 72.Packham MA, Mustard JF. Platelet aggregation and adenosine diphosphate/adenosine triphosphate receptors: a historical perspective. Semin. Thromb. Hemost. 2005;3:129–138. doi: 10.1055/s-2005-869518. [DOI] [PubMed] [Google Scholar]
- 73.Atkinson B, et al. Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: Potential as therapeutic targets. Blood Cells Mol. Dis. 2006;36:217–222. doi: 10.1016/j.bcmd.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 74.Fung CY, et al. P2X(1) receptor inhibition and soluble CD39 administration as novel approaches to widen the cardiovascular therapeutic window. Trends Cardiovasc. Med. 2009;19:1–5. doi: 10.1016/j.tcm.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koszalka P, et al. Targeted disruption of cd73/ecto-5'-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ. Res. 2004;95:814–821. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 76.Salmi M, Jalkanen S. Cell-surface enzymes in control of leukocyte trafficking. Nat. Rev. Immunol. 2005;5:760–771. doi: 10.1038/nri1705. [DOI] [PubMed] [Google Scholar]
- 77.Takedachi M, et al. CD73-generated adenosine restricts lymphocyte migration into draining lymph nodes. J. Immunol. 2008;180:6288–6296. doi: 10.4049/jimmunol.180.9.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zernecke A, et al. CD73/ecto-5'-nucleotidase protects against vascular inflammation and neointima formation. Circulation. 2006;113:2120–2127. doi: 10.1161/CIRCULATIONAHA.105.595249. [DOI] [PubMed] [Google Scholar]
- 79.Ålgars A, et al. Different role of CD73 in leukocyte trafficking via blood and lymph vessels. Blood. 2011;117:4387–4393. doi: 10.1182/blood-2010-11-321646. [DOI] [PubMed] [Google Scholar]
- 80.Kak V, et al. Immunotherapies in infectious diseases. Med. Clin. North. Am. 2012;96:455–474. doi: 10.1016/j.mcna.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Paletta-Silva R, Meyer-Fernandes JR. Adenosine and immune imbalance in visceral leishmaniasis: the possible role of ectonucleotidases. J. Trop. Med. 2012;2012:650874. doi: 10.1155/2012/650874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thammavongsa V, et al. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J. Exp. Med. 2009;206:2417–2427. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Souza MC, et al. The influence of ecto-nucleotidases on Leishmania amazonensis infection and immune response in C57B/6 mice. Acta Trop. 2010;115:262–269. doi: 10.1016/j.actatropica.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 84.Russo-Abrahão T, et al. Biochemical properties of Candida parapsilosis ecto-5'-nucleotidase and the possible role of adenosine in macrophage interaction. FEMS. Microbiol. Lett. 2011;317:34–42. doi: 10.1111/j.1574-6968.2011.02216.x. [DOI] [PubMed] [Google Scholar]
- 85.Fan J, et al. Ecto-5'-nucleotidase: a candidate virulence factor in Streptococcus sanguinis experimental endocarditis. PLoS One. 2012;7:e38059. doi: 10.1371/journal.pone.0038059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Théâtre E, et al. Overexpression of CD39 in mouse airways promotes bacteria-induced inflammation. J. Immunol. 2012;189:1966–1974. doi: 10.4049/jimmunol.1102600. [DOI] [PubMed] [Google Scholar]
- 87.Tasca T, et al. Heterogeneity in extracellular nucleotide hydrolysis among clinical isolates of Trichomonas vaginalis. Parasitology. 2005;131:71–78. doi: 10.1017/s0031182005007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santos RF, et al. Influence of Ecto-nucleoside triphosphate diphosphohydrolase activity on Trypanosoma cruzi infectivity and virulence. PLoS. Negl. Trop. Dis. 2009;3:e387. doi: 10.1371/journal.pntd.0000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Russo-Abrahão T, et al. Giardia duodenalis: biochemical characterization of an ecto-5'-nucleotidase activity. Exp. Parasitol. 2011;127:66–71. doi: 10.1016/j.exppara.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 90.Souza Vdo C, et al. E-NTPDase and E-ADA activities are altered in lymphocytes of patients within determinate form of Chagas' disease. Parasitol. Int. 2012;61:690–696. doi: 10.1016/j.parint.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Oliveira CB, et al. NTPDase activity in lymphocytes of rats infected by Trypanosoma evansi. Parasitology. 2012;139:232–236. doi: 10.1017/S0031182011001879. [DOI] [PubMed] [Google Scholar]
- 92.Sansom FM, et al. A bacterial ecto-triphosphate diphosphohydrolase similar to human CD39 is essential for intracellular multiplication of Legionella pneumophila. Cell. Microbiol. 2007;9:1922–1935. doi: 10.1111/j.1462-5822.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- 93.Yegutkin GG, et al. Disordered lymphoid purine metabolism contributes to the pathogenesis of persistent Borrelia garinii infection in mice. J Immunol. 2010;184:5112–5120. doi: 10.4049/jimmunol.0902760. [DOI] [PubMed] [Google Scholar]
- 94.Alam MS, et al. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J. Infect. Dis. 2009;199:494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kas-Deelen AM, et al. Cytomegalovirus infection increases the expression and activity of ecto-ATPase (CD39) and ecto-5'nucleotidase (CD73) on endothelial cells. FEBS Lett. 2001;491:21–25. doi: 10.1016/s0014-5793(01)02085-3. [DOI] [PubMed] [Google Scholar]
- 96.Leal DB, et al. HIV infection is associated with increased NTPDase activity that correlates with CD39-positive lymphocytes. Biochim. Biophys. Acta. 2005;1746:129–134. doi: 10.1016/j.bbamcr.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Schulze Zur Wiesch J, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J. Virol. 2011;85:1287–1297. doi: 10.1128/JVI.01758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mills JH, et al. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2008;105:9325–9330. doi: 10.1073/pnas.0711175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thompson LF, et al. Regulation of leukocyte migration across endothelial barriers by ECTO-5'-nucleotidase-generated adenosine. Nucleos. Nucleot. Nucl. 2008;27:755–760. doi: 10.1080/15257770802145678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mills JH, et al. Extracellular adenosine signaling induces CX3CL1 expression in the brain to promote experimental autoimmune encephalomyelitis. J. Neuroinflammation. 2012;9:193. doi: 10.1186/1742-2094-9-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Airas L, et al. Mechanism of action of IFN-beta in the treatment of multiple sclerosis: a special reference to CD73 and adenosine. Ann. N. Y. Acad. Sci. 2007;1110:641–648. doi: 10.1196/annals.1423.067. [DOI] [PubMed] [Google Scholar]
- 102.Niemelä J, et al. IFN-beta regulates CD73 and adenosine expression at the blood-brain barrier. Eur. J. Immunol. 2008;38:2718–2726. doi: 10.1002/eji.200838437. [DOI] [PubMed] [Google Scholar]
- 103.Fletcher JM, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J. Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 104.Peelen E, et al. Th17 expansion in MS patients is counterbalanced by an expanded CD39+ regulatory T cell population during remission but not during relapse. J. Neuroimmunol. 2011;240–241:97–103. doi: 10.1016/j.jneuroim.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 105.Moncrieffe H, et al. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J. Immunol. 2010;185:134–143. doi: 10.4049/jimmunol.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Montesinos MC, et al. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5'-nucleotidase: findings in a study of ecto-5'-nucleotidase gene-deficient mice. Arthritis. Rheum. 2007;56:1440–1445. doi: 10.1002/art.22643. [DOI] [PubMed] [Google Scholar]
- 107.Nekooeian AA, Tabrizchi R. Effects of adenosine A2A receptor agonist, CGS 21680, on blood pressure, cardiac index and arterial conductance in anaesthetized rats. Eur. J. Pharmacol. 1996;307:163–169. doi: 10.1016/0014-2999(96)00250-6. [DOI] [PubMed] [Google Scholar]
- 108.Nair PK, et al. Clinical utility of regadenoson for assessing fractional flow reserve. JACC Cardiovasc Interv. 2011;4:1085–1092. doi: 10.1016/j.jcin.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 109.Blume C, et al. Autoimmunity in CD73/Ecto-5'-nucleotidase deficient mice induces renal injury. PLoS One. 2012;7:e3710. doi: 10.1371/journal.pone.0037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedman DJ, et al. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bynoe MS, et al. CD73 is critical for the resolution of murine colonic inflammation. J. Biomed. Biotechnol. 2012;2012:260983. doi: 10.1155/2012/260983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thompson LF, et al. Animal models of airway diseases. Subcel. Biochem. 2011;55:195–234. doi: 10.1007/978-94-007-1217-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schreiber R, et al. Allergen-induced airway hyperresponsiveness is absent in ecto-5'-nucleotidase(CD73)-deficient mice. Pflugers Arch. 2008;457:431–440. doi: 10.1007/s00424-008-0543-0. [DOI] [PubMed] [Google Scholar]
- 114.Ring S, et al. CD4+CD25+ regulatory T cells suppress contact hypersensitivity reactions through a CD39, adenosine-dependent mechanism. J. Allergy Clin. Immunol. 2009;123:1287–1296. doi: 10.1016/j.jaci.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 115.Eckle T, et al. Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 116.Kohler D, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 117.Grenz A, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grenz A, et al. Protective role of ecto-5'-nucleotidase (CD73) in renal ischemia. J. Am. Soc. Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 119.Grenz A, et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB. J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 120.Jian R, et al. CD73 protects kidney from ischemia-reperfusion injury through reduction of free radicals. APMIS. 2012;120:130–138. doi: 10.1111/j.1600-0463.2011.02827.x. [DOI] [PubMed] [Google Scholar]
- 121.Hart ML, et al. Extracellular adenosine production by ecto-5'-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135:1739–1750. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 122.Hart ML, et al. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J. Immunol. 2011;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun X, et al. Liver damage and systemic inflammatory responses are exacerbated by the genetic deletion of CD39 in total hepatic ischemia. Purinergic. Signal. 2011;7:427–434. doi: 10.1007/s11302-011-9239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pommey S, et al. Liver grafts from CD39-overexpressing mice are protected from ischemia reperfusion injury due to reduced numbers of resident CD4(+) T cells. Hepatology. 2012 doi: 10.1002/hep.25985. in press. [DOI] [PubMed] [Google Scholar]
- 125.Hart ML, et al. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB. J. 2008;22:2784–2797. doi: 10.1096/fj.07-103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hart ML, et al. Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5'-nucleotidase (CD73) and the A2B adenosine receptor. J. Immunol. 2011;186:4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.Böring YC, et al. Lack of ecto-5'-nucleotidase (CD73) promotes arteriogenesis. Cardiovasc Res. 2012;97:88–96. doi: 10.1093/cvr/cvs286. [DOI] [PubMed] [Google Scholar]
- 128.Crikis S, et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am. J. Transplant. 2010;10:2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wierda RJ, et al. Epigenetics in atherosclerosis and inflammation. J. Cell. Mol. Med. 2010;14:1225–1240. doi: 10.1111/j.1582-4934.2010.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Di Virgilio F, Solini A. P2 receptors: new potential players in atherosclerosis. Br. J. Pharmacol. 2002;135:831–842. doi: 10.1038/sj.bjp.0704524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reiss AB, Cronstein BN. Regulation of foam cells by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012;32:879–886. doi: 10.1161/ATVBAHA.111.226878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mercier N, et al. Impaired ATP-induced coronary blood flow and diminished aortic NTPDase activity precede lesion formation in apolipoprotein E-deficient mice. Am. J. Pathol. 2012;180:419–428. doi: 10.1016/j.ajpath.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 133.Behdad A, et al. Vascular smooth muscle cell expression of ectonucleotidase CD39 (ENTPD1) is required for neointimal formation in mice. Purinergic Signal. 2009;5:335–342. doi: 10.1007/s11302-009-9158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Buchheiser A, et al. Inactivation of CD73 promotes atherogenesis in apolipoprotein E-deficient mice. Cardiovasc. Res. 2011;92:338–47. doi: 10.1093/cvr/cvr218. [DOI] [PubMed] [Google Scholar]
- 135.Rutsch F, et al. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ. Res. 2011;109:578–592. doi: 10.1161/CIRCRESAHA.111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fish RS, et al. ATP and arterial calcification. Eur. J. Clin. Invest. 2013 doi: 10.1111/eci.12055. in press. [DOI] [PubMed] [Google Scholar]
- 137.Hilaire C, et al. NT5E mutations and arterial calcifications. N. Engl. J. Med. 2011;364:432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Markello TC, et al. Vascular pathology of medial arterial calcifications in NT5E deficiency: implications for the role of adenosine in pseudoxanthoma elasticum. Mol. Genet. Metab. 2011;103:44–50. doi: 10.1016/j.ymgme.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. 1998. [DOI] [PubMed] [Google Scholar]
- 140.Chia JS, et al. The protective effects of CD39 over-expression in multiple low dose streptozotocin-induced diabetes in mice. Diabetes. 2013 doi: 10.2337/db12-0625. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Németh ZH, et al. Adenosine receptor activation ameliorates type 1 diabetes. FASEB. J. 2007;21:2379–2388. doi: 10.1096/fj.07-8213com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Enjyoji K, et al. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes. 2008;57:2311–2320. doi: 10.2337/db07-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.García-Hernández MH, et al. Expression and function of P2X(7) receptor and CD39/Entpd1 in patients with type 2 diabetes and their association with biochemical parameters. Cell Immunol. 2011;269:135–143. doi: 10.1016/j.cellimm.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 144.Burghoff S, et al. Deletion of CD73 promotes dyslipidemia and intramyocellular lipid accumulation in muscle of mice. Arch. Physiol. Biochem. 2013 doi: 10.3109/13813455.2012.755547. in press. [DOI] [PubMed] [Google Scholar]
- 145.Chew V, et al. Immune microenvironment in tumor progression: characteristics and challenges for therapy. J. Oncol. 2012:608406. doi: 10.1155/2012/608406. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yaqub S, Aandahl EM. Inflammation versus adaptive immunity in cancer pathogenesis. Crit. Rev. Oncog. 2009;15:43–63. doi: 10.1615/critrevoncog.v15.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 147.Chalmin F, et al. Phenotypic and functional characteristics of CD4+ CD39+ FOXP3+ and CD4+ CD39+ FOXP3neg T-cell subsets in cancer patients. Immunity. 2012;36:362–373. doi: 10.1002/eji.201142347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun HL, et al. Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World. J. Gastroenterol. 2012;18:3303–3309. doi: 10.3748/wjg.v18.i25.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang B. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS. One. 2013;8:e57114. doi: 10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Clayton A, et al. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 2011;187:676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 151.Zhou X, et al. Effects of ecto-5'-nucleotidase on human breast cancer cell growth in vitro and in vivo. Oncol. Rep. 2007;17:1341–1346. [PubMed] [Google Scholar]
- 152.Sun X, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Künzli BM, et al. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am. J. Physiol. Gastrointest. Liver. Physiol. 2007;292:G223–230. doi: 10.1152/ajpgi.00259.2006. [DOI] [PubMed] [Google Scholar]
- 154.Stagg J, et al. CD73-Deficient Mice Have Increased Antitumor Immunity and Are Resistant to Experimental Metastasis. Cancer Res. 2011;71:2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 155.Serra S, et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 2011;118:6141–6152. doi: 10.1182/blood-2011-08-374728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sadej R, et al. Expression of ecto-5'-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res. 2006;16:213–222. doi: 10.1097/01.cmr.0000215030.69823.11. [DOI] [PubMed] [Google Scholar]
- 157.Stagg J, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl. Acad. Sci. U S A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jackson SW, et al. Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice. Am. J. Pathol. 2007;171:1395–1404. doi: 10.2353/ajpath.2007.070190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feng L, et al. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia. 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Goepfert C, et al. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 161.Wink MR, et al. Thyroid hormone upregulates ecto-5'-nucleotidase/CD73 in C6 rat glioma cells. Mol. Cell. Endocrinol. 2003;205:107–114. doi: 10.1016/s0303-7207(03)00197-7. [DOI] [PubMed] [Google Scholar]
- 162.Mandapathil M, et al. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin. Cancer. Res. 2009;15:6348–6357. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dzhandzhugazyan KN, et al. Ecto-ATP diphosphohydrolase/CD39 is overexpressed in differentiated human melanomas. FEBS Lett. 1998;430:227–230. doi: 10.1016/s0014-5793(98)00603-6. [DOI] [PubMed] [Google Scholar]
- 164.Kondo T, et al. Expression of CD73 and its ecto-5'-nucleotidase activity are elevated in papillary thyroid carcinomas. Histopathology. 2006;48:612–614. doi: 10.1111/j.1365-2559.2005.02277.x. [DOI] [PubMed] [Google Scholar]
- 165.Buffon A, et al. NTPDase and 5' ecto-nucleotidase expression profiles and the pattern of extracellular ATP metabolism in the Walker 256 tumor. Biochim. Biophys. Acta. 2007;1770:1259–1265. doi: 10.1016/j.bbagen.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 166.Zhi X, et al. Potential prognostic biomarker CD73 regulates epidermal growth factor receptor expression in human breast cancer. IUBMB Life. 2012;64:911–920. doi: 10.1002/iub.1086. [DOI] [PubMed] [Google Scholar]
- 167.Künzli BM, et al. Impact of CD39 and purinergic signalling on the growth and metastasis of colorectal cancer. Purinergic Signal. 2011;7:231–241. doi: 10.1007/s11302-011-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu XR, et al. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J. Surg. Oncol. 2012;106:130–137. doi: 10.1002/jso.23056. [DOI] [PubMed] [Google Scholar]
- 169.Stella J, et al. Differential ectonucleotidase expression in human bladder cancer cell lines. Urol. Oncol. 2010;28:260–267. doi: 10.1016/j.urolonc.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 170.Häusler SF, et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer. Immunol. Immunother. 2011;60:1405–1418. doi: 10.1007/s00262-011-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stagg J, et al. CD73-deficient mice are resistant to carcinogenesis. Cancer. Res. 2012;72:2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 172.Pulte D, et al. CD39 activity correlates with stage and inhibits platelet reactivity in chronic lymphocytic leukemia. J. Transl. Med. 2007;5:23. doi: 10.1186/1479-5876-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Perry C, et al. Increased CD39 expression on CD4(+) T lymphocytes has clinical and prognostic significance in chronic lymphocytic leukemia. Ann. Hematol. 2012;91:1271–1279. doi: 10.1007/s00277-012-1425-2. [DOI] [PubMed] [Google Scholar]
- 174.Hilchey SP, et al. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J. Immunol. 2009;183:6157–6166. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]