Abstract
Objective
Modulation of the long-latency reflex (LLR) is important for sensorimotor control during interaction with different mechanical loads. Transcortical pathways usually contribute to LLR modulation, but the integrity of pathways projecting to the paretic and non-paretic arms of stroke survivors is compromised. We hypothesize that disruption of transcortical reflex pathways reduces the capacity for stroke survivors to appropriately regulate the LLR bilaterally.
Methods
Elbow perturbations were applied to the paretic and non-paretic arms of persons with stroke, and the dominant arm of age-matched controls as subjects interacted with Stiff or Compliant environments rendered by a linear actuator. Reflexes were quantified using surface electromyograms, recorded from biceps.
Results
LLR amplitude was significantly larger during interaction with the Compliant load compared to the Stiff load in controls. However, there was no significant change in LLR amplitude for the paretic or non-paretic arm of stroke survivors.
Conclusions
Modulation of the LLR is altered in the paretic and non-paretic arms after stroke.
Significance
Our results are indicative of bilateral sensorimotor impairments following stroke. The inability to regulate the LLR may contribute to bilateral deficits in tasks that require precise control of limb mechanics and stability.
Keywords: stretch reflex, modulation, stroke, brain, arm, long-latency
Introduction
Cortical stroke disrupts descending motor commands and often interferes with one’s ability to stabilize arm postures for various functional tasks such as holding a cup of coffee, driving a car, or walking with a cane. While a healthy motor system is capable of controlling limb muscles in a manner that accurately accounts for the mechanical properties of objects in our environment, this ability is often impaired following stroke. It is well established that the motor system employs flexible control strategies through the development of voluntary motor commands; however, it has recently been suggested that rapid involuntary mechanisms can also compensate for environmental instabilities through regulation of stretch reflex sensitivity (Akazawa et al., 1983, Dietz et al., 1994, Doemges and Rack, 1992, Perreault et al., 2008). In particular, long-latency stretch reflexes (LLRs) are modulated to compensate for instabilities in specific directions during the performance of a given task (Krutky et al., 2010). Compensation is achieved by increasing the sensitivity of LLRs in muscles acting to oppose movement in the direction of instability. A number of studies report increases in the amplitude of LLRs during interactions with compliant loads relative to the amplitude of LLRs during interactions with non-compliant, or stiff loads (Dietz et al., 1994, Doemges and Rack, 1992, Perreault et al., 2008). These results suggest that heightened excitability of LLRs may be important for the maintenance of limb stability when the environment does not provide that stability. Understanding how long-latency responses are regulated following stroke may provide additional insight into the neural pathways responsible for impaired motor function during tasks that require increased stability, and to the deficits that can be expected when those pathways are compromised.
Due to the latency and adaptability of the LLR in muscles of the human arm (e.g., biceps, 50–60 ms), it was suggested that these responses are mediated by a transcortical loop (Hammond, 1956). This proposal has since been corroborated by studies in both humans and primates (Cheney and Fetz, 1984, Evarts, 1973, Kimura et al., 2006, Matthews, 1991, Palmer and Ashby, 1992, Pruszynski et al., 2011). Given the involvement of supraspinal pathways, one might expect that cortical lesions could reduce or even eliminate the LLR. Indeed, this has been observed following stroke (Dietz et al., 1991, Hendrie and Lee, 1978, Marsden et al., 1977). Persons with stroke often exhibit delayed voluntary reaction times (Dickstein et al., 1993) and reflex activity some months after stroke (Marsden, Merton, Morton & Adam, 1977a), suggesting that the circuits responsible for generating the reflex may have been altered and lengthened during the recovery process, resulting in increased processing or transmission delays. Therefore, it also is possible that the LLR in the paretic arm might be delayed if volitional movement and LLRs are mediated through similar neural substrates. This would imply that recovery following stroke involves rerouting of motor and sensory pathways responsible for both volitional movement and postural stability. Our study aimed, in part, to determine if stroke survivors retain the capability for task-specific modulation during this later period, which would help elucidate the efficacy of this rerouting.
Hemispheric stroke often results in bilateral motor deficits in the upper extremity (Desrosiers et al., 1996, Jebsen et al., 1971, Sunderland, 2000). These deficits might be due in part to altered regulation of reflex pathways. There is evidence, for example, that the short latency stretch reflex (SLR) in elbow flexors of the ipsilesional arm is depressed in stroke survivors (Thilmann et al., 1990) and attenuation of the LLR is occasionally observed bilaterally (Marsden et al., 1977). Yet, whether or not stroke survivors retain the ability to modulate LLRs in the ipsilesional arm to account for different physical environments has yet to be determined. If modulation is impaired bilaterally, it could be argued that communication between bilateral brain structures is necessary for the proper regulation of the LLR and efficacy of rerouting after hemispheric stroke. Given the role of the LLR in compensating for environmental instability, impairments of stretch reflex regulation in the ipsilesional upper limb are also likely to have implications for stroke survivors’ ability to effectively interact with directionally unstable objects (e.g. single-point canes) during their daily activities.
The purpose of this study was to examine if stroke survivors retain the ability to modulate LLR amplitude in accordance with the level of mechanical stability provided during a postural task. Based on previous demonstrations of stretch reflex modulation in response to changes in the amount of stability provided by the external environment, we hypothesized that elbow perturbations applied in a stiff (stable) environment would elicit smaller long latency stretch reflexes in neurologically healthy individuals than the same perturbations applied in a compliant (less stable) environment. We also hypothesized that identical perturbations of the paretic arm of stroke survivors would elicit long latency reflexes in stiff and compliant environments not different in amplitude. While the flexibility of reflex control in the non-paretic arm is difficult to predict, motor pathways within and descending from the non-lesioned cerebral hemisphere remain intact following stroke, enabling cortical contributions to reflex control in the non-paretic arm to be preserved. We therefore hypothesized that perturbations of the non-paretic arm of stroke survivors would produce long latency reflexes of smaller amplitude in a stiff relative to a compliant environment. The results of this study have implications for understanding how the stabilizing role of feedback mechanisms is altered following stroke.
Methods
Study Population
Experiments were performed on 8 adults with chronic stroke and 8 healthy, age-matched control subjects (Table 1). Ethical approval for the study was received from the Northwestern University Institutional Review Board (IRB protocol STU00009204); informed consent and HIPAA authorization were obtained from subjects prior to their participation. Control subjects had no history of upper limb or neurological impairments. Stroke subjects underwent an evaluation by a licensed physical therapist to determine their eligibility. Stroke survivors were included if they had sustained a unilateral stroke as defined from chart review, had full passive range of motion of tested shoulder and elbow without pain or shoulder subluxation, some voluntary movement of elbow and shoulder, cortical injury resulting in motor deficits of the upper extremity, no receptive aphasia, and the ability to follow verbal and visual commands. All subjects with stroke had at least mild spasticity in elbow muscles as defined by a cumulative Ashworth score of greater than or equal to 1. Subjects were excluded if they had history of unilateral neglect (spatial and motor), inability to provide informed consent, and/or significant medical complications. We recorded Fugl-Meyer scores (Fugl-Meyer et al., 1975) as a reliable clinical measure of arm motor impairment (Duncan et al., 1983).
Table 1.
Subject Characteristics
| Subject | Age | Gender | Hand Dominance | Years since onset | Lesion Type | Lesion Location | Arm Impairment a | Spasticity F/E b |
|---|---|---|---|---|---|---|---|---|
| 01 | 61 | M | Right | 12 | L. Ischemic | Cortical | 20 | 3/2 |
| 02 | 57 | F | Right | 12 | R. Hemorrhagic | Cortical | 53 | 3/2 |
| 03 | 62 | M | Right | 6 | R. Ischemic | Cortical/Subcortical | 31 | 3/1 |
| 04 | 50 | F | Right | 8 | L. Ischemic | Cortical | 37 | 2/0 |
| 05 | 65 | M | Right | 8 | R. Ischemic | Cortical | 34 | 3/3 |
| 06 | 62 | F | Right | 5 | L. Ischemic | Cortical | 41 | 3/2 |
| 07 | 55 | F | Right | 2 | L. Ischemic | Cortical | 12 | 2/2 |
| 08 | 38 | M | Right | 3 | R. Ischemic | Cortical | 39 | 3/2 |
| 09 | 70 | F | Right | |||||
| 10 | 49 | M | Right | |||||
| 11 | 60 | M | Left | |||||
| 12 | 69 | F | Right | |||||
| 13 | 46 | M | Right | |||||
| 14 | 57 | F | Right | |||||
| 15 | 67 | M | Right | |||||
| 16 | 35 | M | Left |
Based on Fugl-Meyer scale (maximum score = 66)
Modified Ashworth score for the elbow (0 = normal function, 5 = severe spasticity)
F = flexion, E = extension
Shaded rows correspond to age-matched control subjects
Equipment
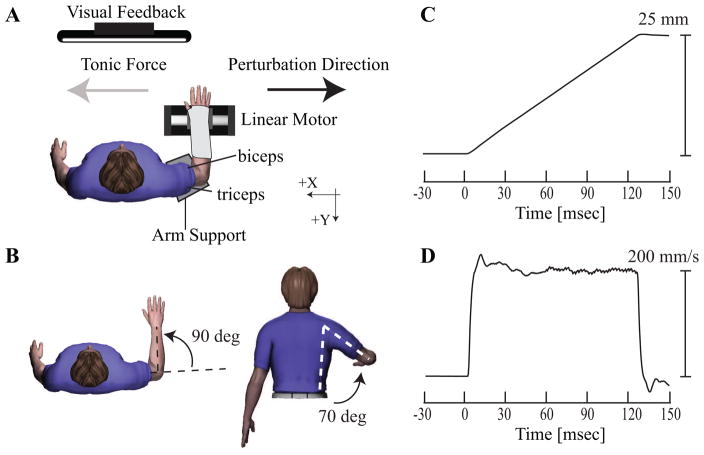
Subjects were seated comfortably with their trunk secured to an adjustable chair (Biodex, Shirley, NY) using padded chest and lap straps. The target arm (paretic or non-paretic arm of stroke subjects; dominant arm of control subjects) was positioned in the horizontal plane with the shoulder at 70 degrees of abduction and 0 degrees of flexion, the elbow joint at 90 degrees, and the forearm slightly pronated (Figure 1 A and B). The upper arm was placed in a height-adjustable trough support to ensure a constant position of the shoulder joint. A fitted orthotic splint extending from the fingers to the middle of the forearm was used to maintain the wrist joint in a neutral position and to attach the forearm to a linear actuator (Copley ThrustTube TB3806; Copley Controls, Canton, MA). A 10 cm steel plate located on the underside of the cast, centered at the wrist joint, was secured to the top surface of the actuator via a precision bearing that allowed rotation in the horizontal plane.
Figure 1.
Experimental protocol. (A) Subjects were seated at a distance of approximately 1 m from a visual display upon which tonic force (gray arrow) or position feedback was provided. As shown in B, subjects maintained arm posture of 70 degrees of shoulder elevation, 0 degrees of shoulder flexion, 90 degrees of elbow flexion, and slightly pronated forearm. (C) Randomly-timed ramp-and-hold perturbations delivered by the linear actuator moved the wrist 25 mm in 125ms along the x-axis (black arrow) with velocity profile shown in (D), thus extending the elbow joint and stretching the biceps brachii muscle.
The linear actuator was used to apply elbow extension perturbations in two different mechanical environments (Compliant and Stiff). The actuator was mounted on an adjustable aluminum frame and was oriented such that perturbations were applied in the horizontal plane in a direction orthogonal to the forearm. This resulted in flexion/extension motions at the elbow joint. The actuator was instrumented with a linear encoder (RGH24; Renishaw, Gloucestershire, UK) to provide position information (resolution 1μm) and was configured during the appropriate portions of the study as either a stiff position servo (250 kN/m) or a compliant load (10 N/m) using an admittance control algorithm implemented in Matlab xPC (The Mathworks Co, Natick, MA, USA). Perturbations were identically matched in each mechanical environment by transiently switching the actuator to a position servo at the start of each perturbation, as we have used in previous studies (Perreault et al., 2008, Shemmell et al., 2009). The manipulandum was controlled by custom software developed using Matlab xPC (The Mathworks Co, Natick, MA). Physical stops limited actuator motion to ±60 mm from the central position, and software limits were implemented to cut power to the motor 10 mm before these limits were reached.
We recorded surface electromyograms (EMGs) from the biceps brachii (biceps) and lateral head of triceps brachii (triceps) muscles. Standard skin preparation techniques were applied prior to the application of bipolar surface electrodes (model #272; Noraxon USA, Inc., Scottsdale, AZ). The resulting signals were amplified using a Bortec® AMT-16 system (Bortec Biomedical, Calgary, AB), which has an input impedance of 10GΩ, a Common Mode Rejection Ratio of 115dB at 60Hz, and a bandwidth between 10–1000Hz. Analog signals were anti-alias filtered using custom-built, differential input, 5th order Bessel filters with a cutoff frequency of 500Hz and then sampled at 2000Hz with an 18-bit data acquisition system (NI PCI-6289; National Instruments, Corp., Austin, TX). The delay introduced by the anti-aliasing filters was taken into account when computing reflex latencies.
Protocols
We hypothesized that stroke survivors have a reduced capacity to modulate the LLR during interaction with different mechanical environments. To test this hypothesis, we quantified the effects of changes in the mechanical properties of the environment on the amplitude of the LLR in the biceps muscle. Stroke subjects and age-matched control subjects interacted with either a Stiff or Compliant environment. Stroke subjects were tested using their paretic and non-paretic arms and age-matched control subjects were tested using their dominant arm. The order of arm testing in the Stroke subjects was randomized. In the Stiff environment, subjects were instructed to generate an endpoint force of 5 ± 1 Newtons (N). The mean maximum voluntary force produced by participants in the control group was 179.1 ± 23 N. This compares to 74.6 ± 8.8 N generated by the paretic arm of stroke survivors and 170.9 ± 14.9 N by the non-paretic arm. During the 5N task, the impaired arm of stroke subjects was therefore required to produce a greater percentage of maximum force (7.6 ± 1.1%) as compared to the control (3.1 ± 0.4%) and unimpaired (3.1 ± 0.2%) arms. Subjects were provided with a visual display of the target force range (4–6 N) during the Stiff condition. The instantaneous force being generated was displayed as a vertical bar on the screen while the target force level was represented as a horizontal bar that changed color when the target was achieved. During the Compliant condition, subjects were provided with a visual display of the endpoint position along with the target position range (2 cm) while overcoming an extension force bias of 4–6 N. The instantaneous position was displayed as a disk on the screen while the target position was represented as a circle; the disk changed color when the target was achieved.
Reflexes were elicited using a set of randomly timed ramp-and-hold perturbations applied to the arm. A common set of instructions was provided for all subjects; they were instructed to maintain a constant effort and not to react to the perturbation, similar to previous studies (Burgess et al., 1995, Crago et al., 1976, Levin and Feldman, 1994). Perturbations were applied to the hand in two directions corresponding to positive and negative directions along the axes. Each perturbation had duration of 125 ms and a velocity of 200 mm/s (Figure 1D), which was sufficient to elicit consistent short and long-latency reflexes in healthy subjects (Lewis et al., 2005). These parameters resulted in a perturbation amplitude of 25 mm (Figure 1C). The endpoint displacement resulted in maximal elbow joint displacement of approximately 5 deg. The corresponding elbow joint velocity was approximately 40 deg/sec for a typical subject. A ramp-and-hold position perturbation was elicited only after the subject maintained the required target force for 1 s. Trials were separated by random intervals ranging from 3 to 5 seconds. Subjects were required to rest for a minimum of 30 seconds between each trial, or longer as needed, to avoid fatigue. The task order was randomized to minimize order effects.
Data Processing and Analysis
All EMG recordings were rectified before further processing. We matched background (BGD) activity of the biceps between Stiff and Compliant conditions; a trial was excluded if the BGD activity for that trial exceeded 3 standard deviations (SD) above the mean BGD of the recorded trials, which resulted in an average of 15–20 (out of 20) trials per condition for each subject. The mean of the retained trials in each condition was calculated to determine the average EMG response. For each perturbation, a 30 ms window of EMG activity was averaged immediately prior to perturbation onset to quantify the average background muscle activation (BGD). The onset of the short-latency response (SLR) was determined as the first point at which the average EMG response exceeded 3 standard deviations (SD) of the background muscle activation for each subject based on data in each experiment (Krutky et al., 2010). The SLR was defined as a 30 ms window average of EMG activity following onset detection. The LLR were defined using 2 windows. The first window (LLR1) was defined as a 30 ms window average of EMG activity that immediately followed the SLR. Because a period of reduced activity was commonly observed following the short latency response in stroke survivors, a second 30 ms window of EMG activity (LLR2) also was averaged immediately after the LLR1. The unscaled SLR, LLR1, and LLR2 amplitudes were expressed as average voltage values above BGD activity.
We tested the null hypothesis that EMG amplitude was equivalent in the stiff and compliant environments. This hypothesis was tested separately for the BGD, SLR, LLR1, and LLR2 time periods using a linear mixed effects model with environment (ENV: stiff or compliant) and arm (ARM: paretic, non-paretic, or control) as fixed factors, and subject (SUBJ) as a random factor; the factor ARM was nested in SUBJ to account for the fact that the paretic and non-paretic arms were tested from each stroke subject. The model considered separate variances across each level of the ARM factor. Planned contrasts were used to make post hoc assessments for the influence of the environment on the EMG measured in each arm; all post hoc tests were corrected for multiple comparisons. These statistical computations were performed in R (Ihaka and Gentleman, 1996) using the nlme and multcomp packages. All statistical comparisons were considered significant at the p ≤ 0.05 level. Data are presented as averages ± 1 standard error.
Results
Reduced modulation of long-latency stretch reflexes following stroke
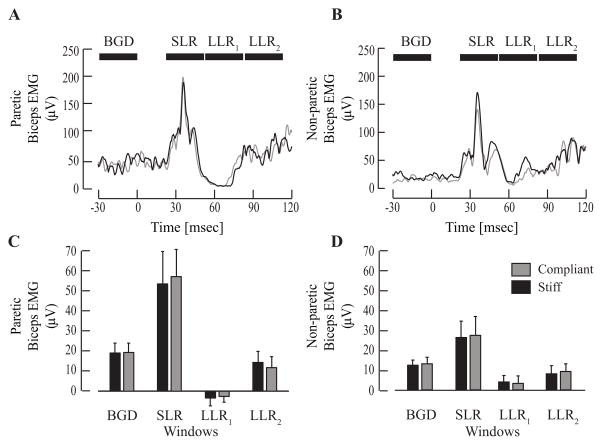
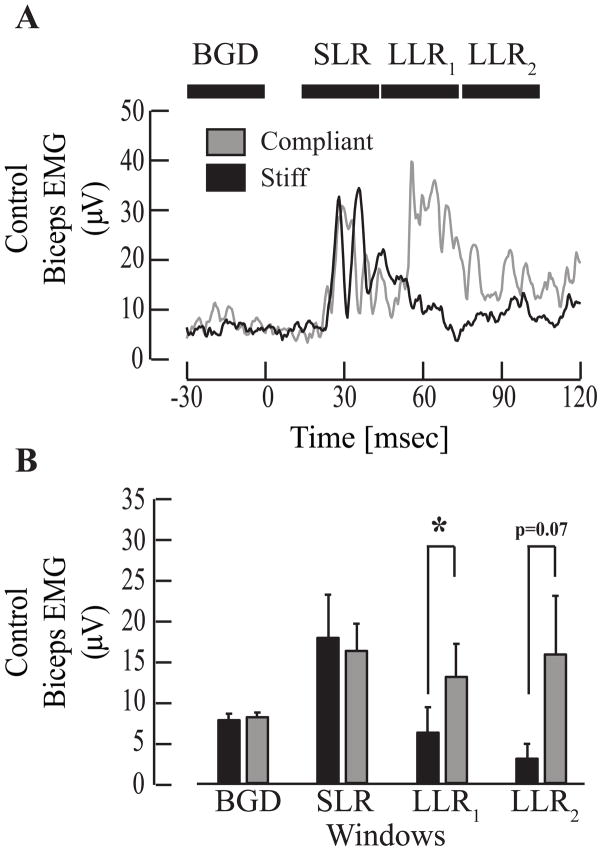
There was no environment-dependent modulation of the LLR in either the paretic or non-paretic limbs of the stroke subjects (Fig 2). This was in contrast to the results from the control subjects (Fig. 3). Considering all subjects, there was no significant effect of environment on the amplitude of the early portion of the LLR (LLR1, F1,21 = 2.5; p = 0.13), but there was a significant interaction between environment and the tested arm (F2,21 = 5.1; p = 0.02). Post hoc comparisons demonstrated that this interaction resulted from a significant modulation of the LLR1 between environments for control subjects (p=0.0016), but not for either limb of the stroke subjects (both p > 0.89). There were similar results for the LLR2 (ENV - F1,21 = 0.00002; p > 0.99; ENV:ARM – F2,21 = 5.3; p = 0.01), though none of the post hoc comparisons testing the influence of the environment for each arm reached significance (paretic: p = 0.13; non-paretic: p = 0.53; control: p = 0.07). The lack of significance for the control arm was due in part to the large variability of the data measured in the LLR2 time period (Fig. 3B). This time period is within the range often associated with the fastest voluntary reaction times for unimpaired subjects.
Figure 2.
Stroke subjects exhibited no modulation of long latency responses (LLR1 and LLR2) with changes in the environment. (A) Representative example of Biceps response in the paretic arm of a single stroke subject (Patient 3 in Table 1). (B) Example of biceps response in the non-paretic limb of the same stroke subject. Black horizontal bars indicate the EMG time periods used for subsequent analysis. (C) Comparison of average (± 1 standard error) SLR, LLR1, and LLR2 amplitudes in the paretic arm of all stroke subjects revealed no differences in reflex responses across conditions. Similarly, the average (± 1 standard error) LLRs in the non-paretic arm shown in (D). Significance was defined at the p < 0.05 level.
Figure 3.
Long latency responses in control subjects adapt to changes in the mechanical environment. (A) Representative examples of average (N=10 trials) biceps EMG activity for an age-matched control subject in the Stiff (black) and Compliant (gray) environments. Black horizontal bars correspond to time periods used to compute background electromyography activity (BGD) prior to perturbation onset (t=0) and 30 ms time windows for the short-latency response (SLR), first long-latency response (LLR1) and second long-latency response (LLR2). (B) Bar plots summarizing group averages (± 1 standard error) of biceps EMG activity corresponding to BGD, SLR, LLR1, and LLR2 time periods. Significance was defined at the p < 0.05 level (*).
No environment-dependent modulation of short latency stretch reflexes
There was no environment-dependent change in the SLR for either the stroke or control subjects, suggesting that the LLR changes observed in the control subjects did not result from environment-dependent changes in the afferent information coming from the spindle. Typical average EMG responses from the paretic, non-paretic, and control arms are shown in Figures 2A, 2B, and 3A, respectively. For the amplitude of the SLR, neither the effect of the environment (F1,21 = 3e–6; p ~ 1), nor the interaction between environment and the tested arm (F2,21 = 0.36; p = 0.70) was significant. Consistent with earlier findings (Pisano et al., 2000, Schmit et al., 2000, Simons and Bingel, 1971, Thilmann et al., 1990, Trumbower et al., 2010, Wolf et al., 1996), there was a tendency for the biceps SLR in both environments to be larger in the paretic limb, but the factor of arm did not reach significance in our data (F2,6 = 4.59; p = 0.06).
Background EMG activity of biceps and triceps did not influence reflex modulation
Reflex modulation was not influenced by differences in the voluntary activity of biceps or triceps muscles in the paretic, non-paretic and control arms. The BGD activity of the biceps was not significantly different across environments (F1,21 = 0.57, p = 0.46), and there was no significant interaction between environment and the tested arm (F2,21 = 0.04, p = 0.96); the same was true for the triceps muscle (ENV - F1,21 = 0.19, p = 0.67; ENV:ARM - F2,21 = 0.64, p = 0.54). There was a non-significant tendency for the background to change with arm (F2,6 = 3.33, p = 0.11). Hence, to rule out the possibility that changes in background across arms contributed to the observed changes in reflex modulation, we repeated our analyses using reflex EMGs normalized by the level of background EMG prior to perturbation onset. This additional analysis led to the same conclusions reported above.
Discussion
This study examined whether stroke survivors retain the ability to modulate the LLR in the biceps in accordance with the level of limb stability provided by the environment. We hypothesized that stroke survivors would have a reduced ability to facilitate long-latency reflexes in their paretic limb during interaction with compliant loads relative to able-bodied individuals. We also hypothesized that task-specific modulation of long-latency reflexes would be retained in the non-paretic limb of stroke survivors. When interacting with a compliant (less stable) mechanical environment, healthy subjects increased the amplitude of the LLR whereas this facilitation was absent in the paretic arm of stroke survivors, supporting our first hypothesis. Contrary to our hypothesis however, environment-dependent modulation of the LLR was also absent in the non-paretic arm. These results demonstrate a reduction in the capacity for environment-dependent modulation of the LLR1 in both arms of stroke survivors. This suggests that task-dependent regulation of the long-latency stretch reflex not only requires the contralateral motor cortex (Pruszynski et al., 2011, Shemmell et al., 2009), but may also require intact interhemispheric balance, which is compromised after hemispheric stroke (Murase et al., 2004, Shimizu et al., 2002) leading to possible disruption of transcortical reflex circuits in the non-injured hemisphere. These bilateral deficits in reflex function have implications for rehabilitation strategies designed to improve bilateral limb stability.
Unilateral stroke disrupts reflex modulation bilaterally
The absence of modulation of the LLR in the paretic and nonparetic limbs of stroke survivors provides support for the proposal that supraspinal structures are involved in the transmission of this response. Early observations of perturbation-induced muscle activity occurring at twice the latency of the monosynaptic reflex led many to suggest that these responses traversed a transcortical loop (Hammond, 1960, Lee and Tatton, 1975, Phillips, 1969). This proposal was corroborated by recordings from motor cortical cells in non-human primates that demonstrated rapid excitatory responses to unexpected perturbations (Cheney and Fetz, 1984, Evarts and Tanji, 1976). More recent evidence has also demonstrated that this perturbation-induced cortical activity is tuned to account for intersegmental dynamics (Pruszynski et al., 2011). In addition, transcranial magnetic stimulation (TMS) has been used to demonstrate that the motor cortex underlies the environment-dependent modulation of the LLR during postural tasks that require increased limb stability (Shemmell et al., 2009). Consistent with this evidence, our results indicate that lesion-related damage to the transcortical reflex pathway renders stroke survivors unable to regulate the LLR in the paretic arm according to the mechanical characteristics of the environment. This deficit is not necessarily exclusive to damage to motor cortex, but could also result from damage to any of the supraspinal networks involved in transmission of the LLR including ascending sensory fibers, primary sensory cortex, or descending motor projections.
Our data suggest that proper regulation of the LLR does not occur in stroke survivors with moderate to more severe motor impairments. No differences in amplitude between Stiff and Compliant environments were observed for either LLR1 or LLR2 responses for the paretic and nonparetic arm of our stroke subjects. These individuals are considered at least moderate in severity with average Fugl-Meyer scores of 33 ± 4 (Fugl-Meyer et al., 1975) and average Ashworth scores of 2 ± 1. A previous study found intact LLRs in a group of mostly mildly impaired stroke survivors with more than half having Brunnström scores > 5 and Ashworth scores of zero (Groenewegen et al., 2012). Dietz et al. (1991) also provided evidence that LLR amplitude was larger when subjects with cerebral damage had to maintain hand position while resisting a sinusoidal torque when compared to responses elicited when subjects maintained a target torque while performing a position tracking task. Although our subjects did not perform a tracking task, one could argue that their torque-targeting task is comparable to our Stiff environment while the former was comparable to our Compliant environment. Taken together, these observations point to post-stroke impairments of reflex gain control that might be associated with the amount of disruption to cortical motor circuits within the lesioned hemisphere. While the motor cortex has been implicated in transmission of the LLR (Shemmell et al., 2009), the precise locus of gain regulation of LLR has yet to be determined.
Role of interhemispheric cortical structures in transmission of the long-latency stretch reflex
In addition to impairment of reflex function in the paretic arm of stroke survivors, our results demonstrate that LLR modulation is impaired in the non-paretic upper limb. The lack of LLR modulation in either arm following monohemispheric stroke eliminates an important involuntary mechanism for responding to unexpected disturbances of posture (Krutky et al., 2010). In addition to abnormalities of involuntary reflex control, it has been reported that voluntary control of both the paretic and non-paretic arms of stroke survivors is subject to deficits of strength (Colebatch and Gandevia, 1989, Noskin et al., 2008) and coordination (Jones et al., 1989, Laufer et al., 2001, Wittenberg et al., 2000, Yarosh et al., 2004). Taken together, these findings suggest that proper motor function within a single limb is reliant on normal processing within both brain hemispheres.
The neural mechanisms responsible for these bilateral deficits are unknown, but may result from direct and indirect effects of lesion-related cortical damage on descending motor pathways. Some pyramidal tract neurons have ipsilateral projections (Davidoff, 1990), the total number of these projections being estimated to range from 10–15% of the total corticospinal tract (Nyberg-Hansen and Brodal, 1963). Damage caused directly to ipsilateral projections descending from the lesioned cortex to the non-paretic limbs may disrupt the transmission of reflex and voluntary motor commands. However, the extent to which ipsilateral corticospinal projections are involved in reflex transmission is unclear. Indirect, interhemispheric effects of lesion-related damage are perhaps more likely to influence transcortical reflex function in the non-paretic arm since it is the non-lesioned cortex that contributes the vast majority of corticospinal fibers descending to the non-paretic arm. Stroke lesions also often disrupt communication between motor cortices in each hemisphere (Carson, 2005, Jenny, 1979), and this disruption may contribute to abnormal reflex sensitivity. Specifically, interhemispheric inhibition from the lesioned motor cortex to the non-lesioned motor cortex is often reduced following stroke (Boroojerdi et al., 1996, Shimizu et al., 2002), and it is possible that this disinhibition alters the function of the non-lesioned motor cortex in a manner that impairs the expression of transcortical reflexes. Our data do not support the idea that reduced transcallosal inhibition results in a simple facilitation of motor circuits in the non-lesioned motor hemisphere, rather they suggest a more complex interaction that can even disrupt reflex function. Similar bilateral interactions could take place within other brain regions such as the cerebellum and basal ganglia, though the evidence for these areas mediating bilateral interactions is less compelling (Carson, 2005).
Bilateral deficits may also be influenced by changes within the spinal cord resulting from stroke. Heightened excitability of short latency reflexes (Powers et al., 1989) and abnormal reflex coordination (Trumbower et al., 2010) have each been observed in the contralesional upper limb. It has been suggested that these abnormalities reflect an increase in spinal excitability resulting from a low-level depolarizing drive to the motoneuron pool (Mottram et al., 2009). Our data demonstrate that short latency stretch reflexes also have a tendency to be enhanced in the non-paretic arm of stroke survivors. In sum, these changes in the regulation of reflex pathways suggest that the properties of the spinal cord may be altered bilaterally following stroke. Thus, even if the correct descending motor commands were sent to either limb, they may not be processed by the spinal cord in a manner, which properly accounts for the mechanical properties of the environment.
Functional Implications
For stroke survivors, bilateral deficits of reflex and voluntary control may lead to an impaired ability to perform activities of daily living. Previous studies on the control of movement in the non-paretic limb have provided evidence of impairments in dexterity (Nowak et al., 2007, Sunderland et al., 1999) and online movement adjustments (Haaland et al., 2004, Schaefer et al., 2012, Winstein and Pohl, 1995). Deficits have also been observed during performance of more functional tasks that mimic activities of daily living such as the Jebsen-Taylor Hand Function Test (Wetter et al., 2005). If stroke survivors rely more heavily on the non-paretic arm for reaching and grasping, then these results suggest that clinical interventions need to address dysfunction in movement control in the non-paretic limb. The disruption in the LLR could manifest as an inability to make rapid corrective adjustments to the position of a walking aide such as a cane when walking over uneven terrain. This might also be revealed during the control of tools that require active stabilization of off-axis movement such as a screw driver. Further work is necessary to determine if the observed impairment in reflex modulation is associated with functional deficits in the control of upper limb posture.
In summary, while our results demonstrate bilateral impairment of environment-dependent, long-latency stretch reflex modulation following monohemispheric stroke, the neurophysiological mechanism underlying impairments in the non-paretic arm are not yet clear. The potential negative effects of reflex invariance on the maintenance of postural stability are worthy of further investigation given the importance of this issue for stroke survivors.
Highlights.
Stroke survivors are unable to adapt their long-latency stretch reflex amplitude during tasks that require increased stability.
Impaired regulation of the long-latency reflex is evident in both the paretic and non-paretic limbs.
The inability to regulate long-latency stretch reflexes to account for the mechanical properties of the environment may contribute to bilateral deficits in tasks that require proprioceptive feedback and rapid changes in muscle activity to maintain stability.
Acknowledgments
This work was supported by NIH grants R01-NS053813 and T32 HD07418. The authors are very grateful to the subjects. The authors would also like to thank Timothy Haswell for his technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akazawa K, Milner TE, Stein RB. Modulation of reflex EMG and stiffness in response to stretch of human finger muscle. J Neurophysiol. 1983;49:16–27. doi: 10.1152/jn.1983.49.1.16. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–70. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Cooper TA, Gottlieb GL, Latash ML. The sense of effort and two models of single-joint motor control. Somatosens Mot Res. 1995;12:343–58. doi: 10.3109/08990229509093667. [DOI] [PubMed] [Google Scholar]
- Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev. 2005;49:641–62. doi: 10.1016/j.brainresrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol. 1984;349:249–72. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112:749–63. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol. 1976;39:925–35. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- Davidoff RA. The pyramidal tract. Neurology. 1990;40:332–9. doi: 10.1212/wnl.40.2.332. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the ‘unaffected’ upper extremity of elderly stroke patients. Stroke. 1996;27:1564–70. doi: 10.1161/01.str.27.9.1564. [DOI] [PubMed] [Google Scholar]
- Dickstein R, Hocherman S, Amdor G, Pillar T. Reaction and movement times in patients with hemiparesis for unilateral and bilateral elbow flexion. Phys Ther. 1993;73:374–80. doi: 10.1093/ptj/73.6.374. discussion 81–5. [DOI] [PubMed] [Google Scholar]
- Dietz V, Discher M, Trippel M. Task-dependent modulation of short- and long-latency electromyographic responses in upper limb muscles. Electroencephalogr Clin Neurophysiol. 1994;93:49–56. doi: 10.1016/0168-5597(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Dietz V, Trippel M, Berger W. Reflex activity and muscle tone during elbow movements in patients with spastic paresis. Ann Neurol. 1991;30:767–79. doi: 10.1002/ana.410300605. [DOI] [PubMed] [Google Scholar]
- Doemges F, Rack PM. Task-dependent changes in the response of human wrist joints to mechanical disturbance. J Physiol. 1992;447:575–85. doi: 10.1113/jphysiol.1992.sp019019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–10. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Motor cortex reflexes associated with learned movement. Science. 1973;179:501–3. doi: 10.1126/science.179.4072.501. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Tanji J. Reflex and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol. 1976;39:1069–80. doi: 10.1152/jn.1976.39.5.1069. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Groenewegen JS, de Groot JH, Schouten AC, Maier AB, Arendzen JH, Meskers CG. Spinal reflex properties in the long term after stroke. J Electromyogr Kinesiol. 2012;22:234–42. doi: 10.1016/j.jelekin.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127:1145–58. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- Hammond PH. The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. J Physiol. 1956;132:17–8P. [PubMed] [Google Scholar]
- Hammond PH. Proceedings of the Third International Conference on Medical Electronics. London: Institute of Electrical Engineers; 1960. An experimental study of servo action in human muscular control; pp. 190–9. [Google Scholar]
- Hendrie A, Lee RG. Selective effects of vibration on human spinal and long-loop reflexes. Brain Res. 1978;157:369–75. doi: 10.1016/0006-8993(78)90044-6. [DOI] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. R: A language for data analysis and graphics. Computational and Graphical Statistics. 1996;5:299–314. [Google Scholar]
- Jebsen RH, Griffith ER, Long EW, Fowler R. Function of “normal” hand in stroke patients. Arch Phys Med Rehabil. 1971;52:170–4. [PubMed] [Google Scholar]
- Jenny AB. Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol. 1979;188:137–45. doi: 10.1002/cne.901880111. [DOI] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM, Parkin PJ. Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain. 1989;112:113–32. doi: 10.1093/brain/112.1.113. [DOI] [PubMed] [Google Scholar]
- Kimura T, Haggard P, Gomi H. Transcranial magnetic stimulation over sensorimotor cortex disrupts anticipatory reflex gain modulation for skilled action. J Neurosci. 2006;26:9272–81. doi: 10.1523/JNEUROSCI.3886-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutky MA, Ravichandran VJ, Trumbower RD, Perreault EJ. Interactions between limb and environmental mechanics influence stretch reflex sensitivity in the human arm. J Neurophysiol. 2010;103:429–40. doi: 10.1152/jn.00679.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer Y, Gattenio L, Parnas E, Sinai D, Sorek Y, Dickstein R. Time-related changes in motor performance of the upper extremity ipsilateral to the side of the lesion in stroke survivors. Neurorehabil Neural Repair. 2001;15:167–72. doi: 10.1177/154596830101500303. [DOI] [PubMed] [Google Scholar]
- Lee RG, Tatton WG. Motor responses to sudden limb displacements in primates with specific CNS lesions and in human patients with motor system disorders. Can J Neurol Sci. 1975;2:285–93. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- Levin MF, Feldman AG. The role of stretch reflex threshold regulation in normal and impaired motor control. Brain Res. 1994;657:23–30. doi: 10.1016/0006-8993(94)90949-0. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Perreault EJ, MacKinnon CD. The influence of perturbation duration and velocity on the long-latency response to stretch in the biceps muscle. Exp Brain Res. 2005;163:361–9. doi: 10.1007/s00221-004-2182-9. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB, Adam J. The effect of lesions of the sensorimotor cortex and the capsular pathways on servo responses from the human long thumb flexor. Brain. 1977;100:503–26. doi: 10.1093/brain/100.3.503. [DOI] [PubMed] [Google Scholar]
- Matthews PB. The human stretch reflex and the motor cortex. Trends Neurosci. 1991;14:87–91. doi: 10.1016/0166-2236(91)90064-2. [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol. 2009;102:2026–38. doi: 10.1152/jn.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Noskin O, Krakauer JW, Lazar RM, Festa JR, Handy C, O’Brien KA, et al. Ipsilateral motor dysfunction from unilateral stroke: implications for the functional neuroanatomy of hemiparesis. J Neurol Neurosurg Psychiatry. 2008;79:401–6. doi: 10.1136/jnnp.2007.118463. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Kust J, Karbe H, Fink GR. Dexterity is impaired at both hands following unilateral subcortical middle cerebral artery stroke. Eur J Neurosci. 2007;25:3173–84. doi: 10.1111/j.1460-9568.2007.05551.x. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R, Brodal A. Sites of termination of corticospinal fibers in the cat. An experimental study with silver impregnation methods. J Comp Neurol. 1963;120:369–91. doi: 10.1002/cne.901200302. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Evidence that a long latency stretch reflex in humans is transcortical. J Physiol. 1992;449:429–40. doi: 10.1113/jphysiol.1992.sp019094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault EJ, Chen K, Trumbower RD, Lewis G. Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination. J Neurophysiol. 2008;99:2101–13. doi: 10.1152/jn.01094.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CG. The Ferrier lecture, 1968. Motor apparatus of the baboon’s hand. Proc R Soc Lond B Biol Sci. 1969;173:141–74. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Pisano F, Miscio G, Del Conte C, Pianca D, Candeloro E, Colombo R. Quantitative measures of spasticity in post-stroke patients. Clin Neurophysiol. 2000;111:1015–22. doi: 10.1016/s1388-2457(00)00289-3. [DOI] [PubMed] [Google Scholar]
- Powers RK, Campbell DL, Rymer WZ. Stretch reflex dynamics in spastic elbow flexor muscles. Ann Neurol. 1989;25:32–42. doi: 10.1002/ana.410250106. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature. 2011;478:387–90. doi: 10.1038/nature10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Mutha PK, Haaland KY, Sainburg RL. Hemispheric specialization for movement control produces dissociable differences in online corrections after stroke. Cereb Cortex. 2012;22:1407–19. doi: 10.1093/cercor/bhr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit BD, Dewald JP, Rymer WZ. Stretch reflex adaptation in elbow flexors during repeated passive movements in unilateral brain-injured patients. Arch Phys Med Rehabil. 2000;81:269–78. doi: 10.1016/s0003-9993(00)90070-4. [DOI] [PubMed] [Google Scholar]
- Shemmell J, An JH, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci. 2009;29:13255–63. doi: 10.1523/JNEUROSCI.0892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Simons DG, Bingel AG. Quantitative comparison of passive motion and tendon reflex responses in biceps and triceps brachii muscles in hemiplegic or hemiparetic man. Stroke. 1971;2:58–66. doi: 10.1161/01.str.2.1.58. [DOI] [PubMed] [Google Scholar]
- Sunderland A. Recovery of ipsilateral dexterity after stroke. Stroke. 2000;31:430–3. doi: 10.1161/01.str.31.2.430. [DOI] [PubMed] [Google Scholar]
- Sunderland A, Bowers MP, Sluman SM, Wilcock DJ, Ardron ME. Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke. 1999;30:949–55. doi: 10.1161/01.str.30.5.949. [DOI] [PubMed] [Google Scholar]
- Thilmann AF, Fellows SJ, Garms E. Pathological stretch reflexes on the “good” side of hemiparetic patients. J Neurol Neurosurg Psychiatry. 1990;53:208–14. doi: 10.1136/jnnp.53.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Ravichandran VJ, Krutky MA, Perreault EJ. Contributions of altered stretch reflex coordination to arm impairments following stroke. J Neurophysiol. 2010;104:3612–24. doi: 10.1152/jn.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter S, Poole JL, Haaland KY. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil. 2005;86:776–81. doi: 10.1016/j.apmr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res. 1995;105:163–74. doi: 10.1007/BF00242191. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Bastian AJ, Dromerick AW, Thach WT, Powers WJ. Mirror movements complicate interpretation of cerebral activation changes during recovery from subcortical infarction. Neurorehabil Neural Repair. 2000;14:213–21. doi: 10.1177/154596830001400307. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Segal RL, Catlin PA, Tschorn J, Raleigh T, Kontos H, et al. Determining consistency of elbow joint threshold angle in elbow flexor muscles with spastic hypertonia. Phys Ther. 1996;76:586–600. doi: 10.1093/ptj/76.6.586. [DOI] [PubMed] [Google Scholar]
- Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J Neurophysiol. 2004;92:3276–85. doi: 10.1152/jn.00549.2004. [DOI] [PubMed] [Google Scholar]