Abstract
OBJECTIVES:
Hydroxychloroquine is an antimalarial agent that has been used in systemic lupus erythematosus and rheumatoid arthritis treatment for many years. Recently, novel mechanisms of action have been proposed, thereby broadening the therapeutic perspective of this medication. The purpose of this study was to evaluate the immunomodulatory activity of hydroxychloroquine in T helper 17 (Th17) cytokines in healthy individuals and patients.
METHODS:
Eighteen female patients with systemic lupus erythematosus (mean age 39.0±12.9 years) and 13 female patients with rheumatoid arthritis (mean age 51.5±7.7 years) were recruited from Universidade Federal de Pernambuco-Brazil. The patients were included after fulfilling four classification criteria for systemic lupus erythematosus or rheumatoid arthritis from the American College of Rheumatology. After being stimulated with phorbol 12-myristate 13-acetate and ionomycin in the absence or presence of different concentrations of hydroxychloroquine, the interleukin 6, 17 and 22 levels were quantified with an enzyme-linked immunosorbent assay in culture supernatants of peripheral blood mononuclear cells from healthy individuals and patients.
RESULTS:
We demonstrated that in peripheral blood mononuclear cells from healthy volunteers and in systemic lupus erythematosus and rheumatoid arthritis patients, there was a significant reduction in the IL-6, IL-17 and IL-22 supernatant levels after adding hydroxychloroquine.
CONCLUSIONS
Our in vitro results demonstrated that hydroxychloroquine inhibits IL-6, IL-17 and IL-22 production and contributes to a better understanding of the mechanism of action of this medication.
Keywords: Systemic Lupus Erythematosus, Rheumatoid Arthritis, Th17 cells, Hydroxychloroquine
INTRODUCTION
Hydroxychloroquine (HCQ) is an antimalarial agent that has been used in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) treatments for many years. Recently, advances in our understanding of its mechanisms of action have expanded the therapeutic prospects of HCQ (1–3). Perhaps the most relevant discovery was of an antagonistic effect of HCQ (inhibiting the immune response) on Toll-like receptors (TLRs) (4). Other mechanisms, such as interfering with antigen presentation and lysosomal acidification, blocking UV light in cutaneous reactions and inhibiting phospholipase A2, have been well described (5,6). All of these molecular effects could partially explain the immunomodulatory effect of HCQ upon proinflammatory cytokines, such as IL-6, IL-1β and TNF-α (7).
Helper T cells (Th) are phenotypically heterogeneous and classified according to the cytokines produced by the innate immune system during the Th cell differentiation process. Four main lineages, Th1, Th2, regulatory T cells (TReg) and a novel cell named Th17, have been well described (8). The activation and maturation of Th17 lymphocytes relies on adequate T cell receptor (TCR) expression, co-stimulatory molecules and cytokines. Combined, TGF-β, IL-1β, IL-6, IL-21 and IL-23 play a pivotal role in properly differentiating naïve CD4 positive cells. TGF-β and IL-6 are essential for inducing transcription factors retinoic acid receptor-related orphan receptor-t (RORγt) and STAT-3 in naïve CD4 positive cells, which are responsible for promoting proper maturation. IL-23 is another important cytokine that is required to expand and stabilize the Th17 cell population (8).
Recently, Th17 cells have been implicated in SLE pathogenesis, which identifies them as potential therapeutic targets (9). Previous studies with SLE patients have demonstrated an increase in serum Th17-related cytokines (IL-17 and IL-23). Th17 appears to infiltrate targeted organs, such as the kidneys and skin, which suggests an important role of these cells in SLE pathogenesis (10–12). Moreover, anti-DNAds and total IgG production in 15 lupus nephritises were increased when the peripheral blood mononuclear cells (PBMCs) were cultured with IL-17 (13). In RA, activated T cells are a characteristic feature of synovial inflammation, which suggests a Th1- and Th17-mediated disease. Elevated levels of IL-17 have been observed in the synovial fluid of these patients (14). Additionally, IL-17A-producing CCR6+ memory T cells have been identified in the synovial fluid of RA patients (15). These studies have clearly shown that the Th17 pathway is implicated in the pathogenesis of SLE and RA, but whether HCQ is able to specifically down-regulate Th17 cytokines is unknown. To our knowledge, our study is the first to address this issue.
MATERIALS AND METHODS
Study Population
Eighteen female SLE patients (mean age 39.0±12.9 years) and 13 female RA patients (mean age 51.5±7.7 years) were recruited from the Rheumatology Division at the Hospital das Clínicas-Universidade Federal de Pernambuco. All patients answered a clinical questionnaire from which demographic, clinical, current medication and laboratorial data were collected (Table 1). Additional information was obtained from hospital records and reviewed by experienced physicians. The patients were included in the study after fulfilling at least four or more of the classification criteria for SLE or RA (16,17) from the American College of Rheumatology (ACR). After excluding any rheumatic disease, five healthy volunteers (mean age 25±1.64 years) were recruited as the control group. Peripheral blood samples were obtained from the patients and healthy volunteers. Tests for serum anti-DNAds and serum complement (C3, C4 and CH50) were conducted using standard methods.
Table 1.
Demographic, clinical and current drug of the patients with RA and SLE.
| Diagnostic | SLE | RA |
| Number of patients | 18 | 13 |
| Age in years: mean ± SD (range) | 39±12.9 (22–61) | 51.5±7.7 (40–69) |
| Disease duration in years: mean (range) | 10.9 (0.1–40) | 8.1 (0.8–29.6) |
| Treatment N(%) | ||
| NSAIDS*) | – | 1 (8%) |
| Steroids | 12 (67%) | 10 (77%) |
| Antimalarial agents | 10 (55%) | 3 (23%) |
| Methotrexate | – | 7 (53.8%) |
| Leflunomide | – | 4 (30.8%) |
| Azathioprine | 4 (22%) | – |
| Mycophenolate Mofetil | 1 (5%) | – |
| Thalidomide | 1 (5%) | – |
Non-steroidal anti-inflammatory drugs.
PBMC purification
The PBMCs were obtained from the heparinized blood of five healthy, non-smoking donors who had not taken any drugs for at least 15 days prior to the sampling. The PBMCs were isolated using the standard Ficoll-Hypaque density-gradient centrifugation (GE Healthcare Biosciences, Pittsburgh, PA, USA) method. The cells were counted in a Neubauer chamber, and cell viability was determined using the Trypan blue (Sigma-Aldrich, St. Louis, USA) exclusion method. Cells were only used when their viability was >98%.
PBMC cultures
The PBMCs (1 × 106 cells/ml) were cultured in RPMI-1640 (Gibco) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), HEPES 10 mM (Gibco, Carlsbad, CA, USA) and penicillin (10.000 U/ml)/streptomycin (10.000 μg/ml) (Gibco, Carlsbad, CA, USA). The cells were stimulated with PMA (Sigma-Aldrich, St. Louis, USA) + Ionomycin (Sigma-Aldrich, St. Louis, USA) in the presence or absence of HCQ (hydroxychloroquine sulfate, Sigma). Methylprednisolone (Pfizer, New York, NY, USA) was used as a positive control. The cells were incubated at 37°C in a humidified 5% CO2 incubator.
Cytokine titration
The cytokine levels in the culture supernatants were determined using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions. In culture, supernatants from PBMCs, IL-6 (BD Biosciences, Franklinlakes, NJ, USA), IFN-γ (BD Bioscience) and TNF-α (BD Bioscience), levels were measured at 24 hours, and for IL-17A (R&D Systems, Minneapolis, MN, USA), IL-22 (eBiosciences, San Diego, CA, USA), and IL-23p19, (eBiosciences, San Diego, CA, USA) levels were measured at 48 hours. The lower detection limits for the ELISA analyses were as follows: 15.625 pg/ml for human IL-17 and human TNF-α, 9.375 pg/ml for human IL-6 and IFN-γ and 31.25 pg/ml for human IL-23p19 and IL-22.
Statistical analysis
All results in this article were analyzed with univariate comparisons using nonparametric tests (Wilcoxon matched-pairs test) with p<0.01 considered a significant association and p<0.05 a suggestive association. All quantitative data were plotted with GraphPad Prism 3.02 software.
Ethics
All subjects signed informed consent forms that were approved by the ethics committee of Universidade Federal de Pernambuco in accordance with the Helsinki Declaration.
RESULTS
Effect of HCQ on the synthesis of cytokines of the Th17 pathway in PBMCs of healthy volunteers
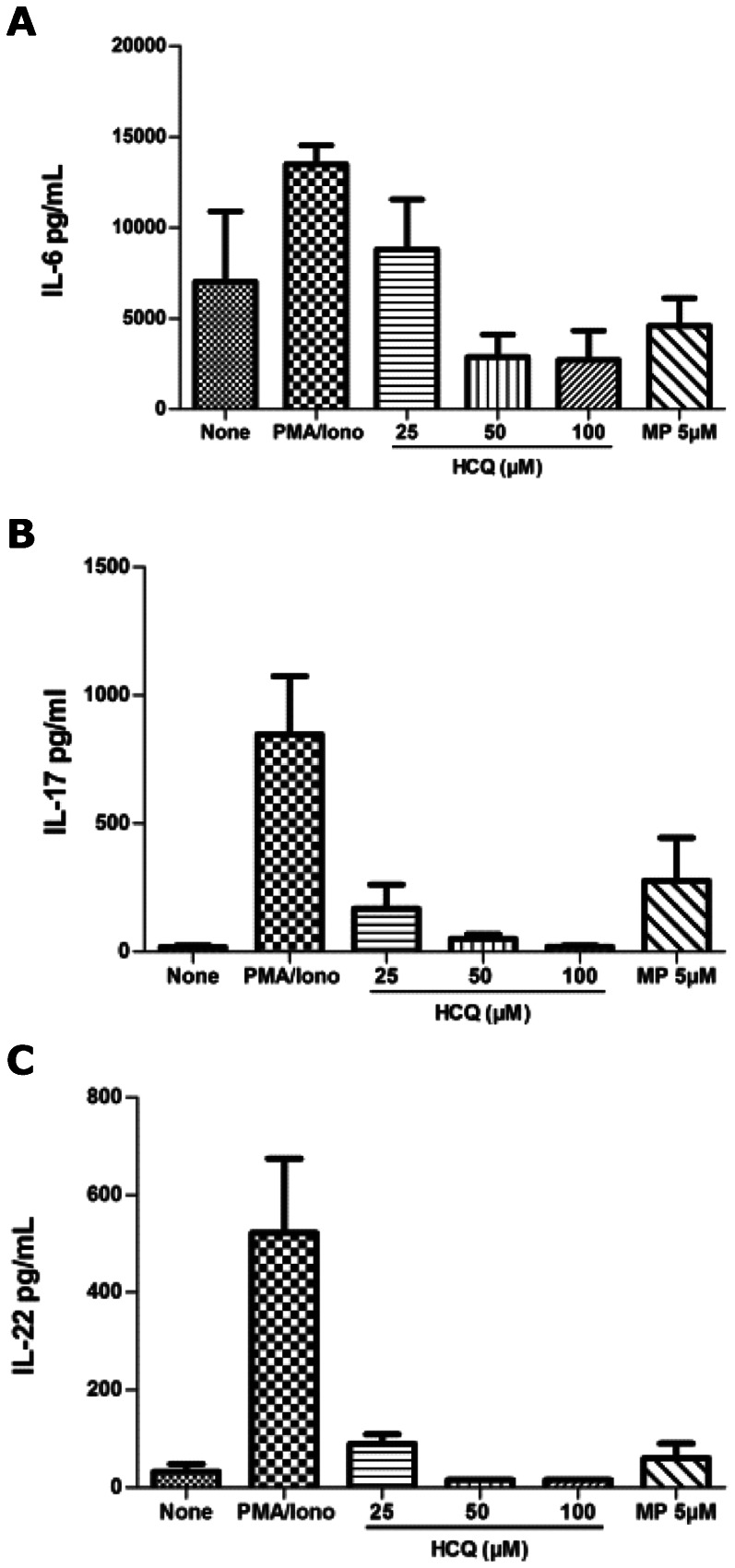
We investigated the effect HCQ on Th17 cytokines (IL-6, IL-17 and IL-22) in the PBMCs of healthy volunteers. After 48 hours of stimulation with PMA and ionomycin, the HCQ inhibited the production of IL-6, IL-17 and IL-22 (Figures 1 A, B and C) in a dose-dependent manner. We did not observe any significant inhibition in the production of these cytokines using the Wilcoxon test (p>0.05). These results are most likely caused by the small number of healthy volunteers.
Figure 1.
Inhibition of IL-17A (A), IL-22 (B) and IL-6 (C) supernatants levels in PBMCs from five healthy individuals using HCQ at 25 μM, 50 μM and 100 μM doses.
Effect of HCQ on the synthesis of Th17-related cytokines in the PBMCs of SLE and RA patients
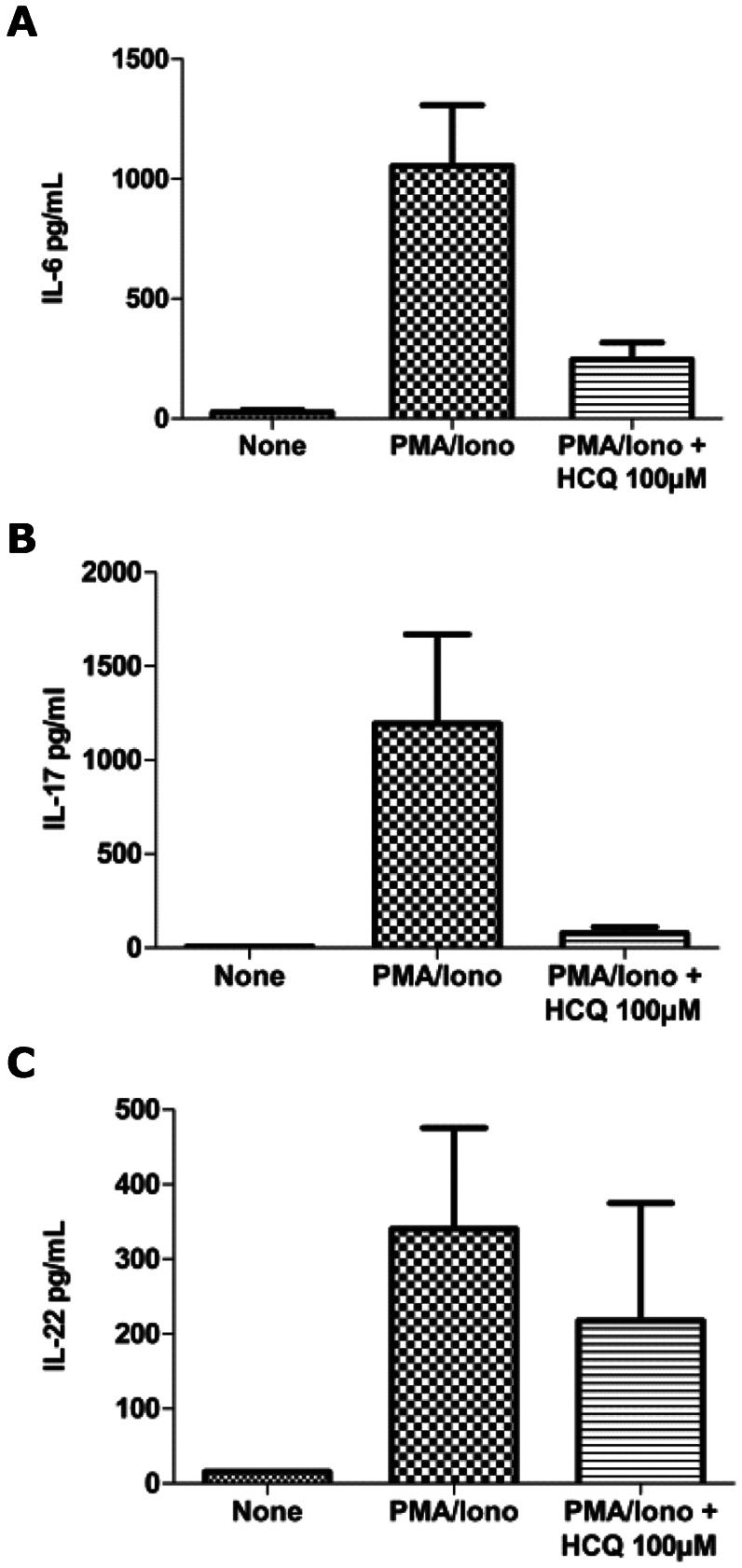
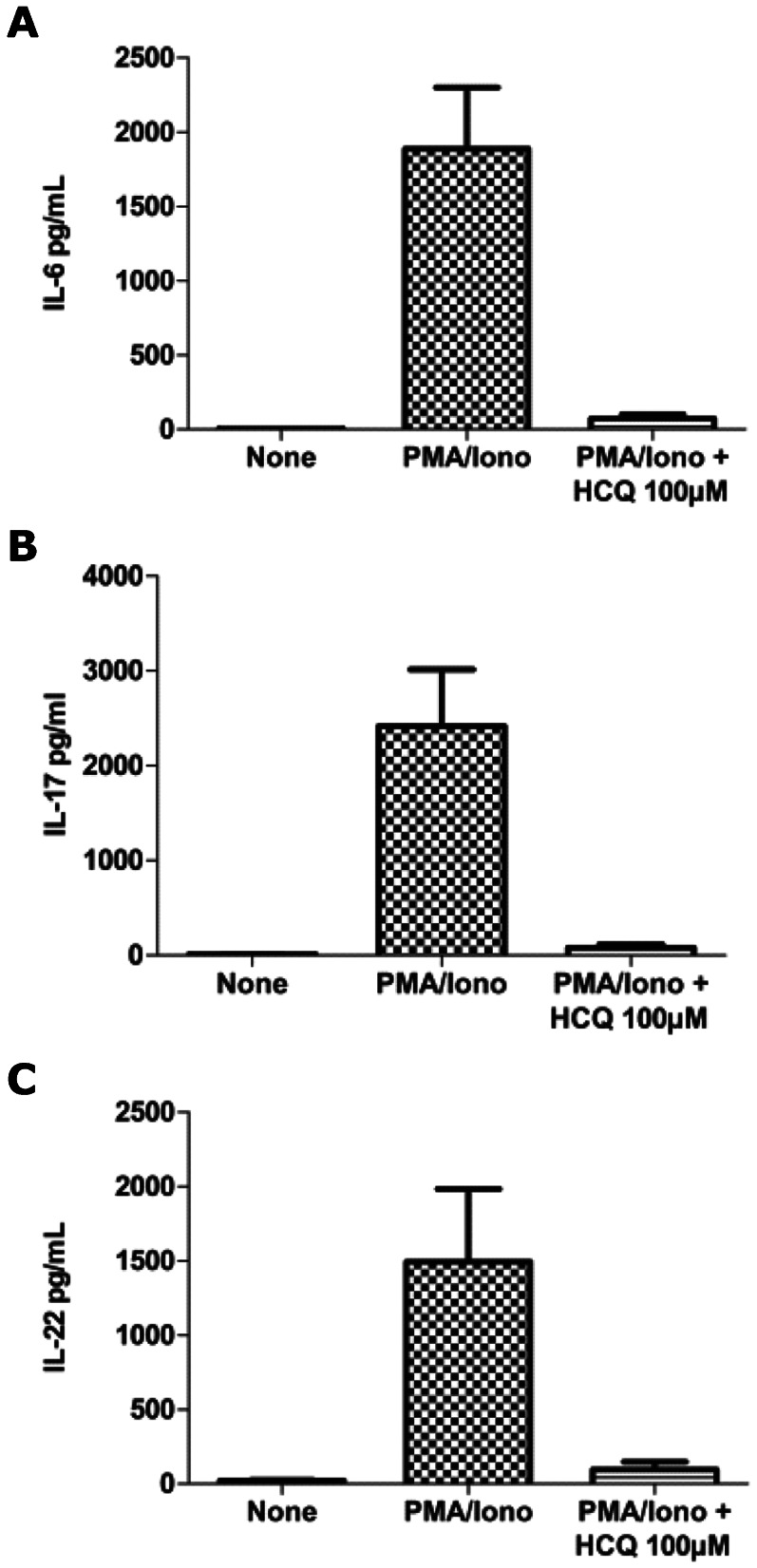
The main objective of this study was to evaluate the effect of HCQ on the cytokine profile of SLE and RA patients. Following our previous experiments in mice and healthy individuals, we analyzed the effect of HCQ (at a concentration of 100 μM) on Th17 cytokine levels from the PBMCs of SLE and RA patients after PMA and ionomycin stimulation. The levels of IL-6, IL-17 and IL-22 were negligible in the non-stimulated cells. There were remarkable increases in the levels of these cytokines in the PBMCs from RA and SLE patients, most likely because of the addition of PMA and ionomycin.
Following HCQ administration in both the SLE and RA patients, we found significant inhibition in the levels of IL-6 (p = 0.0001 for SLE and p = 0.0025 for RA, Figures 2A and 3A, IL-17 (p = 0.0001 for SLE and p = 0.0002 for RA, Figures 2B and 3B and IL-22 (p = 0.0067 for SLE and p = 0.0002 for RA, Figures 2C and 3C.
Figure 2.
Inhibition of IL-6 (A), IL-17 (B) and IL-22 (C) production using HCQ at a dose of 100 μM in PBMCs from SLE patients.
Figure 3.
Inhibition of IL-6 (A), IL-17 (B) and IL-22 production (C) using HCQ at a dose of 100 μM in PBMCs from RA patients.
DISCUSSION
In the present study, we found that the antimalarial medication HCQ decreased pro-inflammatory Th17 cytokines levels in the PBMCs from healthy individuals and SLE/RA patients, suggesting a novel mechanism of action for these antirheumatic drugs. To our knowledge, this study is the first report of an inhibitory effect of antimalarial medication on IL-17 and IL-22 supernatant levels.
Increasingly, experimental and clinical evidence has indicated that IL-17 and Th17 cells play a central role in SLE and RA pathogenesis (10,18). IL-17 amplifies the immune response by increasing target organ inflammation and damage and by augmenting antibody production by B cells, an important player in SLE (19). In RA patients, the IL-17 serum and synovial levels are positively correlated with disease activity and upregulate osteoclast activation, which promotes bone erosion (20). Recently, our group described the upregulation of serum IL-22 levels in RA patients and identified an important correlation with disease activity and the presence of erosion (21).
Antimalarials have been widely used to treat SLE patients for many years, particularly for cutaneous and musculoskeletal manifestations (22,23). HCQ should also be used as a maintenance treatment in patients with stable disease, as previously reported in a randomized controlled trial in which the placebo group presented more lupus flares than the HCQ group (24,25). These results suggest that an immunomodulatory ‘status' is induced by HCQ, which could contribute to adequate disease control over many years. Another important beneficial effect of HCQ is a well-demonstrated reduction in cardiovascular risk in SLE patients. Previous cohort studies have shown a significant reduction in cholesterol levels, carotid plaque formation and thrombosis frequency (26,27). Recently, antimalarials have also improved survival in different populations of SLE patients (28,29).
For RA treatment, controlled trials have demonstrated the efficacy of HCQ in controlling arthritis symptoms as antimalarial agents are classified as disease-modifying antirheumatic drugs (DMARD) (30). Compared with other antirheumatic drugs, DMARDs are relatively well tolerated and safe to use during pregnancy and lactation. Additionally, these medications could improve glycemic control and lipid profiles, thereby decreasing cardiovascular risks in RA patients. An observational study described a 38% risk reduction of developing diabetes in RA patients after a long follow-up period (31).
In the present study, HCQ exhibited an anti-inflammatory effect on Th17-related cytokines in healthy individuals and SLE and RA patients. Previous studies using different cell populations have demonstrated that HCQ inhibits proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-alpha), interferon-gamma (IFN-gamma), IL-1-alpha and IL-6 (32,33). These results are in accordance with our findings, but few studies have evaluated the in vitro effect of HCQ upon supernatant levels of T-cell related cytokines.
An interesting issue regarding our study is whether ‘in vivo' treatment using antimalarial agents could influence the in vitro HCQ effect. In our sample, although only a minority of patients was currently using HCQ, we found no differences when comparing patients with or without HCQ treatment (data not shown). In fact, in our study design, we compared the cytokine supernatant levels before and after administering HCQ to the same patient. Thus, each patient was his or her own control, and ‘in vivo' HCQ use was not a confounding variable.
The exact mechanism by which HCQ reduces IL-6, IL-17 and IL 22 levels is unknown, but one possible explanation is that it occurs by reducing Th17 cells through a decrease in the antigen presentation. Antimalarials classically enter lysosomes, causing an increase in cell pH, which interferes with protein processing and secretion (6). Moreover, the inhibitory effect of HCQ on TLR expression decreases the aberrant immune response typically found in rheumatic diseases. In fact, there is growing evidence that TLRs, particularly TLR 9, play pivotal roles in SLE and RA (34,35).
One limitation of our study was the inability to identify the Th17 cells through a specific technique, such as flow cytometry. However, we identified a consistent inhibitory effect in mice, healthy individuals and patients, suggesting a significant inhibitory effect upon cytokines involved in Th17 activation. Regarding IL-6, our results are in accordance with a previous study of 14 SLE patients, which showed a decrease in IL-6 mRNA expression in skin samples after three months of chloroquine treatment (7).
Although different mechanisms of action have been previously described, it has not been established whether HCQ is able to suppress the cytokines produced by Th17 cells. Our in vitro results demonstrated that this medication can inhibit IL-6, IL-17 and IL-22 production, which may be a novel pathway to understanding the immunomodulatory properties of HCQ.
ACKNOWLEDGMENTS
This study was supported by the Instituto Nacional de Ciência e Tecnologia para Inovação Farmacêutica (INCT_if), Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) and the Financiadora de Estudos e Projetos (FINEP).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42(2):145–53. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz SJ, Russell AS. Re-evaluation of antimalarials in treating rheumatic diseases: re-appreciation and insights into new mechanisms of action. Curr Opin Rheumatol. 2011;23(3):278–81. doi: 10.1097/BOR.0b013e32834456bf. [DOI] [PubMed] [Google Scholar]
- 3.Wallace DJ, Gudsoorkar VS, Weisman MH, Venuturupalli SR. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol. 2012;8(9):522–33. doi: 10.1038/nrrheum.2012.106. [DOI] [PubMed] [Google Scholar]
- 4.Kyburz D, Brentano F, Gay S. Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling. Nat Clin Pract Rheumatol. 2006;2(9):458–9. doi: 10.1038/ncprheum0292. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. 1982;79(1):175–8. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978;75(7):3327–31. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wozniacka A, Lesiak A, Boncela J, Smolarczyk K, McCauliffe DP, Sysa-Jedrzejowska A. The influence of antimalarial treatment on IL-1beta, IL-6 and TNF-alpha mRNA expression on UVB-irradiated skin in systemic lupus erythematosus. Br J Dermatol. 2008;159(5):1124–30. doi: 10.1111/j.1365-2133.2008.08804.x. [DOI] [PubMed] [Google Scholar]
- 8.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181(1):8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 9.Nalbandian A, Crispin JC, Tsokos GC. Interleukin-17 and systemic lupus erythematosus: current concepts. Clin Exp Immunol. 2009;157(2):209–15. doi: 10.1111/j.1365-2249.2009.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9(8):589–93. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Chu Y, Yang X, Gao D, Zhu L, Wan L, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60(5):1472–83. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 12.Kwan BC, Tam LS, Lai KB, Lai FM, Li EK, Wang G, et al. The gene expression of type 17 T-helper cell-related cytokines in the urinary sediment of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2009;48(12):1491–7. doi: 10.1093/rheumatology/kep255. [DOI] [PubMed] [Google Scholar]
- 13.Dong G, Ye R, Shi W, Liu S, Wang T, Yang X, et al. IL-17 induces autoantibody overproduction and peripheral blood mononuclear cell overexpression of IL-6 in lupus nephritis patients. Chin Med J (Engl) 2003;116(4):543–8. [PubMed] [Google Scholar]
- 14.Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42(5):963–70. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–12. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Shah K, Lee WW, Lee SH, Kim SH, Kang SW, Craft J, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(2):R53. doi: 10.1186/ar2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crispin JC, Tsokos GC. Interleukin-17-producing T cells in lupus. Curr Opin Rheumatol. 2010;22(5):499–503. doi: 10.1097/BOR.0b013e32833c62b0. [DOI] [PubMed] [Google Scholar]
- 20.Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Di Padova FE, et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167(1):141–9. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Rocha LF, Jr, Duarte AL, Dantas AT, Mariz HA, Pitta Ida R, Galdino SL, et al. Increased serum interleukin 22 in patients with rheumatoid arthritis and correlation with disease activity. J Rheumatol. 2012;39(7):1320–5. doi: 10.3899/jrheum.111027. [DOI] [PubMed] [Google Scholar]
- 22.Meinao IM, Sato EI, Andrade LE, Ferraz MB, Atra E. Controlled trial with chloroquine diphosphate in systemic lupus erythematosus. Lupus. 5(3):8803897. doi: 10.1177/096120339600500313. 237-41. PubMed PMID. [DOI] [PubMed] [Google Scholar]
- 23.Williams HJ, Egger MJ, Singer JZ, Willkens RF, Kalunian KC, Clegg DO, et al. Comparison of hydroxychloroquine and placebo in the treatment of the arthropathy of mild systemic lupus erythematosus. J Rheumatol. 1994;21(8):1457–62. [PubMed] [Google Scholar]
- 24.Molad Y, Gorshtein A, Wysenbeek AJ, Guedj D, Majadla R, Weinberger A, et al. Protective effect of hydroxychloroquine in systemic lupus erythematosus. Prospective long-term study of an Israeli cohort. Lupus. 2002;11(6):356–61. doi: 10.1191/0961203302lu203ra. [DOI] [PubMed] [Google Scholar]
- 25.The Canadian Hydroxychloroquine Study Group. N Engl J Med. 1991;324(3):150–4. doi: 10.1056/NEJM199101173240303. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. 17. [DOI] [PubMed] [Google Scholar]
- 26.Petri M. Hydroxychloroquine use in the Baltimore Lupus Cohort: effects on lipids, glucose and thrombosis. Lupus. 1996;(Suppl 1):S16–22. [PubMed] [Google Scholar]
- 27.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2399–406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 28.Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L) Ann Rheum Dis. 2007;66(9):1168–72. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Irastorza G, Egurbide MV, Pijoan JI, Garmendia M, Villar I, Martinez-Berriotxoa A, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus. 2006;15(9):577–83. doi: 10.1177/0961203306071872. [DOI] [PubMed] [Google Scholar]
- 30.Clark P, Casas E, Tugwell P, Medina C, Gheno C, Tenorio G, et al. Hydroxychloroquine compared with placebo in rheumatoid arthritis. A randomized controlled trial. Ann Intern Med. 1993;119(11):1067–71. doi: 10.7326/0003-4819-119-11-199312010-00002. [DOI] [PubMed] [Google Scholar]
- 31.Wasko MC, Hubert HB, Lingala VB, Elliott JR, Luggen ME, Fries JF, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187–93. doi: 10.1001/jama.298.2.187. [DOI] [PubMed] [Google Scholar]
- 32.van den Borne BE, Dijkmans BA, de Rooij HH, le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24(1):55–60. [PubMed] [Google Scholar]
- 33.Jeong JY, Choi JW, Jeon KI, Jue DM. Chloroquine decreases cell-surface expression of tumour necrosis factor receptors in human histiocytic U-937 cells. Immunology. 2002;105(1):83–91. doi: 10.1046/j.0019-2805.2001.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202(8):1131–9. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyburz D, Brentano F, Gay S. Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling. Nat Clin Pract Rheumatol. 2006;2(9):458–9. doi: 10.1038/ncprheum0292. [DOI] [PubMed] [Google Scholar]