Abstract
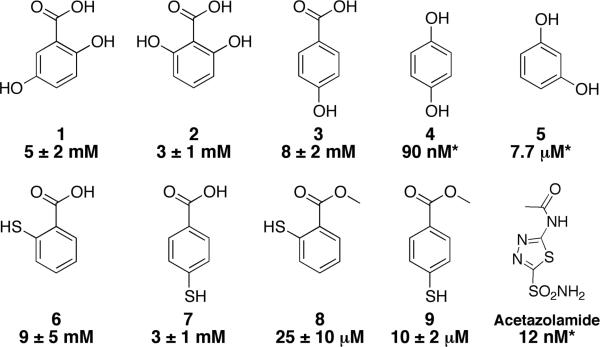
A series of hydroxybenzoic acid derivatives have shown inhibitory activity against carbonic anhydrase (CA). X-ray crystallography shows that these molecules inhibit not by binding the active site metal ion but by strong hydrogen bonding to the metal-bound water nucleophile. The binding mode observed for these molecules is distinct when compared to other non-metal-binding CA inhibitors.
Carbonic anhydrases (CAs, EC 4.2.1.1) are a class of enzymes that catalyze the reversible hydration of CO2 to HCO3- and a proton. CAs are involved in a wide variety of physiological processes including respiration, pH homeostasis, and ion transport.1, 2 The most physiologically abundant isoform, human carbonic anhydrase isoform II (hCAII), is a cytosolic enzyme found largely in red blood cells. The active site of hCAII lies at the bottom of a cone-shaped depression; one side of the depression is hydrophobic and serves as the substrate-binding site while the other is hydrophilic and aids in enzyme turnover through product diffusion and proton transfer.3 The active site consists of a Zn(II) ion bound by three histidine residues and a hydroxide ion. The Zn(II) ion acts to lower the pKa of a bound water molecule and increase its nucleophilicity. A hydrogen bond to a nearby threonine residue (Thr199) positions the nucleophile towards the hydrophobic substrate-binding site.
The vast majority of CA inhibitors (CAi) use a sulfonamide functionality to bind to the Zn(II) ion ( see Acetazolamide, Fig. 1), displacing the water nucleophile and thereby anchoring the inhibitor in the active site. Recently, a distinct class of inhibitors based on phenol has been described; these inhibitors act not by directly coordinating to the catalytic Zn(II) ion, but by hydrogen bonding to the active hydroxide ion and occupying the hydrophobic binding pocket.4-17 This ‘indirect’ inhibition has potential for the development of isoform-specific CAi, something that has proven difficult with sulfonamide-based inhibitors.
Figure 1.
Structure and IC50 inhibitors values of CA. Asterisks denote inhibition constants (Ki) taken from reference 9. The structure of acetazolamide, a clinically approved CA inhibitor, is shown for comparison.
A screen of a chelate-fragment library (CFL-1.1)19 against hCAII using a standard esterase activity assay identified several salicylic acid derivatives, including 2,5-dihydroxybenzoic acid (1, IC50 = 5±2 mM), as inhibitors of hCAII.20 Salicylic acid derivatives have been previously reported as inhibitors of hCAII.6, 7, 9 Although initially hypothesized to bind similar to phenol, recently reported docking studies have suggested that the binding mode of these hydroxybenzoic acids is closer to that of hydrolyzed coumarins.21 In order to determine the mode of inhibition, the crystal structures of several hydroxybenzoic acid and phenol inhibitors bound to hCAII were determined. These structures show that hydroxybenzoic acids and phenols present two, distinct modes of binding that both involve engagement of the nucleophilic, Zn(II)-bound solvent in hydrogen bonding.
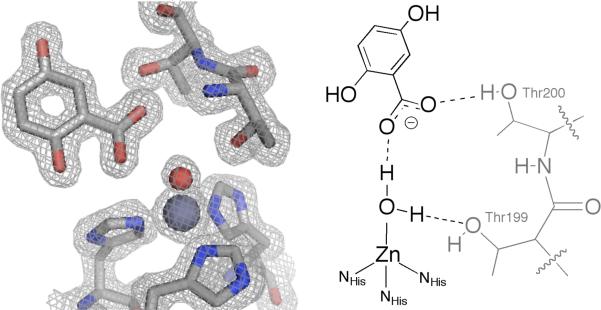
Crystals of inhibitor-free hCAII were obtained by the sitting drop method using ammonium sulfate as a precipitant. Crystals were then transferred to a solution containing inhibitor and allowed to soak for 2-7 days before data collection (see ESI† for details). The binding of 1 in the active site, similar to that of phenol, is anchored by a hydrogen bond with the Zn(II)-bound solvent (Fig. 2). This solvent is assumed to be water (not hydroxide), which would better engage in hydrogen-bonding with the anionic carboxylate. This interaction is through the carboxylate of 1, not the phenol moiety, with an O—O distance of 2.60 Å. One of the carboxylate oxygen atoms does not interact with the catalytic water but for ms a hydrogen bond (2.67 Å) with the hydroxyl group of Thr200. The 2-hydroxyl group of 1 is positioned toward the hydrophobic wall (Val121, Val143, Trp209) of the active site, displacing the ‘deep’ water molecule that normally interacts with those residues.22 The 5-hydroxyl group forms two hydrogen bonds with water molecules in the active site cone with O—O distances of 2.71 and 2.86 Å. A second molecule of 1 was also observed, bound ~ 14 Å from the active site (Fig. S1†). The only interaction of this molecule of 1 with the protein is a long contact (2.94 Å) with the backbone amide of Tyr7.
Figure 2.
Crystal structure of 1 bound in the hCAII active site (left). The Zn(II) ion and bound water are shown as gray and red spheres, respectively. The 2|Fo|-|Fc| electron density map is contoured to 1.2σ. Important interactions with the protein are diagrammed on the right.
The isomer 2,6-dihydroxybenzene 2 showed similar inhibition (IC50 = 3±1 mM) to compound 1 and the crystal structure of 2 bound to hCAII shows a similar binding mode (Fig. 3). The carboxylate functionality still forms a hydrogen bond with the Zn(II)-bound water through one of its oxygen atoms (2.59 Å). One hydroxyl group is positioned towards the hydrophobic wall as in 1 while the other interacts with the hydroxyl group of Thr200 (2.74 Å). The second carboxylate oxygen is left with a weak hydrogen bond (3.13 Å) with the backbone amide nitrogen of Thr200. Another molecule of 2 binds in the same location as the second molecule of 1 described above. A third molecule of 2 binds ~15 Å from the Zn(II) ion near the opening of the active site tunnel (Fig. S1†).
Figure 3.
Crystal structure of 2 bound in the hCAII active site (left). The Zn(II) ion and bound water are shown as gray and red spheres, respectively. The 2|Fo|-|Fc| electron density map is contoured to 1.0σ. Important interactions with the protein are diagrammed on the right.
4-Hydroxybenzoic acid 3 has been previously reported to be a good hCAII inhibitor using a CO2 hydration assay (Ki = 10.6 μM), but a substantially weaker inhibitor using an ester hydrolysis assay (670 μM)9, 14 Under the esterase assay conditions used here, compound 3 showed even weaker potency on the order of that found for compounds 1 and 2 (IC50 = 8±2 mM). This suggests additional studies may be required to resolve the disparity of these different findings with compound 3 (ESI†). Nevertheless, the crystal structure of 3 in the active site of hCAII shows that it binds in the same manner as 1 and 2 (Fig. 4). With no hydroxyl group ortho- to the carboxylate, the hydrophobic water molecule is not displaced, and in fact this water molecule hydrogen bonds to one of the carboxylate oxygen atoms of 3 (2.81 Å). The second carboxylate oxygen has close interactions with both the Zn(II)-bound water and the hydroxyl group of Thr200 (2.59 Å and 2.73 Å, respectively). Interestingly, the para-hydroxyl group of 3 does not appear to make any significant contacts with the protein or active site solvent molecules. Based on the structure of bound 3, it appears unlikely that the compound would be substantially more potent than 1 or 2.
Figure 4.
Crystal structure of 3 bound in the hCAII active site (left). The Zn(II) ion is shown as a gray sphere while the bound and hydrophobic waters are shown as red and magenta spheres, respectively. The 2|Fo|-|Fc| electron density map is contoured to 1.2σ. Important interactions with the protein are diagrammed on the right.
In order to compare the binding mode of hydroxybenzoic acid compounds to the previously reported phenols, the structures of 4 and 5 bound to hCAII were determined. Both compounds crystallize in the active site similarly to the previously described phenol structure;4 one of the hydroxyl groups takes the place of the hydrophobic water and forms a strong hydrogen bond (2.5 Å) with the Zn(II)-bound hydroxide. There are no other close contacts between 4 and either the protein or solvent molecules and as a result, there are two disordered positions for the ligand (Fig. S2†). For compound 5, the second hydroxyl group interacts with a water molecule (2.71 Å) that also accepts a hydrogen bond (2.97 Å) from Gln92 (Fig. 5). Surprisingly, three other molecules of 5 are observed and are well ordered near the active site (Fig. S3†). Two of these are along the hydrophilic side of the cone leading to the active site. Less than 4 Å from the previously described binding site, a molecule of 5 is observed hydrogen-bound to Glu69 (2.52 Å) as well as to a sulfate ion that interacts with both Asn67 and His64. The third binding site is 4 Å furthur away from the active site. One hydroxyl group donates a hydrogen bond to the backbone carbonyl of Phe70 while the other interacts with a carboxylate oxygen atom of Asp72 (2.70 Å for both). The final molecule of 5, located more than 25 Å from the active site, is unlikely to be relevant for inhibition and is anchored by a hydrogen bond to Asp34. Given both the additional interaction and more ordered binding mode, one would expect 5 to be a more potent inhibitor than 4. However, previous studies using a CO2 hydration assay show 5 to be a weaker hCAII inhibitor than 4, with inhibition constants of 90 nM and 7.7 μM for and 4 and 5, respectively.9 It is also surprising that these molecules would have such high potency given the relatively few interactions they have with the protein. Under our assay conditions, neither molecule showed significant inhibitory activity at concentrations up to 9 mM in the esterase assay.
Figure 5.

Crystal structure of 5 bound in the hCAII active site (left). The Zn(II) ion and bound water are shown as gray and red spheres, respectively. The 2|Fo|-|Fc| electron density map is countoured at 1.2σ. Important interactions with the protein are diagrammed on the right.
Although both the phenol and benzoic acid derivatives bind to hCAII via a hydrogen bond to the catalytic hydroxide ion, closer inspection reveals that the two binding modes are in fact quite distinct. The interaction between the inhibitors and the hydrophobic wall of the active site is significantly different. As previously reported, phenols 4 and 5 have several van der Waals interactions (3.5–4 Å) with the hydrophobic residues. The benzoic acid derivatives completely lack these interactions for two reasons: the presence of a hydroxyl group ortho- to the carboxylate and the location of the inhibitor with respect to the active site. In the case of phenols, the hydroxyl group that binds to the catalytic hydroxide also replaces the deep water located near the hydrophobic wall, positioning the inhibitor to the side of the Zn(II) ion with respect to the active site cone. This puts the aromatic ring in a good position to interact with Val121, Val143, and Trp209. The hydroxybenzoic acid derivatives are positioned differently; the ligand is placed more towards the middle of the active site cone. When a hydroxyl group is present ortho- to the carboxylate, it replaces the deep water and is the closest contact the ligand has with the hydrophobic wall. The inability of 3 to replace this water molecule could explain the slight drop in its potency when compared to 1 and 2.
In order to determine the persistence of the hydroxybenzoic acid binding mode described above, thiol-containing molecules 6 and 7 were examined with hCAII. Previous studies have shown that aromatic thiols inhibit hCAII with IC50 on the order of values 1 ~ μM by binding to the Zn(II) ion through a deprotonated, anionic sulphur atom.23 Therefore, a switch in binding mode from carboxylate-based hydrogen bonding to thiol-based Zn(II) binding should be reflected as a significant increase in potency. Neither 6 or 7 appear to bind through their thiol functionality, with IC50 values of 9±5 and 3±1 mM, respectively. The binding mode was confirmed by the crystal structure of 6 bound to hCAII. Compound 6 binds in the same manner as compounds 1-3 (Fig. S4†). Efforts to crystallize 7 bound to hCAII were unsuccessful (poor ligand solubility), but the potency of 7 is consistent with the hydrogen-bonding mode of inhibition.23 The lack of metal binding by the thiol groups of 6 and 7 is likely due to their protonation state. Deprotonation of the aromatic acid acts to an increase in the pKa of the thiol functionality, preventing metal binding. This is supported by the observation that the corresponding methyl esters 8 and 9 have inhibition consistent with thiol binding (IC50 = 25±10 and 10±2 μM, respectively). Crystal structures of esters 8 and 9 bound to hCAII were not obtained due to poor ligand solubility; however, the crystal structure of 8 bound to a tris(pyrazolyl)borate model complex shows coordination of the Zn(II) by the thiolate functionality.24
The structures presented here demonstrate that the hydroxybenzoic acids represent a new class of non-metal-binding inhibitors of hCAII. Recognition of the bound nucleophile may prove to be a viable alternative to direct metal binding for the inhibition of these metalloproteins. Although these fragments inhibit with modest (low millimolar) potency, they are sufficient for initiating fragment-based discovery efforts. By comparison, a simple hydroxamic acid (acetohydroxamic acid) inhibits the Zn(II)-dependent matrix metalloproteinases (MMP) by direct metal coordination with IC50 values of ~25 mM.25 Nonetheless, nanomolar potency MMP inhibitors have been prepared based on the hydroxamic acid fragment.26 Therefore, inhibitor recognition of the metal-bound nucleophile as a functional unit, analogous to the rational incorporation of an interaction with an amino acid sidechain, may present an alternative approach to the development of metalloprotein inhibitors.
Supplementary Material
Figure 6.

Comparison of the binding modes of 1 (blue), 2 (green), and 3 (red) to that of 5 (magenta). The catalytic Zn(II) and hydroxide ions are shown as gray and red spheres, respectively.
Acknowledgments
We thank Dr. Curtis Moore (UCSD) for assistance with X-ray crystallography. This work was supported by a grant from the National Institutes of Health (R01 GM098435). D.P.M. is supported by a SMART scholarship from the Office of the Secretary of Defense - Test and Evaluation (N00244-09-1-0081).
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/b000000x/
references
- 1.Sly WS, Hu PY. Annu. Rev. Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 2.Supuran CT. Nat. Rev. Drug Discov. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 3.Krishnamurthy VM, Kaufman GK, Urbach AR, Gitlin I, Gudiksen KL, Weibel DB, Whitesides GM. Chem. Rev. 2008;108:946–1051. doi: 10.1021/cr050262p. 10.1021/cr050262p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair SK, Ludwig PA, Christianson DW. J. Am. Chem. Soc. 1994;116:3659–3660. 10.1021/ja00087a086. [Google Scholar]
- 5.Innocenti A, Vullo D, Scozzafava A, Casey JR, Supuran CT. Bioorg. Med. Chem. Lett. 2005;15:573–578. doi: 10.1016/j.bmcl.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 6.Bayram E, Şentürk M, Küfrevioğlu O, Supuran CT. Bioorg. Med.Chem. 2008;16:9101–9105. doi: 10.1016/j.bmc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Innocenti A, Hilvo M, Scozzafava A, Parkkila S, Supuran CT. Bioorg. Med. Chem. Lett. 2008;18:3593–3596. doi: 10.1016/j.bmcl.2008.04.077. [DOI] [PubMed] [Google Scholar]
- 8.Innocenti A, Vullo D, Scozzafava A, Supuran CT. Bioorg. Med. Chem. Lett. 2008;18:1583–1587. doi: 10.1016/j.bmcl.2008.01.077. [DOI] [PubMed] [Google Scholar]
- 9.Innocenti A, Vullo D, Scozzafava A, Supuran CT. Bioorg. Med. Chem. 2008;16:7424–7428. doi: 10.1016/j.bmc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Şentürk M, Gülçin I, Daştan A, Küfrevioğlu OI, Supuran CT. Bioorg. Med. Chem. 2009;17:3207–3211. doi: 10.1016/j.bmc.2009.01.067. [DOI] [PubMed] [Google Scholar]
- 11.Davis RA, Innocenti A, Poulsen S, Supuran CT. Bioorg. Med.Chem. 2010;18:14–18. doi: 10.1016/j.bmc.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Innocenti A, Gülçin I, Scozzafava A, Supuran CT. Bioorg. Med. Chem. Lett. 2010;20:5050–5053. doi: 10.1016/j.bmcl.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 13.Innocenti A, Sarikaya SBÖ, Gülçin, C. T. Supuran I. Bioorg.Med. Chem. 2010;18:2159–2164. doi: 10.1016/j.bmc.2010.01.076. [DOI] [PubMed] [Google Scholar]
- 14.Sarikaya SBÖ, Gülçin I, Supuran CT. Chem. Biol. Drug Des. 2010;75:515–520. doi: 10.1111/j.1747-0285.2010.00965.x. 10.1111/j.1747-0285.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 15.Durdagi S, Şentürk M, Ekinci D, Balaydın HT, Göksu S, Küfrevioğlu Öİ, Innocenti A, Scozzafava A, Supuran CT. Bioorg. Med.Chem. 2011;19:1381–1389. doi: 10.1016/j.bmc.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Sarikaya SBÖ, Topal F, Şentürk M, Gülçin I, Supuran CT. Bioorg. Med. Chem. Lett. 2011;21:4259–4262. doi: 10.1016/j.bmcl.2011.05.071. [DOI] [PubMed] [Google Scholar]
- 17.Şentürk M, Gülçin I, Beydemir Ş, Küfrevioğlu Öİ, Supuran CT. Chem. Biol. Drug Des. 2011;77:494–499. doi: 10.1111/j.1747-0285.2011.01104.x. 10.1111/j.1747-0285.2011.01104.x. [DOI] [PubMed] [Google Scholar]
- 18.Winum J-Y, Scozzafava A, Montero J-L, Supuran CT. Curr. Pharm. Des. 2008:615–621. doi: 10.2174/138161208783877848. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen JA, Fullagar JL, Miller MT, Cohen SM. J. Med. Chem. 2011;54:591–602. doi: 10.1021/jm101266s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verpoorte JA, Mehta S, Edsall J. J. Biol. Chem. 1967;242:4421–4229. [PubMed] [Google Scholar]
- 21.Maresca A, Temperini C, Pochet L, Masereel B, Scozzafava A, Supuran CT. J. Med. Chem. 2010;53:335–344. doi: 10.1021/jm901287j. [DOI] [PubMed] [Google Scholar]
- 22.Avvaru BS, Kim CU, Sippel KH, Gruner SM, Agbandje-McKenna M, Silverman DN, McKenna R. Biochemistry. 2010;49:249–251. doi: 10.1021/bi902007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrese A, Genis C, Fisher SZ, Orwenyo JN, Kumara MT, Dutta SK, Phillips E, Kiddle JJ, Tu C, Silverman DN, Govindasamy L, Agbandje-McKenna M, McKenna R, Tripp BC. Biochemistry. 2008;47:3174–3184. doi: 10.1021/bi702385k. 10.1021/bi702385k. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen FE, Cohen SM. Inorg. Chem. 2004;43:3038–3047. doi: 10.1021/ic035388o. [DOI] [PubMed] [Google Scholar]
- 25.Puerta DT, Lewis JA, Cohen SM. J. Am. Chem. Soc. 2004;126:8388–8389. doi: 10.1021/ja0485513. [DOI] [PubMed] [Google Scholar]
- 26.Rao BG. Curr. Pharm. Des. 2005;11:295–322. doi: 10.2174/1381612053382115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.