Abstract
Context:
Animal data suggest that oxytocin is a satiety hormone. We have demonstrated that anorexia nervosa (anorexia), a disorder characterized by food restriction, low weight, and hypoleptinemia, is associated with decreased nocturnal oxytocin secretion. We have also reported functional magnetic resonance imaging (fMRI) hypoactivation in anorexia in brain regions involved in food motivation. The relationships between oxytocin, food-motivation neurocircuitry, and disordered eating psychopathology have not been investigated in humans.
Objective:
The objective of the study was to determine whether the oxytocin response to feeding in anorexia differs from healthy women and to establish the relationship between oxytocin secretion and disordered eating psychopathology and food-motivation neurocircuitry.
Design:
This was a cross-sectional study.
Setting:
The study was conducted at a clinical research center.
Participants:
Participants included 35 women: 13 anorexia (AN), nine weight-recovered anorexia (ANWR), and 13 healthy controls (HC).
Measures:
Peripheral oxytocin and leptin levels were measured fasting and 30, 60, and 120 min after a standardized mixed meal. The Eating Disorder Examination-Questionnaire was used to assess disordered eating psychopathology. fMRI was performed during visual processing of food and nonfood stimuli to measure brain activation before and after the meal.
Results:
Mean oxytocin levels were higher in AN than HC at 60 and 120 min and lower in ANWR than HC at 0, 30, and 120 min and AN at all time points. Mean oxytocin area under the curve (AUC) was highest in AN, intermediate in HC, and lowest in ANWR. Mean leptin levels at all time points and AUC were lower in AN than HC and ANWR. Oxytocin AUC was associated with leptin AUC in ANWR and HC but not in AN. Oxytocin AUC was associated with the severity of disordered eating psychopathology in AN and ANWR, independent of leptin secretion, and was associated with between-group variance in fMRI activation in food motivation brain regions, including the hypothalamus, amygdala, hippocampus, orbitofrontal cortex, and insula.
Conclusions:
Oxytocin may be involved in the pathophysiology of anorexia.
Oxytocin is a nine-amino acid peptide hormone with a range of physiological effects, including modulation of food intake in animals (1). Produced in the supraoptic and paraventricular nuclei of the hypothalamus, oxytocin is stored and released into the peripheral circulation via the posterior pituitary gland. There are central projections of oxytocin neurons to regions throughout the brain, and receptors have been identified in areas involved in food motivation, including the hypothalamus, amygdala, hippocampus, frontal cortex, and insula (2–4). Most data suggest that oxytocin acts as an anorexigenic hormone, inhibiting food intake, although there are conflicting studies and few data defining the role of oxytocin in food motivation pathways in humans. There is evidence that leptin, a key anorexigenic hormone produced by fat cells, signals via oxytocin neurons (5–7).
In anorexia nervosa, a psychiatric illness characterized by severe restriction of food intake, comorbid anxiety and depression, low body weight, hypercortisolemia, and hypoleptinemia, nocturnal serum oxytocin levels are low compared with controls (8). Postprandial oxytocin secretion, however, has not been studied in anorexia nervosa. There is evidence that activation of oxytocinergic appetite pathways in the brain results in inhibition of peripheral oxytocin secretion, as measured in the serum (9–11). We therefore hypothesized that in response to food intake, peripheral oxytocin levels in weight-recovered women with anorexia nervosa would be low, representing an increase in central signaling of satiety as a trait feature of this eating disorder. In contrast, we expected that postprandial peripheral oxytocin secretion in women with active anorexia nervosa would be relatively high, reflecting a lower satiety signal in the starved state. Using a food-related functional magnetic resonance imaging (fMRI) paradigm, we recently demonstrated hypoactivation of regions of the brain involved in food motivation in women with active and, to a lesser extent, weight-recovered anorexia nervosa compared with healthy women (12). In these same subjects, we expected that abnormal postprandial oxytocin secretion in women with anorexia nervosa, even after recovery, would be associated with hypoactivation of food motivation neurocircuitry and disordered eating psychopathology. Hypothalamic-pituitary-adrenal axis activation and subsequent hypercortisolemia is seen in anorexia nervosa, and there is overlap between brain regions involved in the stress response and food motivation pathways (for example, the hypothalamus, amygdala, hippocampus, and insula). We hypothesized that the relationships between oxytocin secretion and disordered eating psychopathology as well as neurocircuitry differences would be independent of cortisol secretion.
Subjects and Methods
Subjects
We studied 35 women between 18 and 28 yr of age: 13 with anorexia nervosa (AN), nine who had recovered from anorexia nervosa [weight-recovered AN (ANWR)], and 13 of normal weight in good health (HC). Clinical characteristics and behavioral and fMRI data have been previously reported (12). All study participants were recruited from the community through advertisements and referrals from health care providers.
Subjects were excluded if they had any contraindication to magnetic resonance imaging such as an implanted medical device, significant orthopedic hardware, or severe claustrophobia. Additional exclusion criteria included active abuse of drugs or alcohol, use of hormones or medications known to affect hormone levels (including estrogen) within 8 wk of the study visit, use of depot medroxyprogesterone within 6 months, diabetes mellitus, history of gastrointestinal tract surgery, pregnancy or breast-feeding within 8 wk of the study, and hematocrit less than 30% or hemoglobin less than 10 g/dl.
We ascertained that subjects met diagnostic criteria for AN with the Structured Clinical Interview for DSM Disorders-IV (SCID), including intense fear of gaining weight, evidence of body image disturbance, significantly low body weight [operationalized as less than 85% of ideal body weight (IBW) as determined by the 1983 Metropolitan Life tables], and amenorrhea for at least 3 consecutive months (13). AN subjects who reported more than one binge and one purge episode per month in the 3 months preceding the study were excluded. Subjects with a history of psychosis by SCID were also excluded.
ANWR were between 90 and 110% IBW and were required to have regular menstrual cycles and stable weight for at least 6 months before the study. ANWR met a diagnosis of AN by Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria other than amenorrhea, as assessed by SCID, in the past. The recovered subjects had not exercised more than 10 h/wk and had not run more than 25 miles/wk in the 3 months preceding the study.
HC were between 90 and 110% of IBW and reported regular menstrual cycles. HC had no history of amenorrhea, no acute or chronic illnesses, and no history of a psychiatric disorder (including an eating disorder) as assessed by SCID. HC were excluded if they had exercised more than 10 h per week or ran more than 25 miles per week in the three months preceding the study.
Materials and Methods
This study was approved by the Partners Human Research Committee. Written informed consent was obtained from all subjects before conducting any procedures. All subjects were admitted to the Massachusetts General Hospital Clinical Research Center for an outpatient screening visit and to the Massachusetts General Hospital Clinical Research Center and Athinoula A. Martinos Imaging Center for a morning, outpatient main visit.
At the screening visit, height, weight, and elbow breadth were measured by research dietitians, blood was drawn for screening laboratory tests, and a comprehensive history and physical examination was performed. Exercise patterns and alcohol intake were assessed. Percent IBW was calculated as above. Body mass index (BMI) was obtained by dividing the weight in kilograms by the square of height in meters. Frame size was determined by comparing elbow breadth to race-specific norms derived from the U.S. Health and Nutritional Examination Survey I (14). The mood episode, psychotic and associated symptoms, mood disorder, anxiety, somatoform, substance abuse and disordered eating modules of the SCID were administered in person during the screening visit or over the telephone before the main visit by a trained psychiatric nurse practitioner or psychologist (13).
At the main visit, percent IBW and BMI were reevaluated. A brief medical history was performed. HC presented during the follicular phase of the menstrual cycle (d 1–10). Subjects were asked to fast for 12 h before the visit. Subjects were given a 400-kcal mixed breakfast meal standardized for micro- and macronutrient content (∼20% calories from protein, 20% from fat, and 60% from carbohydrates) at 0900 h. The participants selected one of the following options of similar macro- and micronutrient content, provided by the Clinical Research Center bionutritionists: cereal, low-fat milk, yogurt, and wheat germ; or minibagel with peanut butter, nonfat Lactaid milk, and Craisins. Participants were asked to eat the entire meal over a 15-min interval. Upon completion of the meal, bionutrition staff weighed the meal to determine exact caloric intake. Blood was drawn at four different time points throughout the morning for hormone levels: a fasting blood draw obtained immediately before the standardized mixed meal, and blood draws at 30, 60, and 120 min after the meal. Disordered eating psychopathology was assessed with the Eating Disorder Examination Questionnaire (EDE-Q). The Beck Depression Inventory-II and Speilberger State-Trait Anxiety Inventory were self-administered. fMRI using a food-related paradigm was performed before and after the meal (see below).
Biochemical analysis
Serum and plasma samples were stored at −80 C until analysis. Oxytocin was measured in extracted serum by ELISA (Assay Designs, Inc., Ann Arbor, MI). Specimens (0.5 ml) were extracted in batches of 20 with an equal volume of 0.1% trifluoroacetic acid (TFA) in water. After centrifugation (17,000 × g per 15 min per 4 C), the organic supernate was subjected to chromatography using C18 Sep-Pak columns preconditioned with 0.1% TFA in water. Extracted oxytocin was recovered by eluting the columns with 3 ml of 60% acetonitrile-40% TFA (0.1%) in water (vol/vol). Extracted oxytocin was stored frozen (−80 C) until all specimens were extracted, using the same lot of columns and reagents, and then lyophilized in a single run, reconstituted in 0.3 ml (i.e. concentrated 1.67-fold) using assay buffer provided by the manufacturer and assayed manually according to the manufacturer's instructions. Plates were read at OD of 405 nm, with correction between 570 and 590, and data calculations were done automatically using an automated ELISA platform (Triturus; Diagnostic Grifols, Barcelona, Spain). The lowest calibrator for this competitive ELISA is 15.6 pg/ml. The limit of detection (lowest dose distinguishable from zero to 3 sd) was 7.0 pg/ml. Analytical recovery after extraction was 91% (range 87–95%) over a range of 125–500 pg/ml final nominal concentrations based on adding kit calibrator to serum pools. Testing was monitored using a pool of serum, which was extracted and tested along with the specimens as well as pools of serum tested directly in the ELISA. The interassay mean, sd, and coefficient of variation (CV) of these measurements were 28.0 pg/ml, 5.0 pg/ml, and 16.5%, respectively.
Serum cortisol levels were measured using a chemiluminescent immunoassay from Beckman-Coulter (Fullerton, CA). The intraassay CV was 4.4–6.7% and the interassay CV was 6.4–7.9%. The sensitivity was 0.4 μg/dl. Plasma leptin levels were measured using an RIA from LINCO Research (St. Charles, MO). The intraassay CV was 3.4–8.3% and the interassay CV was 3.0–6.2%. The sensitivity was 0.5 ng/ml. Area under the curve was calculated using the trapezoidal method.
Assessment of disordered eating psychopathology
The EDE-Q is a well-validated 36-item self-report measure assessing the severity of eating disorder psychopathology in four categories: dietary restraint, eating concern, shape concern, and weight concern. A global score can be calculated to render a dimensional assessment of eating disorder psychopathology. Behaviors such as binge eating, purging, and other compensatory activities such as excessive exercise are also evaluated (15). Normative data (mean ± sd) based on two studies of adult women (16, 17) are as follows: dietary restraint, 1.3 ± 1.4 (16), 1.62 ± 1.54 (17); eating concern, 0.76 ± 1.06, 1.11 ± 1.11; weight concern, 1.79 ± 1.51, 1.97 ± 1.56; shape concern, 2.23 ± 1.65, 2.27 ± 1.54; and global, 1.52 ± 1.25, 1.74 ± 1.30.
Data analysis
JMP Statistical Discoveries (version 9.0; SAS Institute, Inc., Cary, NC) was used for statistical analyses. Hormone levels were not normally distributed and were log transformed before analysis. Clinical characteristics, hormone levels, and disordered eating psychopathology scores were compared using overall ANOVA; variables that were significantly different were then compared by Fisher's least significant difference test. Within-group comparisons of hormone levels at different time points were made using the two-sided paired t test. Linear regression analyses were used to investigate the relationships between oxytocin levels and other hormones as well as disordered eating psychopathology. Multivariate least-square analyses were constructed to control for potential confounders. Statistical significance was defined as a two-tailed P < 0.05. Data are reported as mean ± sem.
fMRI procedures
fMRI procedures have been previously described (12). Briefly, fMRI scanning was performed while subjects viewed high-calorie food stimuli, low-calorie food stimuli, nonfood stimuli, and fixation stimuli in a block design while participants underwent standard gradient-echo echo planar imaging on a Siemens 3T Trio (Malvern, PA).
fMRI data analysis
Full details on fMRI data analysis have been previously described (12). Briefly, fMRI data were preprocessed using Statistical Parametric Mapping (SPM8; Wellcome Trust Centre for Neuroimaging at University College London, 2008) and custom routines in MATLAB (Mathworks, Inc., 2000; Natick, MA). Standard preprocessing steps included realignment and geometric unwarping of echo planar imaging using magnetic field maps, correction for bulkhead motion, nonlinear volume-based spatial normalization using the standard Montreal Neurological Institute (Montreal, Canada) brain template, spatial smoothing with a Gaussian filter (6 mm full-width at half-maximum), and outlier detection and exclusion (18). After preprocessing, statistical analysis was performed at the single-subject level. Specific comparisons of interest (high calorie foods vs. objects, separately for before a meal and after a meal) were tested using linear contrasts, and SPM maps were created based on these contrasts. Results from the single-subject level were submitted to a second-level random effects analysis. Independent-sample t tests were used to compare the size of a particular effect between groups. Clusters were identified within our regions of interest [hypothalamus, amygdala, hippocampus, orbitofrontal cortex (OFC), and anterior insula] in between-group contrasts, significant at P < 0.05 (uncorrected), and P < 0.1 (corrected for multiple comparisons within the search volume using voxel-level family-wise error correction).
Anatomic overlays were used on each subject's statistical maps to acquire signal change values across regions of interest. Values indicated the degree of change in magnetic resonance signal detected between the high-calorie food and object conditions. Average percent signal change values (beta weights averaged across all voxels within an anatomical region) were obtained using the REX toolbox for SPM8 (17) and used for brain-hormone general linear model analyses. Using the PROC MIXED model approach in SAS (version 9.2; SAS Institute), the effect of hormones of interest (oxytocin, cortisol, leptin) on the association between group status and brain activity was assessed. The percent change in the estimate for case status when the model was adjusted for the hormone was calculated as the estimate for case status in the univariate model (b1) minus the estimate for case status in the model adjusted for the hormone (b2) together over the univariate estimate (b1) [(b1 − b2)/b1]. Due to our interest in the mediating effect of each hormone on the case status effect on brain activity, decreases in percent change were of interest, indicating the percent of the case group's effect on brain activity accounted for by the hormone.
Results
Subject characteristics
Subject characteristics are presented in Table 1. Mean age was 22.2 ± 0.4 yr and did not differ between groups. Weight, BMI, and percent ideal body weight were lower in AN, as per study design, and did not differ between ANWR and HC. For AN, mean percent IBW was 80.6 ± 1.3 and time since diagnosis was 52.7 ± 11.2 months. ANWR had a mean percent IBW of 98.3 ± 3.8 and reported weight stability for at least 12 months and regular menstrual cycles for at least 14 months. Menstrual cycle length did not differ between ANWR and HC (31.8 ± 2.5 vs. 29.4 ± 1.8 d, P = 0.44). ANWR had recovered weight a mean of 44.4 ± 12.0 months before the study. Three AN and three ANWR reported a remote (at least 2 yr before the study) history of purging activity, but none were actively binging or purging. Five AN patients were taking psychotropic medications: two were taking venlafaxine, one was taking fluoxetine, one was taking a low dose of amphetamine/dextroamphetamine (5 mg 24 h before the scan), and one was taking escitalopram and aripiprazole. One ANWR was taking a psychotropic medication: fluoxetine. Depressive symptoms as assessed by the Beck Depression Inventory and anxiety trait symptoms as measured by State-Trait Anxiety scores were higher in AN than ANWR or HC. Subjects in each group reported similar mean hours of sleep and hours since last per oral intake. No subjects smoked cigarettes on the morning of the study or consumed caffeine within 12 h of the study. Three AN, one ANWR, and three HC failed to consume the entire mixed meal. Caloric content consumed at the mixed meal did not differ between groups.
Table 1.
Subject characteristics
| AN (n = 13) | ANWR (n = 9) | HC (n = 13) |
P values |
Overall | |||
|---|---|---|---|---|---|---|---|
| AN vs. ANWR | AN vs. HC | ANWR vs. HC | |||||
| Age (yr) | 21.7 ± 0.7 | 23.2 ± 0.8 | 22.0 ± 0.4 | — | — | — | NS |
| Weight (kg) | 48.2 ± 1.1 | 58.4 ± 2.0 | 62.0 ± 1.7 | 0.0001 | <0.0001 | NS | <0.0001 |
| BMI (kg/m2) | 17.7 ± 0.3 | 22.1 ± 0.7 | 22.5 ± 0.4 | <0.0001 | <0.0001 | NS | <0.0001 |
| IBW (%) | 80.6 ± 1.3 | 98.3 ± 3.8 | 97.2 ± 1.7 | <0.0001 | <0.0001 | NS | <0.0001 |
| Exercise (h/wk) | 7.8 ± 1.7 | 3.8 ± 0.8 | 4.1 ± 0.7 | 0.045 | 0.035 | NS | 0.054 |
| Alcoholic drinks per week, n | 0.8 ± 0.4 | 2.8 ± 0.7 | 3.1 ± 0.8 | 0.038 | 0.011 | NS | 0.023 |
| Months since last menstrual period | 50.2 ± 11.1 | — | — | — | — | — | — |
| Duration of illness (months) | 52.7 ± 11.2 | 36.3 ± 6.6 | — | NS | — | — | — |
| Age at diagnosis (yr) | 16.7 ± 0.8 | 15.7 ± 1.0 | — | NS | — | — | — |
| Sleep previous night (h) | 6.0 ± 0.3 | 6.7 ± 0.5 | 6.1 ± 0.3 | — | — | — | NS |
| Hours since last oral intake | 13.9 ± 0.4 | 13.9 ± 0.4 | 14.1 ± 0.2 | — | — | — | NS |
| Calories consumed at breakfast | 378.5 ± 16.2 | 408.5 ± 6.4 | 405.8 ± 2.0 | — | — | — | NS |
| Protein (g) | 18.3 ± 0.7 | 19.4 ± 0.4 | 19.4 ± 0.2 | — | — | — | NS |
| Fat (g) | 9.4 ± 0.8 | 10.4 ± 0.6 | 10.6 ± 0.3 | — | — | — | NS |
| Carbohydrates (g) | 58.2 ± 2.0 | 62.1 ± 1.4 | 61.5 ± 0.5 | — | — | — | NS |
| State-Trait Anxiety trait score | 53.2 ± 3.7 | 33.7 ± 3.7 | 28.0 ± 1.5 | <0.0001 | <0.0001 | NS | <0.0001 |
| Beck Depression Inventory score | 16.8 ± 3.3 | 6.3 ± 1.9 | 0.8 ± 0.4 | 0.004 | <0.0001 | NS | 0.0004 |
Mean ± sem. NS, Nonsignificant; —, not applicable.
Fasting and postprandial oxytocin, cortisol, and leptin levels
Hormone levels are presented in Table 2. Baseline fasting mean levels of oxytocin were comparable in AN and HC but lower in ANWR. Mean oxytocin levels were higher in AN than HC at 60 and 120 min, and lower in ANWR than HC at 0, 30, and 120 min and AN at all time points. Mean oxytocin area under the curve (AUC) was highest in AN, intermediate in HC, and lowest in ANWR. Within the groups, there were no significant differences between fasting and postprandial oxytocin levels.
Table 2.
Hormone levels fasting and after a standardized mixed meal
| AN | ANWR | HC |
P values |
Overall | |||
|---|---|---|---|---|---|---|---|
| AN vs. ANWR | AN vs. HC | ANWR vs. HC | |||||
| Oxytocin (pg/ml) | |||||||
| T 0 fasting | 15.8 ± 1.1 | 8.1 ± 0.6 | 16.4 ± 2.3 | 0.0003 | NS | 0.0006 | 0.0005 |
| T 30 | 17.6 ± 2.5 | 9.3 ± 1.1 | 15.4 ± 1.9 | 0.004 | NS | 0.024 | 0.012 |
| T 60 | 16.6 ± 2.3 | 9.0 ± 1.0 | 12.3 ± 1.9 | 0.003 | 0.049 | NS | 0.009 |
| T 120 | 19.1 ± 3.7 | 7.7 ± 0.4 | 12.0 ± 1.2 | <0.0001 | 0.009 | 0.018 | <0.0001 |
| Area under the curve | 2086 ± 195 | 1040 ± 96 | 1621 ± 125 | <0.0001 | 0.041 | 0.002 | <0.0001 |
| Cortisol (μg/dl) | |||||||
| T 0 fasting | 15.9 ± 1.4 | 12.7 ± 1.1 | 11.7 ± 1.1 | NS | 0.014 | NS | 0.042 |
| T 30 | 15.1 ± 1.6 | 11.3 ± 1.0 | 11.7 ± 0.8 | — | — | — | 0.089 |
| T 60 | 13.3 ± 1.5 | 11.5 ± 0.7 | 10.9 ± 0.8 | — | — | — | NS |
| T 120 | 12.9 ± 1.3 | 11.1 ± 0.8 | 8.8 ± 0.7 | NS | 0.006 | 0.067 | 0.019 |
| Area under the curve | 1679 ± 159 | 1383 ± 83 | 1280 ± 73 | — | — | — | 0.084 |
| Leptin (ng/ml) | |||||||
| T 0 fasting | 3.2 ± 0.4 | 9.3 ± 1.4 | 10.9 ± 1.3 | <0.0001 | <0.0001 | NS | <0.0001 |
| T 30 | 2.9 ± 0.4 | 8.4 ± 1.3 | 9.7 ± 1.2 | <0.0001 | <0.0001 | NS | <0.0001 |
| T 60 | 2.9 ± 0.4 | 8.7 ± 1.3 | 10.3 ± 1.2 | <0.0001 | <0.0001 | NS | <0.0001 |
| T 120 | 2.8 ± 0.4 | 8.3 ± 1.3 | 9.5 ± 1.1 | <0.0001 | <0.0001 | NS | <0.0001 |
| Area under the curve | 347 ± 48 | 1034 ± 159 | 1203 ± 140 | <0.0001 | <0.0001 | NS | <0.0001 |
Mean ± sem. NS, Nonsignificant; —, not applicable.
Mean cortisol levels at 0 and 120 min were higher in AN than HC. At other time points and AUC, there were no significant between-group differences. There were no significant associations between oxytocin AUC and cortisol AUC in any group.
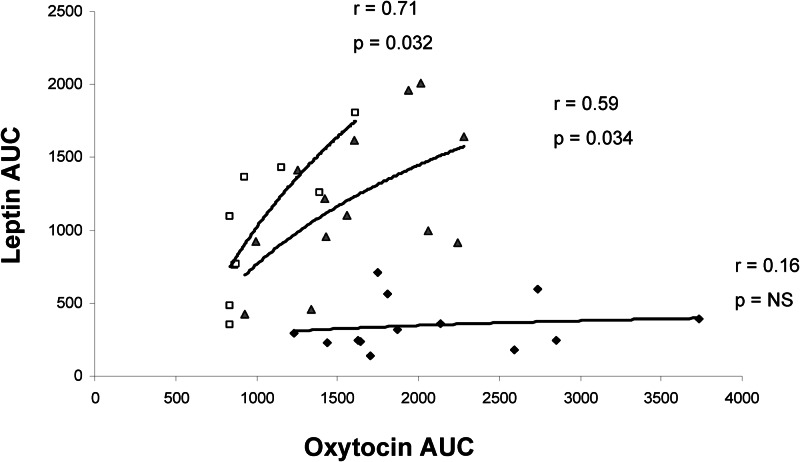
Mean leptin levels at all time points and AUC were lower in AN than HC and ANWR. Oxytocin area under the curve was positively correlated with leptin area under the curve in ANWR (r = 0.71, P = 0.032) and HC (r = 0.59, P = 0.034) but not in AN (r = 0.16, P = NS) (Fig. 1).
Fig. 1.
Relationship between oxytocin and leptin secretion. Oxytocin AUC was positively associated with leptin AUC in ANWR (squares; r = 0.71, P = 0.032) and HC (triangles; r = 0.59, P = 0.034) but not in AN (diamonds; r = 0.16, P = NS).
Relationship between oxytocin secretion and disordered eating core psychopathology
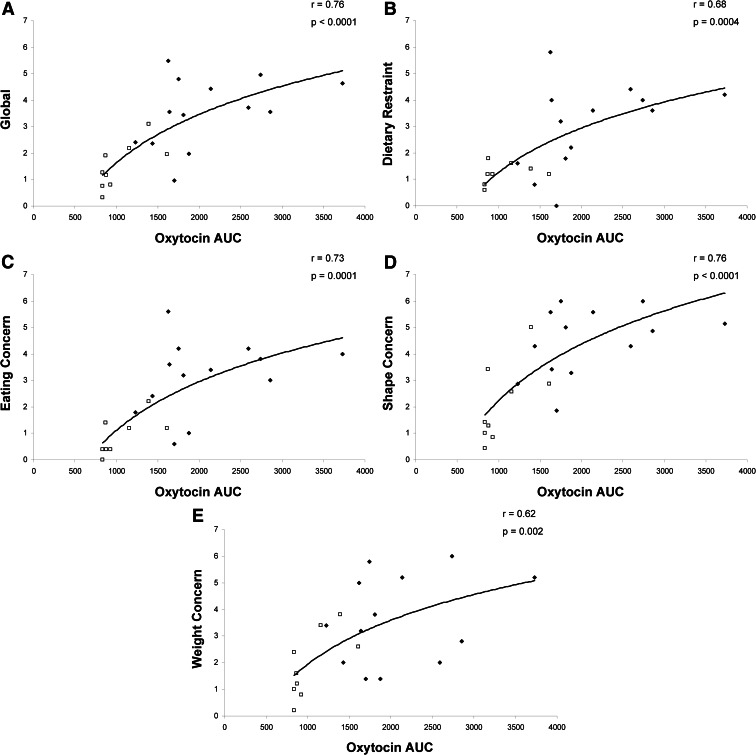
The EDE-Q scores are shown in Table 3. Global EDE-Q and all subscale scores were lowest in HC, intermediate in ANWR, and highest in AN. The relationship between oxytocin secretion and disordered eating psychopathology is shown in Fig. 2. In the women with active and weight-recovered AN, oxytocin AUC was positively associated with global EDE-Q (r = 0.76, P < 0.0001) and all subscale scores (dietary restraint, r = 0.68, P = 0.0004; eating concern, r = 0.73, P = 0.0001; shape concern, r = 0.76, P < 0.0001; weight concern, r = 0.62, P = 0.002). When controlling for cortisol AUC and leptin AUC, the relationships between the oxytocin AUC and the EDE-Q global concern as well as all the subscales remained highly significant (P < 0.002). When the groups are separated, the relationships are consistent, although not all of the correlations are significant, likely due to the small sample size.
Table 3.
Disordered eating core psychopathology
| AN | ANWR | HC |
P values |
Overall | |||
|---|---|---|---|---|---|---|---|
| AN vs. ANWR | AN vs. HC | ANWR vs. HC | |||||
| EDE-Q | |||||||
| Dietary restraint | 3.0 ± 0.5 | 1.2 ± 0.1 | 0.2 ± 0.1 | 0.0003 | <0.0001 | 0.050 | <0.0001 |
| Eating concern | 3.1 ± 0.4 | 0.8 ± 0.2 | 0.0 ± 0.0 | <0.0001 | <0.0001 | 0.050 | <0.0001 |
| Shape concern | 4.5 ± 0.4 | 2.1 ± 0.5 | 0.5 ± 0.1 | <0.0001 | <0.0001 | 0.003 | <0.0001 |
| Weight concern | 3.6 ± 0.5 | 1.9 ± 0.4 | 0.5 ± 0.2 | 0.004 | <0.0001 | 0.017 | <0.0001 |
| Global | 3.6 ± 0.4 | 1.5 ± 0.3 | 0.3 ± 0.1 | <0.0001 | <0.0001 | 0.008 | <0.0001 |
Fig. 2.
Relationship between oxytocin secretion and disordered eating psychopathology in anorexia nervosa. In women with active (diamonds) and weight-recovered (squares) anorexia nervosa, oxytocin AUC was positively associated with global EDE-Q (r = 0.76, p < 0.0001) (A) and all subscale scores, including dietary restraint (r = 0.68, P = 0.0004) (B), eating concern (r = 0.73, P = 0.0001) (C), shape concern (r = 0.76, P < 0.0001) (D), and weight concern (r = 0.62, P = 0.002) (E). When controlling for cortisol AUC and leptin AUC, these associations remained significant.
Relationship between group differences in activation of food motivation neurocircuitry and oxytocin secretion
Table 4 shows how much of the variance in group differences in signal changes in brain activity associated with our significant food motivation circuitry regions (i.e. percent change in betas for group differences in brain activity comparing AN, ANWR, and HC) is related to oxytocin secretion. We previously reported premeal hypoactivation in AN (vs. HC) in the hypothalamus, amygdala, hippocampus, OFC, and insula and in ANWR (vs. HC) in the hypothalamus, amygdala, and insula. Oxytocin was associated with a significant proportion of premeal differences in activation between AN and HC in the hypothalamus (22%), amygdala (42%), hippocampus (13%), and OFC (21%) and 20% of premeal differences in insular activation between ANWR and HC. After the meal, we previously found hypoactivation in AN (vs. HC) in the amygdala and insula; oxytocin secretion was associated with 9–22% of activation differences in these regions. Finally, our prior results demonstrated hyperactivation in AN (vs. ANWR) after the meal in the amygdala, and hypoactivation in AN (vs. ANWR) in the insula. Here we found that oxytocin was associated with 13% of the postmeal difference in insular activation between ANWR and AN. When we entered cortisol AUC into the model, the relationships between oxytocin secretion and between-group variance in brain activation patterns were still evident.
Table 4.
Mediation of group effects at selected brain regions by oxytocin
| Session | Group contrast | Region | Hemisphere | MNI coordinates (x, y, z) | Estimate | Estimate, adjusted for oxytocin | Change in estimate (%) |
|---|---|---|---|---|---|---|---|
| Before a meal | AN vs. HC | Hypothalamus | L | −3, −7, −5 | −0.92 | −0.72 | 21.5 |
| Amygdala | L | −21, −10, −11 | −0.61 | −0.36 | 41.8 | ||
| Hippocampus | L | −9, −40, 1 | −2.27 | −1.97 | 13.3 | ||
| OFC | R | 36, 23, −11 | −1.12 | −0.88 | 20.9 | ||
| Insula | R | 33, 8, 4 | −1.39 | −1.36 | — | ||
| L | −30, 17, 7 | −1.16 | −1.13 | — | |||
| ANWR vs. HC | Hypothalamus | R | 9, −7, −5 | −0.66 | −0.67 | — | |
| L | −6, −10, −5 | −0.90 | −1.08 | — | |||
| Amygdala | L | −24, −10, −11 | −0.68 | −1.11 | — | ||
| Insula | R | 30, 26, −11 | −0.80 | −0.82 | — | ||
| R | 39, 26, −8 | −1.14 | −0.91 | 19.9% | |||
| After a meal | AN vs. HC | Amygdala | L | −24, −10, −14 | −0.37 | −0.30 | 20.5% |
| L | −30, −1, −20 | −0.67 | −0.53 | 21.9% | |||
| Insula | L | −39, −7, 4 | −0.63 | −0.58 | 8.6% | ||
| L | −33, 5, −5 | −0.99 | −1.07 | — | |||
| AN vs. ANWR | Amygdala | R | 15, −1, −17 | 0.61 | 1.22 | — | |
| Insula | R | 36, −10, 13 | −0.99 | −1.19 | — | ||
| L | −39, −7, 4 | −1.49 | −1.30 | 12.8% |
L, Left; R, right; —, not applicable; MNI, Montreal Neurological Institute.
Given the variability in leptin levels in ANWR and HC, we examined the effect of leptin AUC on between-group differences in these brain activation patterns. Leptin secretion was not associated with differences in activations between ANWR and HC in any brain region. However, when oxytocin AUC was entered into the univariate model, oxytocin secretion was associated with a substantial percentage of the variance in premeal activation of the hypothalamus (19%) and the insula (31%) after accounting for variance in leptin secretion.
Discussion
We report altered oxytocin dynamics in women with AN, associated with food-related psychopathology and brain circuitry hypoactivation. We found that postprandial oxytocin secretion was higher in women with active anorexia nervosa and lower in weight-recovered women with anorexia nervosa compared with healthy women. Oxytocin secretion was positively associated with increased severity of disordered eating psychopathology, independent of cortisol and leptin levels. In addition, oxytocin secretion was associated with a substantial proportion of the between-group differences in activation of food motivation brain circuits, independent of cortisol. Moreover, adding leptin secretion to the model strengthened associations between oxytocin secretion and premeal neurocircuitry activation differences between women who had recovered from anorexia nervosa and healthy controls. Taken together, these data in humans support animal studies indicating that oxytocin is an appetite-regulating peptide. Furthermore, our data demonstrate that dysregulation of oxytocin secretion is associated with disordered eating in women with AN.
In animal models, oxytocin is considered an anorexigenic hormone (1, 19). Although there are several investigations reporting contradictory results (20–22), the majority of studies examining the role of oxytocin in feeding behavior indicate that it signals satiety (7, 23–25). In fasting rats, for example, administration of ip or intracerebroventricular oxytocin decreases food intake in a dose-dependent manner, shortens the meal duration, and increases latency before the next meal. When an oxytocin antagonist is administered before intracerebroventricular oxytocin, these effects are reversed (23). Recent animal studies suggest that the effect of oxytocin on satiety may favor limiting carbohydrate rather than fat intake (26–28). Several small studies have looked at the oxytocin response to nutrient intake in healthy humans, with inconsistent results likely due to differences in sample size, meal content, and timing (29, 30). We administered a standardized high-protein, carbohydrate-rich, low-fat mixed meal over 15 min and found that mean oxytocin levels did not significantly change between pre- and postmeal assessments.
However, postprandial oxytocin secretion was higher in women with active anorexia nervosa and lower in weight-recovered women with anorexia nervosa compared with healthy women. There are a number of studies that have suggested a relative oxytocin deficiency in women with anorexia nervosa. We recently found that nocturnal oxytocin secretion, as measured in serum sampled every 20 min for 12 h overnight and then pooled for an integrated measure, was significantly lower in 17 women with anorexia nervosa compared with 19 age-matched healthy controls (8). Low serum levels have also been reported in women with anorexia nervosa in response to stimulation with insulin-induced hypoglycemia or estrogen administration; the oxytocin response to stimulation normalized after weight recovery (31). In our current study, baseline fasting serum levels of oxytocin did not differ between women with anorexia nervosa and controls. It is not surprising that we did not see a between-group difference in fasting oxytocin levels because this represented a single time point in the morning, compared with 36 time points overnight in our prior study. In contrast, we found that women with anorexia nervosa had a relative increase in oxytocin levels compared with healthy women in response to a standardized mixed meal. On the other hand, women who had recovered weight from anorexia nervosa had lower levels of oxytocin than controls at every time point, fasting and in response to food intake. Interestingly, α-MSH, a potent hypothalamic inhibitor of appetite that is stimulated by leptin, has been shown to selectively increase central release and inhibit peripheral release of oxytocin (9–11). Therefore, the lower peripheral oxytocin levels in weight-recovered women may represent an increase in anorexigenic oxytocin signaling in appetite-regulating centers in the brain. This raises the possibility that abnormal secretion of this satiety factor in the brain may be a trait characteristic of anorexia nervosa that contributes to its pathogenesis. Higher postprandial serum oxytocin levels in women with active anorexia nervosa, on the other hand, may indicate an adaptive decrease in the central oxytocinergic signaling of satiety in the starved state.
We assessed hypothalamic-pituitary-adrenal activation using cortisol area under the curve and found that there was no association between cortisol and oxytocin secretion in any group. Furthermore, the relationships between oxytocin and disordered eating psychopathology and brain activation were independent of cortisol secretion.
Notably, we found that oxytocin secretion was positively correlated with leptin secretion in ANWR and HC but not in AN. Although leptin-independent pathways of oxytocinergic effects on appetite have been identified (9, 11, 30, 32), oxytocin is thought to be a mediator of the anorexigenic effects of leptin. Receptors for leptin have been identified on hypothalamic oxytocin neurons (5, 33). In fasting mice, oxytocin mRNA is reduced; giving leptin restores levels of oxytocin mRNA (6). Furthermore, administration of an oxytocin antagonist in rats has been shown to block the anorexigenic effect of leptin (7). There is little known about the relationship between peripheral levels of leptin and oxytocin. It is possible that the relationship we see between leptin and oxytocin levels in ANWR is due to independent factors impacting the release of both hormones. Our data suggest that the relationship between postprandial leptin and oxytocin secretion is disrupted in AN.
In women with anorexia nervosa, oxytocin secretion was positively associated with the severity of core disordered eating psychopathology as measured by the global and all subscales of the EDE-Q. These relationships remained significant after controlling for leptin levels. These data support a potential role for oxytocin in promoting disordered eating symptoms in AN.
We previously reported differences in activation of food motivation neurocircuitry, including the hypothalamus (a key control center for integration of appetitive signals) and the amygdala (a region important for learning satiety cues and assessing the reward value of food) in these same groups of women while viewing high-calorie foods vs. objects (12). The hypothalamus and amygdala are thought to be key loci in oxytocin pathways. Oxytocin receptors are abundant in these brain regions (2). Furthermore, hypothalamic and amygdala volume and the degree of functional coupling during processing of emotional stimuli using fMRI are oxytocin receptor genotype dependent (34, 35). Administration of intranasal oxytocin has been shown to modulate activity in the amygdala in response to socially salient fMRI paradigms (36–40). In the fasting state, we previously reported that activation was lower in women with active anorexia nervosa compared with healthy women in the hypothalamus, amygdala, hippocampus (implicated in the processing of food related memories), OFC (involved in integration of emotion and reward expectation), and insula (which houses the primary taste cortex, integrates visceral signals, and modulates affective tone and motivational behavior) (12).
In these same subjects, we now show that a substantial proportion of the between-group variance in fasting activation of the hypothalamus, amygdala, hippocampus and OFC were associated with differences in oxytocin secretion. After the standardized mixed meal, we previously reported that hypoactivation persisted in the amygdala and insula in the women with active anorexia nervosa (12). Oxytocin secretion was associated with 9–22% of these postprandial between-group differences in the amygdala and insula. We previously demonstrated that weight recovered women with anorexia nervosa had fasting hypoactivation of the hypothalamus, amygdala and insula compared with controls (12). Oxytocin secretion was associated with 20% of the between-group difference in activation of the insula. When we took into account leptin secretion, despite the strong positive correlations between leptin and oxytocin secretion in weight-recovered and control women, oxytocin secretion was associated with an even larger percentage of the ANWR-HC between-group difference in activation in the insula (31%). Furthermore, adding leptin to the model unmasked a relationship between oxytocin secretion and hypothalamic activation (associated with 19% of the between group difference). This argues for leptin-independent effects of oxytocin on feeding pathways. Finally, we previously found that after the meal, women with active anorexia nervosa exhibited increased activation in the amygdala and lower activation in the insula compared with weight-recovered women with anorexia nervosa (12). Oxytocin was associated with 13% of the insular hypoactivation. Given the overlap between areas of the brain involved in food motivation and the stress response, it is important to note that the relationships between oxytocin secretion and the differences in activation remained after entering cortisol AUC into the model.
These findings suggest that oxytocin secretion is substantially associated with abnormal activation of food motivation neurocircuitry in active anorexia nervosa (a state characteristic) as well as in those who have recovered weight (a trait characteristic). Specifically, oxytocin secretion is associated with trait differences in activation of the hypothalamus and insula and state differences in activation of numerous food motivation regions, including the hypothalamus, amygdala, and insula. Importantly, the hypothalamus is central to integration of feeding signals, and the insula integrates interoceptive signaling. A lack of interoceptive awareness, or ability to recognize one's internal state, has been reported in anorexia nervosa (41). Consistent with our data, others have reported insular hypoactivation in anorexia nervosa and hypothesized that an insular defect in this disorder may result in abnormal processing of internal signals, including those involving food motivation (42–44). The hypothesis suggests that due to failure in integration of incoming emotional, visceral, and homeostatic cues, insular dysfunction may result in an inability to appropriately identify the state of hunger vs. satiety. One possibility raised by our data is that insular dysfunction in anorexia nervosa may reflect impaired oxytocin signaling pathways. Interestingly, oxytocin administration resulted in increased insular activation and functional connectivity to the amygdala in prior fMRI studies of healthy individuals (45, 46). Our findings of an association between oxytocin secretion and both state and trait differences in activation of food motivation regions suggests that inherent abnormalities in oxytocin pathways may contribute to underlying deficits that increase susceptibility to developing and sustaining active anorexia nervosa.
This study has several limitations. The sample size was small and may have limited our ability to identify between-group differences. Due to the cross-sectional design, causality cannot be determined. The associations we have identified may be due to other factors that influence oxytocin secretion, disordered eating psychopathology, and fMRI brain activation patterns. Our findings highlight the importance of further research to determine whether oxytocin secretion mediates disordered eating psychopathology and abnormal food motivation neurocircuitry in anorexia nervosa.
In summary, we report abnormal postprandial serum oxytocin dynamics in women with anorexia nervosa, even after recovery. Higher oxytocin secretion in response to a meal, which may reflect attenuated central signaling of satiety, was associated with higher levels of disordered eating psychopathology in women with active and weight-recovered anorexia nervosa, independent of cortisol and leptin levels. Oxytocin secretion was associated with between-group differences in activation of food motivation neural pathways. Taken together, our findings suggest that oxytocin has appetite-regulating functions, and dysregulation in anorexia nervosa may contribute to symptoms of disordered eating. A study administering oxytocin to humans will be an important step in further defining the role of this hormone in appetite and food intake. Further investigation of the clinical significance of oxytocin dysregulation in eating disorders is warranted.
Acknowledgments
This work was supported by Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Institutes of Health Award UL1 RR025758); Harvard Grant K12 HD051959 from the Building Interdisciplinary Research Careers in Women's Health Program supported by the National Institutes of Health Office of Research in Women's Health; and National Institutes of Health Grant K23 MH092560.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AN
- Anorexia nervosa
- ANWR
- weight-recovered AN
- AUC
- area under the curve
- BMI
- body mass index
- CV
- coefficient of variation
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, fourth edition
- EDE-Q
- Eating Disorder Examination Questionnaire
- fMRI
- functional magnetic resonance imaging
- HC
- healthy controls
- IBW
- ideal body weight
- OFC
- orbitofrontal cortex
- SCID
- Structured Clinical Interview for DSM Disorders-IV
- SPM
- Statistical Parametric Mapping
- TFA
- trifluoroacetic acid.
References
- 1. Leng G, Onaka T, Caquineau C, Sabatier N, Tobin VA, Takayanagi Y, Inga DN, Rainer L. 2008. Oxytocin and appetite. In: Neumann ID, Landgraf R, eds. Progress in brain research. Amsterdam: Elsevier; 137–151 [DOI] [PubMed] [Google Scholar]
- 2. Gimpl G, Fahrenholz F. 2001. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683 [DOI] [PubMed] [Google Scholar]
- 3. Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. 2001. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci 21:2546–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell P, Ophir AG, Phelps SM. 2009. Central vasopressin and oxytocin receptor distributions in two species of singing mice. J Comp Neurol 516:321–333 [DOI] [PubMed] [Google Scholar]
- 5. Hâkansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. 1998. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci 18:559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tung YC, Ma M, Piper S, Coll A, O'Rahilly S, Yeo GS. 2008. Novel leptin-regulated genes revealed by transcriptional profiling of the hypothalamic paraventricular nucleus. J Neurosci 28:12419–12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blevins JE, Schwartz MW, Baskin DG. 2004. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287:R87–R96 [DOI] [PubMed] [Google Scholar]
- 8. Lawson E, Donoho D, Blum J, Meenaghan EM, Misra M, Herzog D, Sluss P, Miller K, Klibanski A. 2011. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. J Clin Psychiatry 72:1546–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der Ploeg L, Leng G. 2003. α-Melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci 23:10351–10358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sabatier N. 2006. α-Melanocyte-stimulating hormone and oxytocin: a peptide signalling cascade in the hypothalamus. J Neuroendocrinol 18:703–710 [DOI] [PubMed] [Google Scholar]
- 11. Sabatier N, Leng G. 2006. Presynaptic actions of endocannabinoids mediate α-MSH-induced inhibition of oxytocin cells. Am J Physiol Regul Integr Comp Physiol 290:R577–R584 [DOI] [PubMed] [Google Scholar]
- 12. Holsen LM, Lawson EA, Blum J, Ko E, Makris N, Fazeli PK, Klibanski A, Goldstein JM. 2012. Food motivation circuitry hypoactivation related to hedonic and non-hedonic aspects of hunger and satiety in women with active and weight-restored anorexia nervosa. J Psychiatry Neurosci 37:110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. 2000. Diagnostic and statistical manual of mental disorders. 4th ed.: DSM-IV-TR Washington, DC: American Psychiatric Association [Google Scholar]
- 14. Frisancho AR, Flegel PN. 1983. Elbow breadth as a measure of frame size for U.S. males and females. Am J Clin Nutr 37:311–314 [DOI] [PubMed] [Google Scholar]
- 15. Fairburn CG CZ. 1993. The Eating Disorders Examination. 12th ed In: Fairburn CG, Wilson GT, eds. Binge eating: nature, assessment, and treatment. New York: Guilford Press; 317–360 [Google Scholar]
- 16. Mond JM, Hay PJ, Rodgers B, Owen C. 2006. Eating Disorder Examination Questionnaire (EDE-Q): norms for young adult women. Behav Res Ther 44:53–62 [DOI] [PubMed] [Google Scholar]
- 17. Luce KH, Crowther JH, Pole M. 2008. Eating Disorder Examination Questionnaire (EDE-Q): norms for undergraduate women. Int J Eat Disord 41:273–276 [DOI] [PubMed] [Google Scholar]
- 18. Friston K, Ashburner J, Kiebel S, Nichols T, Penny W, eds. 2007. Statistical parameter mapping: the analysis of functional brain images. Boston: Academic Press [Google Scholar]
- 19. Sabatier N, Rowe I, Leng G. 2007. Central release of oxytocin and the ventromedial hypothalamus. Biochem Soc Trans 35:1247–1251 [DOI] [PubMed] [Google Scholar]
- 20. Björkstrand E, Uvnäs-Moberg K. 1996. Central oxytocin increases food intake and daily weight gain in rats. Physiol Behav 59:947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uvnäs-Moberg K, Alster P, Petersson M, Sohlström A, Björkstrand E. 1998. Postnatal oxytocin injections cause sustained weight gain and increased nociceptive thresholds in male and female rats. Pediatr Res 43:344–348 [DOI] [PubMed] [Google Scholar]
- 22. Uvnäs-Moberg K, Alster P, Petersson M. 1996. Dissociation of oxytocin effects on body weight in two variants of female Sprague-Dawley rats. Integr Physiol Behav Sci 31:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arletti R, Benelli A, Bertolini A. 1989. Influence of oxytocin on feeding behavior in the rat. Peptides 10:89–93 [DOI] [PubMed] [Google Scholar]
- 24. Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. 1991. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12:113–118 [DOI] [PubMed] [Google Scholar]
- 25. Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. 1991. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129:785–791 [DOI] [PubMed] [Google Scholar]
- 26. Miedlar JA, Rinaman L, Vollmer RR, Amico JA. 2007. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am J Physiol Regul Integr Comp Physiol 293:R1063–R1068 [DOI] [PubMed] [Google Scholar]
- 27. Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schiöth HB, Levine AS. 2010. Molecular, immunohistochemical, and pharmacological evidence of oxytocin's role as inhibitor of carbohydrate but not fat intake. Endocrinology 151:4736–4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sclafani A, Rinaman L, Vollmer RR, Amico JA. 2007. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol 292:R1828–R1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stock S, Granström L, Backman L, Matthiesen AS, Uvnäs-Moberg K. 1989. Elevated plasma levels of oxytocin in obese subjects before and after gastric banding. Int J Obes 13:213–222 [PubMed] [Google Scholar]
- 30. Ohlsson B, Forsling ML, Rehfeld JF, Sjölund K. 2002. Cholecystokinin stimulation leads to increased oxytocin secretion in women. Eur J Surg 168:114–118 [DOI] [PubMed] [Google Scholar]
- 31. Chiodera P, Volpi R, Capretti L, Marchesi C, d'Amato L, De Ferri A, Bianconi L, Coiro V. 1991. Effect of estrogen or insulin-induced hypoglycemia on plasma oxytocin levels in bulimia and anorexia nervosa. Metabolism 40:1226–1230 [DOI] [PubMed] [Google Scholar]
- 32. Palasz A, Krzystanek M, Worthington J, Czajkowska B, Kostro K, Wiaderkiewicz R, Bajor G. 2012. Nesfatin-1, a unique regulatory neuropeptide of the brain. Neuropeptides 46:105–112 [DOI] [PubMed] [Google Scholar]
- 33. Ur E, Wilkinson DA, Morash BA, Wilkinson M. 2002. Leptin immunoreactivity is localized to neurons in rat brain. Neuroendocrinology 75:264–272 [DOI] [PubMed] [Google Scholar]
- 34. Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. 2010. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci USA 107:13936–13941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Inoue H, Yamasue H, Tochigi M, Abe O, Liu X, Kawamura Y, Takei K, Suga M, Yamada H, Rogers MA, Aoki S, Sasaki T, Kasai K. 2010. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biol Psychiatry 68:1066–1072 [DOI] [PubMed] [Google Scholar]
- 36. Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. 2005. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25:11489–11493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC. 2007. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry 62:1187–1190 [DOI] [PubMed] [Google Scholar]
- 38. Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. 2008. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58:639–650 [DOI] [PubMed] [Google Scholar]
- 39. Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. 2010. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology 35:83–93 [DOI] [PubMed] [Google Scholar]
- 40. Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. 2010. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35:2403–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pollatos O, Kurz AL, Albrecht J, Schreder T, Kleemann AM, Schöpf V, Kopietz R, Wiesmann M, Schandry R. 2008. Reduced perception of bodily signals in anorexia nervosa. Eat Behav 9:381–388 [DOI] [PubMed] [Google Scholar]
- 42. Brooks SJ, O'Daly O, Uher R, Friederich HC, Giampietro V, Brammer M, Williams SC, Schiöth HB, Treasure J, Campbell IC. Thinking about eating food activates visual cortex with reduced bilateral cerebellar activation in females with anorexia nervosa: an fMRI study. PLoS One 7:e34000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wagner A, Aizenstein H, Mazurkewicz L, Fudge J, Frank GK, Putnam K, Bailer UF, Fischer L, Kaye WH. 2008. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology 33:513–523 [DOI] [PubMed] [Google Scholar]
- 44. Nunn K, Frampton I, Gordon I, Lask B. 2008. The fault is not in her parents but in her insula—a neurobiological hypothesis of anorexia nervosa. Eur Eat Disord Rev 16:355–360 [DOI] [PubMed] [Google Scholar]
- 45. Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. 2012. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology 37:447–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, van Ijzendoorn MH, Rombouts SA. 2011. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol Psychiatry 70:291–297 [DOI] [PubMed] [Google Scholar]