Abstract
Objective:
The objective of the study was to evaluate in premenopausal women the relationships of physically active and sedentary behaviors reported for adulthood and adolescence with a comprehensive profile of estrogen metabolism.
Methodology:
Fifteen estrogens and estrogen metabolites (jointly termed EM) were measured using liquid chromatography-tandem mass spectrometry in luteal phase urines from 603 premenopausal women in the Nurses' Health Study II. Geometric means of individual EM, metabolic pathway groups, and pathway ratios were examined by level of exposure after adjustment for age, body mass index, alcohol intake, menstrual cycle length, and sample collection timing.
Results:
High overall physical activity in adulthood (42+ metabolic equivalent h/wk vs. <3 metabolic equivalent h/wk) was associated with a 15% lower level of urinary estradiol (Ptrend = 0.03) and 15% lower level of 16-hydroxylation pathway EM (Ptrend = 0.03). Levels of 2- and 4-hydroxylation pathway EM did not differ significantly by physical activity. High overall activity was also positively associated with four ratios: 2-pathway EM to parent estrogens (Ptrend = 0.05), 2-pathway catechols to parent estrogens (Ptrend = 0.03), 2-pathway catechols to methylated 2-pathway catechols (Ptrend < 0.01), and 2-hydroxyestrone to 16α-hydroxyestrone (Ptrend = 0.01). Similar patterns of association were noted for walking and vigorous physical activity, but there was little evidence of associations with sedentary behaviors or activity during adolescence.
Conclusions:
High levels of physical activity were associated with lower levels of parent estrogens and 16-hydroxylation pathway EM and preferential metabolism to 2-pathway catechols. The results of our analysis, the largest, most comprehensive examination of physical activity and estrogen metabolism to date, may be useful in future studies investigating the etiology of diseases linked to both physical activity and endogenous estrogen.
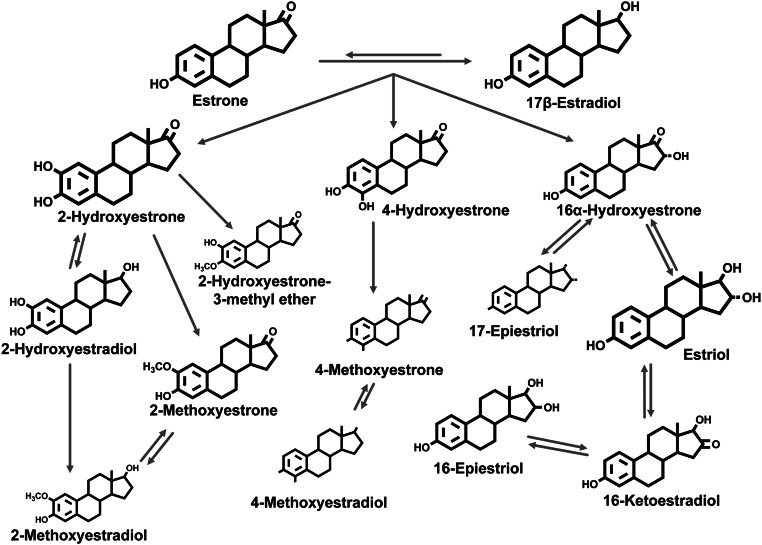
Both physical activity and endogenous estrogen levels have been implicated in several disease processes, including breast cancer and osteoporosis (1–3). However, the influence of physical activity on estrogen metabolism in premenopausal women is complex and not completely understood. In premenopausal women, the ovary is the major source of estrogen, with the parent estrogens (estrone and estradiol) produced by developing follicles and the corpus luteum. Parent estrogens are metabolized in the liver, kidneys, and other tissues by phase I enzymes (CYP450 family) and irreversibly hydroxylated at the 2-, 4-, or 16-position of the steroid ring (Fig. 1). The 2- and 4-pathway catechol estrogens (2-hydroxyestrone, 2-hydroxyestradiol, 4-hydroxyestrone) can be metabolized by the phase II enzyme catechol-O-methyltransferase (COMT) to form five methylated catechol estrogens (4). Metabolism of 16α-hydroxyestrone results in the formation of estriol and three additional 16-pathway estrogen metabolites. In addition to the absolute levels of parent estrogens in circulation, the overall pattern of estrogen metabolism may be an important determinant of the bioavailability of estrogen in target tissues and of estrogen excretion (4).
Fig. 1.
Pathways of endogenous estrogen metabolism. The size of the chemical structures generally reflects the relative urinary concentrations for each EM. Adapted with permission from the American Association for Cancer Research: Eliassen AH, Ziegler RG, Rosner B, Veenstra TD, Roman JM, Xu X, Hankinson SE. 2009 Reproducibility of Fifteen Urinary Estrogens and Estrogen Metabolites over a 2- to 3-Year Period in Premenopausal Women. Cancer Epidemiology Biomarkers & Prevention 18:2860–2868.
High levels of physical activity can result in major disruptions in the menstrual cycle (e.g. amenorrhea, oligomenorrhea) as well as more subtle changes (e.g. anovulation) (5, 6), all of which can influence endogenous estrogen levels and may influence estrogen metabolism. Large cross-sectional studies in premenopausal women (n > 500 subjects) have reported inverse relationships between physical activity and circulating parent estrogen levels, particularly in the luteal phase (7). As for physical activity and estrogen metabolism, most research to date has focused only on 2-hydroxyestrone and 16α-hydroxyestrone because assays for a broad range of metabolites were not available. Two small cross-sectional studies (n < 80 subjects) have reported positive associations between physical activity and urinary 2-hydroxyestrone and the 2-hydroxyestrone to 16α-hydroxyestrone ratio (8, 9), whereas another did not (10). Studies of female athletes that increased their training volume reported mixed results for urinary 2-hydroxyestrone levels (11, 12), and two intervention studies reported that 12–15 wk of aerobic exercise did not change urinary catechol estrogens, 16α-hydroxyestrone, or estriol in the follicular phase (13) or 2-hydroxyestrone or 16α-hydroxyestrone in the luteal phase (14). In contrast, 5 consecutive days of vigorous exercise in eumenorrheic women resulted in a relative increase in circulating levels of methylated catechols (15, 16), possibly due to an increase in COMT activity (17).
The inconsistent results from these studies may be due to the exercise exposure examined (short vs. long term), the timing of estrogen measures within the cycle (follicular, luteal), or the lack of specificity and sensitivity of available assays. There is evidence that high levels of activity are associated with reduced circulating parent estrogen levels in the luteal phase (5, 6). However, information regarding the effects of physical activity on estrogen metabolites other than 2-hydroxyestone and 16α-hydroxyestrone is quite limited (18). Accordingly, this study describes the relationships between usual physical activity in adulthood and adolescence and urinary levels of parent estrogens and 13 additional estrogen metabolites in more than 600 premenopausal women in the Nurses' Health Study II (NHSII).
Materials and Methods
Study population and biospecimen collection
The NHSII was initiated in 1989 when 116,430 female registered nurses enrolled in the cohort by completing and returning a questionnaire. Since that time, participants have completed biennial questionnaires to update exposures, including physical activity, and collect health and disease data. Between 1996 and 1999, 29,611 participants provided blood and urine samples and completed a brief questionnaire at the time of sample collection. At the time of collection, these women were cancer free and between 32 and 54 yr of age. A total of 18,521 premenopausal women who had not used oral contraceptives, been pregnant, or breast-fed during the 6 months before sample collection provided midluteal samples. Women were asked to collect samples approximately 7–9 d before the anticipated onset of the next cycle and return a postcard recording the first day of their next cycle after the collection to allow timing of the sample in the luteal phase using backward dating (19).
Urine samples were collected with no preservatives and shipped with an ice pack via overnight courier; and 93% of samples were received within 26 h of collection. First morning urine samples were obtained from 80% of participants. Samples have been stored in liquid nitrogen freezers since collection.
The current analysis is limited to women who were selected as controls for a nested-case control study of breast cancer (n = 493) (20) or who participated in a biomarker reproducibility study (n = 110) (21). This represents the total number of women in the NHSII with urinary estrogen metabolites (EM) data. The Committee on the Use of Human Subjects in Research at the Brigham and Women's Hospital approved the study.
Measurement of urinary estrogens and EM
For the urinary EM assay, 500 μl of frozen urine was sent to the Laboratory of Proteomics and Analytical Chemistry (SAIC-Frederick, Inc., Frederick, MD). Given that endogenous estrogens and their metabolites are usually present in urine as glucuronide and sulfate conjugates, an initial enzymatic hydrolysis step with β-glucuronidase/sulfatase from Helix pomatia was included. Five deuterated EM (17β-estradiol-d4, estriol-d3, 2-hydroxy-17β-estradiol-d5, 2-methoxy-17β-estradiol-d5, 16-epiestriol-d3) were added as the urine samples were thawed to correct for loss of urinary EM during all steps of the assay procedure and permit accurate quantitation of endogenous EM from the liquid chromatography-tandem mass spectrometry (LC-MS/MS) data. Calibration curves for the 15 EM were constructed by plotting EM/deuterium-labeled EM peak area ratios vs. EM amounts. The amount of each EM in the urine sample was interpolated using a linear function. Details of the assay have been published previously (22).
Masked replicate quality control samples were placed in each batch to assess laboratory variability. The coefficients of variation (CV) for the overall project were less than 7%, except for 4-methoxyestrone (CV of 17%) and 4-methoxyestradiol (CV of 15%), the two EM with the lowest concentrations. The lower level of quantitation for each EM is approximately 150 fmol/ml urine.
Creatinine was measured at three laboratories. In each laboratory, the overall CV was 9.2% or less. Progesterone was measured in plasma by chemiluminescent immunoassay using the Immulite Auto-Analyzer (Diagnostic Products Corp., Los Angeles, CA). The overall CV was 17% or less, and the within-batch, CV of 4% or less.
Physical activity assessment
We examined recreational physical activity using the following question: “During the past year, what was your average time per week spent at each of the following recreational activities?” The activities were walking or hiking outdoors (include walking to work); jogging (slower than 10 min/mile); running (10 min/mile or faster); bicycling (include stationary machine); calisthenics/aerobics; aerobic dance/rowing machine; tennis/squash/racquetball; lap swimming; and other aerobic recreation (e.g. lawn mowing). Duration for each activity was reported as one of nine categories, spanning zero to 11+ h/wk. The number of flights of stairs climbed per day was also assessed. To estimate energy expenditure in metabolic equivalent (MET) hours per week, we multiplied MET values by the reported duration for each activity (23). This approach to assessing recreational physical activity in NHSII has previously been found to have acceptable reliability and validity (24) and has been used to demonstrate associations with several chronic diseases (25–27). The summary exposures examined were total recreational physical activity (all activities), walking (walking and hiking only), and vigorous activity (all activities, including stairs climbed, except walking and hiking). To estimate long-term adult activity during the period when urine was being collected (1996–1999) and to minimize the impact of intraindividual variation over time, we averaged the reports of recreational activity from the surveys completed in 1997 and 2001.
We also examined adult sedentary behaviors. We summed the average duration (hours per week) of sitting at work or away from home and of sitting at home (TV/VCR and other sitting) during the past year (assessed in 1997 only). Finally, the average time spent in moderate and strenuous recreational physical activity and the average time spent watching television during adolescence (grades 9–12), also asked in 1997, were examined.
Assessment of covariates
Data on potential covariates were collected on the biennial study questionnaires as well as the questionnaire completed at urine collection. Women provided updated information on reproductive factors, such as parity and age at first birth, and current alcohol and cigarette use on the biennial questionnaires. In our analyses, we used information from the 1997 biennial questionnaire, the questionnaire administered closest to the initiation of the urine collection (1996–1999). Height and age at menarche were asked in 1989 and usual menstrual cycle length in 1993. In addition, the specimen collection questionnaire asked about the urine collection date and time, whether the urine collected was the first morning urine, and the subject's weight at the time of collection. The postcard returned after the urine collection provided the actual start date of the subsequent menstrual period and allowed for the calculation of the luteal day at collection.
Statistical analysis
We assessed the association between overall physical activity levels and participant characteristics using ANOVA and χ2 tests. Individual EM urinary levels were standardized by creatinine to account for urine concentration, resulting in units of picomoles EM per milligram of creatinine. EM were evaluated individually, in groups defined by metabolic pathways, and as pathway ratios. Given the hypothesized association between the 2-hydroxyestronem to 16α-hydroxyestrone ratio and breast cancer (28), we also examined this ratio. Statistical outliers were identified for individual EM (29). This resulted in the exclusion of up to 10 values, except for 2-methoxyestradiol with 16 outliers.
We calculated geometric means by categories of physical activity for each of the EM measures using generalized linear models. Tests for trend were conducted by modeling the median of the exposure categories as a continuous variable. Minimally adjusted models included age at urine collection (continuous), actual luteal day at collection (≤5, 6–7, 8–9, ≥10 d before next menstrual period), and first morning urine (yes/no). Multivariable models additionally included body mass index (BMI) at urine collection (kilograms per square meter), alcohol consumption (nondrinker, >0–1.44, 1.45–4.80, and >4.80 g/d), and usual menstrual cycle length (<26, 26–31, 32+ d). Age at first birth/parity, age at menarche, and smoking were examined as potential confounders, did not influence our findings, and were not included in our final models. To investigate the potential for adiposity to modify the associations of physical activity with estrogen metabolism, we conducted analyses stratified by BMI (≤25 vs. >25 kg/m2). To examine the influence of women with anovulatory cycles on our results, we also conducted sensitivity analysis by excluding the 61 women with plasma progesterone levels less than 400 ng/dl at the time of urine collection.
All P values are two sided and considered statistically significant if P < 0.05. Analyses were conducted with SAS version 9 (SAS Institute, Cary, NC).
Results
Of the 603 urine samples assayed for this analysis, 79.6% were first morning collections; 89.9% were obtained during an ovulatory cycle defined by circulating progesterone levels 400 ng/dl or greater at the time of urine collection; and 85.6% were collected 4–10 d before the next menstrual cycle. Women reporting higher levels of overall physical activity had lower BMI and reported more alcohol use (Table 1). Urinary creatinine concentrations were not associated with overall physical activity levels (P = 0.65) (Table 1).
Table 1.
Characteristics of NHSII participants (n = 603) at urine collection by overall physical activity levels
| Characteristic | Overall physical activity (MET h/wk) |
||||||
|---|---|---|---|---|---|---|---|
| <3 | 3–8.9 | 9–17.9 | 18–26.9 | 27–41.9 | 42+ | P value | |
| n | 60 | 127 | 148 | 96 | 92 | 80 | |
| Mean age (yr) | 43.2 | 43.1 | 42.7 | 43.2 | 42.5 | 42.5 | 0.57 |
| Mean BMI (kg/m2) | 27.8 | 25.7 | 24.8 | 25.6 | 24.0 | 23.0 | <0.01 |
| Geometric mean creatinine (mg/liter) | 1071 | 959 | 986 | 943 | 935 | 1028 | 0.65 |
| Race (%) | |||||||
| Caucasian | 93.3 | 97.6 | 95.3 | 94.8 | 90.2 | 95.0 | 0.29 |
| Alcohol use (%) | |||||||
| 0 drinks/month | 43.3 | 33.1 | 33.1 | 27.1 | 27.2 | 18.8 | <0.01 |
| >0–1.44 g/d | 28.3 | 25.2 | 20.3 | 13.5 | 17.4 | 13.7 | |
| 1.45–4.80 g/d | 16.7 | 23.6 | 24.3 | 30.2 | 26.1 | 26.2 | |
| > 4.80 g/d | 11.7 | 18.1 | 22.3 | 29.2 | 29.3 | 41.3 | |
| Smoking (% nonsmokers) | 90.0 | 92.1 | 95.3 | 92.7 | 90.2 | 96.0 | 0.48 |
| Parity (%) | |||||||
| 0 | 18.3 | 18.1 | 13.5 | 14.6 | 17.4 | 30.0 | 0.57 |
| 1 | 13.3 | 9.5 | 14.9 | 12.5 | 10.9 | 12.5 | |
| 2 | 45.0 | 44.1 | 41.9 | 41.7 | 42.4 | 31.3 | |
| 3 | 20.0 | 18.1 | 23.0 | 21.9 | 17.4 | 18.8 | |
| ≥4 | 3.3 | 10.2 | 6.8 | 9.4 | 12.0 | 7.5 | |
| Usual menstrual cycle length (%) | |||||||
| <26 d | 22.8 | 14.8 | 23.6 | 18.0 | 15.6 | 25.0 | 0.61 |
| 26–31 d | 64.9 | 74.6 | 68.8 | 69.7 | 73.3 | 62.5 | |
| 32+ d | 12.3 | 10.7 | 7.6 | 12.4 | 11.1 | 12.5 | |
| First morning urine (%) | |||||||
| Yes | 78.3 | 80.3 | 81.8 | 79.2 | 76.1 | 82.5 | 0.90 |
| Ovulatory cycle (%) | |||||||
| Yes | 93.3 | 87.4 | 91.2 | 90.6 | 90.2 | 87.5 | 0.78 |
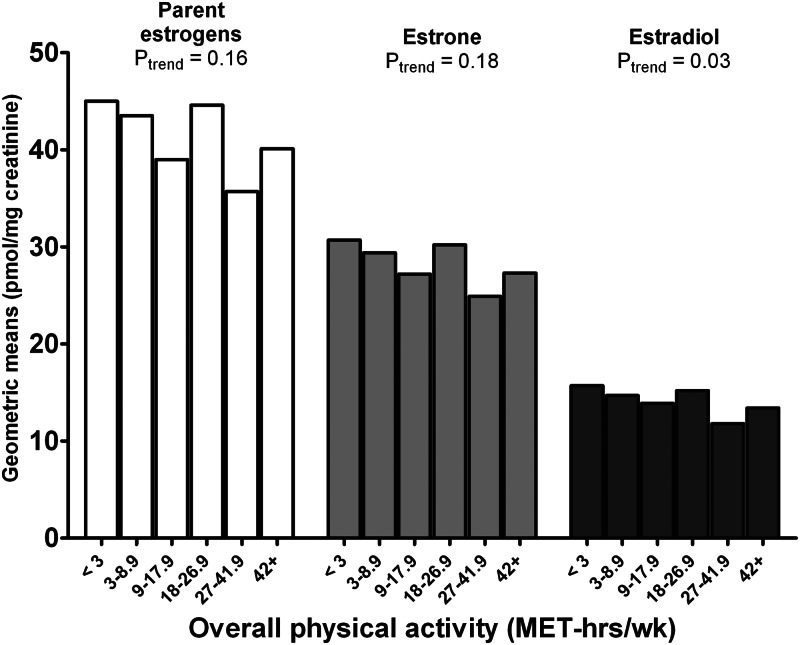
Total EM (all EM combined) was not associated with physical activity (Table 2). Women reporting high levels of physical activity (42+ MET h/wk) had 15% lower levels of estradiol (Ptrend = 0.03) compared with those reporting the least activity (<3 MET h/wk; Fig. 2). Grouped 2-hydroxylation pathway EM and 4-hydroxylation pathway EM were not significantly associated with physical activity, but 16-hydroxylation pathway EM was 15% lower (Ptrend = 0.03) in the most active women (Table 2). In addition, two EM in the 16-pathway were 16–24% lower among the most active women (16α-hydroxyestrone, Ptrend = 0.01; 16-ketoestriol, Ptrend = 0.01), whereas two other EM in this pathway were 7–18% lower but not significantly so (17- and 16-epiestriol, Ptrend = 0.06) (Table 2). There was no significant trend with activity for any of the individual 4-hydroxylation pathway EM. In contrast, overall activity was inversely associated with the methylated two-pathway catechols but not with unmethylated forms. The most active women had 15–19% lower levels for two of the three methylated two-pathway catechols in comparison with the least active women (2-methoxyestrone, Ptrend = 0.04; 2-hydroxyestrone-3-methyl ether, Ptrend = 0.01).
Table 2.
Geometric means for urinary concentrations of individual estrogens and EM and metabolic pathway groups (picomoles per milligram creatinine) and pathway ratios by overall physical activity in the NHSII (n = 603)
| EM measure | Overall physical activity (MET h/wk) |
||||||
|---|---|---|---|---|---|---|---|
| <3 | 3–8.9 | 9–17.9 | 18–26.9 | 27–41.9 | 42+ | Ptrenda | |
| n | 60 | 127 | 148 | 96 | 92 | 80 | |
| Total EM | 201.1 | 207.1 | 204.1 | 203.0 | 174.0 | 200.5 | 0.26 |
| Parent estrogens | 45.0 | 43.5 | 39.0 | 44.6 | 35.7 | 40.1 | 0.16 |
| Estrone | 30.8 | 29.5 | 27.2 | 30.2 | 24.9 | 27.3 | 0.18 |
| Estradiol | 15.7 | 14.7 | 13.9 | 15.2 | 11.8 | 13.4 | 0.03 |
| 2-Hydroxylation pathway EM | 55.3 | 60.3 | 69.2 | 65.8 | 57.7 | 62.5 | 0.99 |
| 4-Hydroxylation pathway EM | 5.6 | 6.2 | 6.3 | 6.3 | 5.4 | 6.2 | 0.83 |
| 16-Hydroxylation pathway EM | 78.5 | 76.9 | 69.5 | 70.3 | 59.7 | 67.1 | 0.03 |
| Catechols | 51.3 | 57.0 | 66.4 | 62.0 | 55.0 | 61.0 | 0.77 |
| 2-Hydroxyestrone | 38.8 | 43.4 | 51.5 | 47.7 | 42.1 | 46.4 | 0.79 |
| 2-Hydroxyestradiol | 4.8 | 5.0 | 5.6 | 5.8 | 5.4 | 5.3 | 0.58 |
| 4-Hydroxyestrone | 5.2 | 6.0 | 5.8 | 6.1 | 4.9 | 5.9 | 0.89 |
| Methylated catechols | 10.6 | 10.7 | 10.4 | 10.5 | 8.6 | 9.1 | 0.03 |
| 2-Methoxyestrone | 8.1 | 8.1 | 8.0 | 7.9 | 6.5 | 6.9 | 0.04 |
| 2-Methoxyestradiol | 0.70 | 0.71 | 0.72 | 0.76 | 0.59 | 0.62 | 0.08 |
| 2-Hydroxyestrone-3-methyl ether | 1.23 | 1.30 | 1.14 | 1.26 | 0.96 | 0.99 | 0.01 |
| 4-Methoxyestrone | 0.09 | 0.14 | 0.09 | 0.12 | 0.11 | 0.12 | 0.80 |
| 4-Methoxyestradiol | 0.05 | 0.04 | 0.05 | 0.05 | 0.05 | 0.06 | 0.16 |
| 16-Hydroxylation pathway EM | |||||||
| 16α-Hydroxyestrone | 13.1 | 13.9 | 11.9 | 13.2 | 10.5 | 10.0 | 0.01 |
| Estriol | 36.2 | 32.5 | 31.2 | 29.4 | 26.2 | 32.3 | 0.30 |
| 17-Epiestriol | 1.9 | 2.0 | 1.6 | 1.8 | 1.4 | 1.6 | 0.06 |
| 16-Ketoestradiol | 15.4 | 15.6 | 14.5 | 14.0 | 12.9 | 13.0 | 0.01 |
| 16-Epiestriol | 6.9 | 7.2 | 6.7 | 6.6 | 5.9 | 6.4 | 0.06 |
| Metabolic pathway ratios | |||||||
| Two-pathway EM to parent estrogens | 1.2 | 1.3 | 1.7 | 1.4 | 1.6 | 1.6 | 0.05 |
| Two-pathway catechols to parent estrogens | 1.0 | 1.1 | 1.4 | 1.2 | 1.4 | 1.3 | 0.03 |
| Methylated two-pathway catechols to parent estrogens | 0.23 | 0.23 | 0.25 | 0.23 | 0.23 | 0.22 | 0.42 |
| Two-pathway catechols to methylated two-pathway catechols | 4.3 | 4.8 | 5.6 | 5.1 | 5.8 | 6.0 | <0.01 |
| Two-pathway EM to four-pathway EM | 10.0 | 9.5 | 10.4 | 10.2 | 10.9 | 10.2 | 0.51 |
| Two-pathway EM to 16-pathway EM | 0.71 | 0.76 | 0.97 | 0.91 | 0.98 | 0.94 | 0.09 |
| 2-Hydroxyestrone to 16α-hydroxyestrone | 3.0 | 3.1 | 4.2 | 3.6 | 4.0 | 4.6 | 0.01 |
| Four-pathway EM to parent estrogens | 0.12 | 0.14 | 0.16 | 0.14 | 0.15 | 0.15 | 0.43 |
| Four-pathway EM to 16-pathway EM | 0.07 | 0.08 | 0.09 | 0.09 | 0.09 | 0.09 | 0.30 |
| Four-pathway catechols to methylated four-pathway catechols | 31.9 | 26.7 | 33.4 | 31.5 | 26.8 | 26.8 | 0.48 |
| 16-Pathway EM to parent estrogens | 1.7 | 1.8 | 1.7 | 1.6 | 1.7 | 1.7 | 0.49 |
Adjusted for age at urine collection, luteal day at collection, first morning urine, BMI (continuous), alcohol consumption, and usual menstrual cycle length.
Ptrend evaluated using the median METhours per week value for each activity category as a continuous variable.
Fig. 2.
Geometric means for urinary concentrations of parent estrogens, estrone, and estradiol (picomoles per milligram creatinine) by level of overall physical activity in the NHSII (n = 603).
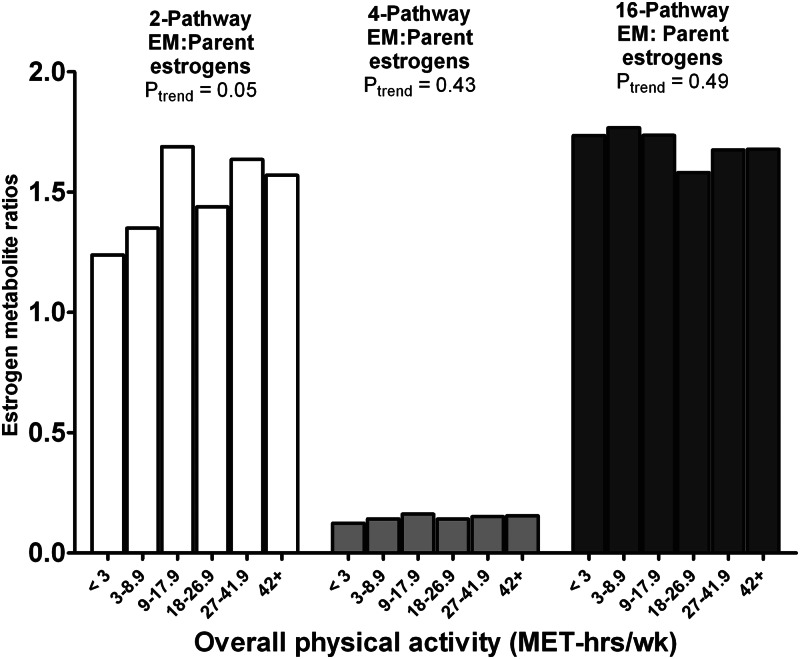
To gain insight into the association between physical activity and patterns of estrogen metabolism, we also systematically examined ratios of metabolic pathways. A significant positive trend was evident for the two-pathway EM to parent estrogens ratio (27% higher for most vs. least active, Ptrend = 0.05) but not for the four-pathway EM to parent estrogens or 16-pathway EM to parent estrogens ratios (Fig. 3). Significant positive trends were also noted for the two-pathway catechols to parent estrogens ratio (33% higher, Ptrend = 0.03) and the two-pathway catechols to methylated two-pathway catechols ratio (40% higher, Ptrend < 0.01) (Table 2). Finally, we noted a 56% elevation in the 2-hydroxyestrone to 16α-hydroxyestrone ratio (Ptrend = 0.01) among the most active women and a nonsignificant 31% elevation in the two-pathway EM to 16-pathway EM ratio (Ptrend = 0.09) (Table 2).
Fig. 3.
Geometric means for the 2-hydroxylation pathway to parent estrogens ratio, 4-hydroxylation pathway to parent estrogens ratio, and 16-hydroxylation pathway to parent estrogens ratio by level of overall physical activity in the NHSII (n = 603).
In sensitivity analyses we excluded 61 women with anovulatory cycles from the analyses shown in Table 2 and found that the trends with overall physical activity were attenuated for most individual EM and metabolic pathway groups. Only one of seven individual and grouped EM (2-hydroxyestrone-3-methyl-ether) remained significant after exclusion of these women (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). In contrast, results for the pathway ratios remained essentially unchanged after excluding anovulatory women. There was little evidence of attenuation of the association and three of the four significant associations for the ratios remained.
To examine the influence of BMI and menstrual cycle length on our results, we fit models excluding these variables and found that results did not differ meaningfully from those presented in Table 2. Analysis of overall physical activity stratified by BMI (≤25 vs. >25 kg/m2) revealed broadly similar results for the parent estrogens and 16-hydroxylation pathway EM. Similar results were also noted in both BMI groups for the two-pathway EM to parent estrogens ratio, two-pathway catechols to methylated two-pathway catechols ratio, two-pathway EM to 16-pathway EM ratio, and 2-hydroxyestrone to 16α-hydroxyestrone ratio. Among the heavier women, however, physical activity appeared to be inversely associated with 4-hydroxyestrone (28% lower in most vs. least physically active, Ptrend = 0.05), which resulted in a significant positive trend with the two-pathway EM to 4-pathway EM ratio (37% higher, Ptrend = 0.02) (Supplemental Table 2).
To examine associations with a common lower intensity activity, we analyzed urinary EM measures and walking in fully adjusted models (Table 3). In general, we observed results similar to those previously noted for overall physical activity. Women reporting the most walking (≥18 MET h/wk) had 20–24% lower levels of parent estrogens (Ptrend = 0.03) and estradiol (Ptrend = 0.02) compared with those reporting the least walking (<3 MET h/wk). There was no clear association with the 2- or 4-hydroxylation pathway EM, and the 16-hydroxylation pathway EM was inversely, but nonsignificantly, associated with walking. In contrast to the results for total physical activity, the two-pathway EM to parent estrogens ratio, two-pathway catechols to parent estrogens ratio, two-pathway EM to 16-pathway EM ratio, and 2-hydroxyestrone to 16α-hydroxyestrone ratio were not significantly associated with walking. However, walking, like total activity, was significantly positively associated with the two-pathway catechols to methylated two-pathway catechols ratio (Ptrend = 0.03). We also examined results for vigorous activities and observed patterns broadly similar to those for overall activity (data not shown).
Table 3.
Geometric means for urinary concentrations of individual estrogens and EM and metabolic pathway groups (picomoles per milligram creatinine) and pathway ratios by level of walking in the NHSII (n = 603)
| EM measure | Walking (MET h/wk) |
||||
|---|---|---|---|---|---|
| <3 | 3–8.9 | 9–17.9 | 18+ | Ptrenda | |
| N | 241 | 219 | 99 | 44 | |
| Total EM | 201.6 | 199.2 | 203.7 | 179.6 | 0.31 |
| Parent estrogens | 42.7 | 41.6 | 41.0 | 33.5 | 0.03 |
| Estrone | 29.0 | 28.2 | 29.7 | 23.1 | 0.11 |
| Estradiol | 14.7 | 14.0 | 14.5 | 11.1 | 0.02 |
| 2-Hydroxylation pathway EM | 59.4 | 68.2 | 62.7 | 50.5 | 0.30 |
| 4-Hydroxylation pathway EM | 5.8 | 6.6 | 5.3 | 6.0 | 0.74 |
| 16-Hydroxylation pathway EM | 74.1 | 67.6 | 71.0 | 63.5 | 0.21 |
| Catechols | 55.8 | 64.9 | 59.7 | 51.2 | 0.64 |
| 2-Hydroxyestrone | 43.0 | 50.1 | 45.0 | 37.7 | 0.41 |
| 2-Hydroxyestradiol | 5.0 | 5.7 | 5.7 | 4.8 | 0.86 |
| 4-Hydroxyestrone | 5.6 | 6.2 | 4.9 | 5.7 | 0.55 |
| Methylated catechols | 10.2 | 10.4 | 9.9 | 7.3 | 0.01 |
| 2-Methoxyestrone | 7.7 | 8.0 | 7.4 | 5.6 | 0.02 |
| 2-Methoxyestradiol | 0.66 | 0.74 | 0.71 | 0.52 | 0.15 |
| 2-Hydroxyestrone-3-methyl ether | 1.15 | 1.19 | 1.20 | 0.85 | 0.07 |
| 4-Methoxyestrone | 0.11 | 0.11 | 0.11 | 0.11 | 0.88 |
| 4-Methoxyestradiol | 0.05 | 0.05 | 0.05 | 0.04 | 0.83 |
| 16-hydroxylation pathway EM | |||||
| 16a-Hydroxyestrone | 13.1 | 11.6 | 12.8 | 9.1 | 0.04 |
| Estriol | 33.4 | 29.0 | 30.8 | 31.9 | 0.65 |
| 17-Epiestriol | 1.8 | 1.7 | 1.7 | 1.5 | 0.34 |
| 16-Ketoestradiol | 14.7 | 14.0 | 14.7 | 11.7 | 0.08 |
| 16-Epiestriol | 6.8 | 6.5 | 6.9 | 6.1 | 0.58 |
| Metabolic pathway ratios | |||||
| Two-pathway EM to parent estrogens | 1.4 | 1.6 | 1.5 | 1.5 | 0.35 |
| Two-Pathway catechols to parent estrogens | 1.1 | 1.3 | 1.2 | 1.3 | 0.22 |
| Methylated two-pathway catechols to parent estrogens | 0.23 | 0.24 | 0.23 | 0.21 | 0.36 |
| Two-pathway catechols to methylated two-pathway catechols | 4.9 | 5.4 | 5.3 | 6.2 | 0.03 |
| Two-pathway EM to four-pathway EM | 10.2 | 10.1 | 11.3 | 8.5 | 0.53 |
| Two-pathway EM to 16-pathway EM | 0.79 | 0.99 | 0.88 | 0.81 | 0.86 |
| 2-Hydroxyestrone to 16α-hydroxyestrone | 3.3 | 4.3 | 3.5 | 4.2 | 0.31 |
| Four-pathway EM to parent estrogens | 0.14 | 0.16 | 0.13 | 0.18 | 0.21 |
| Four-pathway EM to 16-pathway EM | 0.08 | 0.10 | 0.08 | 0.09 | 0.53 |
| Four-pathway catechols to methylated 4-pathway catechols | 28.6 | 31.0 | 27.8 | 33.2 | 0.70 |
| 16-pathway EM to parent estrogens | 1.7 | 1.6 | 1.7 | 1.9 | 0.53 |
Adjusted for age at urine collection, luteal day at collection, first morning urine, BMI (continuous), alcohol consumption, and usual menstrual cycle length.
Ptrend evaluated using the median MET h/wk value for each activity category as a continuous variable.
In contrast to the associations observed with overall physical activity, no consistent associations were found between adult sedentary behaviors and metabolic pathways or pathway ratios. For example, sitting time as an adult was not associated with estradiol (Ptrend = 0.75), 16α-hydroxyestrone (Ptrend = 0.72), or the two-pathway catechols to methylated two-pathway catechols ratio (Ptrend = 0.83). Furthermore, there was little evidence that physical activity or television viewing in adolescence was associated with urinary EM levels during the premenopausal years. For example, moderate/strenuous activity during high school was not associated with estradiol (Ptrend = 0.10), 16α-hydroxyestrone (Ptrend = 0.51), or the two-pathway catechols to methylated two-pathway catechols ratio (Ptrend = 0.14).
Discussion
In this study of premenopausal women, we found that higher physical activity in adulthood was significantly associated with lower urinary estradiol, 16-hydroxylation pathway EM, and methylated two-pathway catechols after controlling for age, BMI and other potential confounders. Physical activity was significantly positively associated with metabolic pathway ratios indicative of increased 2-hydroxylation of parent estrogens relative to 16-hydroxylation, specifically two-pathway EM to parent estrogens, two-pathway catechols to parent estrogens, and 2-hydroxyestrone to 16α-hydroxyestrone. Finally, physical activity was significantly associated with increased levels of two-pathway catechols relative to methylated two-pathway catechols. In contrast, there was little evidence that physical activity in adolescence or adult or adolescent sedentary behavior was associated with estrogen metabolism. To our knowledge, this study of physical activity and estrogen metabolism is the largest and most comprehensive ever conducted.
We examined urinary EM during the luteal phase of the cycle and used the start date of the next menstrual cycle to determine the exact time of urine collection. Our findings for the parent estrogens are largely consistent with two studies of De Souza et al. (5, 6) that collected daily urine samples over several cycles. These studies indicated that the prevalence of luteal phase defects and anovulatory cycles can be as high as 40–50% among highly active premenopausal women and results in reduced urinary parent estrogen levels in these active women in the luteal phase (5, 6). Our findings for urinary parent estrogens are also consistent with the inverse associations between physical activity and circulating parent estrogens, especially in the luteal phase, in two large cross-sectional studies (7, 30), one of which included many of the NHSII participants in the current study (7). Both the inverse association with circulating luteal parent estrogens previously reported in NHSII and the inverse association with urinary luteal parent estrogens in the current analysis were attenuated when women with anovulatory cycles were excluded.
Our results are unique in that we used a novel comprehensive assay for quantitating 15 urinary EM and rigorously assessed participation in physical activity over several years. Intervention studies have examined the effect of 12 wk to 12 months of aerobic exercise on urinary estrogen metabolites (13, 14, 31). The results from these studies and others (10, 12) are consistent with our null results with respect to 2-hydroxyestrone. However, these intervention studies found no effect of exercise on urinary 16α-hydroxyestrone (13, 14, 31) or estriol levels (13). The most consistent association we observed was that physical activity was associated with lower levels of 16-hydroxylation pathway EM and most of the individual EM in this pathway. It may be that participation in physical activity over several years, as assessed in the present study, is necessary for activity to influence the 16-hydroxylation pathway. Little is known about the potential for exercise to modulate activity of the P450 enzymes that regulate estrogen hydroxylation into the two or 16 pathways, but other behaviors, such as smoking, and certain dietary constituents have been shown to influence these enzyme systems (4). Our results suggest this possibility, but more research is needed to understand whether physical activity exerts an influence estrogen metabolism.
We are aware of only a few studies that examined the relation between physical activity and methylation of the catechol estrogens. Our finding of lower levels of methylated two-pathway catechols with higher activity levels is in contrast to the studies of De Cree et al. (15, 16), which reported increases in circulating levels of methylated catechols in the two and four pathways after 5 consecutive days of exhaustive exercise. These discrepant results may be due to differences in exercise exposures (i.e. habitual activity vs. 5 d of exhaustive exercise), or possibly to comparing urinary and circulating levels. It has been proposed that catecholamines, which are elevated with exercise (32), may inhibit COMT activity and reduce formation of methylated catechols (4).
Although this study did not explicitly examine disease risk, our findings may be relevant to health outcomes linked to both physical activity and endogenous estrogen. Several hypotheses about estrogen metabolism and breast carcinogenesis have emerged from laboratory research. Estrogen metabolites vary in their abilities to activate the estrogen receptor and to damage DNA, both of which mechanisms may influence risk (2). In the NHSII nested case-control study from which many of the current study participants originated (20), we reported that higher levels of urinary estrone and estradiol, measured in the luteal phase, were associated with significantly reduced risk for premenopausal breast cancer. There was also a suggestion that higher levels of methylated two-pathway catechols were associated with lower risk. Both of these findings argue against the idea that the physical activity relationships we observed, lower levels of these EM with higher activity, are mechanisms through which physical activity may reduce risk of premenopausal breast cancer (25, 33). In contrast, our finding that physical activity is associated with lower 16-hydroxylation pathway EM levels does provide an explanation. In our prior publication (20), we reported that higher levels of 17-epiestriol and the 16-pathway EM to parent estrogens ratio, both indicating increased estrogen metabolism through the 16-hydroxlylation pathway, are associated with increased breast cancer risk. It is possible that physical activity minimizes exposure to the estrogenic and genotoxic 16-hydroylation pathway EM (4).
Higher levels of circulating parent estrogens are associated with better bone health (3). The few studies that examined estrogen metabolism noted that the more estrogenic 16-pathway EM were associated with higher bone density in both men (34) and postmenopausal women (35). For premenopausal women who experience irregular and anovulatory periods as a consequence of high physical activity and produce less estrogen to support bone health (36), our results suggest that additional loss of the more estrogenic 16-pathway metabolites could also contribute to lower bone density, although this warrants further investigation.
Strengths of our study include collection of carefully timed luteal urine samples from a large number of premenopausal women. We used a novel LC-MS/MS assay that measures with specificity, sensitivity, and precision the absolute concentrations of two parent estrogens and 13 estrogen metabolites. In addition, we used a physical activity assessment instrument determined to be reliable and valid (24) and combined two administrations of the instrument, separated by 4 yr, to estimate long-term adult physical activity. Finally, we evaluated and controlled for a broad range of potential confounders in our multivariate models.
There are also limitations of our study. First, we used a cross-sectional study design; therefore, we cannot unequivocally determine the causal relation between physical activity and estrogen metabolism. Second, we used self-reported information on physical activity and sedentary behaviors, and measurement error in these exposures may have resulted in attenuation of the strength of the associations observed and loss of statistical power (37). Third, we measured EM in urine rather than blood, and the relationships among EM concentrations in urine, blood, and target tissue are not well understood. Fourth, our measures of absolute EM levels in urine were normalized by creatinine levels; and other factors, such as differences in lean body mass, associated with both the excretion of creatine as creatinine and physical activity, could have influenced our results. However, physical activity was not associated with creatinine excretion. In addition, the metabolic pathway ratios did not need to be adjusted for creatinine and gave results consistent with those based on the creatinine-adjusted EM concentrations. Fifth, we examined the association between physical activity and urinary EM in only the luteal phase of the cycle. Sixth, urinary EM were measured only once, and intraindividual variation in these measures may have attenuated our results. However, intraclass correlations for the 15 urinary EM measured in the luteal phase three times over 3 yr are relatively high (0.52–0.72) (21), which is comparable with the reproducibility of blood cholesterol and glucose (38).
Because our LC-MS/MS method measured 15 distinct estrogens and estrogen metabolites for the first time in an epidemiological study of physical activity, we systematically examined associations for each of them as well as associations with a limited number of derived measures, including the sum of all EM, metabolic pathway groups, and metabolic pathway ratios. Evaluation of the groups and ratios were motivated by recognized estrogen metabolism pathways, shared biochemistry, and etiological hypotheses derived from laboratory and clinical research. We did not adjust statistically for multiple comparisons because our primary aim was to identify promising relationships. It is possible that some of our findings are due to chance. We recognize that all of our results require independent confirmation.
In conclusion, the results from this investigation yield new insight into the effects of physical activity on estrogen physiology and suggest mechanisms linking physical activity to chronic diseases, such as breast cancer and osteoporosis. Higher long-term physical activity levels were associated with lower concentrations of estradiol, 16-hydroxylation pathway EM, and methylated two-pathway catechols, and preferential metabolism of parent estrogens to two-pathway catechols. Sedentary behavior during adulthood and physical activity during adolescence were not associated with estrogen metabolism patterns. We view our analysis, the first to systematically explore the relationships between physical activity assessed over several years and all 15 urinary EM, as exploratory, and we have tried, by focusing on recognized metabolic pathways, to interpret the results cautiously. Additional research is required to replicate or refute the current findings, preferably in adequately powered intervention and/or prospective studies and to explore interrelationships with BMI and energy balance. The availability of a robust, accurate, comprehensive measure of estrogen metabolism will aid these investigations.
Supplementary Material
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This work was supported by Research Grants CA67262 and CA50385 from the National Cancer Institute (NCI) and the Intramural Research Program of the NCI Division of Cancer Epidemiology and Genetics and with federal funds of the NCI awarded under Contract HHSN261200800001E (to SAIC-Frederick). R.T.F. is supported, in part, by Grant T32 CA09001.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- BMI
- Body mass index
- COMT
- catechol-O-methyltransferase
- CV
- coefficient of variation
- EM
- estrogen metabolite
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- MET
- metabolic equivalent
- NHSII
- Nurses' Health Study II.
References
- 1. Physical Activity Guidelines Advisory Committee 2008. Physical Activity Guidelines Advisory Committee Report. Washington, DC: U.S. Department of Health and Human Services [Google Scholar]
- 2. Yager JD, Davidson NE. 2006. Estrogen carcinogenesis in breast cancer. N Engl J Med 354:270–282 [DOI] [PubMed] [Google Scholar]
- 3. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. 2002. Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- 4. Zhu BT, Conney AH. 1998. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis 19:1–27 [DOI] [PubMed] [Google Scholar]
- 5. De Souza MJ, Toombs RJ, Scheid JL, O'Donnell E, West SL, Williams NI. 2010. High prevalence of subtle and severe menstrual disturbances in exercising women: confirmation using daily hormone measures. Hum Reprod 25:491–503 [DOI] [PubMed] [Google Scholar]
- 6. De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, Lasley BL. 1998. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab 83:4220–4232 [DOI] [PubMed] [Google Scholar]
- 7. Tworoger SS, Missmer SA, Eliassen AH, Barbieri RL, Dowsett M, Hankinson SE. 2007. Physical activity and inactivity in relation to sex hormone, prolactin, and insulin-like growth factor concentrations in premenopausal women. Cancer Causes Control 18:743–752 [DOI] [PubMed] [Google Scholar]
- 8. Russell JB, Mitchell D, Musey PI, Collins DC. 1984. The relationship of exercise to anovulatory cycles in female athletes: hormonal and physical characteristics. Obstet Gynecol 63:452–456 [PubMed] [Google Scholar]
- 9. Bentz AT, Schneider CM, Westerlind KC. 2005. The relationship between physical activity and 2-hydroxyestrone, 16-α-hydroxyestrone, and the 2/16 ratio in premenopausal women (United States). Cancer Causes Control 16:455–461 [DOI] [PubMed] [Google Scholar]
- 10. Campbell KL, Westerlind KC, Harber VJ, Friedenreich CM, Courneya KS. 2005. Associations between aerobic fitness and estrogen metabolites in premenopausal women. Med Sci Sports Exerc 37:585–592 [DOI] [PubMed] [Google Scholar]
- 11. Russell JB, Mitchell DE, Musey PI, Collins DC. 1984. The role of β-endorphins and catechol estrogens on the hypothalamic-pituitary axis in female athletes. Fertil Steril 42:690–695 [DOI] [PubMed] [Google Scholar]
- 12. Snow RC, Barbieri RL, Frisch RE. 1989. Estrogen 2-hydroxylase oxidation and menstrual function among elite oarswomen. J Clin Endocrinol Metab 69:369–376 [DOI] [PubMed] [Google Scholar]
- 13. Schmitz KH, Warren M, Rundle AG, Williams NI, Gross MD, Kurzer MS. 2008. Exercise Effect on Oxidative Stress Is Independent of Change in Estrogen Metabolism. Cancer Epidemiol Biomarkers Prev 17:220–223 [DOI] [PubMed] [Google Scholar]
- 14. Campbell KL, Westerlind KC, Harber VJ, Bell GJ, Mackey JR, Courneya KS. 2007. Effects of aerobic exercise training on estrogen metabolism in premenopausal women: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 16:731–739 [DOI] [PubMed] [Google Scholar]
- 15. De Crée C, Ball P, Seidlitz B, Van Kranenburg G, Geurten P, Keizer HA. 1997. Effects of a training program on resting plasma 2-hydroxycatecholestrogen levels in eumenorrheic women. J Appl Physiol 83:1551–1556 [DOI] [PubMed] [Google Scholar]
- 16. De Crée C, Van Kranenburg G, Geurten P, Fujimori Y, Keizer HA. 1997. 4-Hydroxycatecholestrogen metabolism responses to exercise and training: possible implications for menstrual cycle irregularities and breast cancer. Fertil Steril 67:505–516 [DOI] [PubMed] [Google Scholar]
- 17. De Crée C, Van Kranenburg G, Geurten P, Fujimura Y, Keizer HA. 1997. Exercise-induced changes in enzymatic O-methylation of catecholestrogens by erythrocytes of eumenorrheic women. Med Sci Sports Exerc 29:1580–1587 [DOI] [PubMed] [Google Scholar]
- 18. Ziegler RG, Faupel-Badger JM, Sue LY, Fuhrman BJ, Falk RT, Boyd-Morin J, Henderson MK, Hoover RN, Veenstra TD, Keefer LK, Xu X. 2010. A new approach to measuring estrogen exposure and metabolism in epidemiologic studies. J Steroid Biochem Mol Biol 121:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. 1998. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. Am J Epidemiol 147:1071–1080 [DOI] [PubMed] [Google Scholar]
- 20. Eliassen AH, Spiegelman D, Xu X, Keefer LK, Veenstra TD, Barbieri RL, Willet WC, Hankinson SE, Ziegler RG. 2012. Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women. Cancer Res 72:696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eliassen AH, Ziegler RG, Rosner B, Veenstra TD, Roman JM, Xu X, Hankinson SE. 2009. Reproducibility of fifteen urinary estrogens and estrogen metabolites over a 2- to 3-year period in premenopausal women. Cancer Epidemiol Biomarkers Prev 18:2860–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, Saavedra JE, Keefer LK, Ziegler RG. 2005. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem 77:6646–6654 [DOI] [PubMed] [Google Scholar]
- 23. Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr 1993. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 25:71–80 [DOI] [PubMed] [Google Scholar]
- 24. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. 1994. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 23:991–999 [DOI] [PubMed] [Google Scholar]
- 25. Maruti SS, Willett WC, Feskanich D, Rosner B, Colditz GA. 2008. A Prospective Study of Age-Specific Physical Activity and Premenopausal Breast cancer. J Natl Cancer Inst 100:728–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. 2004. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 351:2694–2703 [DOI] [PubMed] [Google Scholar]
- 27. Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. 2003. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 289:1785–1791 [DOI] [PubMed] [Google Scholar]
- 28. Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 1996. 2-Hydroxyestrone: the ‘good’ estrogen. J Endocrinol 150(Suppl):259–265 [PubMed] [Google Scholar]
- 29. Rosner B. 1983. Percentage points for a generalized ESD many-outlier procedure. Technometrics 25:165–172 [Google Scholar]
- 30. Verkasalo PK, Thomas HV, Appleby PN, Davey GK, Key TJ. 2001. Circulating levels of sex hormones and their relation to risk factors for breast cancer: a cross-sectional study in 1092 pre- and postmenopausal women (United Kingdom). Cancer Causes Control 12:47–59 [DOI] [PubMed] [Google Scholar]
- 31. Atkinson C, Lampe JW, Tworoger SS, Ulrich CM, Bowen D, Irwin ML, Schwartz RS, Rajan BK, Yasui Y, Potter JD, McTiernan A. 2004. Effects of a moderate intensity exercise intervention on estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev 13:868–874 [PubMed] [Google Scholar]
- 32. Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. 2008. Catecholamines and the effects of exercise, training and gender. Sports Med 38:401–423 [DOI] [PubMed] [Google Scholar]
- 33. Friedenreich CM, Cust AE. 2008. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med 42:636–647 [DOI] [PubMed] [Google Scholar]
- 34. Napoli N, Faccio R, Shrestha V, Bucchieri S, Rini GB, Armamento-Villareal R. 2007. Estrogen metabolism modulates bone density in men. Calcif Tissue Int 80:227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leelawattana R, Ziambaras K, Roodman-Weiss J, Lyss C, Wagner D, Klug T, Armamento-Villareal R, Civitelli R. 2000. The oxidative metabolism of estradiol conditions postmenopausal bone density and bone loss. J Bone Miner Res 15:2513–2520 [DOI] [PubMed] [Google Scholar]
- 36. De Souza MJ, Williams NI. 2004. Physiological aspects and clinical sequelae of energy deficiency and hypoestrogenism in exercising women. Hum Reprod Update 10:433–448 [DOI] [PubMed] [Google Scholar]
- 37. Matthews CE, Moore SC, George SM, Sampson J, Bowles HR. 2012. Improving self-reports of active and sedentary behaviors in large epidemiologic studies. Exerc Sport Sci Rev 40:118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosner B, Spiegelman D, Willett WC. 1992. Correction of logistic regression relative risk estimates and confidence intervals for random within-person measurement error. Am J Epidemiol 136:1400–1413 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.