Abstract
OBJECTIVE:
To compare the efficacy and tolerability of a fixed combination of 0.3% gatifloxacin and 1% prednisolone (Zypred®) versus the individual components used separately (Zymar® and Predfort®) for infection prophylaxis and inflammation control after cataract surgery with intraocular lens implantation.
METHODS:
A prospective, randomized, double-blind, parallel-group study of 108 patients who underwent phacoemulsification and intraocular lens implantation was conducted. After random assignment, 47 eyes received the fixed combination of topical 0.3% gatifloxacin/1% prednisolone drops, and 61 eyes received the same doses of the individual components as separate solutions four times a day for 15 days. Baseline and postoperative assessments were made on postoperative days 1, 7, 15, and 20.
RESULTS:
All objective (best corrected visual acuity, sign of active ocular inflammation, central and incisional corneal edema, the number of cells per high-power field in the anterior chamber, and intraocular pressure) and subjective (eye pain, photophobia, burning sensation, itching, and foreign body sensation) criteria of efficacy were similar in both groups, with no significant differences. Group I included 47 eyes that received the fixed combination of gatifloxacin/prednisolone acetate eye drops and a placebo eye drop solution. Group II included 61 eyes that were treated with 0.3% gatifloxacin and 1% prednisolone acetate eye drops separately. The intraocular pressure was slightly higher in Group II (p<0.05).
CONCLUSION:
Treatment with the fixed-dose combination of gatifloxacin/prednisolone eye drops was as effective as the non-fixed combination in preventing infection and controlling inflammation after phacoemulsification and intraocular lens implantation.
Keywords: Cataract Extraction, Gatifloxacin, Prednisolone, Phacoemulsification
INTRODUCTION
The aging population together with technological advancements in ophthalmic surgery, particularly in cataract surgery, have increased the number of phacoemulsifications around the world (1,2)
Acute endophthalmitis is the most important complication after cataract surgery; the incidence of endophthalmitis among cataract patients ranges from 0.04% to 0.099% in the developed world (3,4). The outcome of this intraocular infection can be devastating to the patient and result in significant loss of vision and even loss of the eye (5). Therefore, prophylaxis with antibiotics and anti-inflammatory agents is mandatory in the cataract surgery postoperative period.
The fourth-generation fluoroquinolone eye drops (gatifloxacin and moxifloxacin) are the most commonly used drugs for prophylaxis against endophthalmitis in cataract surgery. These eye drops increase the spectrum of antibiotic coverage, including resistant strains, and present a lower risk for antibiotic resistance (5-7).
Topical corticosteroids are commonly used as a routine treatment over several weeks to reduce the inflammatory reaction after cataract surgery (8). Corticosteroids are successful at reducing ocular inflammation because of their ability to inhibit nearly all chemical mediators in the inflammatory cascade. They downregulate inflammation by inhibiting deoxyribonucleic acid (DNA) transcription in the cell nucleus and interrupt the inflammatory cascade by increasing histaminase production; histaminase is an enzyme that breaks down unbound histamine into an inactive metabolite by inhibiting the production of phospholipase A2, which produces arachidonic acid from phospholipids in cell walls. Arachidonic acid is the main precursor to inflammatory mediators, such as prostaglandins and leukotrienes (9). Prednisolone acetate 1% has been used for inflammation control in cataract surgery, an important factor in the healing process (10,11). This corticosteroid achieves its highest aqueous level (669.9 ng/ml) within 120 min and maintains a significant level over 24 h; thus, a twice-daily application of prednisolone acetate 1% may be suitable for uncomplicated postoperative cataract cases (12).
Antibiotic and anti-inflammatory eye drops are necessary for cataract surgery postoperative management; combined doses with both drugs were developed to reduce the number of applications required and the potential toxic effects due to preservatives (13). Even at low concentrations, the preservatives and buffering agents cause some degree of cell damage to ocular tissue as evaluated using corneal and conjunctival cells in tissue culture. The toxicity increases with increasing drug concentrations. Corneal toxicity caused by preservatives may cause ocular discomfort and changes in vision and may interfere with patient compliance with the recommended dosage (14).
The aim of this study was to compare the inflammation control and infection prophylaxis after cataract surgery provided by a fixed combination of gatifloxacin 0.3% and prednisolone acetate 1% (Zypred®, Allergan®) versus the individual components (Zymar® and Predfort®).
METHODS
Phacoemulsification and IOL implantation was performed in 129 patients with cataract. Each patient had surgery in only one eye. Exclusion criteria included history of uveitis or chronic ocular inflammation, pseudoexfoliation syndrome, history of ocular trauma, uncontrolled diabetes, pregnant and nursing women, allergy or sensitivity to any component of the medications, serious systemic diseases and perioperative complications, such as anterior capsule rupture and vitreous loss. Patients were randomly assigned using the Research Randomizer software (site: www.randomizer.org); the value 1 was assigned to patients enrolled in Group I, and the value 2 was assigned to patients enrolled in Group II; 64 patients were allocated to Group I, and 65 patients were allocated to Group II.
The group assignment was masked from all patients and investigators. Each patient was given two identical bottles labeled according to their group assignment. All bottles were opaque and patients were instructed to apply one drop from each bottle in the operated eye every 6 h with a 5-min interval between drops, beginning one day prior to the surgery until the 15th day.
All patients underwent monocular cataract removal by phacoemulsification and IOL implantation performed by two experienced surgeons (AA and AAB) using the phaco chop technique under topical anesthesia.
At the screening visit, patients who met the eligibility criteria were informed of the requirements of the study and the risks involved before being asked to sign an informed consent form.
Patients were examined on postoperative days 1, 7, 15, and 20. Best corrected visual acuity (BCVA) was measured with an Early Treatment Diabetic Retinopathy Study (ETDRS) chart and presented in logMAR values. Patients were asked to subjectively rank their eye pain on a five-point scale from 0 (none) to 5 (severe). They were also asked about photophobia, burning sensation, itching, and foreign body sensation. Any sign of active ocular inflammation (redness, edema, tearing, or discharge) was documented. Conjunctival hyperemia and central and incisional corneal edema were evaluated by slit-lamp examination and classified from 0 (no edema) to 4+.
Using the narrowest slit of the slit lamp (0.5-mm wide, 8-mm high), the number of cells per high-power field in the anterior chamber was counted and recorded on a scale where 0 represented ≤5 cells, 1 represented 5 to 10 cells, 2 represented 11 to 20 cells, 3 represented 21 to 50 cells, and 4 represented ≥50 cells. A Goldmann applanation tonometer was used to measure the intraocular pressure on postoperative days 7, 15, and 20. The presence of hypopyon, posterior capsule opacity, and pigments or membrane in front of the IOL were also assessed. At each visit, patients were also questioned regarding treatment compliance and occurrence of any adverse event since the previous examination. On postoperative day 15, patients were instructed to discontinue the medications.
The data for the patients who met the inclusion/exclusion criteria underwent statistical analysis with Fisher's Least Significant Difference post-hoc test, the chi-square test, and parametric non-paired analysis of variance (ANOVA) with two-tailed testing at the 5% confidence interval (p = 0.05). Although all measurements and observations were made in both eyes, only data from the operated eye were analyzed and are reported here.
The hospital's institutional review board approved the study protocol, which followed the principles set forth in the Declaration of Helsinki. It was conducted according to ethical standards for clinical research and surgery and was approved by the Ethics Committee for Analysis of Research Projects (CAPPesq) of the Clinical Board of Hospital das Clínicas, University of São Paulo (Approval number 388/11). Drugs were provided by Allergan Laboratories, Inc.
RESULTS
Sixteen patients enrolled in Group I and 4 patients enrolled in Group II were withdrawn from the study due to absence from a scheduled assessment at any point during the follow-up period. One patient in Group I presented ocular trauma and required another surgery; this patient was excluded from the study.
Group I included 47 eyes that received the fixed combination of gatifloxacin/prednisolone acetate eye drops and a placebo eye drop solution. Group II included 61 eyes that were treated with 0.3% gatifloxacin and 1% prednisolone acetate eye drops separately. The treatment groups were similar regarding patient age, sex, race, and iris color.
Group I comprised 28 female patients (59.6%) and 19 male patients (40.4%), and Group II comprised 45 female patients (73.8%) and 16 male patients (26.2%). The average patient age was 71 years in both groups (Table 1).
Table 1.
Demographic data (gender and age) for patients treated with gatifloxacin 0.3% and prednisolone acetate 1% eye drops in combination (Group I) or separately (Group II).
| Gender | Age | ||||
| Female | Male | Average (± standard deviation) | Min | Max | |
| Group I | 28 (59.6%) | 19 (40.4%) | 71 (±10) | 44 | 88 |
| Group II | 45 (73.8%) | 16 (26.2%) | 71 (±10) | 41 | 88 |
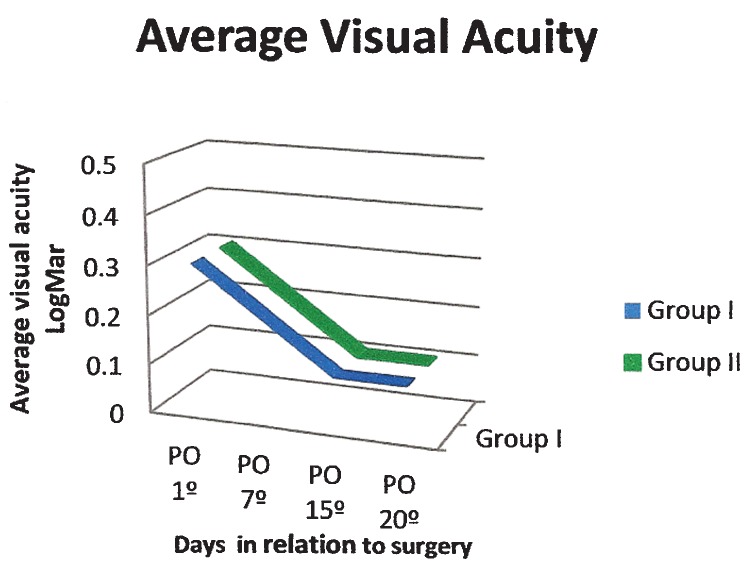
Visual acuity improved from a mean of 0.3 (±0.3) logMAR on postoperative day 1 to 0.1 (±0.2) logMAR on postoperative day 20 in both groups (Figure 1). There was a gradual reduction in pain scores in both groups, but no statistically significant differences were observed.
Figure 1.
Visual acuity (average, logMAR) in patients treated with gatifloxacin 0.3% and prednisolone acetate 1% eye drops combined (Group I) or separately (Group II).
Itching was observed in 11.7% (±4.06%) of patients in Group I and 11.12% (±2.13%) of patients in Group II throughout the follow-up period; there was no statistically significant difference between the groups. Burning sensation was reported by 8.61% (±6.44%) of patients in Group I and 8.22% (±5.17%) of patients in Group II throughout the follow-up period; the maximum values for burning sensation were present at the last assessment (p>0.05).
Photophobia was reported by 0-6% of the patients in both groups (2.15±1.78 for Group I and 5.77±1.66 for Group II) during the assessment period. Group II presented higher photophobia scores during the follow-up period, but no statistically significant difference was observed. Foreign body sensation occurred in 16-32% of the patients in both groups (mean 21.4±5.29 for Group I and 25.5±7.8 for Group II) throughout the follow-up period (p>0.05).
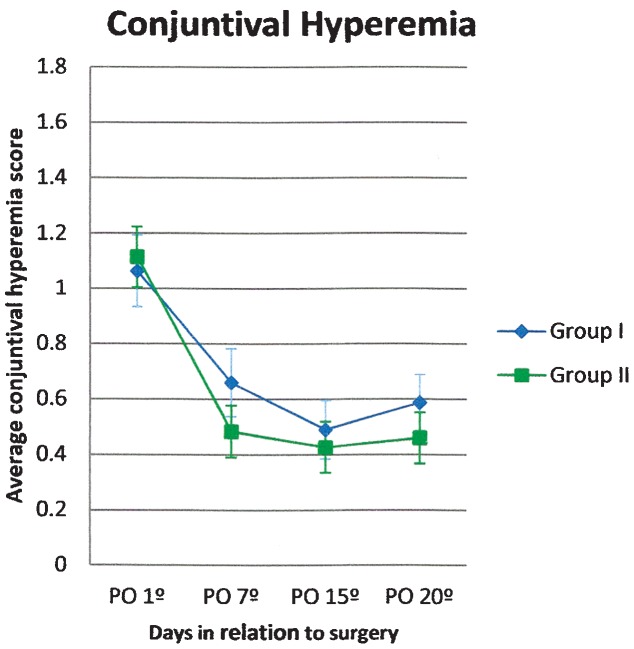
Conjunctival hyperemia was present in both groups until postoperative day 7 and gradually decreased over the evaluation period (Figure 2).
Figure 2.
Conjunctival hyperemia score (average and standard error) in patients treated with gatifloxacin 0.3% and prednisolone acetate 1% eye drops in combination (Group I) or separately (Group II).
Only one patient from Group II presented a pupillary membrane in front of the IOL on postoperative day 20 due to endophthalmitis. None of the patients presented hypopyon or IOL pigmentation. Three patients (6%) from Group I and five patients (8%) from Group II presented opacity of the posterior capsule, but no statistically significant difference was observed between the groups.
Central and incisional corneal edema presented similar results for both groups during the evaluation period, and a progressive decrease was verified, but there was no statistically significant difference between the groups.
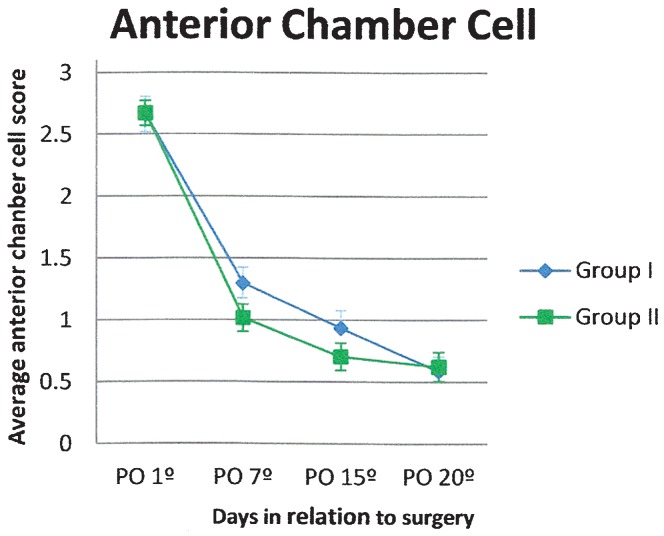
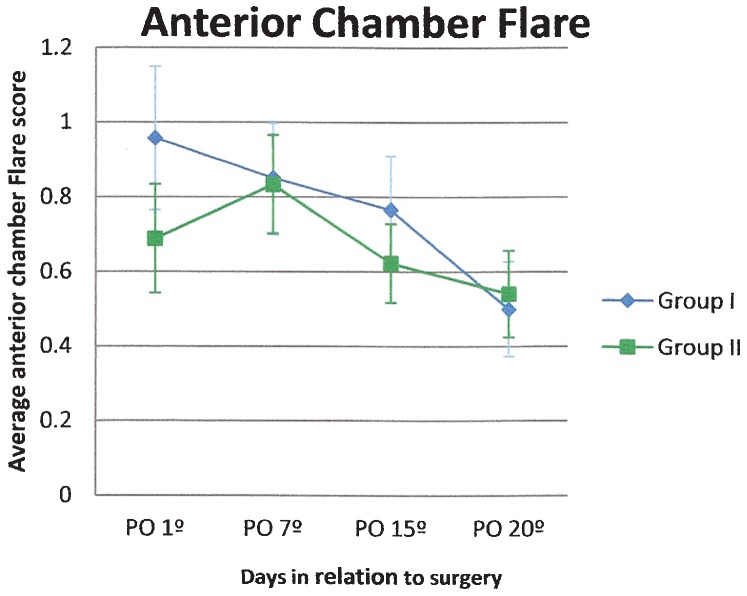
Regarding anterior chamber cell counting, both groups presented an average of 21 to 50 cells (score 3) on postoperative day 1, and a reduction to less than five cells (score 0) was observed in both groups at the end of the follow-up period (Figure 3). Flare was similar for both groups during the study, gradually decreasing over time (Figure 4).
Figure 3.
Cell score in the anterior chamber (average and standard error) in patients treated with gatifloxacin 0.3% and prednisolone acetate 1% eye drops in combination (Group I) or separately (Group II).
Figure 4.
Flare score in the anterior chamber (average and standard error) in patients treated with gatifloxacin 0.3% and prednisolone acetate 1% eye drops in combination (Group I) or separately (Group II).
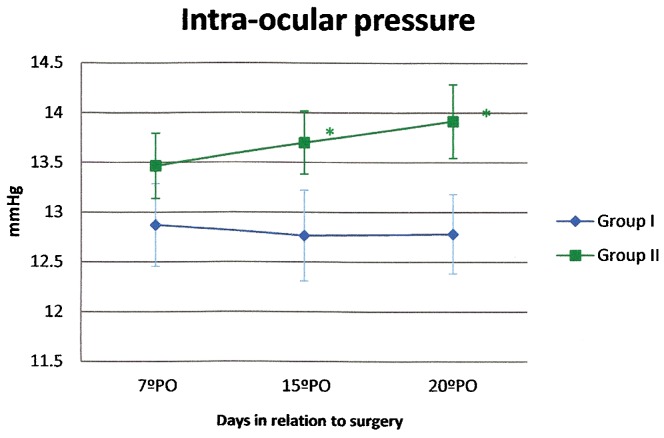
The maximum intraocular pressure was 12.81 (±0.06) mmHg in Group I and 13.67 (±0.23) mmHg in Group II; this difference in pressure was statistically significant (p<0.05) (Figure 5).
Figure 5.
Intraocular pressure (average and standard error) in patients treated with gatifloxacin 0.3% and prednisolone acetate 1% eye drops in combination (Group I) or separately (Group II). (*p<0.05)
Seven patients (15%) from Group I and eight patients (13%) from Group II presented increased anterior chamber cell count, conjunctival hyperemia, and pain score on postoperative day 20 (five days after medication suspension), with no statistically significant difference between the groups.
DISCUSSION
Data were obtained from patients who underwent cataract surgery and IOL implantation at a reference hospital in São Paulo, Brazil. Patient demographics were similar in the study groups.
Gatifloxacin 0.3% is an 8 methoxy-substituted fluoroquinolone agent with greater potency against gram-positive organisms and certain species of atypical mycobacteria than earlier generation fluoroquinolones while retaining excellent coverage against Gram-negative bacteria (5,11). Gatifloxacin 0.3% has also been shown to have excellent penetration into the aqueous (6,15,16). An important factor that contributes to the success of antibiotic therapy is the ability of the molecule to penetrate the target tissues at concentrations greater than the minimum inhibitory concentration (MIC). The concentration of gatifloxacin in the aqueous humor reaches and exceeds the MIC levels for the most common ocular pathogens (6), except for fluoroquinolone-resistant S. aureus. (5). This antibiotic exceeded the known MIC values for most pathogens that cause endophthalmitis (5).
Coagulase-negative Staphylococci resistance to third- and fourth-generation fluoroquinolones has been observed in recent studies. These resistant bacteria emerge rapidly after exposure to the antibiotic and are maintained by periodic reexposure (17,18).
The mechanisms of Staphylococcus resistance to fluoroquinolones consist of mutations in the gyrA and parC genes. Fluoroquinolone-resistant S. epidermidis isolated from clinical samples has emerged rapidly because of the frequent and repeated use of these antibiotics. Approximately 10% of the S. epidermidis strains isolated from ocular infections are resistant to gatifloxacin. The irrational use of gatifloxacin for the treatment of ocular infections could increase the prevalence of resistant strains (18).
The efficacies of the two presentations of the drugs used in this study were assessed by comparing the two treatment groups for signs of inflammation, including hyperemia or edema of the lids or conjunctiva, ocular discharge, structural changes of the cornea, photophobia, pain, and excessive tearing. These findings were similar to our clinical observations of an increased inflammatory response for the first few days after surgery, which gradually decreased and ended by the end of the second week.
The main effectiveness criterion was the rate of anterior chamber cell decrease. A progressive decrease in anterior chamber cell count after the first postoperative day was found on slit-lamp examination, suggesting that the inflammatory reaction was controlled in both groups. After the eye drop treatments were suspended, 15 patients presented an increase in anterior chamber cell count, conjunctival hyperemia, pain, and photophobia complaints, with no statistically significant difference between the groups. This mild intraocular inflammation resolved after the reintroduction and tapering of the prednisolone acetate drops. Therefore, this episode may have been caused by steroid withdrawal syndrome caused by the abrupt suspension of the corticosteroid eye drops. Previous studies have demonstrated that ocular rebound inflammation may develop secondary to rapid tapering or abrupt discontinuation of topical ocular steroid use and is best prevented with gradual tapering (19). The mechanisms by which rebounds in ocular disease may follow steroid withdrawal are still uncertain (20).
Continuous administration of corticosteroid inhibits the feedback mechanism involving hypothalamic-pituitary-adrenal (HPA) functions. Using large doses for a few days or smaller doses for more than two weeks often leads to a prolonged decrease in HPA function. Absorption of any oculary administered corticosteroid through the nasal or pharyngeal mucosa has the potential to produce adverse systemic effects. Tapering the drug, generally over four to six weeks, allows the adrenal glands time to return to their normal secretion patterns (21).
Prednisolone monotherapy after postoperative day 20, instead of the combined formula, can be used to promote inflammation control and avoid corticosteroid withdrawal syndrome and bacteria resistance.
One of our patients presented with a surgically induced intraocular infection on postoperative day 20, likely caused by Propionibacterium acnes. The eye had inflammatory exacerbations and remissions, with recurrence after treatment. Inflammation resolved after vitrectomy with removal of the intraocular lens and capsular bag, but an infection agent could not be isolated from either the aqueous or vitreous humors or capsule bag cultures.
With regard to the safety parameters of visual acuity, ocular pain, and intraocular pressure, eyes that received the fixed combination of gatifloxacin/prednisolone acetate eye drops were similar to those that used the non-fixed combination. There was no adverse reaction related to either drug regimen. Combined and separated gatifloxacin 0.3% and prednisolone acetate 1% were safe and resulted in improved visual acuity, less pain, and intraocular pressure within the normal range (22,23). The intraocular pressure was 1 mmHg lower in Group I than in Group II, but this difference was not clinically significant.
One advantage of a fixed combination of antibiotic and anti-inflammatory agents is the lower exposure of the eye to the toxic effect of preservative agents. Previous in vitro studies have demonstrated the toxic effect in the corneal endothelium, reducing cell survival (13). The detergent properties of BAK (the most common topical ophthalmic medication preservative) interfere with the integrity of the external lipid layer of the precorneal tear film, reduce tear film breakup time, and exacerbate dry eye symptoms. Even in low concentrations, the extended use of eye drops with preservatives may cause adverse reactions, whereas highly concentrated preservatives can cause immediate damage and irritation to the ocular tissue (12,14).
Other advantages of the fixed combination treatment are the ease of use and better treatment compliance, with no administration mistakes and drug washout by concomitant eye drop application within a short time interval (24-26).
In conclusion, the use of a fixed combination is as effective as a non-fixed combination but could be more convenient for the patient, has better treatment compliance, and increases the likelihood of receiving the proper dosage (24-31).
Both fixed and non-fixed combinations of gatifloxacin 0.3% and prednisolone acetate 1% are similarly effective in reducing inflammation and infection prophylaxis after phacoemulsification surgery and intraocular lens implantation.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.O'Brien TP, Arshinoff SA, Mah FS. Perspectives on antibiotics for postoperative endophthalmitis prophylaxis: potential role of moxifloxacin. J Cataract Refract Surg. 2007;33(10):1790–800. doi: 10.1016/j.jcrs.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 2.Chan E, Mahroo OA, Spalton DJ. Complications of cataract surgery. Clin Exp Optom. 2010;93(6):379–89. doi: 10.1111/j.1444-0938.2010.00516.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller JJ, Scott IU, Flynn HW, Jr, Smiddy WE, Newton J, Miller D. Acute-onset endophthalmitis after cataract surgery (2000–2004): incidence, clinical settings, and visual acuity outcomes after treatment. A J Ophthalmol. 2005;139(6):983–7. doi: 10.1016/j.ajo.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Mollan SP, Gao A, Lockwood A, Durrani OM, Butler L. Postcataract endophthalmitis: incidence and microbial isolates in a United Kingdom region from 1996 through 2004. J Cataract Refract Surg. 2007;33(2):265–8. doi: 10.1016/j.jcrs.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, Stark WJ, O'Brien TP, Dick JD. Aqueous Penetration and Biological Activity of Moxifloxacin 0,5% Ophthalmic Solution and Gatifloxacin 0,3% Solution in Cataract Surgery Pacients. Ophthalmology. 2005;112(11):1992–6. doi: 10.1016/j.ophtha.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Ong-Tone L. Aqueous humor penetration of gatifloxacin and moxifloxacin eyedrops given by different methods before cataract surgery. J Cataract Refract Surg. 2007;33(1):59–62. doi: 10.1016/j.jcrs.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Wang NL, Wang YL, Ma C, Ma L, Gao LX, et al. Determination of drug concentration in aqueous humor of cataract patients administered gatifloxacin ophthalmic gel. Chin Med J (Engl) 2010;123(15):2105–10. [PubMed] [Google Scholar]
- 8.Laurell CG, Zetterstrom C. Effects of dexamethasone, diclofenac, or placebo on the inflammatory response after cataract surgery. Br J Ophthalmol. 2002;86(12):1380–4. doi: 10.1136/bjo.86.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilyas H, Slonim CB, Braswell GR, Favetta JR, Schulman M. Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. Eye Contact Lens. 2004;30(1):10–3. doi: 10.1097/01.ICL.0000092071.82938.46. [DOI] [PubMed] [Google Scholar]
- 10.Reddy R, Kim SJ. Critical appraisal of ophthalmic ketorolac in treatment of pain and inflammation following cataract surgery. Clin Ophthalmol. 2011;5:751–8. doi: 10.2147/OPTH.S7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campos M, Muccioli C, Malta JB, Gerade RA, LA Salame A, Belfort R. Efficacy and tolerability of a combined gatifloxacin plus prednisolone formulation for topical prophylaxis after LASIK. Clin Ophthalmol. 2011;5:209–14. doi: 10.2147/OPTH.S17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awan M A, Agarwal PK, Watson D G, McGhee CNJ, Dutton G N. Penetration of topical and subconjunctival corticosteroids into human aqueous humour and its therapeutic significance. Br J Ophthalmol. 2009;93(6):708–13. doi: 10.1136/bjo.2008.154906. [DOI] [PubMed] [Google Scholar]
- 13.Ayaki M, Taguchi Y, Soda M, Yaguchi S, Iwasawa A, Koide R. Cytotoxicity of topical medication used for infection and inflammation control after cataract surgery in cultured corneal endothelial cells. Biocontrol Sci. 2010;15(3):97–102. doi: 10.4265/bio.15.97. [DOI] [PubMed] [Google Scholar]
- 14.Ayaki M, Yaguchi S, Iwasawa A, Koide R. Cytotoxicity of ophthalmic solutions with and without preservatives to human corneal endothelial cells, epithelial cells and conjunctival epithelial cells. Clin Experiment Ophthalmol. 2008;36(6):553–9. doi: 10.1111/j.1442-9071.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 15.Epstein S P, Ahdoot M, Marcus E, Asbell P A. Comparative Toxicity of Preservatives on Immortalized Corneal and Conjunctival Epithelial Cells. J Ocul Pharmacol Ther. 2009;25(2):113–9. doi: 10.1089/jop.2008.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlech BA, Blondeau J. Future of ophthalmic anti-infective therapy and the role of moxifloxacin ophthalmic solution 0.5% (VIGAMOX) Surv Ophthalmol. 2005;50 Suppl 1:S64–7. doi: 10.1016/j.survophthal.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Kim SJ, Toma HS. Antimicrobial Resistance and Ophthalmic Antibiotics1-Year Results of a Longitudinal Controlled Study of Patients Undergoing Intravitreal Injections. Arch Ophthalmol. 2011 Sep;129(9):1180–8. doi: 10.1001/archophthalmol.2011.213. [DOI] [PubMed] [Google Scholar]
- 18.Juárez-Verdayes MA, Parra-Ortega B, Hernández-Rodríguez C, Betanzos-Cabrera G, Rodríguez-Martínez S, Cancino-Diaz ME, et al. Identification and expression of nor efflux family genes in Staphylococcus epidermidis that act against gatifloxacin. Microb Pathog. 2012;52(6):318–25. doi: 10.1016/j.micpath.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 19.McCulley JP, Caudle D, Aronowicz JD, Shine WE. Fourth-generation fluoroquinolone penetration into the aqueous humor in humans. Ophthalmology. 2006;113(6):955–9. doi: 10.1016/j.ophtha.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 20.Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin. 1992;10(3):505–12. [PubMed] [Google Scholar]
- 21.Beltrani VS, Barsanti FA, Pharm, Bielory L. Effects of Glucocorticosteroidis on the Skin and Eye. Immunol Allergy Clin North Am. 2005 Aug;25(3):557–80. doi: 10.1016/j.iac.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Notivol R, Amin D, Whitling A, Wells D, Kennedy M, Cockrum PC, et al. Prophylactic effectiveness of tobramycin-dexamethasone eye drops compared with tobramycin/vehicle eye drops in controlling post-surgical inflammation in cataract patients: prospective, randomised, double-masked, two-arm, parallelgroup, placebo- controlled, multicentre study. Clin Drug Investig. 2004;24(9):523–33. doi: 10.2165/00044011-200424090-00003. [DOI] [PubMed] [Google Scholar]
- 23.Leydhecker W, Akiyama K, Neumann HG. Intraocular pressure in normal human eyesKlin Monbl Augenheilkd Augenarztl Fortbild. 1958;133(5):662–70. [PubMed] [Google Scholar]
- 24.Jakab E, Zbinden R, Gubler J, Ruef C, von Graevenitz A, Krause M. Severe infections caused by Propionibacterium acnes: an underestimated pathogen in late postoperative infections. Yale J Biol Med. 1996;69(6):477–82. [PMC free article] [PubMed] [Google Scholar]
- 25.Eady EA, Ingham E. Propionibacterium acnes—friend or foe. Rev Med Microbiol. 1994;5:163–73. [Google Scholar]
- 26.Smith M A, Alperstein P, France K, Vellozzi E M, Isenberg H D. Susceptibility testing of Propionibacterium acnes comparing agar dilution with E test. J Clin Microbiol. 1996;34(4):1024–6. doi: 10.1128/jcm.34.4.1024-1026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambrano W, Flynn HW, Pflugfelder SC, Roussel TJ, Culbertson WW, Holland S, et al. Management options for Propionibacterium acnes endophthalmitis. Ophthalmology. 1989;96(7):1100–5. doi: 10.1016/s0161-6420(89)32768-0. [DOI] [PubMed] [Google Scholar]
- 28.Costello P, Bakri SJ, Beer PM, Singh RJ, Falk NS, Peters GB, et al. Vitreous penetration of topical moxifloxacin and gatifloxacin in humans. Retina. 2006;26(2):191–5. doi: 10.1097/00006982-200602000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Solomon R, Donnenfeld ED, Perry HD, Snyder RW, Nedrud C, Stein J, et al. Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humor. Ophthalmology. 2005;112(3):466–9. doi: 10.1016/j.ophtha.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Price MO, Price FW, Jr, Maclellan D. Effect of gatifloxacin 0.3%and moxifloxacin 0.5% ophthalmic solutions on human corneal epithelium following 2 dosing regimens. J Cataract Refract Surg. 2005;31(11):2137–41. doi: 10.1016/j.jcrs.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Van Endt JJ, Veraart HG, Kramer R, Janssen AG, Sunder Raj P. A comparison of two ophthalmic steroid-antibiotic combinations after cataract surgery. Eur J Ophthalmol. 1997;7(2):144–8. doi: 10.1177/112067219700700204. [DOI] [PubMed] [Google Scholar]