Abstract
Context:
Polycystic ovary syndrome (PCOS) is a heterogeneous, genetically complex, endocrine disorder of uncertain etiology in women.
Objective:
Our aim was to compare the gene expression profiles in stimulated granulosa cells of PCOS women with and without insulin resistance vs. matched controls.
Research Design and Methods:
This study included 12 normal ovulatory women (controls), 12 women with PCOS without evidence for insulin resistance (PCOS non-IR), and 16 women with insulin resistance (PCOS-IR) undergoing in vitro fertilization. Granulosa cell gene expression profiling was accomplished using Affymetrix Human Genome-U133 arrays. Differentially expressed genes were classified according to gene ontology using ingenuity pathway analysis tools. Microarray results for selected genes were confirmed by real-time quantitative PCR.
Results:
A total of 211 genes were differentially expressed in PCOS non-IR and PCOS-IR granulosa cells (fold change ≥ 1.5; P ≤ 0.001) vs. matched controls. Diabetes mellitus and inflammation genes were significantly increased in PCOS-IR patients. Real-time quantitative PCR confirmed higher expression of NCF2 (2.13-fold), TCF7L2 (1.92-fold), and SERPINA1 (5.35-fold). Increased expression of inflammation genes ITGAX (3.68-fold) and TAB2 (1.86-fold) was confirmed in PCOS non-IR. Different cardiometabolic disease genes were differentially expressed in the two groups. Decreased expression of CAV1 (−3.58-fold) in PCOS non-IR and SPARC (−1.88-fold) in PCOS-IR was confirmed. Differential expression of genes involved in TGF-β signaling (IGF2R, increased; and HAS2, decreased), and oxidative stress (TXNIP, increased) was confirmed in both groups.
Conclusions:
Microarray analysis demonstrated differential expression of genes linked to diabetes mellitus, inflammation, cardiovascular diseases, and infertility in the granulosa cells of PCOS women with and without insulin resistance. Because these dysregulated genes are also involved in oxidative stress, lipid metabolism, and insulin signaling, we hypothesize that these genes may be involved in follicular growth arrest and metabolic disorders associated with the different phenotypes of PCOS.
Polycystic ovary syndrome (PCOS) is a heterogeneous, genetically complex, endocrine disorder of uncertain etiology in women. Its principal features include hyperandrogenism, ovulatory dysfunction, and polycystic ovaries (1–3). Besides the reproductive abnormalities, metabolic complications including insulin resistance, obesity, dyslipidemia, and hypertension are also evident in many PCOS women (4). Compared with the normally cycling women, PCOS patients are at a greater risk for diabetes mellitus and cardiovascular diseases (5).
We hypothesized that modest effects of individual genetic variants may lead to abnormal expression of multiple PCOS, insulin resistance, and cardiovascular disease susceptibility genes in granulosa cells during gonadotropin therapy for in vitro fertilization. Findings presented here demonstrate significant differences in granulosa cell expression of genes involved in diabetes mellitus, inflammation, cardiovascular disease, and infertility in PCOS patients with and without insulin resistance, which may relate to the different PCOS phenotypes.
Subjects and Methods
Experimental subjects
The detailed descriptions of PCOS and control subjects, methods for subject recruitment, ovarian stimulation, and granulosa cell collection are presented in Supplemental Methods (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). We conducted our gene expression study on gonadotropin-stimulated granulosa cells from 12 controls, the normal ovulatory women; 12 women with PCOS without evidence for insulin resistance (PCOS non-IR); and 16 women with PCOS complicated by peripheral insulin resistance (PCOS-IR) who were undergoing in vitro fertilization.
Microarray hybridizations and ingenuity pathway analysis (IPA)
Microarray hybridizations on Affymetrix HG-U133 Plus 2 whole genome GeneChips (Affymetrix, Inc., Santa Clara, CA), were conducted with control and PCOS patient mRNA samples. The detailed descriptions of microarray hybridizations, molecular interaction, and functional analysis using IPA tools and real-time quantitative PCR (RT-qPCR) are presented in the Supplemental Methods. The microarray data are deposited at the Gene Expression Omnibus public repository (http://www.ncbi.nlm.nih.gov/geo) with accession number GSE34526. The criteria for selecting differentially expressed genes were preset as at least fold change (FC) ≥ 1.5 fold (P ≤ 0.001, unpaired t test).
Results
Clinical characteristics of PCOS and control subjects
The clinical characteristics of the control, PCOS non-IR (without insulin resistance), and PCOS-IR (with insulin resistance) are shown in Supplemental Table 1. Levels of LH, LH/FSH, and free testosterone were similar in IR and non-IR groups but significantly higher than controls. Levels of insulin, body mass index, and homeostasis model assessment of insulin resistance were significantly higher in PCOS-IR patients than in the PCOS non-IR group and controls. The PCOS non-IR group and controls had normal insulin levels. All subjects had normal glucose, TSH, and prolactin levels.
Differentially expressed genes in granulosa cells of PCOS women with and without insulin resistance
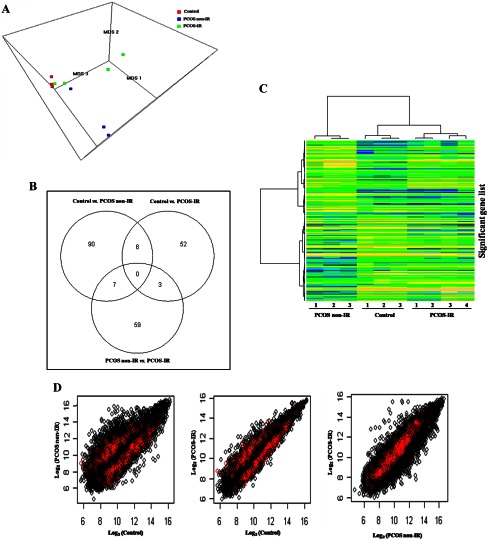
To identify differentially expressed genes, microarray hybridizations were carried out for three control, three PCOS non-IR, and four PCOS-IR subjects. Classical multidimensional scaling was applied in three dimensions to the 17,958 probe sets that remained after filtering, and a 3-dimensional scatterplot showed that the three groups (Fig. 1A and Supplemental 3D Plot) were well separated. Of 17,958 analyzed probe sets, 217 probe sets were identified as differentially expressed (P ≤ 0.001). The Venn diagram (Fig. 1B) showed 103 probe sets in control vs. PCOS non-IR, 61 probe sets in control vs. PCOS-IR, and 69 probe sets in PCOS non-IR vs. PCOS-IR to be differentially expressed. Supervised hierarchical clustering was performed on the dataset of 217 unique probe sets that were significantly expressed in at least one of the three pairwise comparisons, resulting in three distinct clusters on the heat map (Fig. 1C). A total of 211 genes were differentially expressed in PCOS non-IR and PCOS-IR granulosa cells (FC ≥ 1.5; P ≤ 0.001) vs. matched controls. The differentially expressed genes for each pairwise comparison are presented in Supplemental Table 2 (control vs. PCOS non-IR), Supplemental Table 3 (control vs. PCOS-IR), and Supplemental Table 4 (PCOS non-IR vs. PCOS-IR).
Fig. 1.
A, Global gene expression profiles of the control and the two phenotypes of PCOS—PCOS non-IR and PCOS-IR granulosa cells. The 17,958 probe sets left after excluding all control probe sets and probe sets present in less than 80% of the samples were reduced to three dimensions using classical multidimensional scaling (MDS). The figure represents the gene expression data in three dimensions, with each plotted point representing one sample and with color used to represent the sample type: red, control (n = 3); blue, PCOS non-IR (n = 3); and green, PCOS-IR (n = 4). B, Venn diagram illustrating the number of probe sets identified as significantly differentially expressed (FC ≥ 1.5; P ≤ 0.001) in each pairwise comparison (control vs. PCOS non-IR, control vs. PCOS-IR, and PCOS non-IR vs. PCOS-IR) and common between them. A Venn diagram was constructed using limma Bioconductor package. C, Supervised hierarchical clustering for 217 unique probe sets significant (P ≤ 0.001) in at least one of the three pairwise comparisons was performed by Ward's method and 1-(Pearson's correlation) as the distance measure. Each row represents a single gene; each column represents a sample. Expression values were color coded: blue, transcript level below the median; green, equal to median; and yellow, greater than median. D, To compare the results of all PCOS comparison with each pairwise comparison (control vs. PCOS non-IR, control vs. PCOS-IR, and PCOS non-IR vs. PCOS-IR), the mean probe set expression values (on a log2 scale) for all probe sets were plotted. In each scatterplot, 222 differentially expressed probe sets (P ≤ 0.001) in all PCOS groups were overlapped and displayed in red. The distribution of red points showed the specific differences between the differentially expressed probe sets of all PCOS groups and the pairwise comparisons indicating the importance of studying the gene expression profiles of the two phenotypes of PCOS separately.
Differential outcomes of pairwise and all PCOS comparisons
Statistical comparison was also performed for all PCOS (n = 7) vs. control (n = 3) subjects. Among the 17,958 probe sets tested, 222 were differentially expressed in all PCOS groups (P ≤ 0.001) corresponding to 185 unique genes (Supplemental Table 5). To compare the results of all PCOS comparisons with each pairwise comparison (control vs. PCOS non-IR, control vs. PCOS-IR, and PCOS non-IR vs. PCOS-IR), the mean probe set expression values (on a log2 scale) for all probe sets were plotted. In each scatter plot, 222 differentially expressed probe sets (FC ≥ 1.5; P ≤ 0.001) in all PCOS groups were overlapped and displayed in red (Fig. 1D). The distribution of red points showed the specific differences between the differentially expressed probe sets of all PCOS groups and the pairwise comparisons.
Dysregulated interactions in PCOS non-IR and PCOS-IR granulosa cells
The gene lists for each pairwise comparison (P ≤ 0.001; FC ≥ 1.5), control vs. PCOS non-IR (Supplemental Table 2), and control vs. PCOS-IR (Supplemental Table 3) were analyzed by IPA tools. Six networks for PCOS non-IR and four networks for PCOS-IR were generated (Supplemental Table 6). The highest scoring network of PCOS non-IR (Supplemental Fig. 1A) and PCOS-IR (Supplemental Fig. 1B) are given in the Supplemental Data. The major “hubs” dysregulated were NFkB, HNF4A, MAPK1, CAV1, TP53, CTNNB1, MYC, and HTT in PCOS non-IR and NFkB, HNF4A, TNF, HNF1A, and NFYB in PCOS-IR.
PCOS non-IR and PCOS-IR-specific gene ontology
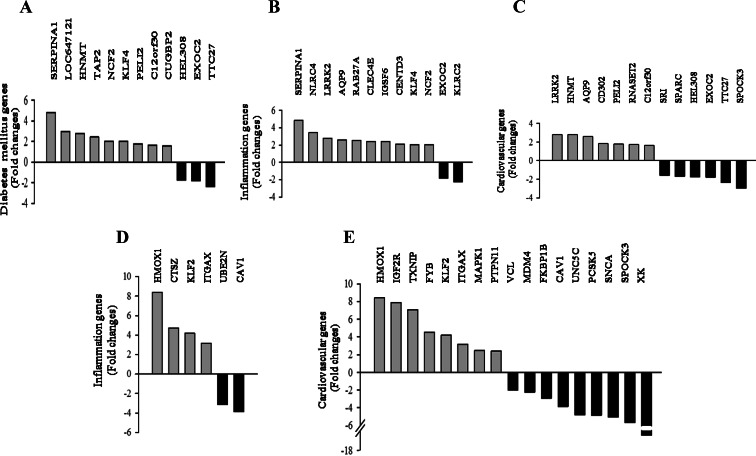
Functional classification of differentially expressed genes identified diabetes mellitus, inflammation, and cardiovascular disease-related genes in granulosa cells from PCOS patients with (Fig. 2, A–C) and without (Fig. 2, D and E) insulin resistance. Microarray results of selected genes were confirmed in additional samples of nine control, nine PCOS non-IR, and 12 PCOS-IR subjects by RT-qPCR (Supplemental Tables 7 and 8).
Fig. 2.
Functional analysis of microarray data by IPA tools indicated differential expression (P ≤ 0.001) of diabetes mellitus, inflammatory response, and cardiovascular disease genes in the granulosa cells of PCOS-IR (n = 4) (A–C) and PCOS non-IR (n = 3) subjects (D and E) vs. matched controls (n = 3). A, Histogram analysis of diabetes mellitus genes in PCOS-IR with at least 1.8-fold change in expression. B, Histogram analysis of inflammation response genes in PCOS-IR with at least 1.58-fold change in expression. C, Histogram analysis of cardiovascular disease genes in PCOS-IR with at least 1.59-fold change in expression. D, Histogram analysis of inflammation response genes in PCOS non-IR with at least 3.11-fold change in expression. E, Histogram analysis of cardiovascular disease genes in PCOS non-IR with at least 1.98-fold change in expression.
Diabetes mellitus genes
Nine up-regulated and three down-regulated diabetes mellitus genes were identified in stimulated granulosa cells of PCOS-IR patients vs. controls (Fig. 2A). The genetic variants of the transcription factor 7-like 2 (TCF7L2) gene are possibly the strongest known genetic risk factor for type 2 diabetes mellitus. Therefore, the expression of TCF7L2 was also studied. Higher expression of neutrophil cytosolic factor 2 (NCF2) (2.13-fold) and TCF7L2 (1.92-fold) was confirmed in PCOS-IR granulosa cells (Supplemental Table 8).
Inflammation genes
Twelve inflammation genes were identified in PCOS-IR granulosa cells, 10 were up-regulated, and two were down-regulated in PCOS-IR (Fig. 2B). RT-qPCR confirmed higher expression of SERPINA1 (5.35-fold) and NCF2 (2.13-fold) (Supplemental Table 8). Four inflammation response genes were up-regulated, and two down-regulated in PCOS non-IR (Fig. 2D). Significantly increased expression of integrin, α X (complement component 3 receptor 4 subunit) (ITGAX) (3.68-fold) and TGF-β-activated kinase 1/MAP3K7 binding protein 2 (TAB2) (1.86-fold) in PCOS non-IR was confirmed (Supplemental Table 8).
Cardiovascular disease genes
Thirteen cardiovascular disease genes were identified in PCOS-IR (seven up-regulated and six down-regulated; Fig. 2C) and the decreased expression of osteonectin (SPARC) (−1.88-fold) was confirmed (Supplemental Table 8). Seventeen cardiovascular disease genes were identified in PCOS non-IR patients (eight up-regulated and nine down-regulated; Fig. 2E), and decreased expression of caveolin-1 (CAV1) (−3.58-fold) was confirmed (Supplemental Table 8).
Differential expression of genes involved in TGF-β signaling [IGF-II receptor (IGF2R), 5.10-fold and 4.30-fold; hyaluronan synthase 2 (HAS2), −5.31-fold and −3.53-fold] and oxidative stress [thioredoxin-interacting protein (TXNIP), 3.32-fold and 2.81-fold] was confirmed in PCOS non-IR and PCOS-IR granulosa cells, respectively (Supplemental Table 8).
Discussion
Our results from whole genome microarray analysis show the differences in gene expression profiles of gonadotropin-stimulated granulosa cells of PCOS patients with and without insulin resistance. The comparison of the differentially expressed probe sets in all PCOS groups with those in the pairwise comparisons demonstrated the specific differences in the outcome. Because the differentially expressed probe sets from pairwise comparisons are not highlighted by all PCOS comparison, it was important to study gene expression profiles of the two phenotypes of PCOS separately. Network analyses of the differentially expressed genes identified “dysregulated hubs” and their interacting molecules in the two phenotypes of PCOS granulosa cells. Microarray analysis and RT-qPCR data demonstrated significant differences in the expression of diabetes mellitus, inflammation, and cardiovascular disease-related genes in PCOS non-IR and PCOS-IR granulosa cells. The identified subsets of genes are also related to oxidative stress, insulin signaling, and infertility. This is the first study to compare the expression profiles of stimulated granulosa cells from PCOS women with and without insulin resistance.
Significantly higher expression of diabetes mellitus genes SERPINA1, NCF2, and TCF7L2 and a significant decrease in the expression of cardiovascular disease-related gene SPARC was confirmed in the PCOS-IR group by RT-qPCR. SERPINA1 encodes for a serine protease inhibitor, an acute phase protein that increases during inflammation (6). NCF2 is a rate-limiting cofactor of NADPH oxidase, and increased expression of NCF2 potentiates NADPH oxidase activity, leading to oxidative stress and hypertension (7). The increased expression of TCF7L2 is reported in adipocytes of insulin-resistant subjects (8), and its overexpression (2-fold) was shown to increase insulin expression in islet cells (2.3-fold) (9). Although the role of SERPINA1, NCF2, and TCF7L2 is not well understood in granulosa cells, we hypothesize that alteration in these genes could contribute to oxidative stress and abnormal glucose metabolism in PCOS follicles. Decreased expression of SPARC, an extracellular matrix glycoprotein, may result in myocardial dysfunction (10). Additionally, SPARC is increased during luteinization and plays an important role in corpus luteum development (11). Its decreased expression in the granulosa cells of PCOS-IR patients may cause defects in proliferative or reorganizational activity during luteinization.
Increased expression of ITGAX and TAB2 and decreased expression of CAV1 were confirmed in PCOS non-IR patients. ITGAX encodes an integrin α X chain protein that mediates cell-cell interactions during inflammation. Increased level of ITGAX is associated with hypertriglyceridemia, hypercholesterolemia and atherosclerosis (12, 13). TAB2 may act as a sensor of inflammatory signals and functions as an adaptor in the IL-1 signaling pathway leading to JNK and nuclear factor κ-B activation (14, 15). CAV1 gene encodes a membrane protein that is involved in lipid and cholesterol transport and steroid and insulin signaling (16). LH/human chorionic gonadotropin is known to increase the expression of CAV1 in granulosa cells of ovulatory follicles (17), and its decreased expression may be deleterious to granulosa cell differentiation. Altered expression of ITGAX, TAB2, and especially CAV1, a dysregulated hub, may contribute to oxidative stress, abnormal lipid metabolism, and follicular growth arrest in PCOS non-IR patients.
Increased expression of IGF2R and TXNIP and decreased expression of HAS2 were confirmed in both PCOS non-IR and PCOS-IR patients. IGF2R encodes IGF-II receptor that activates TGF-β1 and also transports IGF-II to lysosomes, thereby limiting the activation of IGF1R and insulin receptor by IGF-II (18). TXNIP encodes a cytoplasmic protein that inhibits the activity of thioredoxin disulfide reductase and thereby increases oxidative stress (19). A member of the α-arrestin gene family, TXNIP also inhibits glucose uptake and lactate production by destabilizing the hypoxia-induced transcription factor (HIF1α) that regulates the most glycolytic target genes (20). Overexpression of TXNIP is linked to the dysregulation of cardiomyocyte viability (19). HAS2 encodes an enzyme that is stimulated by GDF-9, a TGF-β family member, in cumulus cells leading to hyaluronan-rich matrix during cumulus expansion in the periovulatory period (21, 22). We hypothesize that altered expression of IGF2R, TXNIP, and HAS2 in both groups of patients may contribute to abnormal ovarian follicular growth and cumulus expansion, respectively, in PCOS follicles.
In summary, the current study discloses specific gene expression patterns related to diabetes mellitus, inflammation, cardiovascular diseases, and infertility in the granulosa cells of PCOS women with and without insulin resistance. Because these dysregulated genes are also linked to oxidative stress, lipid metabolism, and insulin signaling, we hypothesize that different genes may be involved in follicular growth arrest and metabolic disorders associated with the different phenotypes of PCOS.
Supplementary Material
Acknowledgments
This work was supported by the INDO-US program on Contraception and Reproductive Health Research of the Department of Biotechnology (Grant BT/PR5379/MED/14/631/2004), New Delhi, India, and National Institutes of Health Grant U54HD034449.
Authors' roles: R.S. conceived the study, assisted with microarrays, analyzed data, drafted and wrote the manuscript. R.S. and S.K. carried out annotation and pathway analysis. S.K. carried out real-time PCR studies. J.F.S. was involved in data analysis and drafting the manuscript. K.J.A. performed statistical analyses on microarray data. M.G.D. and A.K. provided patient data and samples. All authors read and approved the final manuscript.
Disclosure Summary: All authors declare that they have no potential conflict of interest.
Footnotes
- CAV1
- Caveolin-1
- FC
- fold change
- HAS2
- hyaluronan synthase 2
- IGF2R
- IGF-II receptor
- IPA
- ingenuity pathway analysis
- ITGAX
- integrin, α X (complement component 3 receptor 4 subunit)
- NCF2
- neutrophil cytosolic factor 2
- PCOS
- polycystic ovary syndrome
- PCOS-IR
- PCOS with insulin resistance
- PCOS non-IR
- PCOS without insulin resistance
- RT-qPCR
- real-time quantitative PCR
- TAB2
- TGF-β-activated kinase 1/MAP3K7 binding protein 2
- TCF7L2
- transcription factor 7-like 2
- TXNIP
- thioredoxin-interacting protein.
References
- 1. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47 [DOI] [PubMed] [Google Scholar]
- 2. Jabara S, Coutifaris C. 2003. In vitro fertilization in the PCOS patient: clinical considerations. Semin Reprod Med 21:317–324 [DOI] [PubMed] [Google Scholar]
- 3. Cano F, García-Velasco JA, Millet A, Remohí J, Simón C, Pellicer A. 1997. Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women. J Assist Reprod Genet 14:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dokras A, Bochner M, Hollinrake E, Markham S, Vanvoorhis B, Jagasia DH. 2005. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol 106:131–137 [DOI] [PubMed] [Google Scholar]
- 5. Cussons AJ, Stuckey BG, Watts GF. 2007. Metabolic syndrome and cardiometabolic risk in PCOS. Curr Diab Rep 7:66–73 [DOI] [PubMed] [Google Scholar]
- 6. Perlmutter DH, May LT, Sehgal PB. 1989. Interferon β 2/interleukin 6 modulates synthesis of α 1-antitrypsin in human mononuclear phagocytes and in human hepatoma cells. J Clin Invest 84:138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O'Connor PM, Cowley AW., Jr 2012. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahlzén M, Johansson LE, Cervin C, Tornqvist H, Groop L, Ridderstråle M. 2008. Expression of the transcription factor 7-like 2 gene (TCF7L2) in human adipocytes is down regulated by insulin. Biochem Biophys Res Commun 370:49–52 [DOI] [PubMed] [Google Scholar]
- 9. Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P, Sjögren M, Ling C, Eriksson KF, Lethagen AL, Mancarella R, Berglund G, Tuomi T, Nilsson P, Del Prato S, Groop L. 2007. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 117:2155–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d'Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. 2009. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med 206:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bagavandoss P, Sage EH, Vernon RB. 1998. Secreted protein, acidic and rich in cysteine (SPARC) and thrombospondin in the developing follicle and corpus luteum of the rat. J Histochem Cytochem 46:1043–1049 [DOI] [PubMed] [Google Scholar]
- 12. Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, Knowlton AA, Simon SI. 2011. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol 31:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. 2009. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 119:2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K. 2000. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell 5:649–658 [DOI] [PubMed] [Google Scholar]
- 15. Owerbach D, Piña L, Gabbay KH. 2004. A 212-kb region on chromosome 6q25 containing the TAB2 gene is associated with susceptibility to type 1 diabetes. Diabetes 53:1890–1893 [DOI] [PubMed] [Google Scholar]
- 16. Liu P, Rudick M, Anderson RG. 2002. Multiple functions of caveolin-1. J Biol Chem 277:41295–41298 [DOI] [PubMed] [Google Scholar]
- 17. Diouf MN, Lefebvre R, Silversides DW, Sirois J, Lussier JG. 2006. Induction of α-caveolin-1 (αCAV1) expression in bovine granulosa cells in response to an ovulatory dose of human chorionic gonadotropin. Mol Reprod Dev 73:1353–1360 [DOI] [PubMed] [Google Scholar]
- 18. Brown J, Delaine C, Zaccheo OJ, Siebold C, Gilbert RJ, van Boxel G, Denley A, Wallace JC, Hassan AB, Forbes BE, Jones EY. 2008. Structure and functional analysis of the IGF-II/IGF2R interaction. EMBO J 27:265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, De Keulenaer GW, Lee RT. 2002. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem 277:26496–26500 [DOI] [PubMed] [Google Scholar]
- 20. Elgort MG, O'Shea JM, Jiang Y, Ayer DE. 2010. Transcriptional and translational downregulation of thioredoxin interacting protein is required for metabolic reprogramming during G(1). Genes Cancer 1:893–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fülöp C, Salustri A, Hascall VC. 1997. Coding sequence of a hyaluronan synthase homologue expressed during expansion of the mouse cumulus-oocyte complex. Arch Biochem Biophys 337:261–266 [DOI] [PubMed] [Google Scholar]
- 22. Stock AE, Bouchard N, Brown K, Spicer AP, Underhill CB, Doré M, Sirois J. 2002. Induction of hyaluronan synthase 2 by human chorionic gonadotropin in mural granulosa cells of equine preovulatory follicles. Endocrinology 143:4375–4384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.