Abstract
Growth inhibitory molecules in the adult mammalian CNS have been implicated in blocking axonal sprouting and regeneration following injury. Prominent CNS regeneration inhibitors include Nogo-A, OMgp and CSPGs, and a key question concerns their physiological role in the naïve CNS. Emerging evidence suggests novel functions in dendrites and at synapses of glutamatergic neurons. CNS regeneration inhibitors target the neuronal actin cytoskeleton to regulate dendritic spine maturation, long-term synapse stability, and Hebbian forms of synaptic plasticity. This is accomplished in part by antagonizing plasticity-promoting signaling pathways activated by neurotrophic factors. Altered function of CNS regeneration inhibitors is associated with mental illness and loss of long-lasting memory, suggesting unexpected and novel physiological roles for these molecules in brain health.
Keywords: Nogo receptor, Nogo-A, OMgp, proteoglycan, synaptic structure, dendritic spine, synapse stability
Introduction
Proper nervous system function critically depends on the precise assembly and maintenance of an intricate synaptic network. Once the initial scaffold of neuronal connections has been laid down, refinement processes continue to sculpt and transform microcircuits into a mature brain and spinal cord. In many regions of the early postnatal CNS, neuronal activity drives network refinement. A classic example is the experience-driven formation of ocular dominance (OD) columns in the primary visual cortex binocular zone. In the mature brain, neuronal architecture is more stable but it remains subject to changes as part of an adaptive response to learning, aging, injury or disease [1–3]. In adulthood, neuronal structural alterations are largely confined to synapses and typically are the result of prolonged changes in activity at these (or nearby) synapses. Remarkably, many human brain disorders, including schizophrenia, autism, and various forms of mental disability, are correlated with changes in synaptic shape or density and are believed to be caused by an imbalance between neuronal excitation and inhibition. Detailed knowledge of the molecular programs that regulate the strength and number of synapses is important for understanding brain function, and ultimately this will provide insights into how these processes are dysregulated in neurological disorders.
Here we discuss recent findings on the physiological function of CNS regeneration inhibitors and their receptors in the naïve brain. Our primary focus is on the function of Nogo-A, oligodendrocyte myelin glycoprotein (OMgp), and chondroitin sulfate proteoglycans (CSPGs) at the synapse. Emerging evidence suggests that these proteins stabilize synaptic structure and also regulate activity-dependent neurotransmission.
A large and structurally diverse group of neuronal growth inhibitors
The adult mammalian CNS is a rich source of molecularly diverse growth inhibitory cues, including proteinaceous components, carbohydrates and lipids (Figure 1). Many repulsive axon guidance molecules, including semaphorins, ephrins, slits, and netrins, continue to be expressed in the CNS long after the initial scaffold of axonal connections has been established [4]. The extracellular matrix (ECM) components CSPGs and keratan sulfate proteoglycans (KSPGs) function as prominent inhibitors of neuronal growth [5–7]. Sulfatide, a major CNS myelin lipid, strongly inhibits retinal ganglion cell axon outgrowth [8]. Additional important players include the prototypic myelin-associated inhibitors (MAIs) Nogo-A, OMgp, and myelin-associated glycoprotein (MAG) [4, 9]. Nogo-A is the largest splice form of the reticulon 4 gene and comprised of at least two distinct growth inhibitory domains, called NogoΔ20 and Nogo66 (Figure 1). Because of their profound growth inhibitory effects on developing neurites in vitro, collectively these inhibitory cues are thought to contribute to the regenerative failure of injured CNS axons in vivo. Indeed, acute blockage of MAIs or CSPGs in spinal cord injured (SCI) animals has met with some success [5, 6, 10, 11]. However, germline ablation of one or several MAIs in mice results in inconsistent SCI regeneration phenotypes [12, 13]. Here we focus on the physiological role of CNS regeneration inhibitors in the naïve brain. For a more in-depth discussion of these molecules in the injured CNS we refer to recent reviews [4–6, 9, 11, 14].
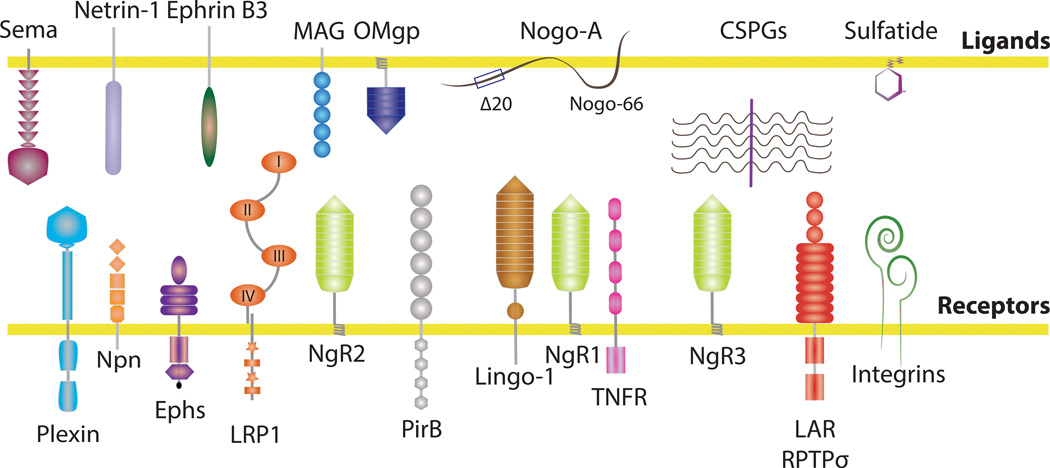
Figure 1. Prominent CNS inhibitors and their receptors.
CNS inhibitors abundantly found in the postnatal and adult brain and spinal cord include canonical axon guidance molecules such as semaphorins, ephrinB3, netrin-1 and the glial inhibitors Nogo-A, OMgp, MAG, CSPGs and sulfatide [4, 9]. Nogo, a member of the reticulon family is strongly expressed by neurons and oligodendrocytes. The splice variant Nogo-A harbors two distinct neurite outgrowth inhibitory regions: NogoΔ20 and Nogo-66. MAG is a sialic-acid binding Ig-lectin expressed by myelinating glia that inhibits neurite outgrowth in a developmental stage-dependent manner. OMgp is a heavily glycosylated lipid-anchored LRR family member expressed by neurons and oligodendrocytes. Inhibitory CSPGs in the extracellular matrix include lecticans (versican, neurocan, brevican, aggrecan), phosphacans and NG2. Sulfatide is a sulfated galactosylceramide that is abundant in CNS myelin. Neuronal surface receptors for CNS inhibitors have been identified. Plexins, neuropilins, and Ephs function as principal receptors for semaphorins and ephrins [4]. NgR1 and PirB support direct binding of Nogo66, MAG and OMgp. NgR1 is a lipid-anchored protein and in some cells is part of a tripartite receptor complex that also includes Lingo-1 and the TNFR family member p75 or Troy/Taj [9]. MAG binds directly to NgR2, β1-integrin and the low-density lipoprotein related receptor LRP1. Receptors for CSPGs include RPTPσ, LAR, NgR1, and NgR3 [19, 20, 22]. Indirect and integrin-dependent mechanisms of growth inhibition have been identified for NogoΔ20 and CSPGs [54, 55]. The neuronal receptors for sulfatide and NogoΔ20 are unknown.
Mechanistic studies have identified a large number of neuronal surface receptors for CNS regeneration inhibitors (Figure 1), some of which operate in a functionally redundant manner. Moreover, depending on the neuronal cell type, the same inhibitory cue may use different receptors [4]. The Nogo66 receptor 1 (NgR1) is the founding member of a small subfamily of lipid-anchored, leucine-rich repeat (LRR) proteins that also includes NgR2 and NgR3. NgR1 supports binding of the Nogo inhibitory peptide Nogo66, OMgp, and MAG. Similar to NgR1, the type-1 transmembrane protein paired Ig-like receptor B (PirB) can form a complex with Nogo66, MAG, or OMgp [4, 9]. Primary neurons deficient for NgR1 or PirB are largely resistant to growth cone collapse induced by acutely applied inhibitors. However, when plated on substrate-bound growth inhibitors, neurons deficient for PirB, but not NgR1, exhibit enhanced neurite outgrowth [15–18]. Sulfated proteoglycans, including HSPGs, KSPGs and CSPGs, are comprised of a protein core with covalently attached glycosaminoglycan (GAG) side chain(s) (Table 1). The chemical composition of the CS-GAG chain greatly influences the inhibitory nature of CSPGs and their binding affinity to the neuronal surface receptors leukocyte common antigen related protein (LAR), its homolog RPTPσ [19–21], or NgR1 and NgR3 [22]. Of particular interest is the di-sulfated GAG CS-E, since it exerts strong inhibitory activity toward primary neurons and binds directly to RPTPσ, NgR1 and NgR3 [21–23]. Additional receptors for CNS regeneration inhibitors have been identified; MAG not only interacts with NgR1 and PirB, but also associates with complex brain gangliosides, NgR2, β1-integrin, and LDL receptor related protein-1 (LRP1) (Figure 1). These molecular interactions contribute to various aspects of neuronal growth inhibition in vitro [24–27]. The biological significance of many of these ligand-receptor complexes in vivo, both in CNS health and injury, is still poorly understood, and remains a major focus of ongoing research efforts.
Table 1.
GAGs form a class of structurally related but functional diverse glycans
| glycosaminoglycans (GAGs) |
adult CNS expression |
upregulation by CNS injury |
inhibitory activity toward neurites in vitro |
neural binding partners/ receptors |
function in the nervous system |
references |
|---|---|---|---|---|---|---|
| CS-A (CS-4) GlcA-GalNAc4S |
most abundant |
no | modest | unknown | unknown | [104] |
| CS-B (dermatan) IdoA-GalNAc4S |
modest | yes | no | RPTPσ, NgR1, NgR3 | fibrotic scar formation |
[21, 22, 105, 106] |
| CS-C (CS-6) GlcA-GalNAc6S |
modest | yes | no | unknown | OD plasticity? | [23, 34, 104] |
| CS-D (CS-2,6) GlcA2S–GalNAc6S |
minor | ? | unknown | NgR1, NgR3 | OD plasticity? | [22, 34, 36] |
| CS-E (CS-4,6) GlcA-GalNAc4S6S |
minor | yes | very strong | RPTPσ, NgR1, NgR3 | OD plasticity? blocks CNS axon regeneration |
[21–23, 34] |
| Keratan sulfate (KS1 and KS2) |
brain, spinal cord, highest in cornea |
yes | strong | unknown | axon guidance, blocks CNS regeneration |
[7] |
| Heparan sulfate (HS) GlcA-GlcNAc GlcA-GlcNS IdoA-GlcNS IdoA(2S)-GlcNS IodA-GlcNS(6S) IdoA(2S)-GlcNS(6S) |
yes, prominent members include glypicans and syndecans |
yes | context- dependent growth promotion or growth inhibition |
NgR1, NgR3, EphB, CAMs, FGFRs, LARs, semaphorins, slits |
axon guidance, synaptogenes is, synaptic structure, neurotransmission |
[22, 42, 44, 57, 66, 68, 71, 73] |
| Hyaluronan GlcA-GlcNAc (non-sulfated) |
abundant in perineuronal nets (PNNs) |
no | largely inert | Toll-like receptor 2 | OD plasticity, myelination |
[23, 40] |
Putting the brakes on neuronal plasticity
In the postnatal and juvenile brain, the structure of many neurons is refined in an experience-dependent manner in order to optimize internal processing of external inputs. Refinement occurs during the critical period (CP), a time-window during which specific inputs result in heightened network plasticity. After the CP, networks are mature and structurally much more stable ensuring optimal information flow and processing. CPs are of fundamental importance in molding microcircuits in various brain regions associated with sensory perception, motor learning, and language. Pharmacological and genetic manipulations that perturb the timing of CPs have begun to shed light on the molecular basis for network stability and how it can be modulated (Figure 2). In the visual system, enhanced maturation of GABAergic interneurons, or local infusion of benzodiazepines, increases intracortical inhibition and expedites the onset and closure of the CP. Conversely, reducing GABA function by genetic ablation of GAD67, or dark-rearing of animals, delays CP onset [28]. Seemingly unrelated manipulations, such as antagonizing inhibitors of neuronal growth, can have a profound impact on CP closure (Figure 2). Extracellular cues that put the brakes on neuronal remodeling after experience-dependent refinement is complete include Nogo-A/B [29] and a small subset of classical MHC1 molecules [30]. Similarly, NgR1- and PirB-deficient mice fail to stabilize neuronal connectivity in the primary visual cortex (V1), resulting in an expansion of the CP into adulthood [29, 31]. Infusion of the CS-GAG-digesting enzyme chondroitinase-ABC (ChaseABC) into the visual cortex of adult rats is sufficient to increase visual experience-driven neuronal plasticity [32]; remarkably, this can also promote recovery from amblyopia inflicted by reverse suture of one eye during the CP [33]. Toward the end of the CP, the extracellular matrix in V1 undergoes substantial remodeling. CSPG levels increase and condense into ternary structures known as perineuronal nets (PNNs). Interestingly, the ratio of sulfated CS-4 to CS-6, and not the overall CSPG expression, is crucial for the closure of the CP [34]. Several independent lines of evidence show that genetic approaches that perturb PNNs lead to an expansion of the CP into adulthood [34–37]. The recent identification of NgR1, and its close homologue NgR3, as receptors for CSPGs suggest that Nogo-A/B and CSPGs share overlapping receptor components and perhaps signal through related receptor complexes [22]. Moreover, tying Nogo-A, CSPGs, NgR1, and possibly PirB and MHC1, to the same receptor complex may explain why individual manipulation of each of these molecules results in increased OD plasticity beyond the CP. As discussed below, Nogo-A, OMgp, and NgR1 negatively regulate neurotransmission, and similar to what is observed following alteration of GABA signaling, they also influence the balance between excitatory and inhibitory transmission, thereby controlling the onset and closure of the CP. Collectively, these studies show that Nogo-A, CSPGs and their receptors restrict neuronal growth at the end of the CP and thereby help to stabilize and maintain the structure of mature microcircuits. Although beneficial for network stability in the naïve CNS, Nogo-A and CSPGs directly contribute to the growth inhibitory nature of adult CNS tissue, and as such negatively influence network repair flowing injury. The close overlap of molecules that consolidate structure of neuronal circuits at the end of the CP and limit axonal growth and sprouting following injury is remarkable, and suggests that one important physiological role of CNS regeneration inhibitors is to ensure long-term network stability.
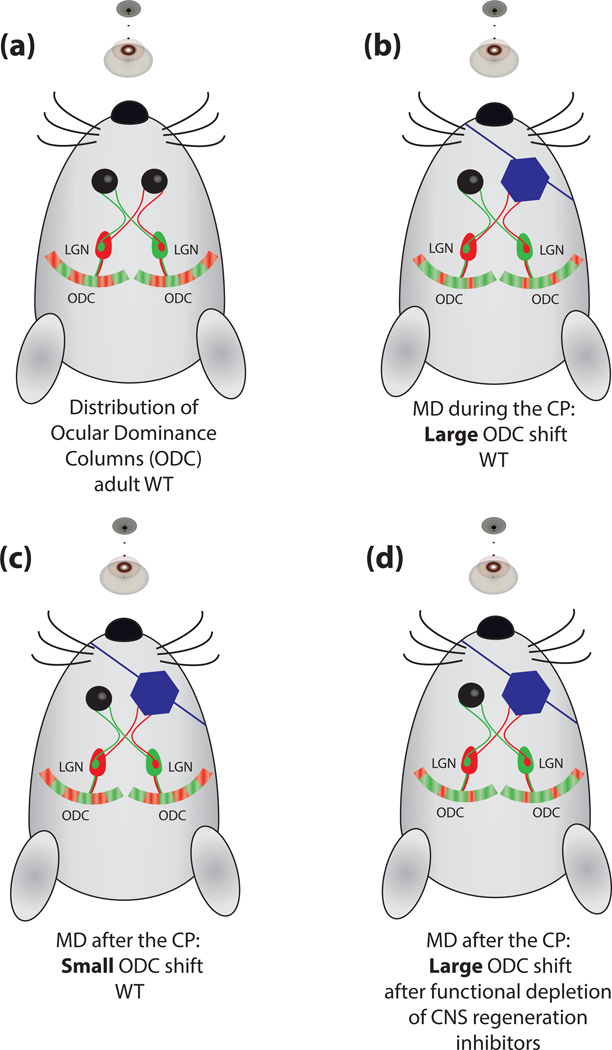
Figure 2. CNS inhibitors consolidate neuronal structure in the visual cortex.
A) In the developing visual system, inputs from both eyes first overlap in the primary visual cortex (V1). Over time, visual experience-driven competition segregates eye specific (red and green) inputs into specific cortical regions, also called ocular dominance columns (ODC), in which one eye will dominate both functionally and anatomically. B) Nearly 50 years ago, David Hubel and Torsten Wiesel observed that following monocular deprivation (MD) during the CP, the non-deprived (green) eye shifts and expands its representations in the binocular zone of V1 at the expense of the deprived (red) eye. C) This shift in ODC occurs in layer IV of V1 and is most robust during the CP, as MD in adulthood results in a comparatively small shift in ODC. D) Toward the end of the CP, the extracellular matrix forms aggregates around fast-spiking parvalbumin-positive interneurons that are called perineuronal nets (PNNs). PNNs are comprised of CSPGs in association with hyaluronic acid, and tenascins. Perturbation of PNNs with pharmacological or genetic manipulations leads to a more pronounced shift in ODC following MD [32, 34–37]. In mice, functional ablation of Nogo-A/B, NgR1, or PirB through the germline results in robust ODC shift following MD throughout adulthood [29, 31].
Nogo-A and CSPGs promote dendritic spine maturation and stability
Is there any evidence that CNS regeneration inhibitors regulate neuronal structure in a cell-autonomous manner? Although many growth inhibitors in the adult CNS are expressed by glia, neuronal expression has clearly been demonstrated (Figure 3). Nogo-A and OMgp, for example, are expressed by excitatory and inhibitory neurons and are found in pre- and postsynaptic density fractions [38, 39]. Several CSPGs are expressed by glia and neurons and are enriched in PNNs and the ECM near synapses [40]. Similarly, synaptic localization of their cognate receptors has been reported, including NgR1 [41, 42], PirB [31], LAR, RPTPσ [43–46], LRP1 [47] and integrins [48]. In hippocampal pyramidal neurons, NgR1 and Nogo-A regulate the complexity of apical and basal dendrites and loss of NgR1 does not alter dendritic spine density but leads to more immature spines in CA1 neurons [42, 49]. Acute knockdown of neuronal Nogo-A in layer V pyramidal neurons of the rat neocortex increases the number of filopodia-like dendritic protrusions and decreases spine density in vivo [50]. This suggests that neuronal Nogo-A can regulate dendritic spine density and morphology in a cell-autonomous manner. In vivo, the combined loss of all three Nogo receptors (NgR1,2,3−/−) results in an increase in dendritic arborization and more dendritic spines in CA1 pyramidal neurons of juvenile mice [51]. Collectively, these studies suggest that the actin cytoskeleton of dendritic spines is a major target for Nogo-A and NgRs to stabilize neuronal architecture in the juvenile and adult CNS.
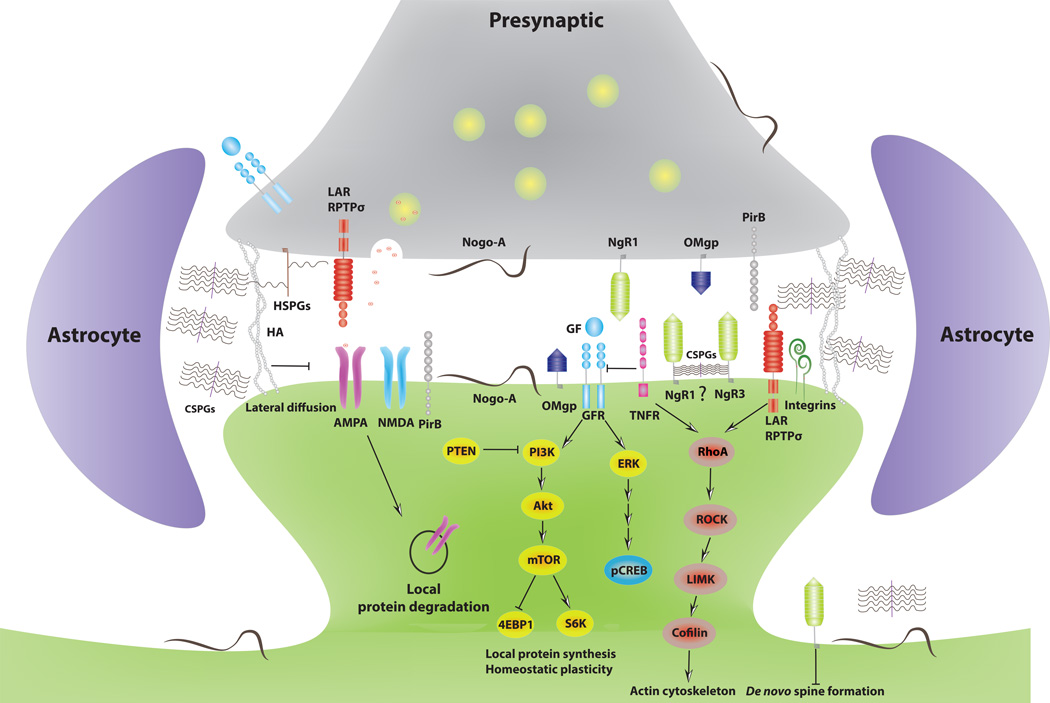
Figure 3. CNS inhibitors regulate synaptic plasticity.
Independent lines of evidence show that many CNS inhibitors and their receptors are present at or near synapses. Nogo receptors suppress dendritic spine formation in the juvenile CNS and promote spine maturation and stability in adulthood [42, 49, 51]. Emerging evidence suggests that the Nogo-A/NgR1 complex antagonizes growth factor (FGF2 and BDNF) signaling by suppressing the activity of the mammalian target of rapamycin complex 1 (mTORC1) and MAP kinase signaling from Erk1/2 to nuclear p-CREB pathway. mTORC1 is activated by PI3K–AKT signaling and promotes translation of synaptic mRNAs, including NMDA receptor and AMPA receptor subunits, via phosphorylation of specific downstream effectors such as elF4E binding protein (4EBP1) and p70S6-kinase [38, 78]. One negative regulatory pathway of mTOR includes phosphatase and tensin homolog (PTEN), a synaptic protein associated with autism spectrum disorders. How exactly NgR1 activates downstream signaling pathways at the synapse is largely unknown but may involve RhoA-ROCK-LIMK-cofilin mediated breakdown of the actin cytoskeleton. The extracellular matrix components hyaluronic acid (HA), CSPGs and HSPGs are found near synapses where they form surface compartments that limit lateral diffusion and exchange of AMPA-type glutamate receptors [40, 81]. The CSPG receptors LAR and RPTPσ are present both pre- and post-synaptically, but their CSPG-dependent function at the synapse has not yet been established. Both NgR1 and NgR3 bind CSPGs, but whether this interaction takes place at or near spine synapses is not known. Integrins have been implicated in the regulation of homeostatic synaptic plasticity, and recent evidence suggests an important role for integrin signaling in CSPG-mediated spine morphogenesis [52].
In mouse hippocampal slice cultures, local digestion of perisynaptic CSPGs in the stratum radiatum preserves PNNs and leads to an increase in CA1 dendritic spine motility and formation of spine head protrusions [52]. Perisynaptic CSPGs may be of neuronal or astrocytic origin. Mechanistic studies revealed that perisynaptic CSPGs restrict spine structural plasticity in a β1-integrin/focal adhesion kinase (FAK-pY397)-dependent manner [52]. β-integrin activation in hippocampal neurons regulates synapse density and spine stability in a RhoA and Ca2+-calmodulin/CamKII-dependent manner [53]. Similar mechanisms operate in the axonal compartment, as Nogo-A and CSPGs block β1- and β3-integrin activation, decrease FAK-pY397 and increase RhoA activity to inhibit axonal growth [54–56]. We still have yet to learn how neuronal Nogo-A and CSPGs influence neuronal structure and synaptic function in the hippocampus and visual cortex (Box 3). Furthermore, it will be important to define the extent to which different types of CNS regeneration inhibitors influence synaptic function, which of their effects are mediated by RPTPσ, LAR, NgR1 or NgR3, and how these interactions regulate integrin function in nervous system health and disease (Box 3).
Box 3: Outstanding questions.
The receptor mechanisms that govern dendritic and axonal functions of Nogo-A, OMgp and CSPGs are still incompletely understood. What is the molecular identity of the neuronal NogoΔ20 receptor? What are the transmembrane-spanning co-receptors that complex with NgR1, NgR2 and NgR3 in the dendritic compartment, and what are the “anti-synaptogenic” ligands of NgR1, NgR2 and NgR3?
What is the functional significance of the interaction between NgR1 and NgR3 with CSPGs and HSPGs in the axonal and dendritic compartments of naïve (uninjured) CNS neurons?
How do CNS regeneration inhibitors regulate protein levels of AMPA and NMDA type glutamate receptors?
Nogo-A/NgR1 and CPSGs consolidate neuronal structure at the end of the CP in the visual system. Do these molecules also regulate duration of the CP in the auditory cortex, somatosensory cortex, and perhaps in brain regions associated with higher-level processing such as language acquisition?
Does manipulation of CNS inhibitors offer therapeutic opportunities following brain or spinal injury, mental illness, or help to erase certain types of memories in the adult brain associated with excessive fears, anxiety, or addiction?
Inhibition of synaptogenesis
Over the past several years, much progress has been made in the identification of neuronal cell-surface molecules and astrocyte-derived secreted factors that promote synaptogenesis [40, 57, 58]. Much less is known about the mechanisms that prevent the formation of supernumerary synapses. Members of the semaphorin family have been found to positively or negatively influence the formation and maturation of both excitatory and inhibitory synapses [59, 60]. Moreover, in acute hippocampal slices, bath-applied Sema3F greatly increases the frequency and amplitude of mEPSCs in dentate granule neurons [61], and it has been shown that the ectodomain of soluble Sema5B triggers rapid synapse elimination in primary hippocampal neurons [62].
Recent work highlights a central role for LRR domain-containing proteins in promoting synaptogenesis [63]. However, NgR1, NgR2, and NgR3 appear to be exceptions, as these LRR proteins inhibit formation of excitatory synapses [51]. In dissociated hippocampal pyramidal neurons, acute RNAi knock-down of individual Nogo receptor family members greatly increases dendritic spine density, whereas their overexpression leads to a significant reduction in spines. Time-lapse studies in hippocampal slice cultures further reveal that NgRs attenuate de novo formation of dendritic spines in CA1 pyramidal neurons but do not alter the rate of dendritic spine elimination [51]. In wild-type mice there is an age-dependent increase in synaptic stability as animals transition from adolescence to adulthood [64]. Conditional ablation of NgR1 in 1-year-old mice is sufficient to significantly increase synaptic turnover and reverse it to adolescent levels [65]. Loss of NgR1 does not increase synapse density in hippocampal CA1 pyramidal neurons; however, juvenile mice null for all three Nogo receptors (NgR1,2,3−/−) show a significant increase in synapse density in vivo [42, 51]. The identity of the “anti-synaptogenic” NgR ligand(s) remains largely elusive (Box 3). Biochemical studies revealed that OMgp and Nogo-A, but not Nogo-B, are present in synaptic density fractions and may function as “anti-synaptogenic” NgR1 ligands [38]. Several additional NgR1 ligands have been identified (Table 2), some of which have been found to antagonize Nogo66 function in the axonal compartment, and it will be interesting to explore their role at synapses. MAG, a ligand for NgR2, is selectively expressed by myelinating glia [24] and as such is an unlikely candidate to influence synaptogenesis. Of interest is the interaction of NgR1 and NgR3 with the GAG portion of neural CSPGs and HSPGs [22]. Numerous studies in invertebrates and vertebrates have established that proteoglycans regulate synaptogenesis [44, 57, 66], synaptic structure [52, 67], and activity-dependent neurotransmission [68, 69]. Some of these functions are exerted by integrins and LAR protein family members. Notably, the function of several repulsive axon guidance molecules can be regulated in a proteoglycan-dependent manner [70–73]. In the presence of HSPGs, Sema5A and RPTPσ promote neurite outgrowth, whereas in the presence of CSPGs, they inhibit growth [70, 71]. Enzymatic digestion of the CSPGs brevican and neurocan promotes the formation of synaptic puncta in neuron-glia co-cultures [74]. Additional studies are needed to establish whether this is caused by a shift in the HSPG to CSPG ratio, and if this leads to altered activation of RPTPσ, NgR1 or NgR3.
Table 2.
Nogo receptor family interactions
| ligand | ligand localization in the CNS |
function of the interaction in the axonal compartment |
function of the interaction in the dendritic compartment |
references | |
|---|---|---|---|---|---|
| NgR1 | Nogo66 | pre- and postsynaptic, myelin, |
growth cone collapse | synaptic strength | [15, 18] |
| NgR1 | MAG | myelin | growth cone collapse | [15, 18] | |
| NgR1 | OMgp | pre- and postsynaptic, myelin |
growth cone collapse | synaptic strength | [15, 18] |
| NgR1 | NgR1, NgR3 | neuron pre- and postsynaptic |
? | ? | [22] |
| NgR1 | Lingo-1 | neuron, pre-synaptic | co-receptor | ? | [9] |
| NgR1 | p75, Troy/Taj | neurons, astrocytes | co-receptor | ? | [9] |
| NgR1 | GT1b | mostly neuronal | co-receptor | ? | [107] |
| NgR1 | APP, Aβ | neurons and glia | decreases Aβ production |
? | [102] |
| NgR1 | FGF2, FGF1 | ECM/proteoglycans | decreased branching | synaptic strength | [42] |
| NgR1 | BLyS | astrocytes, microglia, macrophages |
growth inhibition | ? | [108] |
| NgR1 | LGI1 | neuronal, excitatory synapses |
Nogo66 antagonist | ? | [89] |
| NgR1 | ADAM22 | postnatal neurons | enhances binding of LGI1 |
? | [89] |
| NgR1 | MT3-MMP | microglia, ECM | NgR1 shedding | NgR1 shedding? | [91] |
| NgR1 | Crtac1B/ LOTUS | neurons, lateral olfactory tract axons |
Nogo66 antagonist, LOT fasciculation |
? | [109] |
| NgR1 | CSPGs | ECM, perineuronal nets | inhibition together with NgR3 |
? | [22] |
| NgR1 | HSPGs | neurons and glia | ? | ? | [22] |
| NgR1 | Olfactomedin-1 | DRG and RGC neurons | NgR1 antagonist | ? | [110] |
| NgR2 | MAG | myelin | inhibition when overexpressed in vitro |
[4] | |
| NgR2 | APP | neurons and glia | enhanced APP processing and Aβ production |
? | [103] |
| NgR3 | CSPGs | ECM, perineuronal nets | inhibition together with NgR1 |
? | [22] |
| NgR3 | HSPGs | ECM | ? | ? | [22] |
| NgR3 | APP | neurons and glia | APP processing | ? | [103] |
CNS regeneration inhibitors regulate Hebbian forms of synaptic plasticity
The observation that NgR1 attenuates fibroblast growth factor 2 (FGF2) function in primary neurons [42], coupled with the known role of FGF2 at excitatory synapses [75], prompted investigations of NgR1 at synapses. In acute hippocampal slices, local application of FGF2 to Schaffer-collateral/CA1 synapses enhances LTP induced by theta-burst stimulation (TBS) in NgR1−/−, but not NgR1+/+ slices [42]. Moreover, acute antibody blockade of Nogo-A or NgR1 leads to increased LTP in CA1 neurons [76]. Conversely, acute application of soluble Nogo66 or OMgp to Schaffer-collateral/CA1 synapses decreases LTP in wildtype, but not NgR1−/−, hippocampal slices [38]. Additional studies revealed that p75, a co-receptor in the NgR1 receptor complex for certain inhibitory functions, is dispensable, and PirB plays only a minor role in OMgp-mediated inhibition of LTP [38]. Collectively, these studies provide strong evidence that Nogo-A, OMgp, NgR1 and PirB regulate activity-dependent synaptic strength. The Nogo-A inhibitory peptide NogoΔ20 does not bind to NgR1 or PirB and when applied to Schaffer-collateral/CA1 synapses 5 min before TBS, decreases post-tetanic potentiation and, counter-intuitively, is followed by a rapid and prolonged increase in LTP [76]. Although the underlying mechanism(s) of this enhanced synaptic response remains to be understood, it is known that acute application of NogoΔ20 triggers rapid, EHD4/pincher-dependent endocytosis of surface-bound NogoΔ20 [77]. Rapid internalization of receptor-bound NogoΔ20 could desensitize neurons toward Nogo-A [76].
Interestingly, manipulations that acutely affect Nogo-A/NgR1 signaling have the most pronounced effects on synaptic strength. Germline ablation of NgR1, PirB or Nogo-A in mice does not significantly alter basal synaptic transmission or LTP at Schaffer collateral/CA1 synapses, however, LTD in juvenile NgR1−/− hippocampal slices is absent [38, 42, 76]. The disparity between acute blockage of Nogo-A and NgR1 on LTP and gene ablation through the germline suggests the existence of powerful compensatory mechanisms. Biochemical analysis of primary hippocampal neurons following RNAi knock-down of Nogo-A and NgR1 have begun to provide insight into how these molecules may regulate synaptic strength. Two days following acute knock-down of Nogo-A or NgR1, a global increase in AMPA (GluA1 and GluA2) and NMDA (GluN1, GluN2a and GluN2b) receptor subunit expression is observed [78]. CSPGs are known to influence activity-dependent synaptic strength. Perturbation studies with ChaseABC, hyaluronidase or germline ablation of brevican, neurocan, or RPTPσ, demonstrate a decrease in early or late LTP [40, 79, 80]. One mechanism by which CSPGs may influence synaptic transmission is by functioning as a barrier for lateral mobility of AMPA type glutamate receptors in dendritic spines [81]. Although additional studies are needed to determine the full spectrum of Nogo-A-, OMgp-, and CSPG-mediated regulation of activity-dependent synaptic strength, several independent lines of evidence clearly show that CNS regeneration inhibitors and their receptors not only regulate neuronal structure, but also influence Hebbian forms of synaptic plasticity (Figure 3).
Crosstalk between pro- and anti-plasticity signaling cascades
Finding the right degree of synaptic structural stability and functional plasticity is a life-long challenge for many neurons and of critical importance for proper nervous system function. Mounting evidence suggests that there is extensive crosstalk between “pro-” and “anti-plasticity” signaling pathways (Figure 4). Pretreatment of primary neurons with brain-derived neurotrophic factor (BDNF) attenuates myelin inhibition in a p-CREB-dependent manner [82, 83]. Moreover, NogoΔ20 decreases CREB activation and leads to down-regulation of neuronal growth programs [77]. Conversely, pretreatment of primary neurons with crude myelin or Nogo66 attenuates BDNF-elicited activation of pAKT (Ser473) and p70S6K (Thr389) [38], and NogoΔ20 leads to a decrease in pAKT (Ser473) [84]. In a similar vein, CSPG binding to RPTPσ attenuates TrkB activity [85]. In synaptic density fractions of NgR1−/− hippocampus, activation of Erk1/2 is increased [38] and NgR1−/− neurons are more sensitive to FGF2/FGFR signaling [42]. Collectively, these studies suggest an antagonistic relationship between CNS regeneration inhibitors and neurotrophic factor signaling pathways (Figure 4). Crosstalk between these pathways at the molecular level is only now beginning to be defined. In primary hippocampal neurons, RNAi knock-down of NgR1 or Nogo-A leads to an increase in PSD95, AMPA, and NMDA receptor subunit expression that is blocked in the presence of rapamycin [78]. This suggests that NgR1/Nogo-A signaling inhibits mTOR complex 1 (TORC1)-mediated synthesis of synaptic proteins. Consistent with this idea, Nogo66 attenuates p70S6K (Thr389), a downstream target of TORC1, in primary cortical neurons [38]. mTOR is found at excitatory synapses, where it regulates cap-dependent translation of local mRNAs [86], including BDNF-induced translation and surface expression of GluA1 [87]. Thus, one mechanism for the observed crosstalk between neurotrophic factor and growth inhibitors in neurons is their opposing regulatory influences on mTOR-dependent protein translation. Additional crosstalk may occur at the transcriptional level by opposing regulation of p-CREB. A similar antagonistic interaction between BDNF and CSPGs has recently been reported [85]. The inverse regulation of signaling pathways triggered by CNS regeneration inhibitors and growth factors provides an economical means for neurons to adjust synaptic structure and strength to meet specific demands dictated by network activity.
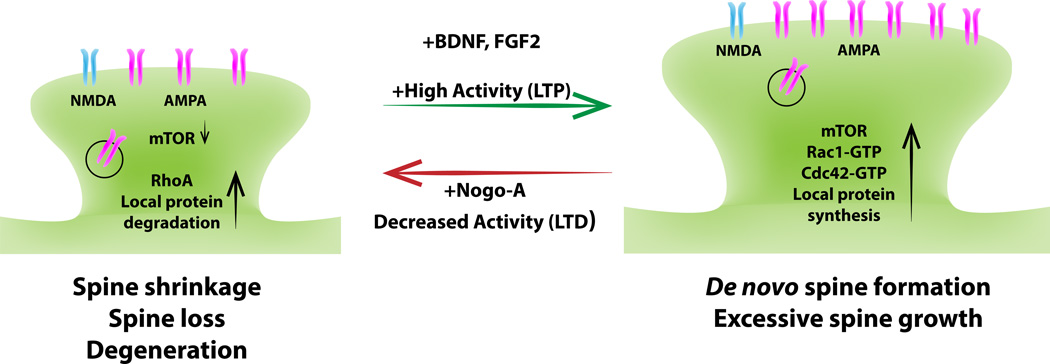
Figure 4. Antagonistic action of growth factors and growth inhibitors at the synapse.
Dendritic spines are small actin-rich protrusions that form the postsynaptic compartment of the majority of excitatory synapses. Depending on their morphological appearance, spines are categorized into different subclasses: thin, stubby, and mushroom-type. Many human brain disorders are associated with abnormal spine density, shape or volume. Long-term potentiation (LTP) of synaptic transmission is correlated with an increase in spine size or formation of new spines, whereas long-term depression (LTD) is associated with spine shrinkage or loss. On a molecular level, changes in spine density, shape or volume are primarily a reflection of reorganization of the actin cytoskeleton. Typically, activation of the small GTPases Rac1 and cdc42 by synaptic GEFs promotes the formation and growth of dendritic spines, whereas activation of RhoA by synaptic GEFs causes spine shrinkage and synapse loss. Neuronal activity regulates local protein turnover at postsynaptic sites. Local protein synthesis can be increased by BDNF-elicited activation of mTORC1-dependent protein translation and protein degradation. Emerging evidence suggests that CNS inhibitors (particularly Nogo-A) antagonize neurotrophic factor signaling cascades at the synapse. Nogo-A/NgR1 signaling negatively regulates mTORC1 dependent local protein synthesis.
Regulation of Nogo receptor function
The prominent role of Nogo-A, OMgp and NgR1 in neuronal plasticity suggests that the NgR1 signaling pathway is not constitutively active but subject to strict regulation. Table 2 lists a growing number of NgR1 binding partners, including molecules with antagonistic action toward Nogo66. Of these, leucine-rich glioma inactivated (LGI1) is of particular interest because it enhances AMPA receptor-mediated synaptic transmission in hippocampal slices [88]. NgR1 and ADAM22 collaborate to form a functional LGI1-binding complex [89]. Both NgR1 and ADAM22 are enriched postsynaptically, and it will be interesting to explore whether LGI1 sequesters NgR1 away from ligands that attenuate activity-dependent synaptic strength. Unlike antibody blocking of NgR1, however, acute application of LGI1 to hippocampal slices does not increase LTP in CA1 neurons, suggesting a more complex mechanism for LGI1 function than simply blocking synaptic NgR1 [88]. ADAM22 shares homology with a large family of transmembrane ADAM metalloproteases but is catalytically inactive. Nevertheless, NgR1 function is regulated by shedding of its ectodomain from the neuronal surface. In cortical neurons, basal shedding of endogenous NgR1 is mediated by membrane-type matrix metalloproteinases-3 (MT3-MMP). In the presence of tissue inhibitor of metalloproteinase-2 (TIMP-2) and TIMP-3, NgR1 shedding is largely absent [90, 91]. The MT3-MMP processing site on NgR1 is located distal to the LRR cluster at position Ala358. Therefore, the released NgR1 ectodomain fragment retains Nogo, MAG, and OMgp (but not GAG) binding activity and may exert a dominant-negative function [91]. Physiological MMP activity is required for dendritic remodeling, synaptogenesis and LTP, and it appears likely that this is accomplished, at least in part, through regulation of Nogo receptor shedding. The discovery of endogenous regulatory mechanisms for these inhibitors and their receptors is of interest from a biological point of view, and may be exploited for therapeutic purposes following CNS injury or disease.
Coordination of structural and functional synaptic plasticity
Prolonged changes in synaptic activity alter neuronal structure. Since growth inhibitors in the adult CNS have the potential to regulate synaptic structure and influence synaptic efficacy, these cues are well suited for linking changes in electrical activity to neuronal structural alterations. In the adult neocortex and hippocampus, neuronal activity regulates the NgR1 promoter [92]. Increased voluntary exercise or administration of kainic acid leads to a reduction of NgR1 and myelin inhibitor expression in vivo [92, 93]. There appears to be an inverse relationship between activity-dependent down-regulation of NgR1 and activity-dependent upregulation of neurotrophic factors such as FGF2 and BDNF [92, 94, 95]. Coordination of activity-induced upregulation of molecules that promote synaptic plasticity with simultaneous downregulation of synaptic plasticity inhibitors may prime neurons for adaptive structural changes. When coupled with more local regulatory mechanisms, such as activity-dependent release of BDNF at synaptic sites or local shedding of NgR1, strengthening of individual synapses and changes in morphology may be achieved rapidly and with high temporal and spatial specificity. Because of the apparent antagonistic effects between growth factors and myelin inhibitors on synaptic structure and function, and their opposite regulation by prolonged neural activity, it is tempting to speculate that similar to BDNF, Nogo-A/NgR1 participate in synaptic scaling and homeostasis (Box 3).
Are some things not meant to be undone?
Recent work indicates that a significant fraction of synapses in the mature brain is structurally stable over a very long time scale [1, 64], thus, it seems quite likely that stable synaptic contacts are required for long-term memory storage. Perturbation studies suggest that CSPGs and Nogo-A play important roles in the stabilization and maintenance of axonal, dendritic and synaptic structure. The diversity and functional redundancy of known CNS growth inhibitors begs the question of why the CNS of higher vertebrates has gone to enormous trouble to evolve mechanisms of turning off adult neuronal growth and regeneration. Although the growth inhibitory nature of adult CNS tissue likely contributes to the regenerative failure of injured neurons, the emerging physiological role of CNS regeneration inhibitors as a stabilizing force that limits exuberant growth in the healthy (uninjured) mature brain may be critical for proper nervous system function and formation of long-lasting memories. Lifting the stabilizing constraints in the adult CNS to promote neuronal plasticity for therapeutic purposes may need to be carefully controlled, both temporally and spatially. Similar to developmental CPs, a more plastic adult CNS may need to be subjected to task-specific rehabilitative training as a means to refine newly formed circuits in an activity-dependent manner and to maximize behavioral outcomes following stroke or other forms of CNS injury [96]. Caution must be exercised, as some forms of neuronal plasticity may be maladaptive, and too much growth and sprouting has been associated with severe complications including allodynia, seizures and epilepsy. Recent evidence suggests that interfering with growth inhibitory signaling cascades may have detrimental consequences for intellectual abilities by impairing key neurologic functions such as long-term memory storage and mental health. Loss of NgR1 does not impair hippocampal learning in the Morris Water Maze (MWM), and forebrain-specific overexpression of NgR1 does not alter short-term memory [97, 98]; however, it does impair the formation of long-lasting memory in the MWM task [98]. In the amygdala, ChaseABC digestion of CS-GAGs renders acquired fear memory susceptible to erasure, supporting the notion that stable synapses are important for long-term memory storage [99]. Key open questions concern the extent to which the plethora of known CNS regeneration inhibitors (Figure 1) exert similar functions, protecting certain types of memory from erasure, and whether these molecules can be targeted for therapeutic purposes following injury or disease without impairing vital neurologic functions (Box 3).
Concluding remarks
Recent work examining the physiological role of CNS regeneration inhibitors in the naïve brain of juvenile and adult rodents reveals novel functions for Nogo-A, OMgp, CSPGs and their receptors in the dendritic compartment of different types of CNS projection neurons. From a biological point of view it will be important to understand how these growth inhibitory molecules cross-talk with growth-promoting mechanisms to strike a delicate balance that keeps a neuron in the “goldilocks zone” of synaptic plasticity (Box 3). Balancing the degree of excitation vs inhibition and finding the right degree of synaptic stability is absolutely critical for proper nervous system function. Too much dendritic remodeling and synaptic turnover may erase memories, and conversely, too much stability in microcircuits may compromise experience-dependent network refinement, formation of new synapses and acquisition of specific types of new memory. As we learn more about CNS regeneration inhibitors, they may in many ways be viewed as the counter players of neurotropic factors. In addition to their role in controlling neuronal architecture, regeneration inhibitors regulate activity-dependent synaptic strength, possibly through blocking mTOR-dependent protein synthesis at synapses. Because altered mTOR activity in the brain has been linked to cognitive and social dysfunction, and mutations in NgR1 and Nogo-A have been associated with schizophrenia [100, 101], a detailed understanding of the physiological role of CNS regeneration inhibitors in the developing and adult brain will be of great interest both from a biological and clinical point of view.
Insights into the physiology of CNS regeneration inhibitors in the naïve brain
CNS regeneration inhibitors serve as negative regulators of synaptic plasticity
Nogo-A restricts synaptic plasticity by antagonizing neurotrophic factor signaling
Box 1: GAGs have evolved as major regulators of neuronal function.
Glycosaminoglycans (GAGs) are comprised of repeating (up to ~20–200) disaccharide units and form long, unbranched polymers. The chemical composition of the disaccharide unit and its sulfation pattern can vary, giving rise to different types of GAGs, including chondroitin sulfate (CS), keratan sulfate (KS), heparan sulfate (HS), hyaluronic acid (HA) and heparin. In proteoglycans, CS-, HS-, and KS-GAGs are covalently linked to a protein core. HA and heparin GAGs are not covalently linked to proteins. CS-GAGs are composed of glucuronic acid (GlcA) and N-acetylgalactosamine (GalNAc) repeats. During biosynthesis, GalNAc is sulfated at position C4 by chondroitin-4 sulfotransferase 1 (C4ST-1) and C6 by chondroitin-6 sulfotransferase-1 (C6ST-1). Depending on the sulfation pattern and epimerization of GlcA at position C5 into iduronic acid (IdoA), CS-GAGs are further classified into CS-A, CS-B, CS–C, CS-D, and CS-E. Of importance for regulation of neuronal structure is the observation that different CS-GAG subclasses contribute to various degrees to the growth inhibitory nature of CSPGs. The receptor binding specificity and biological activity of CS-GAGs is regulated by sulfation [21, 22] and the ratio of CS-4-sulfation/CS-6-sulfation is a critical regulator of OD plasticity in the mouse visual cortex [34]. The CS-GAG binding patterns of RPTPσ, NgR1 and NgR3 are largely overlapping. These receptors strongly interact with CS-B and CS-E but fail to support binding of CS-A and CS-C [21, 22]. Few proteoglycans (e.g. aggrecan) can carry two different types of GAG chains (e.g. KS-GAGs and CS-GAGs). A recent study found that KS-GAGs exert growth inhibitory activity toward growing axons in vitro and regenerating fibers following spinal cord injury in vivo [7]. Heparan sulfate proteoglycans form a large class of HS-GAG proteins, with prominent members including transmembrane syndecans and the lipid-anchored glypicans. HSPGs are often an integral part of large surface receptor complexes with diverse functions. In the nervous system, HSPGs have been shown to participate in axonal growth and guidance, synapse formation and maturation, and synaptic transmission. Hyaluronan (HA) is a major component of the extracellular matrix. In the brain, HA associates with tenascins and CSPGs and is an integral part of perineuronal nets.
Box 2: Candidate ligands that regulate Nogo receptor dependent synaptic function.
A large number of NgR1 binding partners has been identified, including co-receptors and agonistic/antagonistic ligands. Thus far, most studies have focused on the role of these interactions in the axonal compartment. Of interest, many NgR binding partners are found at synapses and have been shown to influence synapse structure, maturation, or efficacy of transmission. Proteins with known synaptic function include Nogo-A, OMgp, FGF2, APP, LGI1, HSPGs and CSPGs. Thus, it will be important to examine which synaptic functions by any of these ligands are exerted through association with Nogo receptor family members. The synaptic function of the NgR1 antagonists LGI1, Crtac1B/LOTUS and olfactomedin-1 is poorly understood, but may offer new approaches to modulate NgR1 function. All three NgR family members interact with and have been implicated in APP processing. Loss of endogenous NgR1 in an Alzheimer disease (AD) mouse model increases Aβ deposition [102]. In the same AD model, the interaction of NgR2 with APP favors processing of APP by BACE1 and loss of endogenous NgR2 reduces Aβ production and formation of amyloid plaques [103].
Acknowledgements
We thank Alex Kolodkin and members of the Giger lab for critical reading of the manuscript. This work was supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (AMRF), the NIH Cellular and Molecular Biology Training Grant T32-GM007315 and R01-NS081281.
Abbreviations
- LOT
lateral olfactory tract
- MAG
myelin-associated glycoprotein
- OMgp
oligodendrocyte myelin glycoprotein, p75
- Troy/Taj
members of the TNF receptor superfamily
- GT1b
complex ganglioside
- APP
amyloid precursor protein
- Aβ
neurotoxic fragment of APP
- FGF
fibroblast growth factor
- LGI1
leucine-rich glioma inactivated
- BLyS/CD253
TNF family member B lymphocyte stimulator
- MT3-MMP
membrane-type matrix metalloproteinases-3
- ADAM22
a disintegrin and metalloproteinase domain-containing protein 22
- Crtac1B/ LOTUS
cartilage acidic protein-1B/ lateral olfactory tract usher substance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest
References
- 1.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 2.Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282(5391):1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 3.Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7(3):269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 4.Giger RJ, Hollis ER. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2(7):a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Alias G, Fawcett JW. Training and anti-CSPG combination therapy for spinal cord injury. Exp Neurol. 2012;235(1):26–32. doi: 10.1016/j.expneurol.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Imagama S, et al. Keratan sulfate restricts neural plasticity after spinal cord injury. J Neurosci. 2011;31(47):17091–17102. doi: 10.1523/JNEUROSCI.5120-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winzeler AM, et al. The lipid sulfatide is a novel myelin-associated inhibitor of CNS axon outgrowth. J Neurosci. 2011;31(17):6481–6492. doi: 10.1523/JNEUROSCI.3004-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7(8):617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24(46):10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zorner B, Schwab ME. Anti-Nogo on the go: from animal models to a clinical trial. Ann N Y Acad Sci. 2010;1198(Suppl 1):E22–E34. doi: 10.1111/j.1749-6632.2010.05566.x. [DOI] [PubMed] [Google Scholar]
- 12.Cafferty WB, et al. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30(20):6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JK, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66(5):663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JK, Zheng B. Role of myelin-associated inhibitors in axonal repair after spinal cord injury. Exp Neurol. 2012;235(1):33–42. doi: 10.1016/j.expneurol.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chivatakarn O, et al. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci. 2007;27(27):7117–7124. doi: 10.1523/JNEUROSCI.1541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atwal JK, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322(5903):967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 17.Zheng B, et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci U S A. 2005;102(4):1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JE, et al. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44(3):439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326(5952):592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher D, et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31(40):14051–14066. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JM, et al. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc Natl Acad Sci U S A. 2012;109(13):4768–4773. doi: 10.1073/pnas.1121318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickendesher TL, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15(5):703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert RJ, et al. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci. 2005;29(4):545–558. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Venkatesh K, et al. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25(4):808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stiles TL, et al. LDL Receptor-related Protein-1 is a sialic acid-independent receptor for myelin-associated glycoprotein (MAG) that functions in neurite outgrowth inhibition by MAG and CNS myelin. J Cell Sci. 2012 doi: 10.1242/jcs.113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vyas AA, et al. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci U S A. 2002;99(12):8412–8417. doi: 10.1073/pnas.072211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goh EL, et al. beta1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Mol Brain. 2008;1:10. doi: 10.1186/1756-6606-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18(1):101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 29.McGee AW, et al. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309(5744):2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datwani A, et al. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64(4):463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syken J, et al. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313(5794):1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 32.Pizzorusso T, et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 33.Pizzorusso T, et al. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103(22):8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyata S, et al. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15(3):414–422. doi: 10.1038/nn.3023. S1–2. [DOI] [PubMed] [Google Scholar]
- 35.Carulli D, et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133(Pt 8):2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 36.Beurdeley M, et al. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci. 2012;32(27):9429–9437. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugiyama S, et al. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134(3):508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 38.Raiker SJ, et al. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 2010;30(37):12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grunewald E, et al. GPR50 interacts with neuronal NOGO-A and affects neurite outgrowth. Mol Cell Neurosci. 2009;42(4):363–371. doi: 10.1016/j.mcn.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11(11):735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, et al. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002;22(13):5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H, et al. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28(11):2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunah AW, et al. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat Neurosci. 2005;8(4):458–467. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- 44.Johnson KG, et al. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49(4):517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi H, et al. Selective control of inhibitory synapse development by Slitrk3-PTPdelta trans-synaptic interaction. Nat Neurosci. 2012;15(3):389–398. doi: 10.1038/nn.3040. S1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida T, et al. IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase delta. J Neurosci. 2011;31(38):13485–13499. doi: 10.1523/JNEUROSCI.2136-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.May P, et al. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24(20):8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGeachie AB, Cingolani LA, Goda Y. Stabilising influence: integrins in regulation of synaptic plasticity. Neurosci Res. 2011;70(1):24–29. doi: 10.1016/j.neures.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zagrebelsky M, et al. Nogo-A stabilizes the architecture of hippocampal neurons. J Neurosci. 2010;30(40):13220–13234. doi: 10.1523/JNEUROSCI.1044-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pradhan AD, et al. Dendritic spine alterations in neocortical pyramidal neurons following postnatal neuronal Nogo-A knockdown. Dev Neurosci. 2010;32(4):313–320. doi: 10.1159/000309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wills ZP, et al. The nogo receptor family restricts synapse number in the developing hippocampus. Neuron. 2012;73(3):466–481. doi: 10.1016/j.neuron.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orlando C, et al. Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci. 2012;32(50):18009–18017. doi: 10.1523/JNEUROSCI.2406-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 2006;26(6):1813–1822. doi: 10.1523/JNEUROSCI.4091-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu F, Strittmatter SM. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci. 2008;28(5):1262–1269. doi: 10.1523/JNEUROSCI.1068-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan CL, et al. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci. 2011;31(17):6289–6295. doi: 10.1523/JNEUROSCI.0008-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou FQ, et al. Neurotrophins support regenerative axon assembly over CSPGs by an ECM-integrin-independent mechanism. J Cell Sci. 2006;119(Pt 13):2787–2796. doi: 10.1242/jcs.03016. [DOI] [PubMed] [Google Scholar]
- 57.Allen NJ, et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486(7403):410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siddiqui TJ, Craig AM, Synaptic organizing complexes. Curr Opin Neurobiol. 2011;21(1):132–143. doi: 10.1016/j.conb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paradis S, et al. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53(2):217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tran TS, et al. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462(7276):1065–1069. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sahay A, et al. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci. 2005;25(14):3613–3620. doi: 10.1523/JNEUROSCI.5255-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Connor TP, et al. Semaphorin 5B mediates synapse elimination in hippocampal neurons. Neural Dev. 2009;4:18. doi: 10.1186/1749-8104-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Wit J, et al. Role of leucine-rich repeat proteins in the development and function of neural circuits. Annu Rev Cell Dev Biol. 2011;27:697–729. doi: 10.1146/annurev-cellbio-092910-154111. [DOI] [PubMed] [Google Scholar]
- 64.Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45(2):279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Akbik FV, et al. Anatomical plasticity of adult brain is titrated by nogo receptor 1. Neuron. 2013;77(5):859–866. doi: 10.1016/j.neuron.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ethell IM, et al. EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron. 2001;31(6):1001–1013. doi: 10.1016/s0896-6273(01)00440-8. [DOI] [PubMed] [Google Scholar]
- 67.Kaufmann N, et al. Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002;34(1):27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- 68.Kaksonen M, et al. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol Cell Neurosci. 2002;21(1):158–172. doi: 10.1006/mcne.2002.1167. [DOI] [PubMed] [Google Scholar]
- 69.Brakebusch C, et al. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol. 2002;22(21):7417–7427. doi: 10.1128/MCB.22.21.7417-7427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kantor DB, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44(6):961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Coles CH, et al. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science. 2011;332(6028):484–488. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inatani M, et al. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302(5647):1044–1046. doi: 10.1126/science.1090497. [DOI] [PubMed] [Google Scholar]
- 73.Cho JY, et al. The extracellular matrix proteoglycan perlecan facilitates transmembrane semaphorin-mediated repulsive guidance. Genes Dev. 2012;26(19):2222–2235. doi: 10.1101/gad.193136.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pyka M, et al. Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. Eur J Neurosci. 2011;33(12):2187–2202. doi: 10.1111/j.1460-9568.2011.07690.x. [DOI] [PubMed] [Google Scholar]
- 75.Li AJ, et al. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. Eur J Neurosci. 2002;16(7):1313–1324. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- 76.Delekate A, et al. NogoA restricts synaptic plasticity in the adult hippocampus on a fast time scale. Proc Natl Acad Sci U S A. 2011;108(6):2569–2574. doi: 10.1073/pnas.1013322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joset A, et al. Pincher-generated Nogo-A endosomes mediate growth cone collapse and retrograde signaling. J Cell Biol. 2010;188(2):271–285. doi: 10.1083/jcb.200906089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng X, et al. Neuronal Nogo-A regulates glutamate receptor subunit expression in hippocampal neurons. J Neurochem. 2011;119(6):1183–1193. doi: 10.1111/j.1471-4159.2011.07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horn KE, et al. Receptor protein tyrosine phosphatase sigma regulates synapse structure, function and plasticity. J Neurochem. 2012;122(1):147–161. doi: 10.1111/j.1471-4159.2012.07762.x. [DOI] [PubMed] [Google Scholar]
- 80.Brenneke F, et al. Mice deficient for the extracellular matrix glycoprotein tenascin-r show physiological and structural hallmarks of increased hippocampal excitability, but no increased susceptibility to seizures in the pilocarpine model of epilepsy. Neuroscience. 2004;124(4):841–855. doi: 10.1016/j.neuroscience.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 81.Frischknecht R, et al. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12(7):897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 82.Gao Y, et al. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44(4):609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 83.Gao Y, et al. Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase. J Neurosci. 2003;23(37):11770–11777. doi: 10.1523/JNEUROSCI.23-37-11770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng K, et al. Overcoming amino-Nogo-induced inhibition of cell spreading and neurite outgrowth by 12-O-tetradecanoylphorbol-13-acetate-type tumor promoters. J Biol Chem. 2010;285(9):6425–6433. doi: 10.1074/jbc.M109.071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kurihara D, Yamashita T. Chondroitin sulfate proteoglycans down-regulate spine formation in cortical neurons by targeting tropomyosin-related kinase B (TrkB) protein. J Biol Chem. 2012;287(17):13822–13828. doi: 10.1074/jbc.M111.314070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44(1):59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 87.Fortin DA, et al. Brain-derived neurotrophic factor activation of CaM-kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1-containing calcium-permeable AMPA receptors. J Neurosci. 2012;32(24):8127–8137. doi: 10.1523/JNEUROSCI.6034-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukata Y, et al. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science. 2006;313(5794):1792–1795. doi: 10.1126/science.1129947. [DOI] [PubMed] [Google Scholar]
- 89.Thomas R, et al. LGI1 is a Nogo receptor 1 ligand that antagonizes myelin-based growth inhibition. J Neurosci. 2010;30(19):6607–6612. doi: 10.1523/JNEUROSCI.5147-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walmsley AR, et al. Zinc metalloproteinase-mediated cleavage of the human Nogo-66 receptor. J Cell Sci. 2004;117(Pt 19):4591–602. doi: 10.1242/jcs.01324. [DOI] [PubMed] [Google Scholar]
- 91.Ferraro GB, et al. Membrane-type matrix metalloproteinase-3 regulates neuronal responsiveness to myelin through Nogo-66 receptor 1 cleavage. J Biol Chem. 2011;286(36):31418–31424. doi: 10.1074/jbc.M111.249169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Josephson A, et al. Activity-induced and developmental downregulation of the Nogo receptor. Cell Tissue Res. 2003;311(3):333–342. doi: 10.1007/s00441-002-0695-8. [DOI] [PubMed] [Google Scholar]
- 93.Chytrova G, Ying Z, Gomez-Pinilla F. Exercise normalizes levels of MAG Nogo-A growth inhibitors after brain trauma. Eur J Neurosci. 2008;27(1):1–11. doi: 10.1111/j.1460-9568.2007.05982.x. [DOI] [PubMed] [Google Scholar]
- 94.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764(1–2):1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Alias G, et al. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12(9):1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- 97.van Gaalen MM, et al. NOGO-66 receptor deficient mice show slow acquisition of spatial memory task performance. Neurosci Lett. 2012;510(1):58–61. doi: 10.1016/j.neulet.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 98.Karlen A, et al. Nogo receptor 1 regulates formation of lasting memories. Proc Natl Acad Sci U S A. 2009;106(48):20476–20481. doi: 10.1073/pnas.0905390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gogolla N, et al. Perineuronal nets protect fear memories from erasure. Science. 2009;325(5945):1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 100.Budel S, et al. Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. J Neurosci. 2008;28(49):13161–13172. doi: 10.1523/JNEUROSCI.3828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Willi R, Schwab ME. Nogo and Nogo receptor: Relevance to schizophrenia? Neurobiol Dis. 2013 doi: 10.1016/j.nbd.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 102.Park JH, et al. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J Neurosci. 2006;26(5):1386–1395. doi: 10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou X, et al. Interaction between amyloid precursor protein and Nogo receptors regulates amyloid deposition. FASEB J. 2011;25(9):3146–3156. doi: 10.1096/fj.11-184325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang H, et al. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121(Pt 18):3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bartolini B, et al. Mouse development is not obviously affected by the absence of dermatan sulfate epimerase 2 in spite of a modified brain dermatan sulfate composition. Glycobiology. 2012;22(7):1007–1016. doi: 10.1093/glycob/cws065. [DOI] [PubMed] [Google Scholar]
- 106.Li HP, et al. Roles of chondroitin sulfate and dermatan sulfate in the formation of a lesion scar and axonal regeneration after traumatic injury of the mouse brain. J Neurotrauma. 2013;30(5):413–425. doi: 10.1089/neu.2012.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams G, et al. Ganglioside inhibition of neurite outgrowth requires Nogo receptor function: identification of interaction sites and development of novel antagonists. J Biol Chem. 2008;283(24):16641–16652. doi: 10.1074/jbc.M802067200. [DOI] [PubMed] [Google Scholar]
- 108.Zhang L, et al. Identification of BLyS (B lymphocyte stimulator), a non-myelin-associated protein, as a functional ligand for Nogo-66 receptor. J Neurosci. 2009;29(19):6348–6352. doi: 10.1523/JNEUROSCI.5040-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sato Y, et al. Cartilage acidic protein-1B (LOTUS), an endogenous Nogo receptor antagonist for axon tract formation. Science. 2011;333(6043):769–773. doi: 10.1126/science.1204144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakaya N, et al. Olfactomedin 1 interacts with the Nogo A receptor complex to regulate axon growth. J Biol Chem. 2012;287(44):37171–37184. doi: 10.1074/jbc.M112.389916. [DOI] [PMC free article] [PubMed] [Google Scholar]