Abstract
Tourette Syndrome (TS) is a neuropsychiatric disorder involving multiple motor and phonic tics. Tics, which usually begin between the ages of 6 and 8, are sudden, rapid, stereotyped, and apparently purposeless movements or sounds that involve discrete muscle groups. Individuals with TS experience a variety of different sensory phenomena, including premonitory urges prior to tics and somatic hypersensitivity due to impaired sensorimotor gating. In addition to other conditions, stress, anxiety, fatigue, or other heightened emotional states tend to exacerbate tics, while relaxation, playing sports, and focused concentration on a specific task tend to alleviate tic symptoms. Ninety percent of children with TS also have comorbid conditions, such as Attention deficit hyperactivity disorder (ADHD), Obsessive-compulsive disorder (OCD), or an impulse control disorder. These disorders often cause more problems for the child both at home and at school than tics do alone. Proper diagnosis and treatment of TS involves appropriate evaluation and recognition, not only of tics, but also of these associated conditions.
Introduction
Tourette syndrome (TS) was first described by the French neurologist, Gilles de la Tourette, in 1885 as a “maladie des tics.” In his original case series describing the syndrome that now bears his name, Gilles de la Tourette wrote about many of the characteristics of the syndrome including: involuntary movements and sounds, markedly enhanced startle reactions, a tendency to repeat both vocalizations (echolalia) and movements (echopraxia), and uncontrollable verbal obscenities (coprolalia) (Lajonchere, Nortz et al. 1996). Since then, our knowledge of TS has progressed significantly, including advances in our understanding of tics, their surrounding sensory phenomena, and the central role that other co-occurring diseases, such as Attention Deficit Hyperactivity Disorder (ADHD) and Obsessive-Compulsive Disorder (OCD), have on the overall clinical course of the disorder. This review will focus on our current understanding of the diagnosis, clinical characterization and assessment of tics as well as their clinical course. Other reviews will focus on the evidence-based treatment and neurobiology of tic disorders.
Definition of Tics
Tics appear as sudden, rapid, purposeless motor movements or sounds that involve discrete muscle groups. They are also stereotyped in that they will occur in a similar manner each time they are performed. In comparison to some movement disorders or psychiatric conditions (e.g. Sterotypies, Chorea, or Dyskinesia), patients with tics report the ability to suppress them, even if only for a short duration. However, they report that suppression often causes discomfort. Almost any movement, sound, or combination therein that the body can make can become a tic. Although some tics are more mild (i.e. eye blinking), others can be more severe to the point of causing pain to the patient (i.e. head or neck jerk). Apart from the physical consequences incurred by them, tics and their associated neuropsychiatric symptoms can diminish patients' quality of life, social and academic function, and lifetime achievements. They can also be very troubling and disruptive to the patients' family, and many times the entire family needs care and counseling (Leckman 2012). Oftentimes, the tics themselves have less adverse effects than the co-occurring disorders. For instance, a 2011 study measuring quality of life (QoL) in fifty youth with TS found that symptoms of depression, OCD, and ADHD appeared to have a widespread negative impact on QoL; however, increased tic severity and poor QoL were not associated (Eddy, Cavanna et al. 2011).
Tourette Syndrome and Other Tic Disorders
The prevalence of TS varies based on study design and location. An international prevalence of 0.6% – 1% has been reported for mainstream schoolchildren, with the disorder being 3–4 times more common in males than in females (Cavanna and Termine 2012). Data from the 2007 National Survey of Children's Health (NSCH) showed an estimated prevalence of 0.3% among U.S. children aged 6–17 years (Scahill, Bitsko et al. 2009). This number may represent an underestimate of TS prevalence since data were gathered from a parent-reported survey, and detection might be imperfect for children with fluctuating levels of symptoms or limited access to specialty health-care services (Scahill, Bitsko et al. 2009). Alternatively, TS prevalence may differ in prevalence worldwide due to either genetic or environmental differences. For example, TS has been reported to be less common in African-American people and has been reported only very rarely in sub-Saharan black African people (Robertson 2008). Regardless, the phenomenology of TS is similar in all cultures in which it has been reported (Robertson 2008).
TS is defined by the pediatric onset of both motor and vocal tics, lasting for at least one year. Although TS is the most notorious cause of chronic tics, there are types of tic disorders that are more common in children. Based on the Diagnostic and Statistical Manual – IV (DSM-IV) of the American Psychiatric Association, other tic disorders include: Chronic motor tic disorder (CMT) and Chronic vocal tic disorder (CVT), which are defined as having motor or phonic tics (but not both) for at least one year; and Transient tic disorder (TTD), which is characterized by tics (either motor and/or vocal) for a duration of less than one year (DSM-IV-TR 2000). Transient tics affect 15–25% of school-aged children with the majority experiencing resolution of tics within several months (Khalifa and von Knorring 2003; Scahill, Sukhodolsky et al. 2005; Robertson 2008; Robertson 2008). With the advent of DSM-V, the category of TTD is likely to be replaced by “Provisional Tic Disorder,” as this designation is more accurate than TTD for patients with ongoing tic symptoms of less than one-year duration since onset (Walkup, Ferrao et al. 2010). Tic Disorders Not Otherwise Specified is the diagnostic term used for tic disorders that begin after age 18 or are secondary to other factors such as substance use (e.g. cocaine), toxins (e.g. carbon monoxide poisoning), or head trauma (e.g. physical trauma, stroke, or encephalitis).
Tics also exhibit several characteristics that distinguish them from other common childhood movement disorder such as stereotypies, choreas and dystonias. The distinguishing characteristics of tics include (1) they wax-and-wane in severity, (2) the character of the movements changes over time, (3) they are temporarily suppressible and (4) they are typically associated with sensory phenomena. Table 2 contrasts TS with other common movement and childhood psychiatric disorders confused with TS.
Table 2.
Differential Diagnosis of Tic Disorders
| Movement | Description | Common Causes |
|---|---|---|
| Tics |
|
|
| Stereotypies |
|
|
| Chorea |
|
|
| Dyskinesia |
|
|
| Athetoid |
|
|
| Myoclonia |
|
|
| Synkinesis |
|
|
Characterization of Tics
Tics are characterized by their anatomical location, number, frequency, and duration. They are also further described by their forcefulness or intensity and by their complexity (ranging from simple to complex). The most widely-used rating scale of tic severity is the Yale Global Tic Severity Scale (YGTSS), which includes separate scores from 0–5 for number, frequency, intensity, complexity, and interference (the degree to which planned actions or speech are interrupted by tics) of both motor and phonic tics (Leckman, Riddle et al. 1989). This tool has allowed for the standardization of tic severity across different studies and research groups, aiding in the characterization and quantification of symptoms.
Additionally, because the clinical characteristics of TS make it hard for clinicians to diagnose and assess the severity of the condition, the Tourette Syndrome Diagnostic Confidence Index (DCI) was created through a collaborative effort of an expert group of clinicians. Based on the range and complexity of tics, their changeable nature, the temporal features of tic expression, and associated subjective and cognitive experiences, the DCI assigns a score from 0 to 100, which reflects the likelihood of having or ever having had TS (Robertson, Banerjee et al. 1999).
Other rating scales include the Shapiro Tourette Syndrome Severity Scale, Tourette's Syndrome-Clinical Global Impression Scale, and the Hopkins Motor and Vocal Tic Scale (Walkup, Rosenberg et al. 1992). Standardized video recordings can also be used to count tics (Tanner, Goetz et al. 1982). See Table 3 for a detailed comparison of various rating scales. For a detailed discussion on these rating scales, we suggest reading a recently published review (McGuire, Kugler et al. 2012).
Table 3.
Tic Rating Scales
| Scale | Citation | Informants | Items | Domains Probed | Strengths | Weaknesses |
|---|---|---|---|---|---|---|
| Yale Global Tic Severity Scale (YGTSS) | (Leckman, Riddle et al. 1989) | Clinician-rated; Semi-structured interview | 10 | Number, frequency, intensity, complexity, and interference from motor and vocal tics, and overall impairment |
|
|
| Tourette's Syndrome Severity Scale (TSSS) | (Shapiro and Shapiro 1984) | Patients and collaterals asked to give ratings | 5 | How much tics are noticed, commented on, seen as odd by others and degree of impairment |
|
|
| Tourette's Disorder Scale-Clinician Rated (TODS-CR) | (Shytle, Silver et al. 2003); (Storch, Merlo et al. 2007) | Clinician-rated; semi-structured interview of parent and child | 15 | Motor and Phonics Tics as well as common comorbid conditions (such as obsessions, compulsions, inattention, hyperactivity, aggression, and emotional disturbances) |
|
|
| Tourette's Disorder Scale-Patient Rated (TODS-PR) | Parent-rated; self-report regarding child | |||||
| Hopkins Motor and Vocal Tic Scale | (Walkup, Rosenberg et al. 1992); (Singer and Rosenberg 1989) | Separate ratings by family member and observer | N/A | Measures overall severity of each individual tic on a visual scale |
|
|
| Tourette's Syndrome Questionnaire (TSQ) | Jagger et al. (1982) (Jagger, Prusoff et al. 1982) | Self-report involving parent and child | 35 pages | Tic history, prenatal and developmental history and family history. |
|
|
| Child Tourette Syndrome Impairment Scale | (Storch, Lack et al. 2007) | Parent-rated, self-report | 37 | Overall impairment (and impairment from tics) in school, home and social activities. |
|
|
| Videotape Ratings and Tic Counts | (Himle, Chang et al. 2006); (Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell, McSwiggan-Hardin et al. 1994)(Chappell et al., 1994) | Videotape subject for at least 5 minutes. Count motor and vocal and total tic frequency. | N/A | Tic frequency |
|
|
Natural History
The natural history of TS has been established based on clinical observations. There is a clear progression of the disorder from the onset of symptoms to, in most cases, full or partial regression of symptoms. Tics usually begin around 6–8 years of age, and 90–95% of TS cases have an onset of tics between the ages of 4–13 (Leckman, Zhang et al. 1998). Simple motor tics involving the eyes or face are usually the first to appear in a child with TS. They are called simple because they involve a single contraction, such as a shoulder shrug or neck stretch. Motor tics will typically progress in a rostral-caudal fashion and over time they have a tendency to become more complex, involving contractions of groups of muscles in a stereotyped, repetitive way (Leckman, Zhang et al. 1998). As such, complex motor tics are often difficult to distinguish from compulsive behaviors.
Phonic tics usually appear after the onset of motor tics and can also progress from simple vocalizations to more complex ones. Although a distinction is made between phonic and motor tics, it is a tenuous one as the sounds produced are a result of contractions of laryngeal, respiratory, oral, or nasal musculature (Jankovic 1997). Simple phonic tics are brief, meaningless vocalizations that often consist of a single sound, such as grunting, squeaking, or sniffing, while complex phonic tics can include uttering different words or phrases. In the same category, echolalia (repeating the words or sounds of others), palilalia (repeating oneself), and coprolalia (saying obscene words or phrases) are types of complex phonic tics. Table 4 describes and gives examples of simple and complex motor and phonic tics.
Table 4.
Types of Tics
| Motor | Phonic | |
|---|---|---|
| Simple | Sudden, brief, short (usually <1 second), one group of muscles (e.g. eye blinking, facial grimacing, head jerk, shoulder shrug) | Fast, meaningless sounds/noises (e.g. sniffing, throat clearing, grunting, or high-pitched squeaks) |
| Complex | Sudden, appear purposive, stereotyped, longer duration, coordinated movements | Syllables, words, or phrases; odd patterns of speech with changes in rate, volume, or rhythm |
| Echopraxia: copying gestures of others | Echolalia: repeating words or phrases of others | |
| Palipraxia: repeating one's own gestures | Palilalia: repeating one's own words or phrases | |
| Copropraxia: lewd and obscene gestures with hands or tongue | Coprolalia: socially inappropriate syllables, words, or phrases expressed in a loud, explosive manner | |
| Dystonic: sustained, gyrating, bending, or twisting movement or posture (e.g. blepharospasm, oculogyric movements, mouth opening, shoulder rotation) | ||
| Tonic: sustained, isometric contraction (e.g. abdominal or limb tensing) | ||
| Self-injurious Behavior: tics that involve injuring oneself (e.g. tongue or lip biting, or hitting one's face) |
Tics tend to wax and wane in severity and frequency. Both motor and phonic tics arise in bouts over the course of the day, and they change in severity over weeks and months. Thus, the amount and length of tic-free intervals throughout the day determines to some extent the severity of the symptom. The tic itself can be more or less forceful, which characterizes its intensity (Leckman 2002).
By contrast, there are no factors known to affect the long-term course of tics. However, the vast majority of children with tics improve. The severity of tics usually peaks at about 10–12 years of age, and in one half to two thirds of cases, symptoms will drastically reduce during adolescence (Bloch, Peterson et al. 2006). In the rare cases in which tic severity persists into adulthood, tic symptoms are most severe, characterized by self-injurious motor tics or coprolalic utterances (Leckman 2002).
In fact, in a recent study by Freeman et al., the overall prevalence of coprophenomena was 19.3% in an international cross-sectional sample of 597 patients. Only 15 of 220 individuals who had mildly-rated tics had coprolalia; whereas 42.6% of the 108 patients with severe tics had coprolalia. The mean age of onset of coprolalia and copropraxia was 5 years 4 months and 4 years 10 months, respectively, after the onset of tics. This delayed onset and greater percentage of coprolalia seen in patients with severe tics is not surprising, as coprophenomena reflects more complex tics and comorbidity patterns (Freeman, Zinner et al. 2009).
Other studies have also associated the presence of certain types of tics with clinical course. A recent study by Martino et al. looked at the prevalence of eye tics in TS patients. They found that of 212 patients, 201 or 94.8%, reported ever having eye tics in their lifetime. They also discovered that overall tic severity positively correlated to lifetime history of eye and/or eyelid/eyebrow movement tics. Furthermore, they found that regardless of the type of tic at onset, patients with a lifetime history of eye movement tics had an earlier onset of TS than those who had never had eye movement tics. These findings suggest the possibility for a difference in the natural history of patients with and without ocular tics. (Martino, Cavanna et al. 2012).
Few studies have examined predictors of long-term outcome on neuropsychological assessment and neuroimaging. One cohort that examined 46 children with TS followed to young adulthood demonstrated that smaller childhood caudate volume and poor Purdue Pegboard performance were associated with increased tic severity in early adulthood (Bloch, Leckman et al. 2005; Bloch, Sukhodolsky et al. 2006). Purdue pegboard performance is a test of fine-motor skill, and poor performance may be a sign of deficits in complex, visually guided or coordinated movement that is likely mediated by circuits involving the basal ganglia. Reduced caudate volume has been previously demonstrated to be a morphological trait of TS on structural MRI (Catafau, Kulisevsky et al. 2000; Peterson, Thomas et al. 2003).
Sensory Phenomena surrounding tics
The outward manifestation of TS represents only a part of the symptomatology experienced by most of our patients. In 1980, Joseph Bliss, articulately described his careful observations from 35 years of self-study of the feelings and subjective events surrounding his own tics. Much of what he described became the basis for future research surrounding the sensory phenomena associated with tics. The term, “sensory phenomena,” is now used as an all-encompassing term to describe such subjective experiences as premonitory urges, “just-right” perceptions, or somatic hypersensitivity in an effort to unify terminology across the literature (Prado, Do Rosário et al. 2007).
Premonitory Urges
Premonitory urges (PU) are uncomfortable sensory phenomena that typically precede and are subjectively experienced as being the initiators of tics. Premonitory urges, formerly deemed, “sensory tics,” can be experienced by individuals with tics and are likened to the need to sneeze or itch or an inner feeling of restlessness, pressure or mounting tension (Kurlan, Lichter et al. 1989). In a questionnaire administered to 135 patients with tic disorders, it was shown that the anatomical regions with the greatest density of urges were the palms, shoulders, midline abdomen, and throat (Leckman, Walker et al. 1993). Thus, premonitory urges are focal in character and limited to specific anatomical locations. They can also vary in frequency, intensity, and location. The performance of the tic itself is usually associated with a momentary feeling of relief from this uncomfortable urge.
The premonitory urge has been studied in comparison with other normal physiological urges, such as the urge to urinate, cough, blink or sleep. An urge is one mode of processing internal or external sensory input into motor output. However, an urge is not always perceived. Often the motor action can be triggered by sensory input alone outside of our awareness, and the action would thus be perceived as involuntary (Belluscio, Tinaz et al. 2011).
Similarly, Bliss writes when describing the process of a tic that, “the inception and emergence of a single action and its passage into the overt phase is so faint, subtle, surrep titious, and lightening fast that rarely is it known to the subject that it exists at all” (Bliss 1980).
If the action is delayed, an urge develops. This feeling of a need to act is different from the sensation of the sensory input itself. Typically, the discomfort associated with the premonitory urge builds up until the tic is performed. Some patients state that they will voluntarily make tics in response to the urge in order to relieve themselves of the mounting discomfort.
In 1994, Kane, then a graduate student with TS, wrote in reference to premonitory urges, “these sensations are not mere precursors to tics; […] more than providing a signal of imminence, the pre-tic sensation acts as the aversive stimulus toward which tics are directed” (p. 806) (Kane 1994).
Patients with TS have the ability to suppress tics temporarily but only at the expense of mounting discomfort like suppressing a sneeze, itch, or the urge to urinate. In fact, with prolonged suppression, the urge to tic can become so great that the action occurs beyond the patients' control. In this way, tics have been called “un-voluntary,” since they are neither voluntary nor involuntary. In contrast to normal urges, the urge to tic is different in that the sensory input that generates the urge to tic is unknown, tics are not key to survival – in fact, they are both nonessential and nonproductive –, and the execution of a tic only temporarily reduces the intensity of the urge to tic (Belluscio, Tinaz et al. 2011). Also, individuals with tics sometimes report the need to perform tics until they get the feeling associated with it being “just right.”
It remains possible that abnormal perception or filtering of these sensory phenomena may be central to the pathogenesis of TS (see “Sensorimotor gating” below). Several individuals with tics have suggested that these premonitory urges may be as characteristic of TS and as disruptive and distracting as the tics themselves. Some individuals perceive premonitory urges and other sensory phenomena as being the “core” of TS (Hollenbeck 2001).
Furthermore, patients have reported an awareness of the premonitory urge helps them suppress imminent tics because they are fore-warned of their arrival and can take measures to suppress them. Along these lines, certain types of behavioral therapies have been developed in order to take advantage of this awareness. Premonitory urges are utilized in cognitive-behavioral interventions that include empirically supported behavioral therapy (Piacentini, Woods et al. 2010) and exposure and response prevention (Verdellen, Hoogduin et al. 2008).
Awareness of premonitory urges typically increases as children with TS become older (Banaschewski, Woerner et al. 2003). Individuals with TS have reported that they first became aware of their premonitory urges on average 3.1 years after the onset of tic symptoms (Leckman, Walker et al. 1993). The delayed onset of awareness of urges most likely represents the normal development self-awareness and the fact that younger children are less able to recognize and describe bodily urges. Premonitory urges are experienced by most adolescents and adults with TS. Eighty-two to ninety-two percent of patients will report experiencing premonitory urges prior to motor and vocal tics (Cohen and Leckman 1992) (Kwak, Dat Vuong et al. 2003).
Whether a tic is voluntary or involuntary has been the topic of much study. Some have said, the tic is a voluntary action performed in an attempt to relieve an involuntary urge (Bliss 1980). Furthermore, in a 2003 study, 68% of 50 TS subjects described a motor tic as a voluntary motor response to an involuntary sensation, as opposed to a completely involuntary movement (Kwak, Dat Vuong et al. 2003). Also, in a study involving 135 individuals with TS, 92% of individuals indicated that their tics were either fully or partially a voluntary response to their premonitory urges. Also, in the same study, 84% of these subjects reported that their tics were associated with a momentary feeling of relief (Leckman, Walker et al. 1993).
The Premonitory Urge for Tics Scale (PUTS) is a rating scale designed to measure the strength of these premonitory urges in tic disorders. Although premonitory urges have been difficult to recognize and consistently report for youth under the age of 10, the scale was found to have excellent psychometric properties for children above the age of 10 years, with PUTS scores correlating with tic severity as measured by the YGTSS (Woods, Piacentini et al. 2005).
Somatic Hypersensitivity
Sensorimotor gating describes the neurological processes of filtering out redundant or unnecessary sensory stimuli from all possible environmental stimuli. Individuals with TS (and schizophrenia) have consistently demonstrated deficits in sensorimotor gating as compared to healthy controls. Prepulse inhibition (PPI) of startle to a high-intensity stimulus is an experimentally measurable indication of sensorimotor gating. Prepulse inhibition of startle is defined as the inhibitory effect of a low-intensity stimulus or “prepulse,” on the startle response to the subsequent same, but high-intensity stimulus (Baldan Ramsey, Xu et al. 2011). The prepulse is believed to activate brain mechanisms which suppress or “gate” the processing of that stimulus for a brief window of time. Impaired PPI has been shown in patients with TS, and recently lesions in the dorsomedial striatum have been implicated in their diminished capacity for PPI (Baldan Ramsey, Xu et al. 2011). Swerdlow has demonstrated PPI is regulated by both norepinephrine and dopamine substrates, and clonidine can repair PPI disrupted by cirazoline (Swerdlow, Bongiovanni et al. 2006).
As hypothesized by these sensorimotor gating deficits observed in patients with TS, many individuals describe hypersensitivity as being an important phenomenon intertwined with other aspects of the disorder. A salient example of this phenomenon is the extreme sensitivity to tags in new clothing experienced by some children with TS.
“Because of the state of sensitization (combined with memory recall and attention targeting), this site is the most difficult to extinguish. Paradoxically, for the same reasons it is the one most likely to be extinguished first in any period of remission.” (Bliss 1980)
“All these sensory actions can dart from one to another with great speed and varying intensities, at times escalating to a fever pitch of intensity and at other times fading quickly away, to recur some other time. Often the effort to control these wild sensations seems to be more than the human spirit can bear; there are really only two choices: let it all hang out or keep fighting. However great the confusion and diversity of sensory-related actions and sensations, only one of these is active at any given moment. All others, residual and secondary, stand in the wings, with their entrances and exits following so quickly on after the other that it is very hard at times to be aware of their single movements.” (Bliss, 1980)
“Perhaps the best description for the sensory state of TS is a somatic hyper-attention: It is not as itch-like as it is an enduring somatosensory bombardment. I experience the TS state as one of keen bodily awareness, or a continual consciousness of muscle, joint, and skin sensations. For example, when sitting in a chair, I do not lose awareness of the tactile sensation of the seat against my body, nor can I ignore the deeper somatic sensations of what my back and legs feel like” (Kane 1994).
“How does a new tic get started? The activation of TS sites is dependent on a combination of (1) attention direction and (2) various precipitants such as stress, tactile and kinesthetic perceptions, previous sensitization of a site, inadvertent pressure points anywhere on the body, memory recall of the earlier sites, and phantom fixations. […] The subject's attention, for any of a multitude of chance reasons, can fall on any potential site. Over seconds, minutes, or hours, the attention shifts to numberless places via sounds, sights, touch, pressure, discomfort, pain, temperature, or thoughts. In the normal person, these attention-exciting events can go relatively unnoticed. In the person with TS, any one can set off a TS action even though that person may be completely unaware of the stimulating factor” pg 1346 (Bliss 1980).
In 2011, Belluscio et al. studied in detail the experience of sensitivity to external stimuli in a case-control study of 19 TS patients and 19 age-matched healthy volunteers. An in-depth interview and questionnaire revealed that 80% of TS patients reported heightened sensitivity to external stimuli, with examples among all sensory modalities, but with statistically significant heightened sensitivity to 4 of 5 sensory modalities (sound, light, smell, and touch) as compared to the healthy volunteers (Belluscio, Jin et al. 2011). They found bothersome stimuli were characterized as “faint, repetitive or constant, and nonsalient, whereas intense stimuli were well tolerated” (Belluscio, Jin et al. 2011). Examples of such bothersome stimuli include: rough fabrics, the constant pressure exerted by a shirt collar or a waistband, the pressure of a chair or another person's arm. Patients also described a preference for strong tactile stimuli such as having their skin scratched or receiving a massage. Furthermore, these investigators did not observe in TS patients any greater ability to detect different intensities of olfactory and tactile stimuli as compared to healthy volunteers. This led them to suggest that the perceived sensitivities were the result of altered or impaired central processing (Belluscio, Jin et al. 2011).
Several rating scales have been designed to measure this hypersensitivity experienced by those with TS. The University of Sao Paulo Sensory Phenomena Scale (USP-SPS) was designed in 2005 in order to assess the severity and frequency of sensory phenomena that precede, accompany, or follow tics and other repetitive behaviors, such as compulsions or rituals (Sutherland Owens, Miguel et al. 2011). Furthermore, in 2009 it was validated against other established scales, such as the Yale-Brown Obsessive-Compulsive Scale, Dimensional Yale-Brown Obsessive-Compulsive Scale, Yale Global Tic Severity Scale, Beck Anxiety Inventory, and Beck Depression Inventory, as a reliable instrument for measuring the presence and severity of sensory phenomena in individuals with OCD (Rosario, Prado et al. 2009).
In addition to PPI as an experimental measure of sensorimotor gating, the Structured Interview for Assessing Perceptual Anomalies (SIAPA) and the Sensory Gating Inventory (SGI) are rating scales that were developed in order to quantify sensorimotor gating impairment seen in TS and schizophrenic patients. SIAPA was developed in 1999 as a way to measure perceptual anomalies, such as flooding or inundation of sensory stimuli in individuals with schizophrenia. The interview employs Likert ratings of perceived hypersensitivity, inundation, and selective attention to external sensory stimuli (Bunney, Hetrick et al. 1999).
Furthermore, Hetrick et al. created the self-report rating scale, Sensory Gating Inventory (SGI) in an effort to expand upon the SIAPA scale by employing an empirical, factor analytic procedure to assess and systematically identify the phenomenology and major dimensions of sensory gating. The self-report rating scale also employs Likert ratings of subjective experiences, such as: perceptions of heightened stimulus sensitivity, sensory inundation, disturbances in the processes of focal and radial attention, and exacerbation of sensory gating-like anomalies by fatigue and stress. The SGI scale demonstrated strong reliability and validity (Hetrick, Erickson et al. 2012).
Exacerbating/Alleviating Factors
Tic symptoms vary in frequency and intensity, and in addition to potential neurological variation, it has been shown that certain environmental or contextual factors will either exacerbate or alleviate tic symptoms in individuals with TS.
The results of 6 different descriptive studies looking at the effects of different antecedent variables on tic severity show stress and anxiety appear to be the most common factors associated with an increase in TS symptoms, while fatigue and boredom also rank high on the list (Conelea and Woods 2008). On the other hand, relaxation, concentration, and physical exercise were antecedent factors shown to contribute to tic attenuation (Conelea and Woods 2008). These studies are limited by the fact that they describe aggregate data, thus removing individual experiences from the descriptions, and they are subject to bias because data were collected by self report and parental observation.
Experimental designs studying the impact of various antecedent factors on tic expression show tic expression occurs more frequently in cases of direct, overt observation, during easy reading assignments, and when the tics themselves are spoken about. For instance, more tics were observed when children were overtly, as opposed to covertly, observed by a video camera; and the presence of another person in the room did not affect overall tic counts (Piacentini, Himle et al. 2006). Also, direct observation revealed tics are aggravated by easy reading assignments, reading in a quiet classroom, and by the period between assignments (Watson, Dufrene et al. 2005). Conversely, it has been shown that periods of focused attention to tasks and reduced peripheral sympathetic tone inhibit tic expression (Nagai, Cavanna et al. 2009). Another study revealed tic-related conversations increase the frequency of phonic tics (not motor tics) as compared to conversations that do not have to do with tics (Woods, Watson et al. 2001). Additionally, instructions to suppress tics have been shown to modestly reduce tic frequency, at least for 30 minutes, with adults demonstrating suppression more frequently. In this same study of 7 adults and children, tic suppression did not lead to the rebound effect of increased tic frequency after the period of suppression, but the impact of suppression instructions on strength of premonitory urges ratings remains unclear (Meidinger, Miltenberger et al. 2005).
Furthermore, taken together, multiple studies have suggested stress, anxiety, frustration, and tension are emotional variables often associated with an increase in tics (Conelea and Woods 2008). However, it remains unclear as to why certain emotions exacerbate tics and what their effect is on premonitory urges. With regard to consequent factors that affect tic expression, it has been shown reinforcing tic-free periods acts to reduce tic frequency, while paying attention to the tics themselves or publicly commenting on tics increases these symptoms (Conelea and Woods 2008).
Suppressing Tics
One of the characteristics of tic symptoms is that they are suppressible, even if only for a short while. However, as stated earlier, the act of suppression can lead to the build-up of uncomfortable premonitory urges. In one study, 3 of 4 children who demonstrated reliable suppression showed a pattern of higher subjective urge ratings during suppression as compared to baseline (Himle, Woods et al. 2007).
Although tics can be suppressed, to do so requires more attention and energy from the individual. For instance, in a study involving 9 children with TS, ages 9–15, accuracy and performance on a distraction task was reduced while children were simultaneously told to suppress tics as compared to free-to-tic conditions (Conelea and Woods 2008). However, no significant difference was demonstrated between tic frequencies during periods of reinforced suppression and reinforced suppression plus a distraction task. This study demonstrates accuracy on an attention-demanding task may be impacted if a child is simultaneously trying to suppress their tics: a finding that has strong implications on school performance for children with TS. This finding suggests school performance of children with TS may be impacted not only by tics but by the attention devoted to suppressing tics and highlights the importance of a supportive environment where negative feedback from their peers and teachers in response to tics is minimized.
Stress has been shown to be one of the major factors associated with tic exacerbation. In a study involving 10 youth with TS, ages 9–17, it was demonstrated that stress impacts children's ability to suppress tics but not necessarily their baseline tic frequency. Tic frequency was greater during periods of reinforced suppression plus a stressor as compared to just reinforced suppression (Conelea, Woods et al. 2011). However, tic frequency was not different between free-to-tic baseline levels and periods when applied stress was added to this condition (Conelea, Woods et al. 2011).
Additionally, it has been shown that tic suppression rewarded for tic-free intervals is more successful at reducing tic frequency than is just being told to suppress tics. For instance, in a study design in which tokens were delivered contingent on the absence of tics and non-contingently, tic frequency was lower in 3 of 4 children during the former condition. The success of reinforced tic suppression could be one of the reasons children are seen to tic more at home than in the classroom because tic absence is reinforced in the classroom by the avoidance of teasing from peers (Himle, Woods et al. 2008). Alternatively, it is possible tic frequency is greater at home than in the classroom because children become more tired by the end of the day when they return home from school.
Finally, one concern with the use of reinforced tic suppression as a model for therapy is the potential for a tic rebound effect, which describes an increase in frequency of tics after suppression. However, studies have not supported such concerns. Although tic frequencies have been shown to increase post-suppression as compared to during suppression, they do not increase above pre-suppression levels (Himle and Woods 2005). Another study demonstrated similar findings after repeated 2-hour sessions of Exposure and Response Prevention (ER), a behavioral treatment program, consisting of habituation to premonitory sensory experiences during prolonged tic suppression. The study demonstrated successful ER as this treatment resulted in a reduction of tics by 91% as compared to baseline. However, comparison of 15 minute pre- and post-suppression measurements did not result in a significant increase in tic frequency (Verdellen, Hoogduin et al. 2007). Additionally, one study noted the absence of the rebound effect in the 5 minutes following reinforced tic suppression during periods of up to 40 consecutive minutes (Woods, Himle et al. 2008).
Comorbidities
The description of behavioral and emotional disturbances in patients with TS has occurred since 1899, around the time the disorder was first described by Georges Gilles de la Tourette himself (Coffey and Park 1997). In fact, comorbid neuropsychiatric disorders, the majority being ADHD and OCD, have been shown to occur in up to 90% of TS patients in both clinic and community settings (Wright, Rickards et al. 2012).
Obsessive-Compulsive Disorder
Roughly one-third to one-half of individuals with TS experience recurrent obsessive-compulsive (OC) symptoms (Leckman, Grice et al. 1994; Leckman, Grice et al. 1997; Khalifa and von Knorring 2005). Genetic, neurobiological, and treatment response studies suggest there may be qualitative differences between tic-related forms of OCD and cases of OCD not related to tics. Specifically, tic-related OCD has a male preponderance, an earlier age of onset, a poorer level of response to standard anti-obsessional medications, and a greater likelihood of first-degree family members with a tic disorder (Hounie, do Rosario-Campos et al. 2006). Symptomatically the most common obsessive-compulsive symptoms encountered in TS patients are obsessions concerning a need for symmetry or exactness, repeating rituals, counting compulsions, and ordering/arranging compulsions (Leckman, Grice et al. 1997). Also, obsessive-compulsive symptoms, when present, in children with TS, appear more likely to persist into adulthood than the tics themselves (Bloch et al., 2006). OCD with comorbid tics is less responsive to SSRI pharmacotherapy and more responsive to antipsychotic augmentation than OCD in patients without tics (Bloch, Landeros-Weisenberger et al. 2006; March, Franklin et al. 2007). OCD patients with and without tic disorders appear equally responsive to cognitive-behavioral therapy (March, Franklin et al. 2007).
Baseline data from a study of 158 youth with a chronic tic disorder (TD) showed children with comorbid OCD (53% of subjects) experienced more severe tics, increased levels of depressive and anxious symptoms, heightened psychosocial stress and poorer global functioning (Lebowitz, Motlagh et al. 2012). The authors concluded TD with OCD is a more severe subtype of TD and describes children with more internalizing disorders than those without OCD (Lebowitz, Motlagh et al. 2012). By contrast, another exploratory study involving 306 children with TD, OCD, or TD + OCD, failed to show that those with TD + OCD exhibited increases in tic severity as compared to those with TD alone (Lewin, Chang et al. 2010).
Attention-Deficit Hyperactivity Disorder
Roughly 30–50% of children with TS are diagnosed with comorbid ADHD (Khalifa and von Knorring 2005). This rate of comorbid ADHD is higher in clinical samples. Although the etiological relationship between TS and ADHD is unclear, it is clear individuals with both TS and ADHD are at a much greater risk for a variety of poor outcomes including greater academic and social impairment (Carter, O'Donnell et al. 2000); (Peterson, Staib et al. 2001); (Sukhodolsky, Scahill et al. 2003; Sukhodolsky, do Rosario-Campos et al. 2005). They are often regarded as less likeable, more aggressive, and more withdrawn than their classmates (Stokes, Bawden et al. 1991). These social difficulties are amplified in a child with TS who also has ADHD (Bawden, Stokes et al. 1998; Sukhodolsky, Scahill et al. 2003; Sukhodolsky, do Rosario-Campos et al. 2005). Surprisingly, levels of tic severity are less predictive of peer acceptance than is the presence of ADHD (Bawden, Stokes et al. 1998). Comorbid ADHD symptoms in children with tics are responsive to similar pharmacological treatment as ADHD in children without tics (Bloch, Panza et al. 2009). Therefore, prompt screening of ADHD symptoms in children with tic disorders is imperative. We suggest examination of recent practice parameters for a thorough review of the diagnosis, assessment, and treatment of ADHD (Pliszka 2007; Wolraich, Brown et al. 2011).
Impulse control disorders
In addition to the high frequency of such comorbid conditions as ADHD and OCD, many children with TS have been noted to exhibit rage attacks, self-injurious behavior, inappropriate sexual activity, discipline problems, sleep disturbances, and other forms of impulse control disorders. Impulse-control disorders are currently classified as an individual category within the DSM-IV-TR (DSM-IV-TR 2000). “Impulsivity is defined as the failure to resist an impulse, drive, or temptation that is potentially harmful to oneself or others. It is evidenced behaviorally as carelessness; an underestimated sense of harm; extroversion; impatience, including the inability to delay gratification; and a tendency toward risk-taking and pleasure- and sensation-seeking” (Wright, Rickards et al. 2012). Wright et al. review TS as it relates to impulse-control disorders, specifically, intermittent explosive disorder (IED), self-injurious behavior (SIB), and other forms of impulse-control disorder.
This type of disinhibited behavior is inextricably linked to tics. For instance, some individuals will have the urge to make a loud vocal tic in a quiet library upon seeing the sign, “Quiet Please.” Similarly, one can feel the need to jerk his shoulder after someone lightly puts their hand on it. This type of behavior could represent the disrupted sensory gating in that the light stimulus is bothersome and can create a site of unpleasant urge. Furthermore, there is the example of a physicist during WWII, who had to relinquish his job in a high energy physics laboratory because whenever he saw the sign, “Danger High Voltage,” he had the strong urge to touch the apparatus. These types of tics are seen as reflexive tics to specific sensory clues, but often appear as disinhibited or impulsive behavior.
It is estimated between 23% and 40% of clinically-referred TS subjects report distressing behavioral symptoms, such as sudden unpredictable anger, irritability, temper outbursts, and aggression (Wright, Rickards et al. 2012). A part of intermittent explosive disorder, rage attacks have been linked to TS as early as 1998, when it was suggested individuals with TS and another comorbid condition, such as ADHD or OCD, are more likely to also experience rage attacks (Budman, Bruun et al. 1998). Since then, a study in 2008 showed that of 314 children in a Danish cohort of TS patients, 109 experienced rage attacks. Interestingly, when examining the presence of rage attacks within different subgroups, it was noted rage attacks were present in the greatest percentage (70.6%) of children who have TS with both ADHD and OCD. In those with TS and ADHD, 56.7% experienced rage, which is similar to the 50.9% of children with TS and OCD who experience rage. In those children who have TS without any other comorbidity, 36.7% exhibited rage attacks (Mol Debes, Hjalgrim et al. 2008). This data could support the suggestion that impulsivity and compulsivity are interlinked. Another hypothesis as to why OCD is linked to rage attacks in TS patients is that the sudden, impulsive outbursts of anger are a result of a disruption to routines that are linked to the compulsivity present in these patients (Wright, Rickards et al. 2012). In 2003, a questionnaire was developed in order to screen TS patients for episodic rage according to their symptoms. In this study, 48 children with TS, ages 7–17, were screened to explore rage attack phenomenology, and the investigators used a cluster analysis to identify four potential subgroups of TS with rage: specific urge resolution, environmentally secure reactivity, nonspecific urge resolution, or labile non-resolving (Budman, Rockmore et al. 2003).
Furthermore, self-injurious behavior (SIB) has been consistently associated with a subgroup of TS patients. Of the 9 patients described by Gilles de la Tourette in 1885, 2 of them were described as exhibiting SIB. Self-injurious behavior has been reported in anywhere between 14.8% and 29% of TS subjects (Freeman 2007) (Mathews, Waller et al. 2004). Additionally, the proportion of SIB present in those with TS is higher in those with comorbid ADHD and who are older in age. In those patients with ADHD and TS, age of onset of SIB was 7.4 years, as compared to 10 years in those without ADHD (Freeman 2007). Examples of types of SIB noted are biting one's tongue or lip, head-banging, body punching/slapping, head or face punching/slapping, body-to-hard-object banging, and poking sharp objects into one's body (Wright, Rickards et al. 2012).
The co-occurrence of impulse-control disorders with those patients with TS has further implications on the cognitive aspects of these individuals. They can exhibit the inability to delay gratification, making decisions based on immediate reward, they are distractible, and they are generally disinhibited, which can lead to behavior that does not comply with cultural norms. If impulsivity and compulsivity are thought to be opposite ends of a spectrum, TS would be considered a mixture of the two. While compulsions are driven by an attempt to reduce anxiety, impulsions are driven by an attempt to obtain arousal and gratification (Wright, Rickards et al. 2012).
Conclusions
Tourette's syndrome is a neuropsychiatric disorder characterized by multiple motor and vocal tics. However, for many individuals with TS, the tics are neither the most prominent nor distressing part of the disorder. In the majority of children with TS, tic symptoms diminish significantly during adolescence. Most individuals with TS experience associated sensory phenomena such as premonitory urges and somatic hypersensitivity that are often as distressing as the tics themselves. The majority of individuals with TS reaching clinical attention have common comorbid conditions such as ADHD, OCD and impulse control disorders. Proper diagnosis and treatment of TS involves appropriate evaluation and recognition, not only of tics, but also of these associated conditions.
Figure 1. Average Tic Severity from Age 2–18 years.
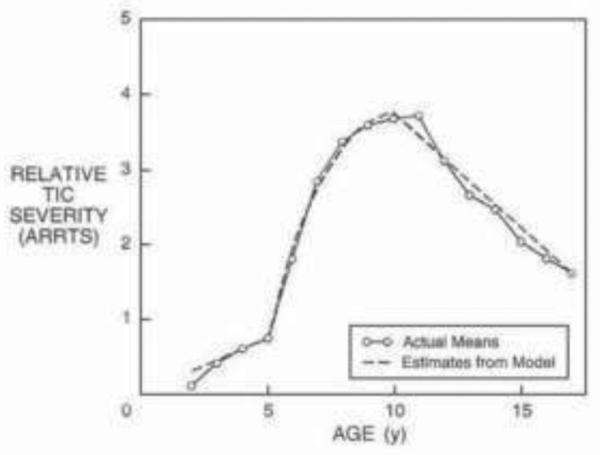
Adapted with permission from (Leckman, Zhang et al. 1998)
Figure 2. Clinical Course of Tourette syndrome and Associated Conditions.
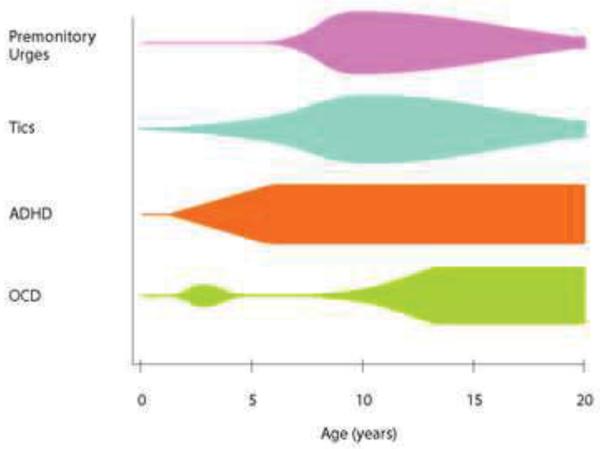
Figure depicts severity of tics and comorbid conditions associated with Tourette syndrome. Width of bars correspond to severity of symptoms. Adapted with permission from (Leckman 2002).
Table 1.
Tic Disorders According to DSM IV
| Diagnosis | Type of Tics | Timing of Tics | Age of Onset |
|---|---|---|---|
| Tourette's Syndrome (TS) | Multiple motor and one or more vocal tics | Nearly every day for >1 year, with no more than 3 consecutive months tic free | <18 years of age |
| Chronic Motor Tic Disorder (CMT) | Single or multiple motor tics | Nearly every day for >1 year, with no more than 3 consecutive months tic free | <18 years of age |
| Chronic Vocal Tic Disorder (CVT) | Single or multiple vocal tics | Nearly every day for >1 year, with no more than 3 consecutive months tic free | <18 years of age |
| Transient Tic Disorder (TTD) | Single or multiple motor and/or vocal tics | Nearly every day for at least 4 weeks, but no more than 12 consecutive months | <18 years of age |
| Tic Disorder NOS | Motor or vocal | Not specified; often used when current tics have been present for less than a year | Not specified, often used for individuals that have onset at >18 years of age |
Table 5.
Sensory Phenomena Rating Scales
| Measure | Citation(s) | # Items | Domains Probed | Strengths | Limitations |
|---|---|---|---|---|---|
| Premonitory Urge for Tics Scale (PUTS) | (Woods, Piacentini et al. 2005) | 9 items | Frequency of specific pre-tic related sensory symptoms along with relief after tic completion |
|
|
| University of Sao Paulo Sensory Phenomena Scale (USP-SPS) | (Rosario, Prado et al. 2009) | 2 parts: checklist and severity scale | Frequency, interference and distress of sensory phenomena that precede, accompany, or follow tics and other obsessive-compulsive spectrum behaviors |
|
|
| Sensory Gating Inventory (SGI) | (Hetrick, Erickson et al. 2012) | 124 items | 6-point Likert ratings assessing 4 factors: perceptual modulation, distractibility, over-inclusion or hyper-attention, and fatigue and stress vulnerability |
|
|
| Structured Interview for Assessing Perceptual Anomalies (SIAPA) | (Bunney, Hetrick et al. 1999) | 15 items | 5-point Likert ratings of hypersensitivity, inundation and flooding, and selective attention to external sensory stimuli for each of the 5 sensory modalities |
|
|
Table 6.
Exacerbating and Alleviating Factors for Tics.
| Tic Attenuation | Tic Exacerbation |
|---|---|
| Relaxation | Stress, anxiety, worry, frustration |
| Physical exercise, sports | Fatigue, tiredness |
| Concentration, study activity | Returning to school |
| Habitual, automatic actions | Boredom, waiting |
| Reading for pleasure | Emotional trauma |
| Leisure activity | Holidays, birthdays |
| Talking to friends | Working under pressure |
| Doctor visits | Overstimulation, multitasking |
| Verbal instructions to suppress tics and rewarding /reinforcing tic-free periods | Tic-related conversation |
| Interaction with familiar people | Being alone |
| Socialization (30%), social gatherings (25%) | Social gatherings (42%), socialization (50%) (presence of others/overt observation) |
| Transportation |
Adapted from data in (Conelea and Woods 2008)
Acknowledgements
The authors acknowledge the National Institute of Mental Health support of the Trichotillomania Learning Center (MHB), the Yale Child Study Center Research Training Program (MHB), the National Institutes of Health (K23MH091240 (MHB), T32MH018268-26 (JFL) and R25 MH077823 (JFL)), the APIRE/Eli Lilly Psychiatric Research Fellowship (MHB), the AACAP/ Eli Lilly Junior Investigator Award (MHB), , NARSAD (MHB), and UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research (MHB).
Footnotes
Disclosures: The authors have no conflicts of interest to disclose
References
- Baldan Ramsey LC, Xu M, et al. Lesions of the dorsomedial striatum disrupt prepulse inhibition. Neuroscience. 2011;180:222–228. doi: 10.1016/j.neuroscience.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T, Woerner W, et al. Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: developmental aspects in children and adolescents. Developmental Medicine & Child Neurology. 2003;45(10):700–703. doi: 10.1017/s0012162203001294. [DOI] [PubMed] [Google Scholar]
- Bawden HN, Stokes A, et al. Peer relationship problems in children with Tourette's disorder or diabetes mellitus. J Child Psychol Psychiatry. 1998;39(5):663–668. [PubMed] [Google Scholar]
- Belluscio BA, Jin L, et al. Sensory sensitivity to external stimuli in Tourette syndrome patients. Mov Disord. 2011;26(14):2538–2543. doi: 10.1002/mds.23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio BA, Tinaz S, et al. Similarities and differences between normal urges and the urge to tic. Cognitive Neuroscience. 2011;2(3–4):245–246. doi: 10.1080/17588928.2011.618630. [DOI] [PubMed] [Google Scholar]
- Bliss J. Sensory experiences of Gilles de la Tourette syndrome. Arch Gen Psychiatry. 1980;37(12):1343–1347. doi: 10.1001/archpsyc.1980.01780250029002. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Landeros-Weisenberger A, et al. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006;11:622–632. doi: 10.1038/sj.mp.4001823. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, et al. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65(8):1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Panza KE, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884–893. doi: 10.1097/CHI.0b013e3181b26e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Peterson BS, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65–69. doi: 10.1001/archpedi.160.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Sukhodolsky DG, et al. Fine-motor skill deficits in childhood predict adulthood tic severity and global psychosocial functioning in Tourette's syndrome. J Child Psychol Psychiatry. 2006;47(6):551–559. doi: 10.1111/j.1469-7610.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- Budman CL, Bruun RD, et al. Rage attacks in children and adolescents with Tourette's disorder: a pilot study. Journal of Clinical Psychiatry. 1998;59(11):576–580. doi: 10.4088/jcp.v59n1103. [DOI] [PubMed] [Google Scholar]
- Budman CL, Rockmore L, et al. Clinical phenomenology of episodic rage in children with Tourette syndrome. J Psychosom Res. 2003;55(1):59–65. doi: 10.1016/s0022-3999(02)00584-6. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Jr., Hetrick WP, et al. Structured Interview for Assessing Perceptual Anomalies (SIAPA) Schizophr Bull. 1999;25(3):577–592. doi: 10.1093/oxfordjournals.schbul.a033402. [DOI] [PubMed] [Google Scholar]
- Carter AS, O'Donnell DA, et al. Social and emotional adjustment in children affected with Gilles de la Tourette's syndrome: associations with ADHD and family functioning. Attention Deficit Hyperactivity Disorder. J Child Psychol Psychiatry. 2000;41(2):215–223. [PubMed] [Google Scholar]
- Catafau AM, Kulisevsky J, et al. Relationship between cerebral perfusion in frontal-limbic-basal ganglia circuits and neuropsychologic impairment in patients with subclinical hepatic encephalopathy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2000;41(3):405–410. [PubMed] [Google Scholar]
- Cavanna AE, Termine C. Tourette syndrome. Advances in Experimental Medicine & Biology. 2012;724:375–383. doi: 10.1007/978-1-4614-0653-2_28. [DOI] [PubMed] [Google Scholar]
- Chappell PB, McSwiggan-Hardin MT, et al. Videotape tic counts in the assessment of Tourette's syndrome: stability, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1994;33(3):386–393. doi: 10.1097/00004583-199403000-00013. [DOI] [PubMed] [Google Scholar]
- Coffey BJ, Park KS. Behavioral and emotional aspects of Tourette syndrome. Neurol Clin. 1997;15(2):277–289. doi: 10.1016/s0733-8619(05)70312-1. [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Leckman JF. Sensory phenomena associated with Gilles de la Tourette's syndrome. Journal of Clinical Psychiatry. 1992;53(9):319–323. [PubMed] [Google Scholar]
- Conelea CA, Woods DW. Examining the impact of distraction on tic suppression in children and adolescents with Tourette syndrome. Behaviour Research & Therapy. 2008;46(11):1193–1200. doi: 10.1016/j.brat.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Conelea CA, Woods DW. The influence of contextual factors on tic expression in Tourette's syndrome: a review. J Psychosom Res. 2008;65(5):487–496. doi: 10.1016/j.jpsychores.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Conelea CA, Woods DW, et al. The impact of a stress induction task on tic frequencies in youth with Tourette Syndrome. Behaviour Research & Therapy. 2011;49(8):492–497. doi: 10.1016/j.brat.2011.05.006. [DOI] [PubMed] [Google Scholar]
- DSM-IV-TR . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- Eddy CM, Cavanna AE, et al. Clinical correlates of quality of life in Tourette syndrome. Mov Disord. 2011;26(4):735–738. doi: 10.1002/mds.23434. [DOI] [PubMed] [Google Scholar]
- Freeman RD. Tic disorders and ADHD: answers from a world-wide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):15–23. doi: 10.1007/s00787-007-1003-7. [DOI] [PubMed] [Google Scholar]
- Freeman RD, Zinner SH, et al. Coprophenomena in Tourette syndrome. Dev Med Child Neurol. 2009;51(3):218–227. doi: 10.1111/j.1469-8749.2008.03135.x. [DOI] [PubMed] [Google Scholar]
- Hetrick WP, Erickson MA, et al. Phenomenological dimensions of sensory gating. Schizophr Bull. 2012;38(1):178–191. doi: 10.1093/schbul/sbq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himle MB, Chang S, et al. Establishing the feasibility of direct observation in the assessment of tics in children with chronic tic disorders. J Appl Behav Anal. 2006;39(4):429–440. doi: 10.1901/jaba.2006.63-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himle MB, Woods DW. An experimental evaluation of tic suppression and the tic rebound effect. Behaviour Research & Therapy. 2005;43(11):1443–1451. doi: 10.1016/j.brat.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Himle MB, Woods DW, et al. Evaluating the role of contingency in differentially reinforced tic suppression. J Appl Behav Anal. 2008;41(2):285–289. doi: 10.1901/jaba.2008.41-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himle MB, Woods DW, et al. Investigating the effects of tic suppression on premonitory urge ratings in children and adolescents with Tourette's syndrome. Behaviour Research and Therapy. 2007;45(12):2964–2976. doi: 10.1016/j.brat.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ. Insight and hindsight into Tourette syndrome. Adv Neurol. 2001;85:363–367. [PubMed] [Google Scholar]
- Hounie AG, do Rosario-Campos MC, et al. Obsessive-compulsive disorder in Tourette syndrome. Adv Neurol. 2006;99:22–38. [PubMed] [Google Scholar]
- Jagger J, Prusoff BA, et al. The epidemiology of Tourette's syndrome: a pilot study. Schizophr Bull. 1982;8(2):267–278. doi: 10.1093/schbul/8.2.267. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Tourette syndrome. Phenomenology and classification of tics. Neurol Clin. 1997;15(2):267–275. doi: 10.1016/s0733-8619(05)70311-x. [DOI] [PubMed] [Google Scholar]
- Kane MJ. Premonitory urges as “attentional tics” in Tourette's syndrome. J Am Acad Child Adolesc Psychiatry. 1994;33(6):805–808. doi: 10.1097/00004583-199407000-00005. [DOI] [PubMed] [Google Scholar]
- Khalifa N, von Knorring AL. Prevalence of tic disorders and Tourette syndrome in a Swedish school population. Dev Med Child Neurol. 2003;45(5):315–319. doi: 10.1017/s0012162203000598. [DOI] [PubMed] [Google Scholar]
- Khalifa N, von Knorring AL. Tourette syndrome and other tic disorders in a total population of children: clinical assessment and background. Acta Paediatr. 2005;94(11):1608–1614. doi: 10.1111/j.1651-2227.2005.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Kurlan R, Lichter D, et al. Sensory tics in Tourette's syndrome. Neurology. 1989;39(5):731–734. doi: 10.1212/wnl.39.5.731. [DOI] [PubMed] [Google Scholar]
- Kwak C, Dat Vuong K, et al. Premonitory sensory phenomenon in Tourette's syndrome. Movement Disorders. 2003;18(12):1530–1533. doi: 10.1002/mds.10618. [DOI] [PubMed] [Google Scholar]
- Lajonchere C, Nortz M, et al. Gilles de la Tourette and the discovery of Tourette syndrome. Includes a translation of his 1884 article. Arch Neurol. 1996;53(6):567–574. doi: 10.1001/archneur.1996.00550060111024. [DOI] [PubMed] [Google Scholar]
- Lebowitz ER, Motlagh MG, et al. Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2012 doi: 10.1007/s00787-012-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF. Tourette's syndrome. Lancet. 2002;360(9345):1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- Leckman JF. Tic disorders. BMJ. 2012;344:d7659. doi: 10.1136/bmj.d7659. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Grice DE, et al. Tic-related vs. non-tic-related obsessive compulsive disorder. Anxiety. 1994;1(5):208–215. [PubMed] [Google Scholar]
- Leckman JF, Grice DE, et al. Symptoms of obsessive-compulsive disorder. Am J Psychiatry. 1997;154(7):911–917. doi: 10.1176/ajp.154.7.911. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, et al. Premonitory urges in Tourette's syndrome. American Journal of Psychiatry. 1993;150(1):98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Zhang H, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102(1 Pt 1):14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- Lewin AB, Chang S, et al. Comparison of clinical features among youth with tic disorders, obsessive-compulsive disorder (OCD), and both conditions. Psychiatry Res. 2010;178(2):317–322. doi: 10.1016/j.psychres.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Franklin ME, et al. Tics moderate treatment outcome with sertraline but not cognitive-behavior therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2007;61(3):344–347. doi: 10.1016/j.biopsych.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Martino D, Cavanna AE, et al. Prevalence and phenomenology of eye tics in Gilles de la Tourette syndrome. J Neurol. 2012 doi: 10.1007/s00415-012-6470-1. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Waller J, et al. Self injurious behaviour in Tourette syndrome: correlates with impulsivity and impulse control. J Neurol Neurosurg Psychiatry. 2004;75(8):1149–1155. doi: 10.1136/jnnp.2003.020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Kugler BB, et al. Evidence-Based Assessment of Compulsive Skin Picking, Chronic Tic Disorders and Trichotillomania in Children. Child Psychiatry Hum Dev. 2012 doi: 10.1007/s10578-012-0300-7. [DOI] [PubMed] [Google Scholar]
- Meidinger AL, Miltenberger RG, et al. An investigation of tic suppression and the rebound effect in Tourette's disorder. Behav Modif. 2005;29(5):716–745. doi: 10.1177/0145445505279262. [DOI] [PubMed] [Google Scholar]
- Mol Debes NM, Hjalgrim H, et al. Validation of the presence of comorbidities in a Danish clinical cohort of children with Tourette syndrome. J Child Neurol. 2008;23(9):1017–1027. doi: 10.1177/0883073808316370. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Cavanna A, et al. Influence of sympathetic autonomic arousal on tics: Implications for a therapeutic behavioral intervention for Tourette syndrome. J Psychosom Res. 2009;67(6):599–605. doi: 10.1016/j.jpsychores.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Staib L, et al. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58(5):427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, et al. Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60(4):415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Himle MB, et al. Reactivity of tic observation procedures to situation and setting. Journal of Abnormal Child Psychology. 2006;34(5):649–658. doi: 10.1007/s10802-006-9048-5. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA. 2010;303(19):1929–1937. doi: 10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Prado HDS, Do Rosário MC, et al. Sensory phenomena, “just-right” and “not just-right” experiences in ocd patients: Looking for a consensus. CNS Spectrums. 2007;12(2):95–96. [PubMed] [Google Scholar]
- Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: the epidemiological and prevalence studies. J Psychosom Res. 2008;65(5):461–472. doi: 10.1016/j.jpsychores.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 2: tentative explanations for differing prevalence figures in GTS, including the possible effects of psychopathology, aetiology, cultural differences, and differing phenotypes. J Psychosom Res. 2008;65(5):473–486. doi: 10.1016/j.jpsychores.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Banerjee S, et al. The Tourette syndrome diagnostic confidence index: development and clinical associations. Neurology. 1999;53(9):2108–2112. doi: 10.1212/wnl.53.9.2108. [DOI] [PubMed] [Google Scholar]
- Rosario MC, Prado HS, et al. Validation of the University of Sao Paulo Sensory Phenomena Scale: initial psychometric properties. CNS Spectr. 2009;14(6):315–323. doi: 10.1017/s1092852900020319. [DOI] [PubMed] [Google Scholar]
- Scahill L, Bitsko RH, et al. Prevalence of diagnosed Tourette syndrome in persons aged 6–17 years - United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;58(21):581–585. [PubMed] [Google Scholar]
- Scahill L, Sukhodolsky DG, et al. Public health significance of tic disorders in children and adolescents. Adv Neurol. 2005;96:240–248. [PubMed] [Google Scholar]
- Shapiro AK, Shapiro E. Controlled study of pimozide vs. placebo in Tourette's syndrome. J Am Acad Child Psychiatry. 1984;23(2):161–173. doi: 10.1097/00004583-198403000-00007. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, et al. The Tourette's Disorder Scale (TODS): development, reliability, and validity. Assessment. 2003;10(3):273–287. doi: 10.1177/1073191103255497. [DOI] [PubMed] [Google Scholar]
- Singer HS, Rosenberg LA. Development of behavioral and emotional problems in Tourette syndrome. Pediatr Neurol. 1989;5(1):41–44. doi: 10.1016/0887-8994(89)90008-8. [DOI] [PubMed] [Google Scholar]
- Stokes A, Bawden HN, et al. Peer problems in Tourette's disorder. Pediatrics. 1991;87(6):936–942. [PubMed] [Google Scholar]
- Storch EA, Lack CW, et al. A measure of functional impairment in youth with Tourette's syndrome. J Pediatr Psychol. 2007;32(8):950–959. doi: 10.1093/jpepsy/jsm034. [DOI] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, et al. Further psychometric examination of the Tourette's Disorder Scales. Child Psychiatry Hum Dev. 2007;38(2):89–98. doi: 10.1007/s10578-006-0043-4. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG, do Rosario-Campos MC, et al. Adaptive, emotional, and family functioning of children with obsessive-compulsive disorder and comorbid attention deficit hyperactivity disorder. Am J Psychiatry. 2005;162(6):1125–1132. doi: 10.1176/appi.ajp.162.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolsky DG, Scahill L, et al. Disruptive behavior in children with Tourette's syndrome: association with ADHD comorbidity, tic severity, and functional impairment. J Am Acad Child Adolesc Psychiatry. 2003;42(1):98–105. doi: 10.1097/00004583-200301000-00016. [DOI] [PubMed] [Google Scholar]
- Sutherland Owens AN, Miguel EC, et al. Sensory gating scales and premonitory urges in Tourette syndrome. TheScientificWorldJournal. 2011;11:736–741. doi: 10.1100/tsw.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Bongiovanni MJ, et al. Separable noradrenergic and dopaminergic regulation of prepulse inhibition in rats: implications for predictive validity and Tourette Syndrome. Psychopharmacology (Berl) 2006;186(2):246–254. doi: 10.1007/s00213-006-0374-7. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Goetz CG, et al. Cholinergic mechanisms in Tourette syndrome. Neurology. 1982;32(11):1315–1317. doi: 10.1212/wnl.32.11.1315. [DOI] [PubMed] [Google Scholar]
- Verdellen CW, Hoogduin CA, et al. Habituation of premonitory sensations during exposure and response prevention treatment in Tourette's syndrome. Behav Modif. 2008;32(2):215–227. doi: 10.1177/0145445507309020. [DOI] [PubMed] [Google Scholar]
- Verdellen CWJ, Hoogduin CAL, et al. Tic suppression in the treatment of Tourette's syndrome with exposure therapy: The rebound phenomenon reconsidered. Movement Disorders. 2007;22(11):1601–1606. doi: 10.1002/mds.21577. [DOI] [PubMed] [Google Scholar]
- Walkup JT, Ferrao Y, et al. Tic disorders: some key issues for DSM-V. Depress Anxiety. 2010;27(6):600–610. doi: 10.1002/da.20711. [DOI] [PubMed] [Google Scholar]
- Walkup JT, Rosenberg LA, et al. The validity of instruments measuring tic severity in Tourette's syndrome. J Am Acad Child Adolesc Psychiatry. 1992;31(3):472–477. doi: 10.1097/00004583-199205000-00013. [DOI] [PubMed] [Google Scholar]
- Watson TS, Dufrene B, et al. Brief antecedent assessment and treatment of tics in the general education classroom: a preliminary investigation. Behav Modif. 2005;29(6):839–857. doi: 10.1177/0145445505279252. [DOI] [PubMed] [Google Scholar]
- Wolraich M, Brown L, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DW, Himle MB, et al. Durability, negative impact, and neuropsychological predictors of tic suppression in children with chronic tic disorder. Journal of Abnormal Child Psychology. 2008;36(2):237–245. doi: 10.1007/s10802-007-9173-9. [DOI] [PubMed] [Google Scholar]
- Woods DW, Piacentini J, et al. Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. Journal of Developmental & Behavioral Pediatrics. 2005;26(6):397–403. doi: 10.1097/00004703-200512000-00001. [DOI] [PubMed] [Google Scholar]
- Woods DW, Watson TS, et al. Analyzing the influence of tic-related talk on vocal and motor tics in children with Tourette's syndrome. J Appl Behav Anal. 2001;34(3):353–356. doi: 10.1901/jaba.2001.34-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A, Rickards H, et al. Impulse-control disorders in gilles de la tourette syndrome. J Neuropsychiatry Clin Neurosci. 2012;24(1):16–27. doi: 10.1176/appi.neuropsych.10010013. [DOI] [PubMed] [Google Scholar]


