Abstract
OBJECTIVES:
MiRNAs are intrinsic RNAs that interfere with protein translation. Few studies on the synergistic effects of miRNAs have been reported. Both miR-424 and miR-381 have been individually reported to be involved in carcinogenesis. They share a common putative target, WEE1, which is described as an inhibitor of G2/M progression. Here, we studied the synergistic effects of miR-424 and miR-381 on renal cancer cells.
METHODS:
The viability of 786-O cells was analyzed after transfection with either a combination of miR-424 and miR-381 or each miRNA alone. We investigated cell cycle progression and apoptosis with flow cytometry. To confirm apoptosis and the abrogation of G2/M arrest, we determined the level of pHH3, which is an indicator of mitosis, and caspase-3/7 activity. The expression levels of WEE1, Cdc25, γH2AX, and Cdc2 were manipulated to investigate the roles of these proteins in the miRNA-induced anti-tumor effects. To verify that WEE1 was a direct target of both miR-424 and miR-381, we performed a dual luciferase reporter assay.
RESULTS:
We showed that the combination of these miRNAs synergistically inhibited proliferation, abrogated G2/M arrest, and induced apoptosis. This combination led to Cdc2 activation through WEE1 inhibition. This regulation was more effective when cells were treated with both miRNAs than with either miRNA alone, indicating synergy between these miRNAs. WEE1 was verified to be a direct target of each miRNA according to the luciferase reporter assay.
CONCLUSIONS:
These data clearly demonstrate that these two miRNAs might synergistically act as novel modulators of tumorigenesis by down-regulating WEE1 expression in renal cell cancer cells.
Keywords: miRNA, WEE1, miR-424, miR-381, Synergistic Effect
INTRODUCTION
MiRNAs (miRs) are small endogenous RNAs that interfere with protein translation by binding to target mRNAs (1). MiRs can bind to the 3' untranslated region (3'-UTR) of their targets and silence gene expression in a sequence-specific manner. The modulation of gene expression by miRs is involved in the regulation of many crucial biological events, such as apoptosis (2), cell cycle distribution (3), cellular differentiation (4), and cellular proliferation (5). A considerable body of evidence has confirmed that miRs play roles as tumor suppressors. Moreover, miRs have emerged as key regulators in renal cancer and may be potential therapeutic targets (6).
The control of entry into mitosis is highly conserved in all eukaryotic cells (7). The key mitotic inducer is the mitotic cyclin-dependent kinase Cdc2, and its activity is controlled by a reversible inhibitory phosphorylation event (8). This phosphorylation of Cdc2 is catalyzed by WEE1, which has been described as an inhibitor of G2/M transition (9). WEE1 was recently reported to be a safeguard against mitotic catastrophe in instances of sensitive cell division. Accordingly, WEE1 overexpression causes G2 arrest by promoting the inhibitory phosphorylation of cyclin-dependent kinases (CDKs) (10). This regulation of CDKs by WEE1 has been confirmed in various cancer types, such as glioblastoma (11), lung carcinoma (12), and breast cancer (13). These previous studies demonstrated that WEE1 depletion reduced carcinoma cell survival and was correlated with better prognosis. WEE1 expression was further correlated with survival in patients with mantle cell lymphoma (14). Moreover, in tumors in which the G1 checkpoint is defective, the tumor cells are more sensitive to the abrogation of the G2 checkpoint (15). Therefore, WEE1 has become an interesting target in the development of a “cell cycle G2 checkpoint abrogation” treatment strategy.
We used five algorithms in starBase (http://starbase.sysu.edu.cn/(16) to determine whether WEE1 is a target of miR regulation in renal clear cell carcinoma. We hypothesized that miRNA-424 and miRNA-381 might be involved in WEE1 inactivation.
It was previously determined that miR-424 played a critical role in the development of cervical carcinomas through its modulation of Chk1, a cell cycle regulator; therefore, this miR is also considered a prognostic indicator and an important therapeutic target (17). MiR-424 has also been shown to be involved in the VEGF and FGF signaling pathways by modulating VEGFR2 and FGFR1, leading to downstream effects on angiogenesis (18). Another study demonstrated that miR-424 deregulation is involved in the pathogenesis of CN-AML patients with NPM1 mutations (19). In addition, miR-424 expression has been reported to distinguish cancers from high-grade intraepithelial neoplasia in colon tissues, which suggests that miR-424 may have considerable clinical value in the early diagnosis of colorectal cancer (20). The deregulation of miR-381 and its target ID1 contributes to the metastasis of lung adenocarcinomas, and a novel regulatory “miR-381-/LRRC4-MEK/ERK/AKT” network has been thoroughly elucidated in glioma (21). MiR-381 has also been identified as an important serum marker that could potentially help diagnose brain tumors and monitor their response to therapy (21). Moreover, miRNA expression profiling in renal cell carcinoma demonstrated that miR-381 was significantly down-regulated in tumors compared with normal kidney tissue (22). Therefore, we believe that both miR-424 and miR-381 play important suppressive roles in renal cell carcinoma; however, their exact effect and mechanism of action are unknown.
There have been an increasing number of studies investigating the anti-tumor effect of miRs and their mechanism of regulation. A majority of target genes are modulated by the same miR. This finding raises the following question: why do so many different miRs control the same targets? A potential explanation is that various miRs act together more efficiently. In the present study, we investigated the synergistic effect of miR-381 and miR-424 on the proliferation and apoptosis of 786-O cells in vitro. We also investigated the mechanism through which miR-381 and miR-424 act as novel and potent inhibitors of WEE1 in 786-O cells.
MATERIALS AND METHODS
Cell culture and miR transfections
Double-stranded miRNA mimics (miR-381 and miR-424) were synthesized by Shanghai GenePharma Company in China and stored at -20 °C. They were transfected into cells using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. A random miR sequence (non-targeting oligonucleotide) (Genepharma, Shanghai, China) was used as a negative control (miR-NC). A transfection efficiency of approximately 80% was achieved using 6-carboxyfluorescein-labeled miR. Cells were harvested for total RNA and protein extraction. A renal clear cell carcinoma cell line (786-O) and a renal tubular epithelial cell line (HK-2) were procured from ATCC (Mannassas, VA, USA) and cultured in RPMI-1640 supplemented with 10% FBS in an incubator at 5% CO2 and 37 °C.
Cell survival and proliferation---MTT assay
786-O cells were plated in a 96-well flat-bottomed plate. They were transfected with miR-NC, miR-381, and miR-424 and incubated for 24 h, 48 h, or 72 h before adding MTT. The cells were subsequently incubated for an additional 4 h at 37 °C and 5% CO2. The absorbance at 540 nm was determined, and the cell viability was defined as the percentage of untreated cells.
Assessment of caspase 3/7 activity
Caspase-3/7 activity was evaluated using a caspase-3/7 assay (Promega, Madison, WI, USA). Cells (1×105 cells per well) were plated in a 96-well plate and incubated at 37 °C overnight. The cells were lysed after treatment with miR-381 and miR-424. The caspase-3/7 reagent (100 μl/well) was added, and the plates were examined using a plate-reading luminometer (Bio-Rad, Hercules, CA, USA) at an excitation wavelength range of 485±20 nm and an emission wavelength range of 530±25 nm.
Mitotic index
The 786-O cells were stained with DAPI (Auragene Bioscience, Changsha, Hunan, China). We assessed mitotic morphology according to duplicated chromatid pairs, which align during cell division. The mitotic index of 786-O cells was determined using the following formula: mitotic index % = (number of mitotic cells/total number of cells) ×100.
Cell cycle analysis
Following treatment with miR-381 and miR-424 for 48 h, logarithmically growing cells were harvested and fixed with 70% ethanol at 4 °C overnight. After extensive washing with PBS, the cells were suspended in Hank's balanced salt solution containing 75 μg/ml RNase A and 50 μg/ml propidium iodide (Sigma, St.Louis, MO, USA). The cells were incubated for 0.5 h at 37 °C before immediate analysis using a FACS Canto II instrument (BD, San Jose, CA, USA). They were further analyzed using the ModFit 2.0 cell cycle analysis program (BD, San Jose, CA, USA).
Apoptosis Assay
An Annexin V/FITC apoptosis detection kit was purchased from BD Biosciences (BD, San Jose, CA, USA). 786-O cells were treated with miR-381 and miR-424 in 24-well culture plates for 48 h. The cells were trypsinized and centrifuged at 2500 g for 5 min, followed by resuspension in buffer. Annexin V-FITC solution (Key Gene, JiangSu, Nanjing, China) and PI (Sigma, St.Louis, MO, USA) were added, and the cells were analyzed using a FACS Canto II instrument (BD, San Jose, CA, USA).
Prediction of modulated miRs targeting WEE1
To identify miRs that target WEE1, we used five algorithms to computationally predict potential modulated miRs. The miRs were predicted using the starBase (16) database, which combines gene predictions from the TargetScan, PicTar, RNA22, PITA, and miRanda algorithms. Only targets that were predicted by at least three algorithms were included. Potential miRs were chosen based on their possible functions, the number of predicted target sites, and target prediction by multiple algorithms.
RT-PCR and Western blotting
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and its purity was determined at 260 nm. cDNA was synthesized through reverse transcription using ReverTra Ace (Toyobo, Osaka, Japan). WEE1 primers were purchased from Sangon (Shanghai, China), and the SYBR Green PCR Master Mix (YRBIO, Changsha, Hunan, China) was used for RT-PCR. The reaction volume was 20 μl, and PCR was performed in triplicate. Gene expression was quantified using the comparative Ct method (the ΔΔCt method). 786-O cells were harvested for the analysis of protein expression 48 h after treatment, followed by Western blotting as previously described. Antibodies against WEE1, Cdc25, MYT1, PARP, Bcl-2, and γH2AX were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies against Cdc2 and p-Cdc2 were obtained from Abcam (Cambridge, MA, USA). The histone H3 ps10 antibody was purchased from Millipore (Billerica, MA, USA). β-Actin (Santa Cruz, California, USA) was used as an internal control.
Validation of WEE1 as a target of miR-424 and miR-381 using a luciferase reporter assay
WEE1 was predicted to be regulated by both miR-424 and miR-381 using a miR target prediction tool (starBase). We cloned the 3'-UTR of WEE1, which contained the putative binding sites of miR-424 and miR-381, into an empty luciferase reporter vector, pGL4.13 (Promega, Madison, WI, USA). Mutations were introduced into the seed region of miR-424 or miR-381 in the 3'-UTR of WEE1, and the mutants were termed WEE1-3'-UTR-mut-1, WEE1-3'-UTR-mut-2, WEE1-3'-UTR-mut-3, and WEE1-3'-UTR-mut-4. The sequences of the constructs were verified with DNA sequencing. 786-O cells seeded in 24-well plates were transfected with the reporter plasmid and miR mimics or a NC oligo using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Lysates were collected 48 h after transfection. The luciferase activity was measured using a TD 20/20 luminometer (Promega, Madison, WI, USA) according to the manufacturer's protocol. The luminescence intensity of firefly luciferase was normalized to that of renilla luciferase.
Statistical analysis
Data analysis was performed with SPSS 17.0 software (SPSS Inc., Chicago, IL). Numerical values were expressed as the mean ± SEM. The significance of inter-group differences was evaluated with a one-way analysis of variance, and the significance of single comparisons was determined with Student's t-test. All tests performed were two sided. A p-value less than 0.05 was considered to be statistically significant.
Ethics
Ethical approval for this investigation was obtained from the Research Ethics Committee, Third Xiang-Ya Hospital of Central South University.
RESULTS
The levels of both miR-424 and miR-381 were decreased in 786-O cells, and simultaneous transfection with both miRs inhibited the proliferation of 786-O cells more efficiently than transfection with either miR alone in vitro.
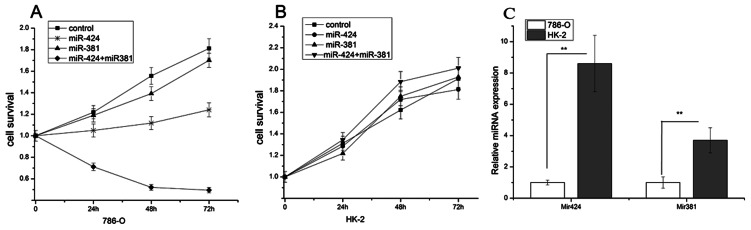
We determined the expression levels of miR-424 and miR-381 using real-time PCR. We demonstrated a significantly lower level of miR-424 (approximately 10-fold) in 786-O cells compared with HK-2 cells, and miR-381 showed similar results (3.7-fold) (Figure 1C). To assess the effects of these two miRs on 786-O survival, we determined cell viability following combined transfection or transfection with either miR alone. Cell survival was assessed after incubation for 24 h, 48 h, and 72 h. There was an obvious inhibition of proliferation by approximately 4-fold after the combined transfection of the two miRs, whereas either miR alone had less influence on the survival of 786-O cells (Figure 1 A). Cell death reached a maximum at 48 h after transfection, and there was no significant increase in cell death at 72 h compared with 48 h. To ascertain whether the two miRs inhibited normal cells, the survival of HK-2 was assessed using a similar MTT assay. In contrast to 786-O cells, no inhibitory effect was observed in HK-2 cells (Figure 1B). These data indicate that the combination of miR-381 and miR-424 was effective in inhibiting the proliferation of 786-O cells. Treatment with the combination of miR-381 and miR-424 promoted mitotic entry and induced apoptosis.
Figure 1.
MiR-424 and miR-381 are poorly expressed in 786-O cells, and the combination of miR-424 and miR-381 significantly inhibited the proliferation of 786-O cells. (A) Cell survival curves of 786-O cells treated with a combination of miR-424 and miR-381 and with either miR alone. The lines represent three independent experiments, which were performed in triplicate. Synergistic inhibition was significant in 786-O cells, and cell survival was decreased by approximately 4-fold compared with the control (p<0.01). (B) No similar effects were observed in the normal cell line HK-2. (C) Both miRs (miR-424 and miR-381) were analyzed with real-time PCR using total RNA from 786-O and HK-2 cells. We calculated ΔCt (miR Ct-U6 Ct) from the Ct values of each gene for normalization. The ΔCt values were then converted into relative gene expression levels using the 2−ΔΔCt method. The graph shows the relative fold difference in gene expression between 786-O and HK-2 cells. **p<0.01.
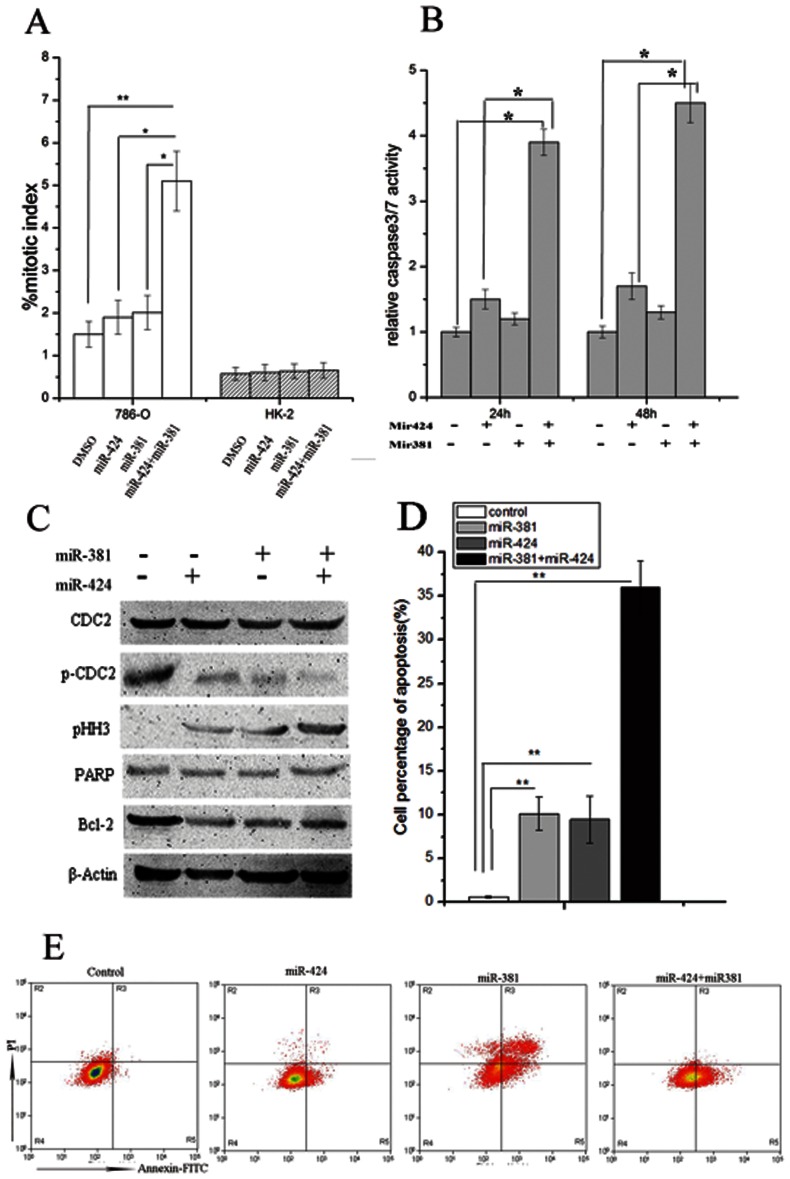
To confirm that the combination of the two miRs induced mitotic entry, the mitotic index following combined transfection was determined and compared with the transfection of either miR alone. The number of mitotic cells was significantly higher for 786-O cells treated with the combination of the two miRs (Figure 2A). The percentage of cells in mitosis following combined transfection was approximately 5%, compared with 1.5%, 1.9%, and 2.0% in the other three groups (Figure 2A). To investigate how mitotic entry was modulated by combined treatment, 786-O cells treated with both miR-424 and miR-381 for 48 h were examined to assess the activation status of Cdc2. Upon co-inhibition with miR-424 and miR-381, Cdc2 was unphosphorylated (Figure 2C, lane 2). The subsequent activation of Cdc2 resulted in a higher percentage of mitotic cells, which reached as high as 5%, in cells treated with both miRs compared with cells treated with either miR alone (Figure 2A). Phospho-histone H3 (pHH3) is considered a reliable biomarker of the mitotic count (23). The combination of both miRs led to an increased pHH3 level (Figure 2C), lane 3), which reaches a maximum during mitosis and reflects premature mitotic entry (24). These data indicate that the two miRs modulated Cdc2 activation and augmented mitotic entry by inhibiting Cdc2 phosphorylation.
Figure 2.
MiR-381 and miR-424 synergistically promoted mitotic entry and induced apoptosis. (A) The mitotic index (%) was determined in both 786-O cells and HK-2 cells. The combination of miRs significantly increased the mitotic index. (B) Cell death was analyzed through caspase-3/7 activation in cells treated with miR-424, miR-381, the combination, or DMSO for 48 h. Caspase 3/7 activity increased only in the presence of both miRs compared with the control. (C) Cell lysates collected from control cells and cells treated with miR-424, miR-381, and the combination of miR-381 and miR-424 were assessed with SDS-PAGE. The combined treatment strongly reduced the level of p-Cdc2, increased the level of pHH3, and increased the percentage of mitotic cells without DNA damage repair, which caused mitotic catastrophe and apoptosis. The combination of miRs also down-regulated Bcl-2 expression compared with the control cells. β-Actin was used as a loading control. (D) and (E) The fraction of apoptotic 786-O cells was investigated after the cells were stained with both Annexin V-FITC and PI. (D) The means ± SDs of three independent experiments. (E) Representative results. *p<0.05, **p<0.01.
To evaluate the synergistic effect of these miRs on apoptosis, cells were analyzed with flow cytometry following labeling with Annexin V-FITC and PI. The combination of miR-381 and miR-424 augmented the percentage of apoptotic 786-O cells significantly more than monotherapy (Figure 2D, E). This finding was confirmed through the analysis of the apoptosis-related protein Bcl-2 (Figure 2C), lane 5). As shown in Figure 2B, when 786-O cells were treated with the combination of miRs for 48 h, there was a significant decrease in caspase-3/7 activity, suggesting that this combination may result in synergistic cytotoxicity through the activation of the caspase pathway.
The combination of miR-381 and miR-424 inhibited Cdc2 phosphorylation at Tyr15 by inhibiting WEE1, thereby abrogating the G2/M block.
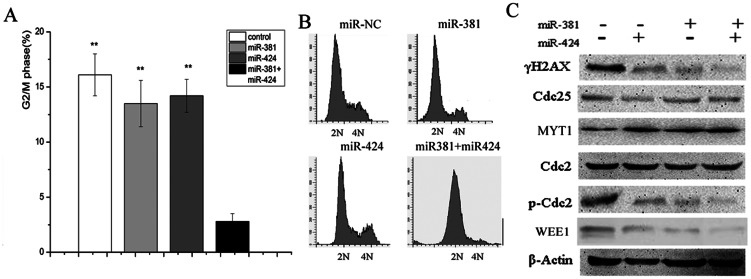
It has been reported that Cdc2/cyclin B kinase is activated prior to the onset of mitotic entry, which could trigger G2/M transition (25). It has been clearly established that in addition to multiple mutations, endogenous DNA damage is accumulated in the majority of carcinomas. WEE1 is crucial for coordinating the DNA damage response and genomic replication and for avoiding mitotic catastrophe caused by the abrogation of G2 checkpoint arrest (26). Thus, miR-381 and miR-424, putative regulators of WEE1, are vital for the fate of 786-O cells. We therefore transfected 786-O cells with a combination of miR-381 and miR-424 to test whether G2/M arrest was abrogated. Accordingly, flow cytometry results showed that G2/M arrest was abrogated when 786-O cells were pretreated with both miRs for 48 h but not when the cells were treated with either one alone (Figures 3A and B). Treatment with miR-424 and miR-381 resulted in an obvious down-regulation of G2/M-arrested cells, which accounted for approximately 3% of cells. Neither miR-424 nor miR-381 led to a significant decrease in the percentage of cells arrested in G2. The percentages of cells arrested in G2 by miR-424 and miR-381 alone were 14% and 13%, respectively (Figures 3A and B). Therefore, it is clear that the simultaneous transfection of miR-381 and miR-424 abrogated G2 arrest in 786-O cells.
Figure 3.
(A) and (B) Cell cycle progression was analyzed with FACS. Cells were treated with miR-381, miR-424, or the combination of miR-381 and miR-424. Based on the DNA contents of the cells, the percentage of cells in G2/M decreased significantly after the combination treatment compared with the control. (B) Representative results. (C) The combination of miR-381 and miR-424 abolished the phosphorylation of Cdc2 at Tyr15. Both MYT1 and Cdc25 were ruled out in the regulation of this phosphorylation event. The cells were treated as described above. **p<0.01.
To explore the molecular mechanisms underlying the abrogation of G2/M cell cycle arrest induced by miR-381 and miR-424, we determined the expression levels of WEE1. Figure 3C shows that there was a marked decrease in WEE1 activity following the transfection of 786-O cells with a combination of miR-381 and miR-424 compared with the control. As described above, Western blotting also demonstrated that p-Cdc2 expression was decreased.
Thus, the above data suggest that the combined treatment results in the synergistic inhibition of WEE1 and the abrogation of the G2/M block, which are related to the persistent inhibitory effect of WEE1 and Cdc2 activation.
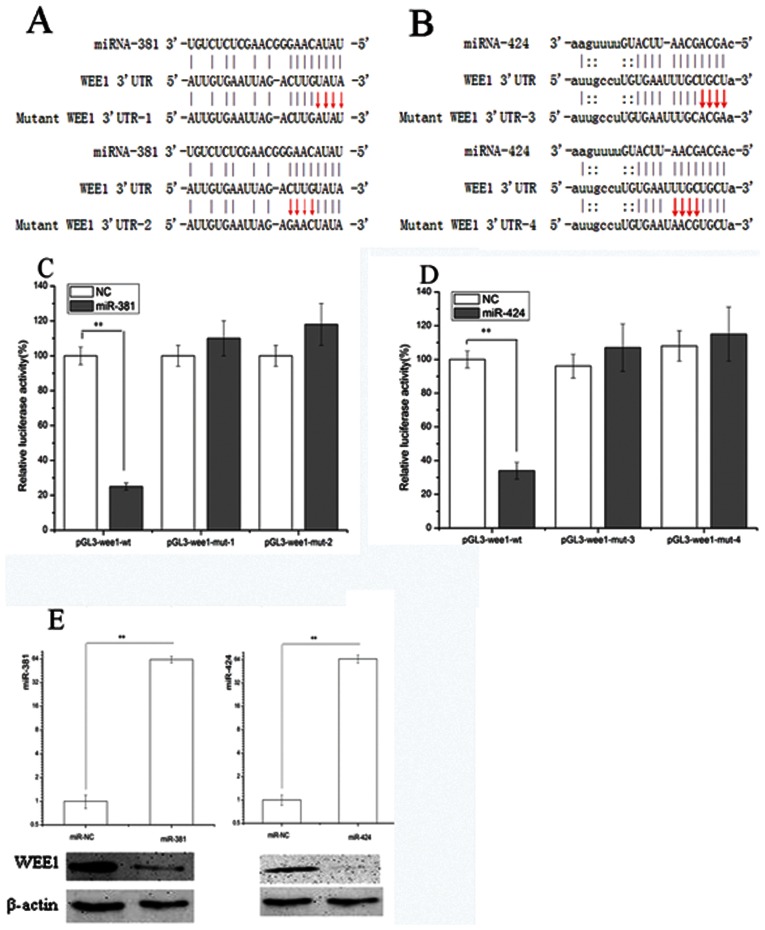
We next determined whether miR-381 and miR-424 directly target WEE1, which in turn regulates the cell cycle progression and apoptosis of 786-O cells. A bioinformatics search (http://starbase.sysu.edu.cn) (16) revealed that the 3'-UTR of WEE1 (Figures 4A and B) was complementary to the nucleotide bases of miR-424 and miR-381, which might be involved in WEE1 inactivation.
Figure 4.
(A) An illustration depicting the base pairing between miR-381 and the 3'-UTR of WEE1. Mutations in the 3'-UTR of WEE1 were designed for the first four consecutive bases (UAUA to AUAU) or the last four bases (CUUG to GAAC), generating two mutants termed mutant-WEE1 3'-UTR-1 and mutant-WEE1 3'-UTR-2, respectively. (B) The base pairing between miR-424 and the 3'-UTR of WEE1 is shown. Similar to mutant 3'-UTR WEE1-1, mutant-WEE1 3'-UTR-3 and mutant-WEE1 3'-UTR-4 were designed according to the base pairing between miR-424 and the 3'-UTR of WEE1. (C) and (D) Dual-luciferase reporter gene assay. 786-O cells were co-transfected with the indicated reporter constructs and Renilla luciferase plasmids. The cells were also transfected with miR-NC, miR-381 mimic, or miR-424 mimic. Forty-eight hours later, the luciferase activity was determined. The relative activity was normalized to the Renilla luciferase activity. Significant differences in the luciferase activity are shown. (E) The increased expression levels of miR-424 and miR-381 upon transfection were confirmed with real-time PCR. Western blotting showed that WEE1 was inhibited by both miR-424 and miR-381. **p<0.01.
To determine whether miR-424 and miR-381 act directly on the WEE1 3'-UTR, we constructed a luciferase reporter containing either the wild-type or a mutant WEE1 3'-UTR (Figures 4A and B). The increased levels of miR-424 and miR-381 following transfection were confirmed with real-time PCR (Figure 4E) and significantly affected the relative luciferase activity (Figures 4C and D). The results revealed a statistically significant down-regulation of luciferase activity when these cells were simultaneously transfected with both miRs and 3'-UTR-WEE1-wt. However, this regulation was significantly alleviated when four nucleotides in the seed region were mutated (Figures 4A and B), indicating that both miRs directly regulate WEE1 together.
To provide further evidence that WEE1 down-regulation is induced by both miRs, we transfected 786-O cells with another mutant of the 3'-UTR of WEE1 and either miR. A similar decrease in luciferase activity was observed (Figures 4C and D). In addition, WEE1 expression decreased significantly following transfection with either miR. Thus, these data clearly show that miR-381 and miR-424 up-regulation could induce WEE1 inhibition.
DISCUSSION
A recent study demonstrated that miR-424 up-regulation induced the inhibition and phosphorylation of Chk1, which in turn modulated WEE1 and Cdc25c phosphorylation (17). The activated WEE1 and inactivated Cdc25c coordinay prevented Cdc2/cyclin B activation and mitotic entry in cancer cells (27). However, we observed different results following the treatment of 786-O cells with miR-424 or miR-381 alone; we showed that either miR alone had no marked effect on 786-O cells. The regulatory effects of miRs on apoptosis and proliferation have been clearly validated in many reports, which demonstrate the targeting of various cell cycle regulatory proteins by miRs (28). Moreover, convincing evidence has shown that WEE1 inhibitors synergize to achieve tumor regression (29). These observations led to the original hypothesis that the synergistic inhibition of WEE1 could limit the repair of endogenous DNA damage and therefore sensitize cancer cells to this damage. WEE1 is considered the putative target of both miR-424 and miR-381 according to the combination of gene predictions from the miRBase, TargetScan, miRanda, RNA22, and PicTar algorithms. We demonstrated that these two miRs function synergistically to inhibit WEE1, and this inhibition has an enhanced anti-tumor effect by modulating Cdc2/cyclin B activity.
Our research showed that the combination of miR-424 and miR-381, which is also involved in the pathogenesis and the drug resistance of leukemia (19), potentiated the anti-tumor effect of each miR. The up-regulation of miR-424 was also previously shown to inhibit the proliferation and cord formation of endothelial cells by targeting VEGF and VEGF receptor-2 in vitro (18). Interestingly, miR-424 has also been previously investigated to determine its role in cell cycle regulation. The expression of miR-424 was shown to block cell cycle progression. When miR-424 was combined with miR-503, additional effects were observed. We similarly demonstrated that miR-424 combined with miR-381 could augment the apoptosis of 786-O cells and directly affect cell cycle progression by targeting WEE1. Thus, miR-424 and miR-381 could be novel therapeutic targets for a majority of individuals with renal carcinomas.
The Cdc2/cyclin B complex undergoes inhibitory phosphorylation by WEE1 and myelin transcription factor 1 (MYT1). In contrast, Cdc25 activates this complex through dephosphorylation. Thus, WEE1, MYT1, and their counterpart Cdc25 coordinate to regulate the activity of the Cdc2/cyclin B complex and constitute the main switch for mitosis (29). In this study, neither Cdc25 nor MYT1 was activated or inhibited (Figure 3C), further confirming that the deregulation of Cdc2 activity was induced by the synergistic effect of both miRs on the WEE1/Cdc2 pathway (29).
In addition, the siRNA-mediated depletion of WEE1 augments DNA damage during cell cycle progression (30). Recent studies have demonstrated that the WEE1 3'-UTR is a direct target of miR-195, miR-128a, miR-155, and miR-516 (31). The results of our study support our hypothesis that a post-translational mechanism involving miR-424 and miR-381 may attenuate WEE1 expression. We demonstrated that these two miRs interacted directly with the WEE1 3'-UTR.
As recent publications have highlighted, many genes have multiple binding sites for many miRs. Therefore, various combinations of miRs might overcome the relative weakness of each individual miR (32). Further validation of other predicted targets is being performed, and this analysis may yield a more complex explanation for the interaction between miR-424 and miR-381. We believe that an interaction between miR-381 and miR-424 may help strengthen the association between the two miRs and the WEE1 3'-UTR. Neither miR-424 nor miR-381 alone significantly killed human tumor cells. One possible explanation for the synergistic effect is that each miR has unique targets in addition to WEE1 (17,18). When combined, their unique mRNA targets are able to enhance the binding effect of each miR with WEE1, which was demonstrated previously (33). Moreover, consistent with the synergistic effect of these two miRs, the binding site proximity of miR-424 and miR-381 may be a crucial factor in the regulation of WEE1, which potentially inhibits protein translation. Third, WEE1 plays important roles in DNA duplication, chromosome condensation, and anaphase entry (9,10). The explanation for the synergistic inhibition may lie in any of these effects. Few studies have investigated the synergistic inhibition of cancers by miRs, and the various mechanisms need to be further investigated (34). We currently have no definitive explanation for the observed synergistic effect. Determining the mechanism will require additional experiments that were beyond the scope of this study. Clearly, further research should be performed until this surprising biological phenomenon is fully understood.
Our study demonstrated that carcinoma cells were more susceptible to the combined miRs than normal cells. It is therefore crucial to further validate this biological phenomenon and identify a significant therapeutic target. It was also reported that the inhibition of WEE1 activity combined with DNA damage resulted in increased cytotoxicity in p53-deficient cells (30). Therefore, we will benefit from further studies demonstrating a similar p53 status-dependent mechanism for the effects of the combined treatment.
Various studies have shown that WEE1 depletion leads to S phase block, increased apoptotic morphology, and caspase-induced apoptosis (13,29). We also observed caspase-induced apoptosis after combination treatment in 786-O cells. The mechanism underlying caspase activation and subsequent apoptosis is unknown. Further study is needed to determine the mechanism through which miR-424 and miR-381 synergistically induce caspase activation.
Few studies have been performed to assess the synergistic effects of miRs, and thus, it is important to investigate new combination treatment methods. We confirmed that miR-424 in combination with miR-381 inhibited cell proliferation and induced apoptosis in a synergistic manner. Our data suggest that combining miR-381 and miR-424 may provide therapeutic benefits to patients. This synergy may increase the response rate and provide a clinical opportunity to achieve anti-tumor effects.
ACKNOWLEDGMENTS
This work was supported by a grant from the 125 Talents Program (Yin Guangming) of Third Xiang-Ya Hospital in China.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Cho WC. MicroRNAs as therapeutic targets and their potential applications in cancer therapy. Expert Opin Ther Targets. 2012;16(8):747–59. doi: 10.1517/14728222.2012.696102. [DOI] [PubMed] [Google Scholar]
- 2.Hilgers V, Bushati N, Cohen SM. Drosophila microRNAs 263a/b confer robustness during development by protecting nascent sense organs from apoptosis. PLoS Biol. 2010;8:e1000396. doi: 10.1371/journal.pbio.1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang LH, He XH. Macro-management of microRNAs in cell cycle progression of tumor cells and its implications in anti-cancer therapy. Acta Pharmacol Sin. 2011;32(11):1311–20. doi: 10.1038/aps.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciaudo C, Servant N, Cognat V, Sarazin A, Kieffer E, Viville S, et al. Highly dynamic and sex-specific expression of microRNAs during early ES cell differentiation. PLoS Genet. 2009;5(8):e1000620. doi: 10.1371/journal.pgen.1000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Corrigan-Cummins M, Hudson J, Maric I, Simakova O, Neelapu SS, et al. MicroRNA profiling of follicular lymphoma identifies microRNAs related to cell proliferation and tumor response. Haematologica. 2012;(97(4)):586–94. doi: 10.3324/haematol.2011.048132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang A, Liu Y, Shen Y, Xu Y, Li X. miR-21 modulates cell apoptosis by targeting multiple genes in renal cell carcinoma. Urology. 2011;78(2):474.e13–9. doi: 10.1016/j.urology.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185(2):193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashita K. Cdc2 activation by phosphorylation. Seikagaku. 1996;68(5):367–71. [PubMed] [Google Scholar]
- 9.Fasulo B, Koyama C, Yu KR, Homola EM, Hsieh TS, Campbell SD, et al. Chk1 and Wee1 kinases coordinate DNA replication, chromosome condensation, and anaphase entry. Mol Biol Cell. 2012;23(6):1047–57. doi: 10.1091/mbc.E11-10-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen CS, Syljuasen RG. Safeguarding genome integrity: the checkpoint kinases ATR, CHK1 and WEE1 restrain CDK activity during normal DNA replication. Nucleic Acids Res. 2012;40(2):477–86. doi: 10.1093/nar/gkr697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mir SE, De Witt HP, Krawczyk PM, Balaj L, Claes A, Niers JM, et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell. 2010;18(3):244–57. doi: 10.1016/j.ccr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Wang Y, Sun Y, Lawrence TS. Wild-type TP53 inhibits G(2)-phase checkpoint abrogation and radiosensitization induced by PD0166285, a WEE1 kinase inhibitor. Radiat Res. 2002;157:322–330. doi: 10.1667/0033-7587(2002)157[0322:wttigp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Garimella SV, Rocca A, Lipkowitz S. WEE1 inhibition sensitizes basal breast cancer cells to TRAIL-induced apoptosis. Mol Cancer Res. 2012;10(1):75–85. doi: 10.1158/1541-7786.MCR-11-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blenk S, Engelmann JC, Pinkert S, Weniger M, Schultz J, Rosenwald A, et al. Explorative data analysis of MCL reveals gene expression networks implicated in survival and prognosis supported by explorative CGH analysis. BMC Cancer. 2008;8:106. doi: 10.1186/1471-2407-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon H, Norbury CJ. Therapeutic exploitation of checkpoint defects in cancer cells lacking p53 function. Cell Cycle. 2002;1(6):362–8. doi: 10.4161/cc.1.6.257. [DOI] [PubMed] [Google Scholar]
- 16.Yang JH, Li JH, Shao P, Zhou H, Chen YQ, Qu LH. starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res. 2011;39(Database issue):D202–9. doi: 10.1093/nar/gkq1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Li Y, Wang F, Wang X, Cheng B, Ye F, et al. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene. 2013;32(8):976–87. doi: 10.1038/onc.2012.121. [DOI] [PubMed] [Google Scholar]
- 18.Chamorro-Jorganes A, Araldi E, Penalva LO, Sandhu D, Fernandez-Hernando C, Suarez Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler Thromb Vasc Biol. 2011;31(11):2595–606. doi: 10.1161/ATVBAHA.111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faraoni I, Laterza S, Ardiri D, Ciardi C, Fazi F, Lo-Coco F. MiR-424 and miR-155 deregulated expression in cytogenetically normal acute myeloid leukaemia: correlation with NPM1 and FLT3 mutation status. J Hematol Oncol. 2012;5:26. doi: 10.1186/1756-8722-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Wang L, Bayaxi N, Li J, Verhaegh W, Janevski A, et al. A microRNA panel to discriminate carcinomas from high-grade intraepithelial neoplasms in colonoscopy biopsy tissue. Gut. 2013;62(2):280–9. doi: 10.1136/gutjnl-2011-301554. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Shen S, Wu M, Li X, Xiong W, Lu J, et al. Transcriptomic regulation and molecular mechanism of polygenic tumor at different stages. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36(7):585–91. doi: 10.3969/j.issn.1672-7347.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Nakada C, Matsuura K, Tsukamoto Y, Tanigawa M, Yoshimoto T, Narimatsu T, et al. Genome-wide microRNA expression profiling in renal cell carcinoma: significant down-regulation of miR-141 and miR-200c. J Pathol. 2008;216(4):418–27. doi: 10.1002/path.2437. [DOI] [PubMed] [Google Scholar]
- 23.Kim YJ, Ketter R, Steudel WI, Feiden W. Prognostic significance of the mitotic index using the mitosis marker anti-phosphohistone H3 in meningiomas. Am J Clin Pathol. 2007;128(1):118–25. doi: 10.1309/HXUNAG34B3CEFDU8. [DOI] [PubMed] [Google Scholar]
- 24.Tapia C, Kutzner H, Mentzel T, Savic S, Baumhoer D, Glatz K. Two mitosis-specific antibodies, MPM-2 and phospho-histone H3 (Ser28), allow rapid and precise determination of mitotic activity. Am J Surg Pathol. 2006;30(1):83–9. doi: 10.1097/01.pas.0000183572.94140.43. [DOI] [PubMed] [Google Scholar]
- 25.Jin ZH, Kurosu T, Yamaguchi M, Arai A, Miura O. Hematopoietic cytokines enhance Chk1-dependent G2/M checkpoint activation by etoposide through the Akt/GSK3 pathway to inhibit apoptosis. Oncogene. 2005;24(12):1973–81. doi: 10.1038/sj.onc.1208408. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura T, Shimaoka T, Kano K, Naito K. Insufficient amount of Cdc2 and continuous activation of Wee1 B are the cause of meiotic failure in porcine growing oocytes. J Reprod Dev. 2009;55(5):553–7. doi: 10.1262/jrd.09-072a. [DOI] [PubMed] [Google Scholar]
- 27.Indovina P, Giordano A. Targeting the checkpoint kinase WEE1: selective sensitization of cancer cells to DNA-damaging drugs. Cancer Biol Ther. 2010;9(7):523–5. doi: 10.4161/cbt.9.7.11276. [DOI] [PubMed] [Google Scholar]
- 28.Rivas MA, Venturutti L, Huang YW, Schillaci R, Huang TH, Elizalde PV. Downregulation of the tumor-suppressor miR-16 via progestin-mediated oncogenic signaling contributes to breast cancer development. Breast Cancer Res. 2012;14(3):R77. doi: 10.1186/bcr3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Witt HP, Mir SE, Noske D, Van Noorden CJ, Wurdinger T. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011 1. 17(13):4200–7. doi: 10.1158/1078-0432.CCR-10-2537. [DOI] [PubMed] [Google Scholar]
- 30.Carrassa L, Chila R, Lupi M, Ricci F, Celenza C, Mazzoletti M, et al. Combined inhibition of Chk1 and Wee1: in vitro synergistic effect translates to tumor growth inhibition in vivo. Cell Cycle. 2012;11(13):2507–17. doi: 10.4161/cc.20899. [DOI] [PubMed] [Google Scholar]
- 31.Qi J, Yu JY, Shcherbata HR, Mathieu J, Wang AJ, Seal S, et al. microRNAs regulate human embryonic stem cell division. Cell Cycle. 2009;8(22):3729–41. doi: 10.4161/cc.8.22.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peter ME. Targeting of mRNAs by multiple miRNAs: the next step. Oncogene. 2010;29(15):2161–4. doi: 10.1038/onc.2010.59. [DOI] [PubMed] [Google Scholar]
- 33.Bandi N, Vassella E. miR-34a and miR-15a/16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner. Mol Cancer. 2011;10:55. doi: 10.1186/1476-4598-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nachmani D, Lankry D, Wolf DG, Mandelboim O. The human cytomegalovirus microRNA miR-UL112 acts synergistically with a cellular microRNA to escape immune elimination. Nat Immunol. 2010;11(9):806–13. doi: 10.1038/ni.1916. [DOI] [PubMed] [Google Scholar]