Abstract
We conducted a meta-analysis of randomized, placebo-controlled trials to determine the efficacy of antipsychotic and alpha-2 agonists in the treatment of chronic tic disorders and examine moderators of treatment effect. Meta-analysis demonstrated a significant benefit of antipsychotics compared to placebo (standardized mean difference (SMD)= 0.58 (95% confidence interval (CI): 0.36–0.80). Stratified subgroup analysis found no significant difference in the efficacy of the 4 antipsychotic agents tested (risperidone, pimozide, haloperidol and ziprasidone). Meta-analysis also demonstrated a benefit of alpha-2 agonists compared to placebo (SMD = 0.31 (95% confidence interval CI: 0.15–0.48). Stratified subgroup analysis and meta-regression demonstrated a significant moderating effect of co-occurring ADHD. Trials which enrolled subjects with tics and ADHD demonstrated a medium-to-large effect (SMD=0.68 (95%CI: 0.36–1.01) whereas trials that excluded subjects with ADHD demonstrated a small, non-significant benefit (SMD=0.15 (95%CI: −0.06–0.36). Our findings demonstrated significant benefit of both antipsychotics and alpha-2 agonists in treating tics but suggest alpha-2 agonists may have minimal benefit in tic patients without ADHD.
Keywords: Antipsychotic Agents, Alpha-2 Adrenergic Agonist, Tic Disorders, Attention-Deficit/Hyperactivity Disorder, Meta-Analysis
INTRODUCTION
Tourette Syndrome (TS) is a neurodevelopmental tic disorder characterized by the presence of both motor and vocal (phonic) tics for at least a year in duration.1 Tic symptoms typically have an onset around the age 5 or 6 years and reach their worst-ever severity around 10–12 years of age.2 Approximately one half to two thirds of adolescents with TS will have a decrease in tic severity by early adulthood.2, 3 For the rest of these adolescents, the persistence of tics into adult life may have detrimental effects on quality of life. Some tics may be self-injurious, while others, such as coprolalia, may be disruptive in the social environment.4
Antipsychotics are generally recognized by experts as the most effective pharmacological treatment for tics.5–8 Two antipsychotic medications, haloperidol and pimozide, are the only two FDA approved treatments for tics, although they are not currently recommended as the first-line pharmacotherapy because of their adverse side-effect profile. Possible side effects of antipsychotics include weight gain, sedation and cognitive blunting, parkinsonism, dyskinesia, and akathisia.7
Although generally recognized as not as effective as antipsychotic medications, alpha-2 agonists including clonidine and guanfacine are often used as the first-line pharmacological treatment for tics because of their more benign safety profile.6, 7, 9–11 As written by the Tourette Syndrome Medical Advisory Board, “For tics of moderate or greater severity, guanfacine or clonidine may be considered as the first line given the favorable safety margin of these medications.”7 Guanfacine although widely utilized for the treatment of tics in the United States, is not available in many European countries. Alpha-2 agonists also have the advantage of being effective in the treatment of ADHD in patients with and without tics.12, 13 In clinically-ascertained samples, more than half of children with TS also have ADHD.14 Historically, using clonidine as the prototype, the alpha-2 agonists were presumed to exert therapeutic befits by turning down arousal resulting in more optimal regulation of norepinephrine subcortical and cortical circuits.15 Accumulated evidence from animal studies suggest that the alpha-2 agonists enhance the functional connectivity of prefrontal cortical networks through stimulation of post-synaptic alpha-2A receptors on the dendritic spines of prefrontal cortical pyramidal cells.15, 16 This mechanism, which may apply more specifically to guanfacine than clonidine, implies that alpha-2 agonists may increase the effectiveness of the frontal cortex in regulating attention and suppressing tics.15 Although alpha-2 agonists are commonly used as first-line treatment for children with tics, research to date has not rigorously examined the efficacy of these medications in treating tics in children with and without ADHD.
Several influential professional organizations, the American Academy of Child and Adolescent Psychiatry (AACAP), the Canadian Academy of Child and Adolescent Psychiatry (CACAP) and the European Society for the Study of Tourette Syndrome (ESSTS) have recently or are currently developing guidelines in the pharmacological treatment of TS.11, 17 These treatment guidelines have or are currently considering making treatment recommendations between and within these classes of pharmacological agents. Therefore quantitative meta-analysis summarizing the current evidence of efficacy of different anti-tic medications is timely. Previous systematic reviews in the area have been narrow in scope (confined to atypical or typical antipsychotics separately), and have not performed quantitative synthesis of data from available trials.17, 18
The purpose of this meta-analysis is to compare existing randomized, controlled trials of alpha-2 agonists and antipsychotics to determine their efficacy in treating tic disorders. In meta-analysis of trials involving antipsychotic agents, our goal was to determine the average effect size (compared to placebo) of antipsychotics as a class and determine if there was any evidence that individual antipsychotic agents differ in efficacy. We also conducted stratified subgroup analysis and meta-regression to determine if dose and duration of antipsychotic treatment or trial methodological quality influenced the estimated efficacy of antipsychotics. In meta-analysis of trials involving alpha-2 agonists, we sought to determine the average effect size (compared to placebo) of alpha-2 agonists as a class and examine moderators of treatment effect. We hypothesized that alpha-2 agonists would be significantly more efficacious in treating tics of patients accompanied by ADHD compared to those without ADHD.
METHODS
Search Strategy
Two reviewers (HW and MHB) searched PubMED (1965-October 2011) (for relevant trials using the search strategy ("Antipsychotic Agents" [Pharmacological Action] OR "Antipsychotic Agents" [Mesh]) AND tic disorders) to locate trials of antipsychotic agents and (“Adrenergic alpha-2 Receptor agonists [Pharmacological Action" AND "Tic Disorders"[Mesh]) to locate trials of alpha-2 agonist medications. The results of the search were further limited to randomized control trials. The references of eligible trials as well as any appropriate review articles in this area were additionally searched for citations of further relevant published and unpublished research. There were no language limitations on our search strategy.
Criteria for Inclusion of Studies in this Review
Studies were included in this meta-analysis if they were randomized, controlled trials examining the efficacy of FDA-approved antipsychotic agent medications or alpha-2 adrenergic agonists medications for treating tic disorders. Trials that compared alpha-2 agonists or antipsychotic agents to each other, placebo or other medications in the same class (e.g. a head-to-head comparison of two different antipsychotic agents) were included in this meta-analysis. Trials were considered randomized when investigators explicitly represented them as such in the methods section of their published manuscript. Both crossover and parallel group trials were included in this review. Trials in which other psychoactive substances were started at the same time as antipsychotics or alpha-2 agonists were excluded. Discontinuation trials were also excluded.
Meta-Analytic Methods
Data extraction was performed on specially designed Microsoft Excel spreadsheets. Data were collected on methods, participants, intervention and outcome measurements, and other relevant attributes and results of the studies. Any missing information was requested from the study investigators when possible. The outcome measure selected from each included trial was difference in tic severity rating between the medication and placebo group at endpoint. For antipsychotic trials, active medication was compared to other antipsychotics (in all cases pimozide) or alpha-2 agonists (in all cases clonidine). Preferred rating scales for rating of tic severity (in order of preference) were the Yale Global Tic Severity Scale (total tic score or global severity score), Tourette Syndrome Global Scale, Shapiro Tourette Syndrome Severity Scale, Hopkins Motor/Vocal Tic Severity Scale.19–22 If none of these scales were available we would then use any rating scale that specifically measured tic severity. A hierarchy of preferred tic rating scale for our primary outcome was established a priori (as opposed to using the tic rating scale identified as primary by the trial investigator) in order to avoid any possible inflation of treatment effects caused by possible reporting bias via selection of measures that showed the greatest efficacy.
Standard mean difference (SMD) was chosen as the summary statistic for meta-analysis and calculated by pooling the standardized mean difference using Comprehensive Meta-Analysis Version 2.23 SMD was favored over weighted mean difference because the tic severity rating scales differed across studies. A fixed effects model was chosen for meta-analysis because this method is favored for testing subgroup differences in stratified meta-analysis. Publication bias was assessed by plotting the effect size against standard error for each trial (funnel plot).24 Publication bias was also statistically tested by using the Egger’s test and also testing the association between sample size and effect size in meta-regression. Heterogeneity of treatment response was assessed visually from the forest plot of standardized mean differences and relative risk of individual studies. Statistical estimates of heterogeneity were also assessed using the I-square heterogeneity statistic in Comprehensive Meta-Analysis.23 We conducted a sensitivity analysis to examine our decision to use a fixed-effects rather than random effects model for meta-analysis.
For alpha-2 agonist trials we also examined the moderating effects of ADHD. We conducted a subgroup analysis by stratifying trials based on comorbid ADHD status of subjects (some trials excluded comorbid ADHD, some trials only included subjects with comorbid ADHD). For the alpha-2 agonist trials only we also conducted a meta-regression where the association between the proportion of subjects with comorbid ADHD and effect size was examined. We tested for subgroup differences in Comprehensive Meta-Analysis by examining whether subgroups significantly reduced overall heterogeneity.25 Meta-regression was performed in Comprehensive Meta-Analysis. Trials were weighted using the generic inverse variance method. Effect size (SMD) of trials was the dependent variable with the variables of interest being entered as moderating variables. We used meta-regression techniques to examine the association between measured efficacy in trials and naturally continuous variables such as (1) trial duration, (2) trial methodological quality and (3) proportion of sample with Tourette syndrome (as opposed to transient or chronic tic disorders). Overall methodological quality of trials was assessed using the Jadad Scale.26, 27 We did not examine proportion of subjects with Tourette syndrome as a moderating variable in antipsychotic trials as greater than 95% of participants in these trials had a diagnosis of TS.
RESULTS
Antipsychotic Agents
Selection of Studies
Our PubMED search identified 29 manuscripts that were potentially eligible for inclusion in this meta-analysis. Figure 1 is a flow diagram that depicts our selection procedure. A total of 10 trials were included in this review. Five trials compared antipsychotics to placebo.28–32 Five trials (including 2 that were also placebo controlled) were head-to-head comparisons of antipsychotic medications.31–35 Two trials compared an antipsychotic medication to an alpha-2 agonist medication.36 Table 1 depicts the characteristics of included trials. Trials compared 4 different antipsychotic medications to placebo – haloperidol, pimozide, risperidone and ziprasidone. Trials compared 2 different antipsychotic agents to pimozide – haloperidol and risperidone. We identified no controlled trials that measured the efficacy of several common antipsychotics used to treat tics. These medications included fluphenazine, aripiprazole and quetiapine. One crossover trial comparing haloperidol to pimozide was excluded because it relied on unvalidated measures of tic counts rather than rating scales.37 One trial that compared olanzapine to pimozide was excluded because it included 4 subjects.34 We also excluded one large trial that compared aripiprazole to tiapride because tiapride is not an FDA approved medication in the United States. Because we believed this trial was informative and important although outside the scope of our planned systematic review we included trial data in the discussion section.38
Figure 1. Selection of Studies for Meta-analysis of Antipsychotic Agents in the Treatment of Tics.
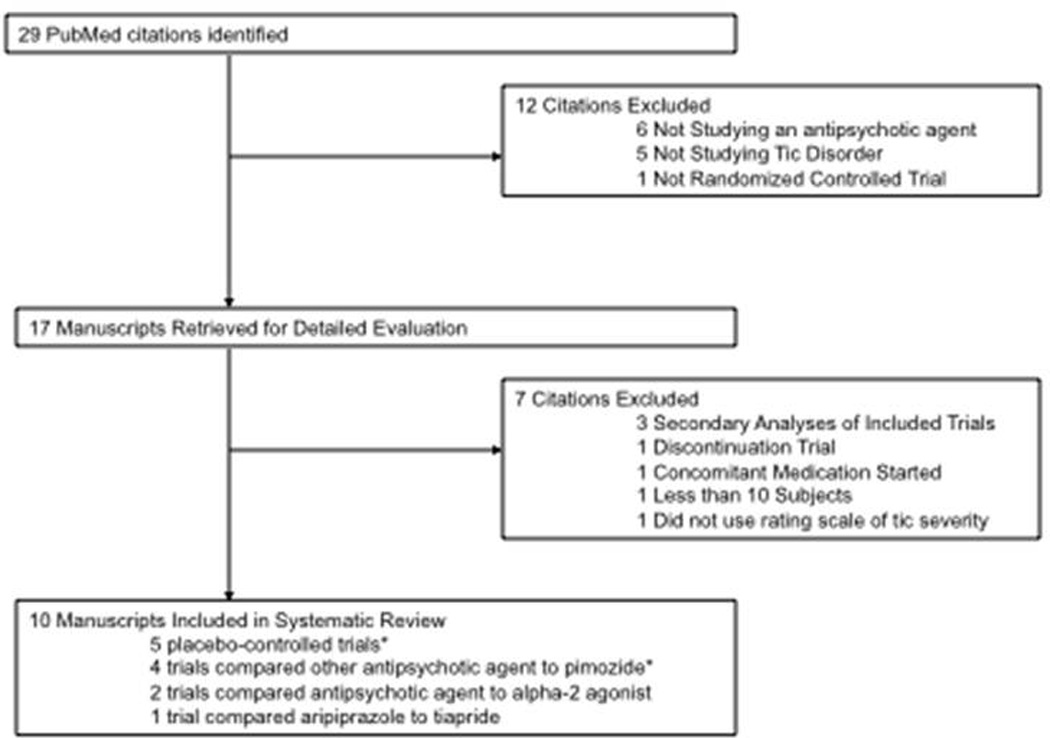
Flow diagram depicting reasons for exclusion of several trials identified in our search but not included in meta-analysis.
Table 1.
Randomized, Controlled Trials of Antipsychotic Agents for the Treatment of Tics
| Author | Year | Medication | Average Dose (mg) |
Design | Duration (in weeks) |
N | % TS | Age Range (in years) |
JADAD |
|---|---|---|---|---|---|---|---|---|---|
| Placebo-Controlled Trials | |||||||||
| Shapiro* | 1989 | Haloperidol | 10.6 | Parallel group | 6 | 57 | 100 | 8–46 | 3 |
| Pimozide | 4.5 | ||||||||
| Sallee* | 1997 | Haloperidol | 3.5 | Crossover | 6 | 22 | 100 | 7–16 | 3 |
| Pimozide | 3.4 | ||||||||
| Sallee | 2000 | Ziprasadone | 28.2 | Parallel group | 8 | 24 | 96 | 7–17 | 4 |
| Dion | 2002 | Risperidone | 2.5 | Parallel group | 8 | 48 | 100 | 14–49 | 4 |
| Scahill | 2003 | Risperidone | 2.5 | Parallel group | 8 | 26 | 100 | 6–62 | 5 |
| Head-to-Head Comparison of Antipsychotic Agents | |||||||||
| Bruggeman | 2001 | Risperidone | 3.8 | Parallel group | 7 | 41 | 100 | 11–65 | 4 |
| Pimozide | 2.9 | ||||||||
| Gilbert | 2004 | Risperidone | 2.5 | Crossover | 4 | 13 | 84 | 7–17 | 5 |
| Pimozide | 2.4 | ||||||||
| Comparison of Antipsychotic Agents to Alpha-2 Agonists | |||||||||
| Gaffney | 2002 | Risperidone | 1.5 | Parallel group | 8 | 20 | 100 | 7–17 | 4 |
| Clonidine | 0.175 | ||||||||
| Kang | 2009 | Haloperidol | 1.2 | Parallel group | 4 | 119 | 100 | 5–17 | 2 |
| Clonidine Patch | 1.5 | ||||||||
Antipsychotic efficiency
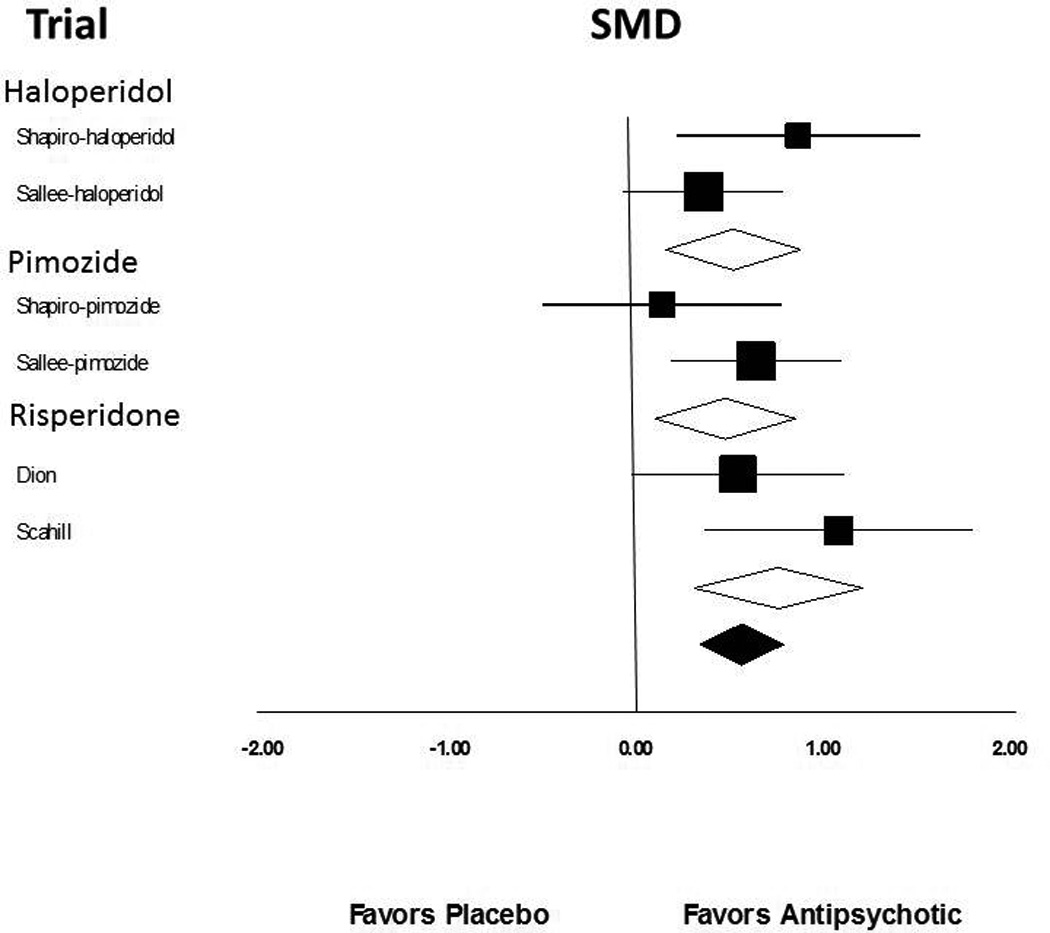
Meta-analysis demonstrated a significant, medium-to-large treatment effect of antipsychotic agents in improving tic symptoms compared to placebo (standardized mean difference (SMD)= 0.58 (95% confidence interval (CI): 0.36–0.80), z=5.27, p<0.0001). There was also a no significant heterogeneity between trials (Q=5.65, df=6, p=0.46, I2=0%). Figure 2 depicts a forest plot demonstrating the efficacy of antipsychotics compared to placebo. Visual inspection of the funnel plot and the Egger’s test did not demonstrate any evidence of publication bias (t=1.18, df=5, p=0.29). Sensitivity analysis using a random-effects rather than fixed effects model demonstrated similar efficacy of antipsychotics (SMD=0.58 (95%CI: 0.36–0.80), z=5.26, p<0.0001).
Figure 2. Efficacy of Antipsychotic Agents Compared to Placebo for the Treatment of Tics.
Meta-analysis demonstrated a significant, medium-to-large treatment effect of antipsychotic agents in improving tic symptoms compared to placebo (standardized mean difference (SMD)= 0.61 (95% confidence interval (CI): 0.36–0.86), z=4.80, p=0.00001). There was also a no significant heterogeneity between trials (Q=4.51, df=6, p=0.61, I2=0%).
Differential Efficacy of Antipsychotic Agents
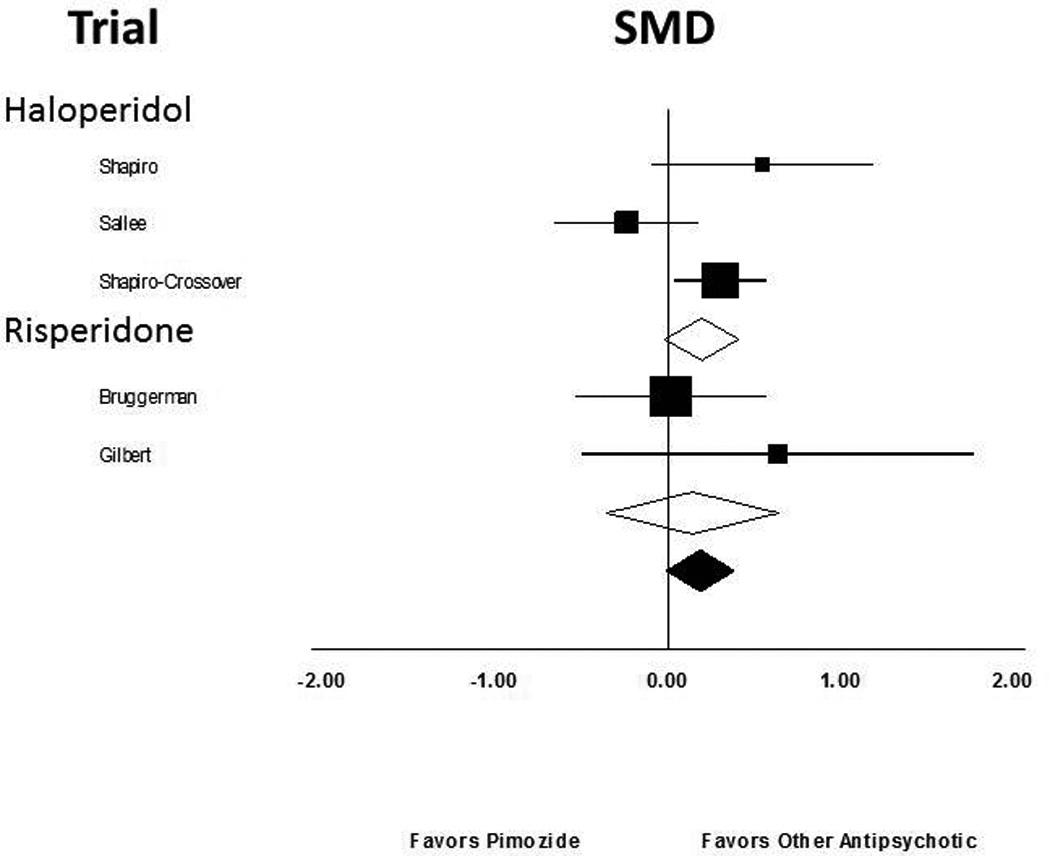
Stratified subgroup analysis comparing the efficacy of different antipsychotic agents to placebo provided no evidence of differences in comparative efficacy (test for subgroup differences α2 =1.2, df=3, p=0.75). The effect sizes of different antipsychotic agents compared to placebo were haloperidol (SMD= 0.52 (95% CI: 0.16–0.88)), pimozide, (SMD=0.48 (95% CI: =0.04–1.04)), risperidone (SMD= 0.76 (95% CI: 0.31–1.21)), and ziprasidone (SMD= 0.76 (95% CI: −0.02–1.53)). The differences in measured effect sizes between antipsychotic agents were not statistically significant but were consistent with random variation.
Five trials compared the efficacy of FDA approved antipsychotic agents to pimozide. Meta-analysis demonstrated no evidence that other antipsychotics differed in efficacy from pimozide. Stratified subgroup analysis demonstrated no evidence of differences in comparative efficacy compared to pimozide (Test for subgroup differences α2 =0.04, df=1, p=0.85).
Trial Duration
In exploratory analysis, meta-regression demonstrated no significant difference between trial duration and reported efficacy of antipsychotic medications compared to placebo (β=0.130 (95% CI: −0.104–0.363), t=1.09, p=0.28).
Antipsychotic Dose (in chlorpromazine equivalents)
Meta-regression demonstrated no significant difference between the average dose of antipsychotic medications in chlorpromazine equivalents and reported effect size (β=0.01 (95% CI: −0.02–0.04), t=−0.63, p=0.53).
Age
Meta-regression demonstrated no association between participant age in trials and efficacy of antipsychotic medications compared to placebo (β=−0.004 (95% CI: −0.024–0.033), t=0.31, p=0.75).
Trial Methodological Quality
Meta-regression demonstrated no association between trial methodological quality as measured by the Jadad scale and antipsychotic effect size compared to placebo (β=0.24 (95% CI: −0.09–0.58), t=1.4, p=0.16).
Alpha-2 Agonists
Selection of Studies
Our PubMED search identified 12 manuscripts that were potentially eligible for inclusion in this meta-analysis. Figure 1 is a flow diagram that depicts our selection procedure. Six trials involving 631 participants were eligible for inclusion. Table 2 depicts the characteristics of included trials. Four eligible trials examined the efficacy of clonidine39–42 and two trials examined the efficacy of guanfacine.43, 44 Four trials demonstrated a significant beneficial effect of alpha-2 agonists compared to placebo,39–41, 44 whereas two trials did not.42, 43 For one trial we report week 3 as endpoint rather than the week 4, which was reported in the trial. By design, this trial withdrew a substantial proportion of study subjects at week 3 who did not demonstrate a beneficial response.40
Table 2.
Randomized, Controlled Trials of Alpha-2 Agonists for the Treatment of Tics
| Author | Year | Medication | Design | Duration (in weeks) |
N | Percent with TS |
Age Range (in years) |
Primary Efficacy Scale | Method of Analysis | JADAD |
|---|---|---|---|---|---|---|---|---|---|---|
| Leckman | 1991 | Clonidine | Parallel | 12 | 40 | 100% | 7–48 | TSGS | Completers | 4 |
| Singer | 1995 | Clonidine | Crossover | 6 | 34 | 100% | 7–14 | Parent Linear Anologue | Completers | 4 |
| Scahill | 2001 | Guanfacine | Parallel | 8 | 34 | 59% | 7–14 | YGTSS | ITT | 4 |
| Tourette's Syndrome Study Group | 2002 | Clonidine | Parallel | 16 | 65 | 89% | 7–14 | YGTSS | ITT | 5 |
| Cummings | 2002 | Guanfacine | Parallel | 4 | 24 | 96% | 6–16 | YGTSS | Completers | 4 |
| Du | 2008 | Clonidine | Parallel | 3 | 427 | 55% | 6–18 | YGTSS | ITT | 4 |
Alpha-2 Agonist Efficacy
Meta-analysis demonstrated a significant benefit of alpha-2 agonists in the treatment of tic symptoms compared to placebo (standardized mean difference (SMD)=0.31 (95% confidence interval (CI): 0.15–0.48), z=3.64, p<0.001). Figure 2 depicts a forest plot demonstrating the efficacy of alpha-2 agonists compared to placebo. There was modest, although not statistically significant, amount of heterogeneity between trials (Q=7.85, df=5, p=0.17, I2 =36%). Visual inspection of the funnel plot and the meta-regression of the association between effect size and sample size demonstrated significant evidence of publication bias (α =−0.0012 (95%CI: −0.0021–(−0.0003)), z=−2.65, p=0.008). The Egger’s test (p=0.06) was equivocal. Figure 3 depicts a funnel plot suggesting possible publication bias in the literature. When publication bias was adjusted for using the Duval and Tweedie trim-and-fill method, alpha-2 agonists still demonstrated a modest, but significant, benefit compared to placebo (SMD=0.18 (95%CI: 0.03–0.33)). Sensitivity analysis also demonstrated a significant benefit of alpha-2 agonists when a random-effects (SMD=0.43 (95%CI: 0.17–0.69), z=3.22, p=0.001) rather than a fixed-effects model for meta-analysis was used.
Figure 3. Efficacy of Other Antipsychotic Agents Compared to Pimozide for the Treatment of Tics.
Five trials compared the efficacy of FDA approved antipsychotic agents to pimozide. Meta-analysis demonstrated no evidence that other antipsychotics differed in efficacy from pimozide.
Impact of ADHD
Stratified subgroup analysis demonstrated that trials requiring the presence of ADHD demonstrated a significantly greater effect of alpha-2 agonists in reducing tics than trials that excluded subjects with ADHD (α 2=7.27, df=1, p=0.007). Trials that enrolled subjects with tics and ADHD demonstrated a medium-to-large effect of alpha-2 agonists in reducing tics (SMD=0.68 (95%CI: 0.36–1.01), z=4.10, p<0.001). Trials that excluded subjects with ADHD demonstrated a small, non-significant benefit (SMD=0.15 (95%CI: −0.06–0.36), z=1.40, p=0.16). The one trial that enrolled subjects with and without ADHD demonstrated an intermediate effect of alpha-2 agonists in reducing tic symptoms (SMD=0.43 (95%CI: −0.18–1.04)).
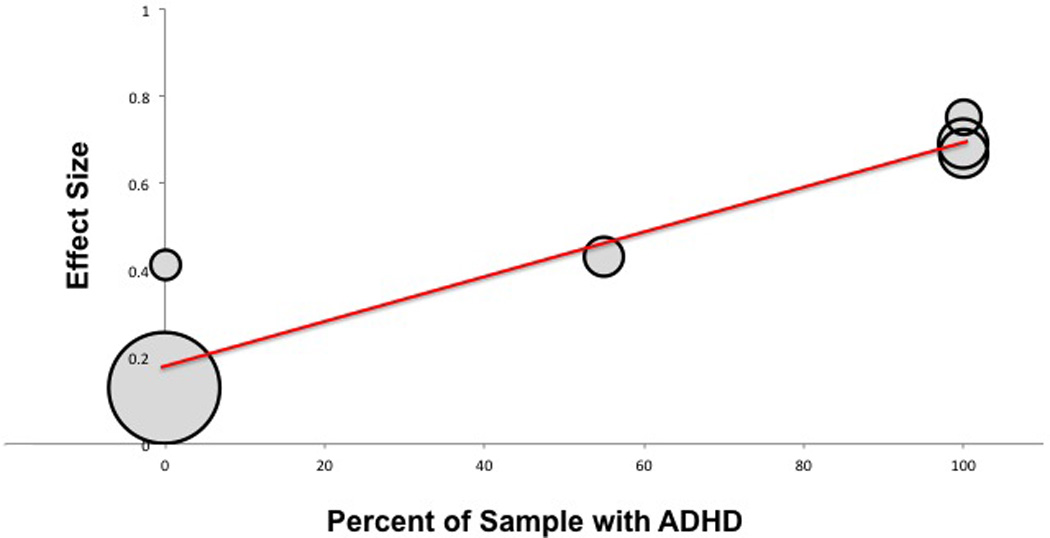
Meta-regression also demonstrated a significant moderating effect of ADHD on the efficacy of alpha-2 agonists in treating tics. Trials enrolling greater proportion of subjects with ADHD reported greater efficacy of alpha-2 agonists in the treatment of tics (α =0.0053 (95% CI: 0.0015–0.0091), z=−2.72, p=0.006). Figure 7B plots the proportion of subjects with ADHD in trials versus reported effect size.
Figure 7.
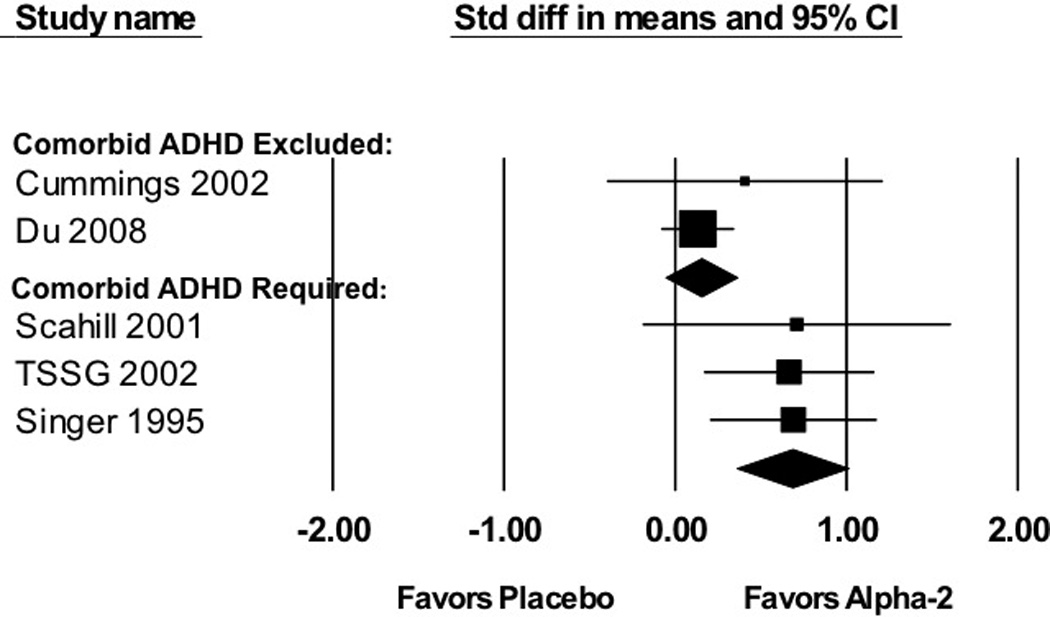
A: Efficacy of Alpha2-Agonists for the Treatment of Tics in Trials Stratified by ADHD Comorbidity. Trials that required tic patients to have comorbid ADHD (SMD=0.68 (95%CI: 0.36–1.01), z=4.10, p<0.001) demonstrated a significantly greater effect (Test for subgroup differences α2=7.27, df=1, p=0.007) of alpha-2 agonists in reducing tic symptoms than trials that excluded subjects with comorbid ADHD (SMD=0.15 (95%CI: −0.06–0.36), z=1.40, p=0.16).
B: Meta-Regression of Alpha-2 Agonist Efficacy in Treating Tics versus Percent of Subjects with Comorbid ADHD in Trial. Meta-regression demonstrated that trials enrolling a larger proportion of subjects with comorbid ADHD reported a greater efficacy of alpha-2 agonists in treating tics. (α=0.0053 (95%CI: 0.0015–0.0091), z=−2.72, p=0.006).
Proportion of Subjects with Tourette Syndrome
Meta-regression demonstrated a significant association between proportion of subjects with Tourette syndrome and reported efficacy of alpha-2 agonists in the treatment of tic symptoms (α =0.010 (95% CI: 0.002–0.019), z=−2.36, p=0.018).
Age
Meta-regression demonstrated no significant relationship between baseline age of subjects and reported efficacy of alpha-2 agonists (α =0.027 (95% CI: −0.088–0.143), z=0.47, p=0.64).
Medication Type
Stratified subgroup analysis demonstrated no significant difference between the reported efficacy of clonidine and guanfacine (α 2=0.60, df=1, p=0.44). Clonidine (SMD=0.29 (95%CI: 0.12–0.47), z=3.28, p=0.001) and guanfacine (SMD=0.54 (95%CI: − 0.06–1.14), z=1.76, p=0.08) demonstrated similar efficacy in trials, perhaps because of the limited number of guanfacine trials.
Trial Duration
In exploratory analysis, meta-regression demonstrated a significant association between trial duration and reported efficacy of alpha-2 agonist medications (α =−0.041 (95%CI: 0.003–0.079), z=2.11, p=0.035). Longer duration trials reported greater effects of alpha-2 agonist medications in improving tics.
Trial Methodological Quality
Meta-regression demonstrated no association between trial methodological quality as measured by the Jadad scale and trial effect size (α =0.40 (95%CI: −0.13–0.93), z=1.46, p=0.14).
Comparative Efficacy of Antipsychotic Agents versus Alpha-2 Agonists
Two trials examined the efficacy of antipsychotics compared to alpha-2 agonists (clonidine). One trial examined the efficacy of an antipsychotic agent (risperidone) to an alpha-2 agonist (clonidine).36 This 8-week parallel trial of 21 adults/children demonstrated no significant difference between treatments. Clonidine performed non-significantly better in improving tic symptoms than risperidone.36 The other was a larger, randomized but unblinded trial of 119 children randomized to clonidine adhesive patch or haloperidol for 4 weeks.45 This study demonstrated a significant benefit of the clonidine adhesive patch compared to haloperidol but was not blinded and involved a shorter duration of treatment then is standard in antipsychotic efficacy trials.
DISCUSSION
Meta-analysis of 5 randomized, placebo-controlled trials demonstrated a significant benefit of antipsychotics in the treatment of tic disorders. Meta-analysis demonstrated a medium effect size (SMD=0.58 (95% CI: 0.36–0.80) of antipsychotics compared to placebo with no evidence of publication bias or significant heterogeneity between trials. We found no evidence of differential efficacy of different antipsychotic agents based on comparison of their effect sizes in both placebo-controlled and pimozide-controlled trials. Although adverse effect profiles may be different across the various antipsychotic medications, it does not appear that any antipsychotic agent is more effective in reducing tics than any other antipsychotic agent. Similarly, a randomized, controlled trial of 65 children with TS that compared aripiprazole to tiapride demonstrated no significant difference between agents after 12 weeks of treatment (SMD=0.04 (95%CI: −0.45–0.53, z=0.2, p=0.87, favoring aripiprazole).38 This trial was not included in this systematic review because tiapride is not an FDA approved medication in the United States. The medium treatment effect of antipsychotics is favorable compared to alpha-2 agonist medications in the treatment of tics (SMD=0.31 (95%CI: 0.15–0.48). Despite the positive effects on tics, however, the adverse effect profile of the antipsychotic medications also warrant consideration. Given the potential adverse effects of antipsychotic medications such as acute dystonia, weight gain, cardiometabolic consequences and tardive dyskinesia, it is difficult to recommend this medication class as a first-line treatment for any child with TS despite their considerable evidence of efficacy. The only blinded head-to-head trial that compared an antipsychotic to an alpha-2 agonist showed no difference between the two classes of agents. The small sample size and uncertain aim of the trial limits any interpretation of this finding of no difference.33
Meta-analysis of six randomized, placebo-controlled trials demonstrated a modest but significant benefit of alpha-2 agonists in the treatment of children with chronic tics. Stratified subgroup analysis and meta-regression demonstrated that alpha-2 agonists had a medium-to-large effect (ES=0.68) in reducing tic symptoms in trials in which participants also had ADHD. In the absence of ADHD, however, the efficacy of these agents was small (ES=0.15) and non-significant. This finding calls into question existing treatment guidelines for TS that recommend alpha-2 agonists as first-line pharmacological treatment of tics.6, 7, 9 10, 11 Despite the clear trend in our findings, however, the available studies for review were few in number and sample sizes were small. Thus, firm conclusions cannot be drawn due the less than adequate state of current evidence.
Other treatment options for the treatment of tics include cognitive behavioral intervention based on habit reversal training.46 Habit Reversal Training (HRT) and closely related inventions such as Comprehensive Behavioral Intervention for Tics (CBIT) is recommended as a first-line intervention for youth with chronic tics in recently published guidelines.11, 17 In a multi-site randomized trial, CBIT showed superiority to supportive treatment in 126 children with moderate or greater tic severity. The effect size of (ES=0.68) is slightly lower than the effect of antipsychotic medications.25,27 However, direct comparison of treatment effects between pharmacological and behavioral treatments is hindered by the fact that participants are not blinded in behavioral intervention trials and the control condition may not be equivalent to placebo. Future trials may examine whether the presence of ADHD has a negative impact on CBIT.47 If children with tics and ADHD are less likely to make use of CBIT, it would be the converse of the findings in the current meta-analysis.
Alpha-2 agonists function by stimulating post-synaptic alpha-2A receptors on dendritic spines of the prefrontal cortical pyramidal cells, and by increasing the functional connectivity of the prefrontal cortical networks.15 The brain regions that are activated during tic suppression belong to a neural circuit that participates in the inhibition of unwanted impulses.48 Cognitive control of the prefrontal cortex may be important in controlling tic severity and in the pathogenesis of TS.49 An fMRI study of adults with TS demonstrated that tic suppression is associated with activation of the frontal cortex. Magnitude of frontal activation during tic suppression in this study was associated with increased activity in the caudate and decreased activity in the putamen, globus pallidus and thalamus.50 The magnitude of activity in the caudate (increased) or putamen, globus pallidus and thalamus (decreased) was significantly associated with severity of tics in the preceding month. Thus alpha-2 agonists may enhance prefrontal corticostriatal circuits and contribute to tic suppression. Children with ADHD may have delayed maturation of the frontal cortex.51 The proposed enhancement of prefrontal function by the alpha 2 agonists may explain why this class of medications is more successful in reducing tics in children with co-existing ADHD. These neurobiological clues raise intriguing questions about the mechanism of behavioral interventions and the common practice of tic suppression in patients with TS.52–54
There are several limitations of our meta-analysis that warrant mention. Our meta-analysis had a small number of trials with large differences in sample sizes, which limited our power to detect the effects of moderators and publication bias. This lack of power may be particularly important when comparing the effect sizes of clonidine and guanfacine (or between antipsychotic agents). Some researchers have hypothesized that clonidine and guanfacine may have different side effect profiles and efficacy for treating ADHD and tics based on differences in affinity for subtypes of alpha-2 adrenergic receptors.15 This meta-analysis is underpowered to detect even fairly large differences in efficacy in treating tics between these two agents. Head-to-head trials may be used to compare efficacy and adverse effects between two medications. However, results of active comparator trials are often ambiguous.55 Unfortunately, the finding of no difference does not prove no difference. Moreover, investigators often fail to articulate whether the head-to-head trial is a test of superiority, equivalence or non-inferiority. In the absence of a clearly stated purpose, the results are difficult to interpret – as in the case of the active comparator trials reviewed here. Similar problems relate to the comparison of different antipsychotic agents as well. Meta-analysis is also not the best tool to examine the impact of moderating variables because potential moderating variables are often correlated within trials. For example, the trials in our analysis that contained smaller samples and had longer durations also tended to include patients with ADHD. It is difficult to disentangle the effects of publication bias, ADHD, initial disease severity and trial duration separately in this context. We also intended to examine the effects of medication on motor and vocal tics separately but were unable to do so because these data were not uniformly reported across trials. A related issue is that some of the trials included in the review did not specify tic severity as the primary outcome. Although three trials clearly focused on ADHD as the primary outcome and tics severity was generally mild in these trials,41, 42, 44 other trials were less clear about the primary outcome.43 Larger randomized trials with tic severity as the primary treatment target are needed to improve the precision of the measured effects of the alpha-2 agonists on tics. Additional studies will also be needed in order to draw any firm conclusions about the relative benefits of these agents in adults with TS accompanied by ADHD given that only one study included adult subjects.
Our meta-analysis suggests that antipsychotic agents have the greatest demonstrated effect of reducing tics in randomized, placebo-controlled trials. Our meta-analysis and our comparison of treatments available for patients with tics concentrated solely on efficacy. Treatment decisions for patients with tics must be based on considering both the risks and benefits of any intervention. Antipsychotics agents have a considerably worse side-effect profile compared to alpha-2 agonist medications and behavioral therapy. Alpha-2 agonists demonstrated similar or slightly larger benefit in reducing tics but only among subjects with comorbid ADHD. Alpha-2 agonists demonstrated minimal benefits in reducing tics in subjects without ADHD. This meta-analysis highlights the need for further research into effective treatments for tics and to quantify how well commonly used existing treatments work. Clinical trials that examine agents with novel mechanisms of action are urgently needed. Candidate mechanisms of action that are of particular interest in TS include histaminergic agents56, 57, dopamine 1 receptor antagonists such as ecopipam, glutamatergic compounds58 and endocannabinoids.59 Treatment guidelines for TS currently recommend the use of antipsychotic agents such as aripiprazole and fluphenazine, which have not been tested in double-blind, placebo-controlled trials. Similarly, guidelines recommend alpha-2 agonists as the first-line pharmacological treatment for tics in most patients despite the meager evidence. This recommendation appears to be driven by concern about the use of antipsychotic medications in TS.
Figure 4. Selection of Trials for Alpha-2 Agonists for the Treatment of Tics.
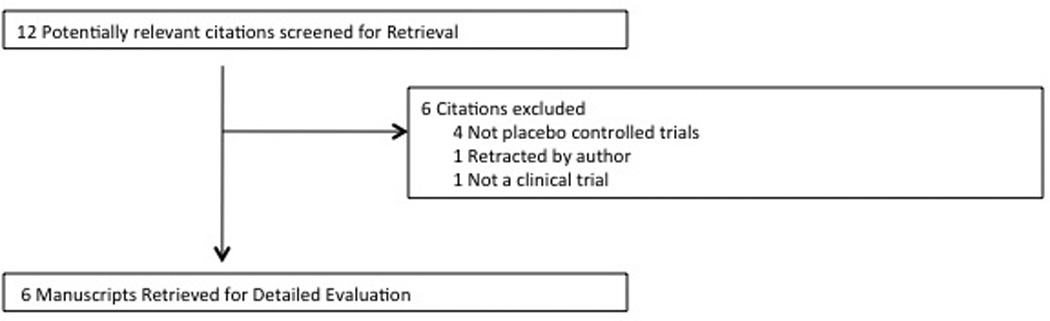
A flow diagram depicting reasons for exclusion of 6 articles identified by our search strategy. One trial was excluded because the journal editorial staff retracted the article after there was evidence that the trial and its data was plagiarized from an earlier included trial.
Figure 5. Alpha-2 Agonists for the Treatment of Tics.
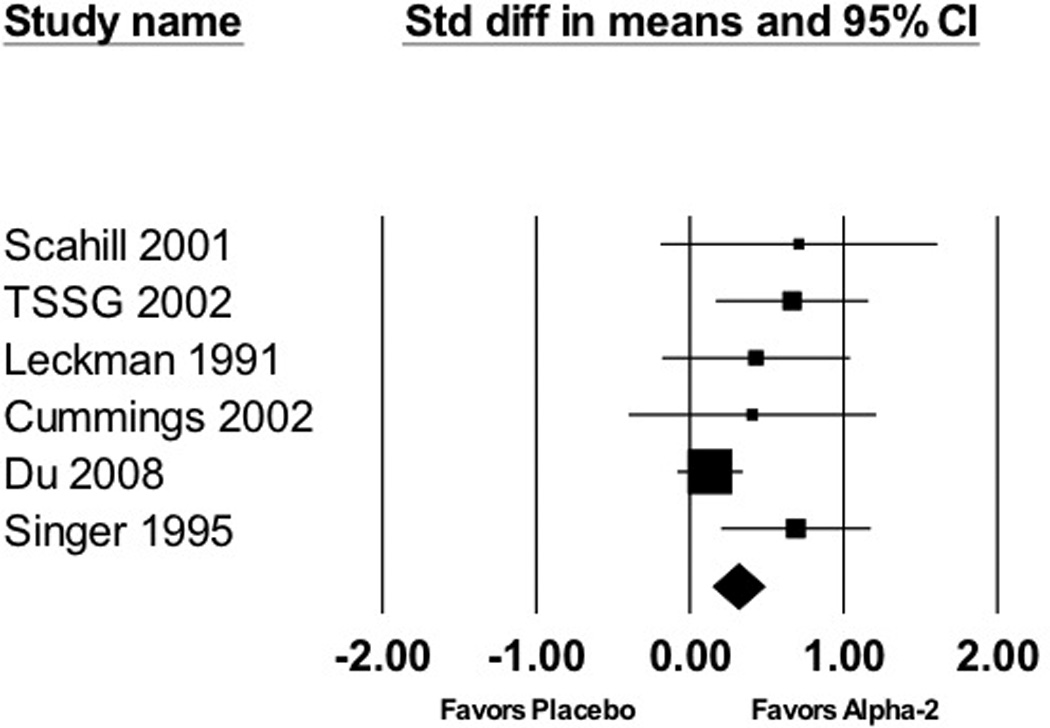
Meta-analysis of 6 trials demonstrated a significant effect of alpha-2 agonists in reducing tic severity (standardized mean difference (SMD)=0.31 (95% confidence interval (CI): 0.15–0.48), z=3.64, p<0.001).
Figure 6. Funnel Plot Depicting Publication Bias in Alpha-2 Agonist Trials in the Treatment of Tics.
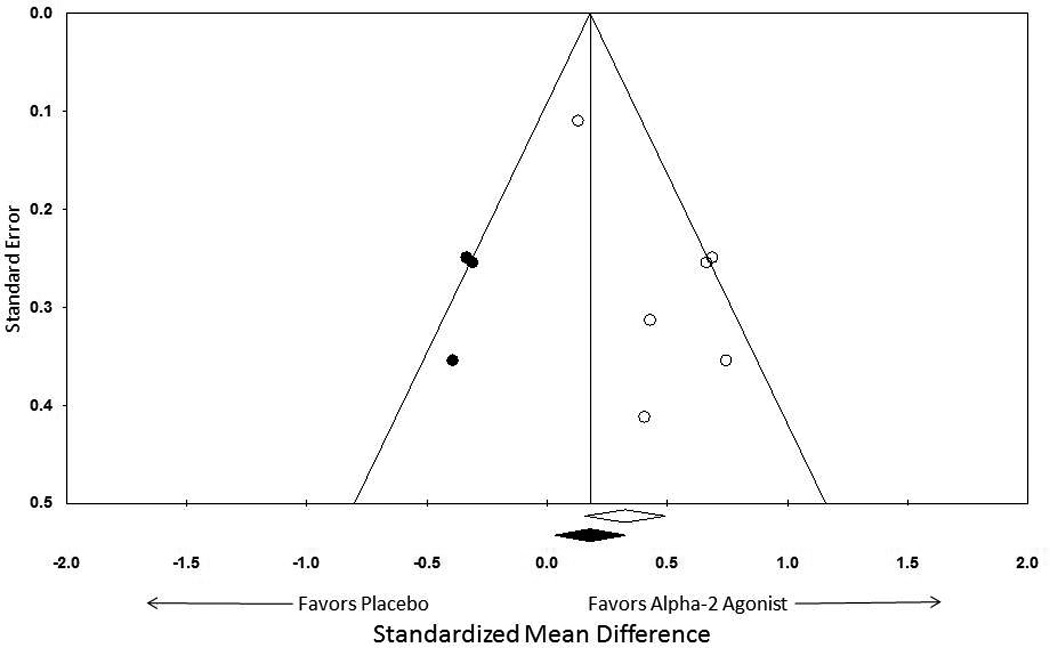
Visual inspection of the funnel plot and the meta-regression of the association between effect size and sample size demonstrated significant evidence of publication bias (α=−0.0012 (95%CI: −0.0021–(−0.0003)), z=−2.65, p=0.008). When publication bias was adjusted for using the Duval and Tweedie trim-and-fill method, alpha-2 agonists still demonstrated a significant benefit compared to placebo (SMD=0.18 (95%CI: 0.03–0.33)).
Highlights.
-
!
Both antipsychotics and alpha-2 agonists have efficacy in reducing tic symptoms when compared to placebo.
-
!
There is no evidence from controlled trials suggesting any particular antipsychotic agent is more effective than any other for the treatment of tic disorders.
-
!
Alpha-2 Agonist agents appear particularly effective in alleviating tic symptoms in children with comorbid ADHD.
-
!
There is limited evidence to demonstrate alpha-2 agonist agents are effective in reducing tic symptoms in children without comorbid ADHD
Acknowledgements
The authors acknowledge the National Institutes of Health grants (K23MH091240 (MHB), U10MH66764 (LS); R01 MH083707 (LS)), the APIRE/Eli Lilly Psychiatric Research Fellowship (MHB), the AACAP/ Eli Lilly Pilot Research Award (MHB), the Trichotillomania Learning Center (MHB), NARSAD (MHB), Investigator-initiated award from Shire Pharmaceuticals (LS), and UL1 RR024139 (MHB, LS) from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research (MHB).
This study was funded in part by the National Institutes of Health (K23MH091240 and UL1 RR024139) and AACAP/Eli Lilly Pilot Research Award.
Lawrence Scahill has received research funding from the National Institutes of Health and the Tourette Syndrome Association; he has served as a consultant for Boehringer-Ingelheim, NeuroSearch, Pfizer, Roche, and BioMarin. Lawrence Scahill has received research support from Roche, Pfizer and Shire.
James Leckman has received research funding from the National Institutes of Health, Tourette Syndrome Association, Grifols (formerly Talecris Biotherapeutics) and C8Sciences, Klingenstein Third Generation Foundation (medical student fellowship program). James F. Leckman has received book royalties from John Wiley and Sons, McGraw Hill, Oxford University Press.
Michael Bloch has received funding from the National Institutes of Health the APIRE/Eli Lilly Psychiatric Research Fellowship (MHB), the AACAP/ Eli Lilly Pilot Research Award (MHB), the Trichotillomania Learning Center (MHB), NARSAD (MHB), Investigator-initiated award from Shire Pharmaceuticals (LS), and UL1 RR024139 (MHB, LS) from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research (MHB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Hannah Weisman and Imraan A. Qureshi have no disclosures.
Author Contributions:
Hannah Weisman, Imraan Qureshi and Michael H. Bloch were involved in all aspects of this manuscript (design, analysis and interpretation of the data and drafting/revising this manuscript). Michael H. Bloch takes primary responsibility for all statistical analyses.
James F. Leckman and Lawrence Scahill were involved in the interpretation of data and revising of manuscript.
References
- 1.DSM-IV-TR. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. Washington D.C.: American Psychiatric Association; 2000. [Google Scholar]
- 2.Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102(1 Pt 1):14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Zhang H, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65–69. doi: 10.1001/archpedi.160.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erenberg G, Cruse RP, Rothner AD. The natural history of Tourette syndrome: a follow-up study. Ann Neurol. 1987;22(3):383–385. doi: 10.1002/ana.410220317. [DOI] [PubMed] [Google Scholar]
- 5.Waldon K, Hill J, Termine C, Balottin U, Cavanna AE. Trials of pharmacological interventions for Tourette Syndrome: A systematic review. Behav Neurol. 2012 doi: 10.3233/BEN-2012-120269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer HS. Treatment of tics and tourette syndrome. Curr Treat Options Neurol. 2010;12(6):539–561. doi: 10.1007/s11940-010-0095-4. [DOI] [PubMed] [Google Scholar]
- 7.Scahill L, Erenberg G, Berlin CM, Jr, Budman C, Coffey BJ, Jankovic J, et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3(2):192–206. doi: 10.1016/j.nurx.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roessner V, Schoenefeld K, Buse J, Bender S, Ehrlich S, Munchau A. Pharmacological treatment of tic disorders and Tourette Syndrome. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Bloch MH. Emerging treatments for Tourette's disorder. Curr Psychiatry Rep. 2008;10(4):323–330. doi: 10.1007/s11920-008-0052-z. [DOI] [PubMed] [Google Scholar]
- 10.Swain JE, Scahill L, Lombroso PJ, King RA, Leckman JF. Tourette syndrome and tic disorders: a decade of progress. J Am Acad Child Adolesc Psychiatry. 2007;46(8):947–968. doi: 10.1097/chi.0b013e318068fbcc. [DOI] [PubMed] [Google Scholar]
- 11.Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry. 2011;20(4):173–196. doi: 10.1007/s00787-011-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884–893. doi: 10.1097/CHI.0b013e3181b26e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attentiondeficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(12):1551–1559. doi: 10.1097/00004583-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Khalifa N, von Knorring AL. Psychopathology in a Swedish population of school children with tic disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(11):1346–1353. doi: 10.1097/01.chi.0000251210.98749.83. [DOI] [PubMed] [Google Scholar]
- 15.Arnsten AF. The use of alpha-2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2010;10(10):1595–1605. doi: 10.1586/ern.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129(2):397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, et al. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry. 2012;57(3):133–143. doi: 10.1177/070674371205700302. [DOI] [PubMed] [Google Scholar]
- 18.Pringsheim T, Marras C. Pimozide for tics in Tourette's syndrome. Cochrane Database Syst Rev. 2009;(2):CD006996. doi: 10.1002/14651858.CD006996.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Harcherik DF, Leckman JF, Detlor J, Cohen DJ. A new instrument for clinical studies of Tourette's syndrome. J Am Acad Child Psychiatry. 1984;23(2):153–160. doi: 10.1097/00004583-198403000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro AK, Shapiro ES, Young JG. Signs, symptoms and clincal course. In: Shapiro AK, Shapiro ES, Young JG, editors. Gilles de la Tourette Syndrome. 2nd ed. New York: 1988. pp. 127–193. [Google Scholar]
- 22.Walkup JT, Rosenberg LA, Brown J, Singer HS. The validity of instruments measuring tic severity in Tourette's syndrome. J Am Acad Child Adolesc Psychiatry. 1992;31(3):472–477. doi: 10.1097/00004583-199205000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Comprehensive Meta-Analysis. 2 ed. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deeks J, Higgins J, Altman D. Cochrane Reviewers' Handbook 4.2.1. John Wiley & Sons, Ltd; 2003. [Google Scholar]
- 26.Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16(1):62–73. doi: 10.1016/0197-2456(94)00031-w. [DOI] [PubMed] [Google Scholar]
- 27.Moncrieff J, Churchill R, Drummond C, McGuire H. Development of a quality assessment instrument for trials of treatments for depression and neurosis. International Journal of Methods in Psychiatric Research. 2001;10:126–133. [Google Scholar]
- 28.Scahill L, Leckman JF, Schultz RT, Katsovich L, Peterson BS. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60(7):1130–1135. doi: 10.1212/01.wnl.0000055434.39968.67. [DOI] [PubMed] [Google Scholar]
- 29.Dion Y, Annable L, Sandor P, Chouinard G. Risperidone in the treatment of tourette syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2002;22(1):31–39. doi: 10.1097/00004714-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Sallee FR, Kurlan R, Goetz CG, Singer H, Scahill L, Law G, et al. Ziprasidone treatment of children and adolescents with Tourette's syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292–299. doi: 10.1097/00004583-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Sallee FR, Nesbitt L, Jackson C, Sine L, Sethuraman G. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette's disorder. Am J Psychiatry. 1997;154(8):1057–1062. doi: 10.1176/ajp.154.8.1057. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro E, Shapiro AK, Fulop G, Hubbard M, Mandeli J, Nordlie J, et al. Controlled study of haloperidol, pimozide and placebo for the treatment of Gilles de la Tourette's syndrome. Arch Gen Psychiatry. 1989;46(8):722–730. doi: 10.1001/archpsyc.1989.01810080052006. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert DL, Batterson JR, Sethuraman G, Sallee FR. Tic reduction with risperidone versus pimozide in a randomized, double-blind, crossover trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(2):206–214. doi: 10.1097/00004583-200402000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Onofrj M, Paci C, D'Andreamatteo G, Toma L. Olanzapine in severe Gilles de la Tourette syndrome: a 52-week double-blind cross-over study vs. low-dose pimozide. J Neurol. 2000;247(6):443–446. doi: 10.1007/s004150070173. [DOI] [PubMed] [Google Scholar]
- 35.Bruggeman R, van der Linden C, Buitelaar JK, Gericke GS, Hawkridge SM, Temlett JA. Risperidone versus pimozide in Tourette's disorder: a comparative double-blind parallel-group study. J Clin Psychiatry. 2001;62(1):50–56. doi: 10.4088/jcp.v62n0111. [DOI] [PubMed] [Google Scholar]
- 36.Gaffney GR, Perry PJ, Lund BC, Bever-Stille KA, Arndt S, Kuperman S. Risperidone versus clonidine in the treatment of children and adolescents with Tourette's syndrome. J Am Acad Child Adolesc Psychiatry. 2002;41(3):330–336. doi: 10.1097/00004583-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Ross MS, Moldofsky H. A comparison of pimozide and haloperidol in the treatment of Gilles de la Tourette's syndrome. Am J Psychiatry. 1978;135(5):585–587. doi: 10.1176/ajp.135.5.585. [DOI] [PubMed] [Google Scholar]
- 38.Liu YY, Chen YH, Chen H, Liu ZS. [A control study of aripiprazole and tiapride treatment for tic disorders in children] Zhongguo Dang Dai Er Ke Za Zhi. 2010;12(6):421–424. [PubMed] [Google Scholar]
- 39.Leckman JF, Hardin MT, Riddle MA, Stevenson J, Ort SI, Cohen DJ. Clonidine treatment of Gilles de la Tourette's syndrome. Arch Gen Psychiatry. 1991;48(4):324–328. doi: 10.1001/archpsyc.1991.01810280040006. [DOI] [PubMed] [Google Scholar]
- 40.Du YS, Li HF, Vance A, Zhong YQ, Jiao FY, Wang HM, et al. Randomized double-blind multicentre placebo-controlled clinical trial of the clonidine adhesive patch for the treatment of tic disorders. Aust N Z J Psychiatry. 2008;42(9):807–813. doi: 10.1080/00048670802277222. [DOI] [PubMed] [Google Scholar]
- 41.Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58(4):527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- 42.Singer HS, Brown J, Quaskey S, Rosenberg LA, Mellits ED, Denckla MB. The treatment of attention-deficit hyperactivity disorder in Tourette's syndrome: a double-blind placebo-controlled study with clonidine and desipramine. Pediatrics. 1995;95(1):74–81. [PubMed] [Google Scholar]
- 43.Cummings DD, Singer HS, Krieger M, Miller TL, Mahone EM. Neuropsychiatric effects of guanfacine in children with mild tourette syndrome: a pilot study. Clin Neuropharmacol. 2002;25(6):325–332. doi: 10.1097/00002826-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158(7):1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- 45.Kang H, Zhang YF, Jiao FY, Guo XY, Gao XM. [Efficacy of clonidine transdermal patch for treatment of Tourette's syndrome in children] Zhongguo Dang Dai Er Ke Za Zhi. 2009;11(7):537–59. [PubMed] [Google Scholar]
- 46.Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA. 2010;303(19):1929–1937. doi: 10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyon GJ, Samar SM, Conelea C, Trujillo MR, Lipinski CM, Bauer CC, et al. Testing tic suppression: comparing the effects of dexmethylphenidate to no medication in children and adolescents with attention-deficit/hyperactivity disorder and Tourette's disorder. J Child Adolesc Psychopharmacol. 2010;20(4):283–289. doi: 10.1089/cap.2010.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13(7):244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 49.Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166(6):664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55(4):326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 51.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attentiondeficit/ hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verdellen CW, Hoogduin CA, Kato BS, Keijsers GP, Cath DC, Hoijtink HB. Habituation of premonitory sensations during exposure and response prevention treatment in Tourette's syndrome. Behav Modif. 2008;32(2):215–227. doi: 10.1177/0145445507309020. [DOI] [PubMed] [Google Scholar]
- 53.Verdellen CW, Hoogduin CA, Keijsers GP. Tic suppression in the treatment of Tourette's syndrome with exposure therapy: the rebound phenomenon reconsidered. Mov Disord. 2007;22(11):1601–1606. doi: 10.1002/mds.21577. [DOI] [PubMed] [Google Scholar]
- 54.Verdellen CW, Keijsers GP, Cath DC, Hoogduin CA. Exposure with response prevention versus habit reversal in Tourettes's syndrome: a controlled study. Behav Res Ther. 2004;42(5):501–511. doi: 10.1016/S0005-7967(03)00154-2. [DOI] [PubMed] [Google Scholar]
- 55.Leon AC. Comparative Effectiveness Clinical Trials in Psychiatry: Superiority, Noninferiority and the Role of Active Comparators. J Clin Psychiatry. 2011;72:1344–1349. doi: 10.4088/JCP.10m06089whi. [DOI] [PubMed] [Google Scholar]
- 56.Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O'Roak BJ, Mason CE, et al. L-histidine decarboxylase and Tourette's syndrome. N Engl J Med. 2010;362(20):1901–1908. doi: 10.1056/NEJMoa0907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandez TV, Sanders SJ, Yurkiewicz IR, Ercan-Sencicek AG, Kim YS, Fishman DO, et al. Rare copy number variants in tourette syndrome disrupt genes in histaminergic pathways and overlap with autism. Biol Psychiatry. 2012;71(5):392–402. doi: 10.1016/j.biopsych.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singer HS, Morris C, Grados M. Glutamatergic modulatory therapy for Tourette syndrome. Med Hypotheses. 2010;74(5):862–867. doi: 10.1016/j.mehy.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 59.Muller-Vahl KR, Schneider U, Prevedel H, Theloe K, Kolbe H, Daldrup T, et al. Delta 9- tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. J Clin Psychiatry. 2003;64(4):459–465. doi: 10.4088/jcp.v64n0417. [DOI] [PubMed] [Google Scholar]


