Abstract
Context:
Coronary heart disease (CHD) is the leading cause of death in the United States.
Objective:
This study compares differences in risk factors for CHD in diabetic vs. nondiabetic Strong Heart Study participants.
Design:
This was an observational study.
Setting:
The study was conducted at three centers in Arizona, Oklahoma, and North and South Dakota.
Participants:
Data were obtained from 3563 of 4549 American Indians free of cardiovascular disease at baseline.
Intervention(s):
CHD events were ascertained during follow-up.
Main Outcome Measure:
CHD events were classified using standardized criteria.
Results:
In diabetic and nondiabetic participants, 545 and 216 CHD events, respectively, were ascertained during follow-up (21,194 and 22,990 person-years); age- and sex-adjusted incidence rates of CHD were higher for the diabetic group (27.5 vs. 12.1 per 1,000 person-years). Risk factors for incident CHD common to both groups included older age, male sex, prehypertension or hypertension, and elevated low-density lipoprotein cholesterol. Risk factors specific to the diabetic group were lower high-density lipoprotein cholesterol, current smoking, macroalbuminuria, lower estimated glomerular filtration rate, use of diabetes medication, and longer duration of diabetes. Higher body mass index was a risk factor only for the nondiabetic group. The association of male sex and CHD was greater in those without diabetes than in those with diabetes.
Conclusions:
In addition to higher incidence rates of CHD events in persons with diabetes compared with those without, the two groups differed in CHD risk factors. These differences must be recognized in estimating CHD risk and managing risk factors.
Coronary heart disease (CHD) is the leading cause of mortality in the United States and was responsible for 405,309 (approximately one of every six) deaths in 2008 (1). Each year, an estimated 785,000 persons in the United States have a first coronary event, and approximately 470,000 have a recurrent event (1). American Indians previously were thought to have low CHD rates (2–4). However, systematic surveillance from the Strong Heart Study (SHS) shows that rates of CHD in this population are higher than those of other U.S. populations, with the majority of events occurring in persons with diabetes (5, 6). This pattern of increasing CHD following increasing diabetes rates has been observed in other ethnic groups and in developing countries (7–9). To our knowledge, no studies have compared associations between risk factors and incident CHD events among nondiabetic and diabetic individuals in a population with high diabetes prevalence. The SHS, begun in 1989, is a longitudinal study of CHD and its associated risk factors in a population-based sample of American Indians from three geographic regions, 46% of whom had diabetes at the baseline examination. Extended follow-up has permitted examination of a sufficient number of CHD events to identify risk factors among those with and without prevalent or incident diabetes. The aims of this article are: 1) to compare incidence rates of CHD among those with and without diabetes; and 2) to identify risk factors and compare the strength of these risk factors for incident CHD events in diabetic and nondiabetic SHS participants.
Subjects and Methods
Details of the SHS design, survey methods, and laboratory techniques have been reported (5, 6). Briefly, the SHS cohort was composed of 4549 American Indians, ages 45 to 74 yr at the baseline examination (1989–1991). Cohort survivors were reexamined in 1993–1995 and 1998–1999. Morbidity and mortality have been reviewed and tabulated annually. Institutional Review Boards of the Indian Health Service and participating institutions and tribes approved the study. Informed consent was obtained from all participants.
Of 4549 participants at the baseline examination, 24 (0.5%) had unknown diabetic status and 327 (7.2%) had cardiovascular disease (CVD) [definite myocardial infarction (MI), CHD, stroke, or congestive heart failure]. After exclusion of these individuals, 4198 (1872 with diabetes and 2326 without diabetes) participants remained in the analysis. Because some CHD events identified in our follow-up for nondiabetic individuals could have been in persons who developed diabetes after their last SHS examination, we reviewed medical records for all individuals with CHD events to determine whether diabetes had been diagnosed before the event. Only those without a diabetes diagnosis were included as nondiabetic in the current analyses. Among 2326 initially nondiabetic participants, we excluded 599 (14.3%) who developed diabetes during follow-up and 36 (2.1%) who had macroalbuminuria at the baseline examination, a predictor of subsequent diabetes. Thus, this report analyzed 3563 participants (1872 with diabetes and 1691 without prevalent or incident diabetes) who were free of CVD at baseline.
Baseline measurements
The baseline examination included a personal interview to obtain demographic data, personal medical history, health habits and family history of CVD and diabetes, and a physical examination. The physical examination consisted of a 12-lead resting electrocardiogram and a fasting blood sample for laboratory measurements, including plasma total cholesterol, low-density lipoprotein (LDL) cholesterol (LDL-C), high-density lipoprotein (HDL) cholesterol (HDL-C), glucose, and creatinine. Non-HDL-C was calculated as total cholesterol minus HDL-C. A random morning urine sample was collected for measurements of creatinine and albumin. Plasma lipoproteins (total triglycerides, HDL, LDL, and total cholesterol) were measured by β quantification. Cholesterol, triglyceride, and glucose concentrations were determined by an enzymatic method using a Hitachi autoanalyzer and reagents from Boerhinger Mannheim Diagnostics (Indianapolis, IN). HDL-C was isolated by precipitation with heparin and manganese chloride. LDL particle size was measured by a gradient gel electrophoresis procedure using nondenaturing polyacrylamide gels. Serum and urine creatinine were measured by the alkaline picrate method (10). Each measurement was performed in a central laboratory and standardized to outside reference values.
Height and weight were measured with the participant wearing lightweight clothing and without shoes. BMI was calculated as weight in kilograms/(height in meters)2. Systolic and diastolic arterial blood pressures were measured three times with the participants sitting; the mean of the last two measurements was used. Cigarette smoking was assessed by questionnaire. Physical activity (including leisure and occupational activity) during the past year and past week was assessed by questionnaire and expressed in hours per week (11).
Definitions
Hypertension was defined as systolic blood pressure (SBP) of at least 140 mm Hg, diastolic blood pressure (DBP) of at least 90 mm Hg, or use of antihypertensive medication (12). Prehypertension was defined as SBP of 120 to 139 mm Hg or DBP of 80 to 89 mm Hg. Normal BP was defined as SBP/DBP of less than 120/80 mm Hg. Diabetes was defined as taking antidiabetic medication or having a fasting glucose of at least 126 mg/dl or glycated hemoglobin of at least 6.5% (13). Microalbuminuria was defined as urinary albumin/creatinine ratio (UACR) of at least 30 mg/g but less than 300 mg/g, and macroalbuminuria as UACR of at least 300 mg/g. Chronic kidney disease was defined as Modification of Diet in Renal Disease (MDRD)-estimated glomerular filtration rate (eGFR) of less than 60 ml/min/1.73 m2. The abbreviated MDRD equation is eGFR = 186.3 × Scr−1.154 × age−0.203 × (0.742 if female) × 1.210 (if African-American) (14), where Scr is serum creatinine measured in milligrams per deciliter and age is in years.
Determination of diabetic status during follow-up
Diabetic status during follow-up was determined at the second (1993–1995) and third (1998–1999) SHS examinations, at the first SHS family study visit (2001–2003) for the subset (520 of 4549) of participants who were included in that study, and from medical records data abstracted from morbidity and mortality review for participants who experienced a CHD event.
Ascertainment of incident events
All CHD events were defined as the first nonfatal or fatal event during follow-up for each participant (from baseline examination to December 31, 2007). Nonfatal CHD events included definite MI, definite CHD, and definite electrocardiographic MI. Fatal CHD was defined as fatal MI, sudden death due to CHD, and definite and possible fatal CHD. The criteria and definitions used have been described (5, 15). Incident CHD events were ascertained by annual mortality and morbidity surveillance or at the SHS/Strong Heart Family Study examinations. Medical records were abstracted, and CHD events were ascertained and confirmed by mortality and morbidity review committees using specific criteria (16). Mortality and morbidity follow-up were 99.8 and 99.2% complete, respectively.
Statistical analysis
Baseline characteristics were summarized by CHD and diabetic status and presented as means ± sd values for continuous variables, as medians [interquartile ranges (IQR)] for skewed continuous variables, and as numbers (percentages) for categorical variables. Tests for differences between CHD and diabetic status were based on χ2 or Fisher's exact test for categorical variables and t test or Wilcoxon rank sum test for continuous variables.
Person-time incidence rates of CHD by diabetic status and study center were calculated in men, women, and both sexes combined. Age- and sex-specific incidence rates, age-adjusted rates, age- and sex-adjusted rates, and their 95% confidence intervals (CI) were calculated. Age-adjusted incidence rates of CHD were calculated by the direct method using the 1990 U.S. Bureau of the Census population distribution as the standard population and expressed as events per 1000 person-years (17). Person-years were based on length of follow-up, calculated as number of years from baseline examination to first event or end of follow-up. The log-rank test was used for comparisons among age groups, study centers, between men and women, and between diabetic and nondiabetic participants. The significance of trend with age was evaluated using the Mantel trend statistic, modified for person-time denominators (18).
Data on time to CHD event were analyzed using statistical methods for survival data analysis (19). Cox proportional hazards models were used to identify risk factors for incident CHD in those with and without diabetes, selected by a backward selection with a retention criterion of 5%. The full model included the following risk factors: age, sex, study center (Arizona, Oklahoma, and North and South Dakota), BMI, education (< high school or ≥ high school), SBP, DBP, antihypertensive medication use, or BP by category (normal, prehypertension, and hypertension), LDL-C (or non-HDL-C), HDL-C, triglycerides, fasting glucose, albuminuria (normal, microalbuminuria, and macroalbuminuria for diabetes), eGFR, smoking (never, past, and current), and physical activity. Diabetes treatment and diabetes duration were included for the diabetes group. Because the use of lipid-lowering medications was very low (about a 3% usage rate) at the time of the baseline exam, we did not adjust for lipid medication use. Interactions between sex and each risk factor were assessed. The Cox proportionality assumption was verified by including time-dependent interactions of covariates with survival time in the models. The strength of the associations [hazard ratios (HR)] was compared between the diabetic and nondiabetic groups (20). Analyses were performed with STATA version 11 (StataCorp, College Station, TX) (21). Statistical significance was defined as two-tailed P < 0.05 for all tests.
Results
Baseline characteristics of participants
Comparing the diabetic and nondiabetic participants at baseline, 64 and 56% of participants were female, 30 and 39% were overweight, and 60 and 36% were obese, respectively. Baseline characteristics are presented by diabetic status and follow-up CHD status (Table 1). In unadjusted comparisons stratified by diabetes status, those who developed CHD during follow-up were older and more likely to be male, have hypertension, use antihypertensive medications, reside in North or South Dakota, and have higher SBP, total cholesterol, non-HDL-C, LDL-C, and lower HDL-C than those who did not develop CHD. Diabetic participants who developed CHD during follow-up also had higher BMI and total triglycerides, were more likely to be current smokers, had longer diabetes duration (median, 10 vs. 6 yr; P < 0.001), and were more likely to have taken insulin or oral hypoglycemic agents (77.4 vs. 60.2%; P < 0.001) and have macroalbuminuria than those who did not.
Table 1.
Baseline characteristics by diabetic status and follow-up CHD status in 3563 American Indian men and women, ages 45–74 yr (SHS, 1989–2007)
| With diabetes |
Without diabetes |
|||||
|---|---|---|---|---|---|---|
| With CHD | Without CHD | P value | With CHD | Without CHD | P value | |
| n | 545 | 1327 | 216 | 1475 | ||
| Age (yr) | 57.5 ± 7.7 | 56.5 ± 7.9a | 0.008 | 58.4 ± 8.4 | 55.3 ± 8.1 | <0.001 |
| Male sex (%) | 39.5b | 33.9a | 0.02 | 63.4 | 40.6 | <0.001 |
| Study center (%)a,b | <0.001 | <0.001 | ||||
| Arizona | 38.7 | 51.9 | 9.3 | 20.8 | ||
| Oklahoma | 28.1 | 27.0 | 30.1 | 40.3 | ||
| North/South Dakota | 33.2 | 21.1 | 60.7 | 38.9 | ||
| At least high school (%) | 56.0b | 56.2a | 0.93 | 67.4 | 67.7 | 0.94 |
| BMI (kg/m2) | 31.6 ± 5.5b | 32.4 ± 6.6a | 0.008 | 29.4 ± 5.1 | 28.8 ± 5.7 | 0.13 |
| Physical activity (h/wk) | 8 (1–24)b | 7 (1–24)a | 0.88 | 17 (4–33) | 14 (3–30) | 0.07 |
| Current smoking (%) | 33.9b | 24.7a | <0.001 | 45.4 | 41.1 | 0.25 |
| SBP (mm Hg) | 134 ± 21.7b | 129 ± 19.7a | <0.001 | 128 ± 18.8 | 123 ± 17.5 | 0.001 |
| DBP (mm Hg) | 77.8 ± 9.7 | 77.1 ± 10.1a | 0.12 | 77.1 ± 10.5 | 75.7 ± 10.0 | 0.05 |
| Antihypertensive medication use (%) | 33.4b | 27.5a | 0.001 | 21.8 | 13.0 | 0.001 |
| BP (%)a,b | <0.001 | <0.001 | ||||
| Normal | 16.0 | 24.0 | 24.6 | 39.7 | ||
| Prehypertension | 31.6 | 31.7 | 35.7 | 33.1 | ||
| Hypertension | 52.4 | 44.3 | 38.9 | 27.2 | ||
| Fasting glucose (mg/dl) | 220 ± 79.5b | 204 ± 79.0a | <0.001 | 101 ± 9.8 | 100 ± 10.7 | 0.20 |
| Total cholesterol (mg/dl) | 202 ± 44.8 | 185 ± 42.9b | <0.001 | 200 ± 35.3 | 192 ± 37.2 | 0.007 |
| HDL-C (mg/dl) | 41 ± 10.8b | 44 ± 11.7a | <0.001 | 46 ± 12.9 | 50 ± 15.0 | <0.001 |
| Non-HDL-C (mg/dl) | 160 ± 46.1 | 141 ± 43.6 | <0.001 | 154 ± 37.2 | 142 ± 37.6 | <0.001 |
| LDL-C (mg/dl) | 121 ± 33.8b | 110 ± 32.9a | <0.001 | 130 ± 33.6 | 119 ± 33.1 | <0.001 |
| Total triglycerides (mg/dl) | 165 (114–240)b | 130 (94–187)a | <0.001 | 107 (74–142) | 102 (71–147) | 0.45 |
| UACR (mg/g) | 52.2 (12.3–327.1)b | 27.0 (9.1–142.4)a | <0.001 | 6.2 (3.3–12.4) | 6.3 (3.4–12.1) | 0.82 |
| Albuminuria (%)a,b | <0.001 | 0.69 | ||||
| Normal | 42.7 | 51.7 | 92.4 | 91.6 | ||
| Microalbuminuria | 30.8 | 30.9 | 7.6 | 8.5 | ||
| Macroalbuminuria | 26.5 | 17.4 | ||||
| eGFR (ml/min/1.73 m2) | 82 ± 30.9 | 84 ± 26.6 | 0.15 | 81 ± 15.1 | 82 ± 17.0 | 0.25 |
Data were presented as means ± sd for continuous variables, and median (IQR) if continuous variables were skewed. P value was based on χ2 or Fisher's exact test for categorical variables and t test or Wilcoxon rank sum test for continuous variables for comparisons between diabetic participants who developed CHD and those who did not and nondiabetic participants who developed CHD and those who did not.
P < 0.05 for comparison between diabetic and nondiabetic participants who did not develop CHD.
P < 0.05 for comparison between diabetic and nondiabetic participants who developed CHD.
Incidence rates of CHD in those with and without diabetes
Participants with and without diabetes were followed for a median (IQR) of 12.2 (6.3–16.8) and 16.3 (9.9–17.4) yr, respectively. During follow-up (21,194 and 22,990 person-years, respectively), 545 and 216 CHD events were ascertained in participants with and without diabetes, respectively. Age- and sex-specific incidence rates of CHD by center and diabetic status are presented (Table 2). As expected, participants with diabetes had significantly higher CHD incidence than those without diabetes within each age stratum and at each center (all P < 0.001). When combining all three centers, the crude incidence of CHD increased with age in all participants (p for trend = 0.002 and <0.001 in diabetic men and women, and p for trend < 0.001 and = 0.002 in nondiabetic men and women, respectively). Age-adjusted incidence rates of CHD also were higher in those with diabetes than those without diabetes when combining all three centers (P < 0.001 in both men and women). CHD rates were significantly higher in North and South Dakota than in the other two centers in men and women and in those with and without diabetes. Age- and sex-adjusted incidence rates of CHD in Arizona, Oklahoma, and North and South Dakota were 22.2, 26.5, and 40.2 per 1000 person-years for participants with diabetes and 6.5, 9.3, and 17.7 per 1000 person-years for participants without diabetes, respectively. Combining all three centers, age- and sex-adjusted incidence rates of CHD were 27.5 and 12.1 per 1000 person-years in those with and without diabetes, respectively.
Table 2.
Age- and sex-specific incidence rates, age-adjusted rates (95% CI), and age- and sex-adjusted rates (95% CI) (per 1000 person-years) of CHD by diabetic status and study center in 3563 American Indians, ages 45–74 yr (the SHS)
| Arizona |
Oklahoma |
North/South Dakota |
All centers |
|||||
|---|---|---|---|---|---|---|---|---|
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |
| Diabetes | ||||||||
| Men | ||||||||
| 45–54 yr | 41 | 20.5 | 29 | 27.5 | 34 | 37.9 | 104 | 26.4 |
| 55–64 yr | 16 | 19.5 | 28 | 40.1 | 28 | 52.3 | 72 | 35.1 |
| 65–74 yr | 12 | 33.6 | 17 | 46.1 | 10 | 75.8 | 39 | 45.5 |
| Total | 69 | 21.7 | 74 | 34.9 | 72 | 46.0 | 215 | 31.4 |
| Age-adjusted rate (95% CI) | 23.6 (20.8–26.5) | 36.6 (32.3–40.8) | 52.6 (46.4–58.8) | 34.2 (31.9–36.6) | ||||
| Women | ||||||||
| 45–54 yr | 58 | 14.5 | 29 | 19.0 | 40 | 29.9 | 127 | 18.5 |
| 55–64 yr | 58 | 23.5 | 27 | 18.4 | 47 | 36.9 | 132 | 25.4 |
| 65–74 yr | 26 | 28.4 | 23 | 27.8 | 22 | 41.8 | 71 | 31.3 |
| Total | 142 | 19.2 | 79 | 20.7 | 109 | 34.7 | 330 | 23.0 |
| Age-adjusted rate (95% CI) | 21.6 (19.8–23.4) | 21.4 (19.0–23.8) | 35.8 (32.3–39.2) | 24.5 (23.2–25.9) | ||||
| Men and women | ||||||||
| 45–54 yr | 99 | 16.5 | 58 | 22.5 | 74 | 33.1 | 231 | 21.4 |
| 55–64 yr | 74 | 22.5 | 55 | 25.4 | 75 | 41.5 | 204 | 28.1 |
| 65–74 yr | 38 | 29.8 | 40 | 33.4 | 32 | 48.6 | 110 | 35.2 |
| Total | 211 | 20.0 | 153 | 25.8 | 181 | 38.5 | 545 | 25.7 |
| Age- and sex-adjusted rate (95% CI) | 22.2 (20.7–23.8) | 26.5 (24.4–28.7) | 40.2 (37.2–43.2) | 27.5 (26.3–28.6) | ||||
| Nondiabetes | ||||||||
| Men | ||||||||
| 45–54 yr | 4 | 3.8 | 12 | 5.3 | 37 | 17.5 | 53 | 9.8 |
| 55–64 yr | 5 | 12.6 | 17 | 17.8 | 26 | 20.9 | 48 | 18.5 |
| 65–74 yr | 5 | 21.4 | 10 | 24.9 | 21 | 40.9 | 36 | 31.3 |
| Total | 14 | 8.4 | 39 | 10.7 | 84 | 21.7 | 137 | 14.9 |
| Age-adjusted rate (95% CI) | 11.3 (8.3–14.4) | 14.5 (12.2–16.9) | 24.7 (22.0–27.4) | 18.3 (16.7–19.8) | ||||
| Women | ||||||||
| 45–54 yr | 3 | 1.9 | 9 | 3.1 | 18 | 5.7 | 30 | 4.0 |
| 55–64 yr | 2 | 2.6 | 12 | 6.1 | 16 | 11.3 | 30 | 7.2 |
| 65–74 yr | 1 | 2.4 | 5 | 5.0 | 13 | 19.1 | 19 | 9.1 |
| Total | 6 | 2.2 | 26 | 4.5 | 47 | 9.0 | 79 | 5.7 |
| Age-adjusted rate (95% CI) | 2.3 (1.4–3.2) | 4.7 (3.7–5.6) | 11.5 (9.8–13.2) | 6.5 (5.8–7.3) | ||||
| Men and women | ||||||||
| 45–54 yr | 7 | 2.7 | 21 | 4.1 | 55 | 10.5 | 83 | 6.4 |
| 55–64 yr | 7 | 6.0 | 29 | 9.9 | 42 | 15.8 | 78 | 11.5 |
| 65–74 yr | 6 | 9.2 | 15 | 10.7 | 34 | 28.5 | 55 | 17.0 |
| Total | 20 | 4.5 | 65 | 6.9 | 131 | 14.4 | 216 | 9.4 |
| Age- and sex-adjusted rate (95% CI) | 6.5 (5.1–8.0) | 9.3 (8.1–10.5) | 17.7 (15.9–19.7) | 12.1 (11.2–12.9) | ||||
Risk factors for CHD in persons with and without diabetes
In the Cox proportional hazards models, interactions between sex and each risk factor were not statistically significant in diabetic or nondiabetic participants; thus, the models were not stratified by sex. The proportionality assumption of the Cox models was verified by the lack of significant interactions for any covariate with survival time. In multivariate Cox proportional hazards models with a backward covariate selection procedure adjusting for center (Table 3), older age, male sex, prehypertension, hypertension, lower HDL-C, higher LDL-C, current smoking, macroalbuminuria, lower eGFR, use of oral hypoglycemic agent and/or insulin, and longer diabetes duration were each identified as having independent and significant association with development of CHD in diabetic participants. In nondiabetic participants, older age, male sex, higher BMI, prehypertension, hypertension, and higher LDL-C were each independently and significantly associated with CHD. The strength of the association of CHD with male sex was greater in those without diabetes than in those with diabetes (P = 0.014). Among diabetic participants, when SBP and use of antihypertensive medication were included in the full model instead of BP by category (normal, prehypertension, hypertension), SBP was a significant predictor of incident CHD [for each 5 mm Hg increase in SBP, risk of developing CHD increased by 5% (HR = 1.05; 95% CI, 1.02–1.07; P < 0.001)] independent of other risk factors. Among nondiabetic participants, in a similar model, SBP also significantly predicted incident CHD (per 5 mm Hg, HR = 1.04; 95% CI, 1.00–1.08; P = 0.049), as did antihypertensive treatment (HR = 1.62; 95% CI, 1.14–2.30; P = 0.007), independent of other risk factors. When non-HDL-C was included in the full model instead of LDL-C, non-HDL-C significantly predicted incident CHD (per 10 mg/dl, HR = 1.04; 95% CI, 1.02–1.05; P < 0.001 for diabetes; and HR = 1.03; 95% CI, 1.01–1.04; P = 0.004 for nondiabetes), independent of other risk factors.
Table 3.
Multivariate risk factor selection results for CHD in 3563 American Indians, ages 45–74 yr, by diabetic status (the SHS)a
| With diabetes |
Without diabetes |
Comparison between diabetic and nondiabetic groups |
||||
|---|---|---|---|---|---|---|
| HRD (95% CI) | P value | HRN (95% CI) | P value | HRN/HRD ratio (95% CI) | P value | |
| Age (per 5 yr) | 1.16 (1.08–1.23) | <0.001 | 1.31 (1.20–1.42) | <0.001 | 1.07 (1.00–1.15) | 0.063 |
| Male sex | 1.58 (1.28–1.95) | <0.001 | 2.93 (2.19–3.93) | <0.001 | 1.54 (1.09–2.17) | 0.014 |
| North/South Dakota vs. Arizona and Oklahoma | 1.91 (1.54–2.38) | <0.001 | 2.56 (1.93–3.40) | <0.001 | 1.26 (0.88–1.79) | 0.208 |
| BMI (kg/m2) | 1.05 (1.02–1.08) | <0.001 | ||||
| Prehypertension | 1.48 (1.11–1.98) | 0.008 | 1.61 (1.13–2.29) | 0.009 | 1.06 (0.67–1.66) | 0.815 |
| Hypertension | 1.53 (1.16–2.03) | 0.003 | 1.90 (1.32–2.74) | 0.001 | 1.32 (0.84–2.09) | 0.228 |
| HDL–C (per 5 mg/dl) | 0.95 (0.91–0.99) | 0.040 | ||||
| LDL–C (per 10 mg/dl) | 1.07 (1.04–1.11) | <0.001 | 1.07 (1.03–1.12) | 0.001 | 1.04 (1.00–1.09) | 0.062 |
| Current smoking | 1.48 (1.20–1.82) | <0.001 | ||||
| Macroalbuminuria | 1.54 (1.20–1.98) | 0.001 | ||||
| eGFR (per 5 ml/min/1.73 m2) | 0.97 (0.94–0.99) | 0.018 | ||||
| Oral hypoglycemic agent alone | 1.52 (1.16–2.00) | 0.003 | ||||
| Insulin treatment | 1.84 (1.34–2.52) | <0.001 | ||||
| Diabetes duration (5–10 yr) | 1.93 (1.44–2.57) | <0.001 | ||||
| Diabetes duration >10 yr | 1.79 (1.37–2.35) | <0.001 | ||||
HRD, HR in diabetic participants; HRN, HR in nondiabetic participants.
The variables in the Cox proportional hazards models were selected by backward selection. The full model included age, BMI, study center (Arizona, Oklahoma, North/South Dakota), education (less than high school and at least high school), LDL-C, HDL-C, triglycerides, fasting glucose, BP (normal, prehypertension, hypertension), albuminuria (normal, microalbuminuria, macroalbuminuria), eGFR, smoking (never, past, current), and physical activity. Diabetes treatment and diabetes duration were added to the model for the group with diabetes.
Discussion
This is the first study comparing incidence rates and risk factors for CHD in nondiabetic vs. diabetic persons in a single population with high prevalence of diabetes. CHD incidence was significantly higher in individuals with diabetes; rates of CHD were lower in Arizona and higher in North and South Dakota for both the nondiabetic and diabetic groups. Some risk factors for CHD were similar between the groups, including older age, male sex, prehypertension or hypertension, and higher LDL-C. Other risk factors were particular to the specific group, including lower HDL-C, current smoking, macroalbuminuria, lower eGFR, use of diabetes medication, and longer duration of diabetes for the diabetic group and higher BMI for the nondiabetic group. The association with male sex was stronger in those without diabetes.
It is difficult to compare CHD incidence rates among studies of different populations. To our knowledge, the Atherosclerosis Risk in Communities (ARIC) Study, which included population-based samples of blacks and whites (22), is the study best suited to comparisons with our data, because of its use of comparable methods for determining rates of CHD. A comparison of CHD incidence rates for diabetic and nondiabetic women and men, ages 45–64 yr, in the two studies is shown (Fig. 1). CHD incidence rates in diabetic and nondiabetic American Indian men were similar to those of men in the ARIC population (P = 0.47 and 0.23, respectively). In contrast, both diabetic and nondiabetic American Indian women had significantly higher CHD incidence rates compared with those of women in the ARIC population (P < 0.001 and P = 0.004, respectively). One explanation for the higher CHD rates in nondiabetic American Indian women may be the high level of obesity and insulin resistance in the SHS population; this reasoning is supported by the observation that BMI is an independent risk factor in nondiabetic SHS participants. The higher rate of CHD in the diabetic American Indian women indicates that diabetes may have a greater adverse effect on CVD in women in this population. This finding may be related to the lower age of onset of diabetes or to the poorer glycemic control (23, 24).
Fig. 1.
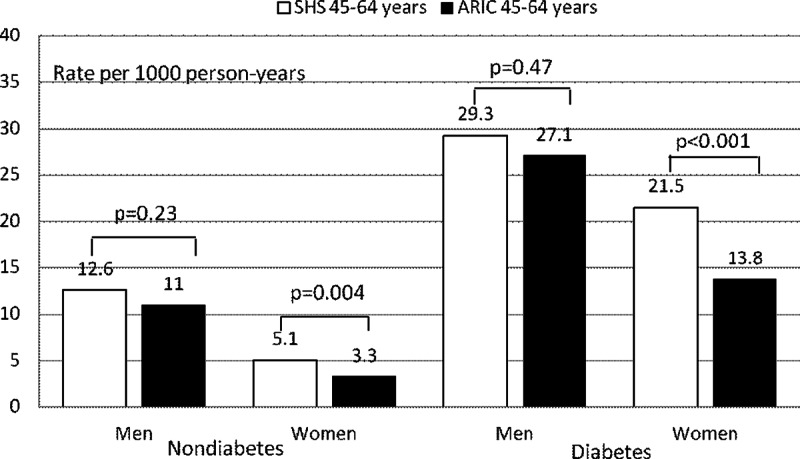
Crude incidence rates (per 1000 person-years) of CHD (fatal and nonfatal) in diabetic and nondiabetic American Indians, ages 45–64 yr, in the SHS compared with those in the same age group from the AIRC Study (22). Average follow-up was 11 yr.
BMI was a significant independent predictor of CHD in nondiabetic individuals. This finding contrasts with evidence that BMI has not been an independent risk predictor in most other populations (25–27). However, our population now represents many groups in whom obesity and diabetes rates are rapidly increasing. Interestingly, obesity rates are highest in Arizona, which has the lowest rates of CHD. Considering the high prevalence of metabolic syndrome in the SHS population, obesity in the nondiabetic group may be an indicator of insulin resistance. Thus, the influence of obesity may be strong in the other two centers (P = 0.063 for interaction between center and BMI) or BMI may parallel other lifestyle factors in this population that predispose to CHD.
No association was observed between eGFR or microalbuminuria and development of CHD in nondiabetic men and women, either in univariate or multivariate models, whereas renal disease strongly predicted CHD in those with diabetes (28, 29). Although the few individuals (n = 36) with macroalbuminuria were eliminated to avoid inclusion of undiagnosed diabetes in the nondiabetic group, a substantial number (n = 139) had microalbuminuria; thus, any association should have been detected. It has been proposed that a significant increase in CHD events in American Indians can be attributed to the progression of diabetic nephropathy to end-stage renal disease (30). The measures of renal function were not independently associated with kidney disease in the nondiabetic individuals in the current study. It is not clear why smoking was not independently associated with CHD in those without diabetes. Smoking rates are low in this population, and the number of cigarettes used per day is low; thus, the association may not have been strong enough to reach significance in the nondiabetic group, which had lower numbers of cases.
In addition to hypertension as an independent risk factor, we found that prehypertension defined by JNC 7 (The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) criteria significantly predicted incident CHD in both nondiabetic and diabetic participants, independent of other risk factors. Nondiabetic and diabetic participants with prehypertension had 65 and 48% higher risk of developing CHD than their counterparts with normal BP. Prehypertension was a significant risk factor for CHD in participants free from hypertension and CVD at baseline in both the SHS (31) and the Framingham Heart Study (32).
LDL-C was an independent risk factor for CHD in the nondiabetic and diabetic participants. It was a stronger predictor (as shown by a higher slope) of CHD in SHS participants than it is in conventional risk calculators (33). In the current study, for every 10 mg/dl increase in LDL-C, risk of CHD was significantly increased (by 7%) in the diabetic group. This finding can be explained by the fact that persons with diabetes have more triglyceride-rich lipoproteins with smaller and more numerous LDL particles (34). The Adult Treatment Panel III, the American Diabetes Association, and the American College of Cardiology Foundation reached a consensus that non-HDL-C is a better marker than LDL-C for identifying patients at high risk for CHD (35). The results of the current study show that non-HDL-C is a strong marker in those with and without diabetes. For every 10 mg/dl increase in non-HDL-C, CHD risk increased by 3% in the nondiabetic group and by 4% in the diabetic group.
American Indians with and without diabetes in North and South Dakota tended to have the highest rates of CHD, whereas the lowest rates were found in Arizona. The reasons for these center differences are not apparent; however, a number of factors exist, including differences in cultural backgrounds, historical circumstances, and socioeconomic conditions (36). Tribes in the three areas differ both genetically and culturally, but no readily available covariates reflect these differences. Levels of LDL-C and smoking were higher in North and South Dakota, and either of these factors could explain the regional difference. Rates of hypertension did not differ by center (37). Similar variations between American Indians in North and South Dakota and Arizona have been noted in previous SHS analyses (38, 39). Further examination of such regional differences, over more time, will provide additional insight into the pathophysiological processes and is likely to have practical importance for those designing health-care programs.
The strengths of the present study are its direct comparison of participants with and without diabetes within the same populations and communities, use of a common protocol and identical prospective design, large sample size, long and continuous surveillance with almost no loss to follow-up, and standardized ascertainment and confirmation of prevalent and incident diabetes and CHD events. This study was limited by the wide CI for CHD rates among nondiabetic women in Arizona, due to the small number of events in this group. This study was further limited because the nondiabetic group may have included some persons with undiagnosed diabetes not detectable by chart review. Because glucose screening is common in this population, however, undiagnosed diabetes is not common among the older adults who comprise this cohort. An additional limitation is the exclusion of nondiabetic participants with macroalbuminuria, who were eliminated to lower the possibility of inclusion of undiagnosed diabetic persons in the nondiabetic group.
In conclusion, in this population with a high prevalence of diabetes, CHD rates were significantly lower in those without diabetes, and risk factors differed for diabetic and nondiabetic individuals. Higher BMI was an independent determinant of CHD only in those without diabetes, whereas measures of renal function, smoking, and low HDL-C were independent determinants only in those with diabetes. For risk factors common to both groups, male sex was a significantly stronger risk factor in those without diabetes. These data suggest that equations estimating CHD risk should be developed separately for those with and without diabetes; risk factor management programs should recognize the enhanced potential for CHD in obese persons, even in those without diabetes, and should focus on management of BP and LDL-C as well as weight loss.
Acknowledgments
The authors thank the Indian Health Service hospitals and clinics at each center; the directors of the Strong Heart Study clinics—Dr. Darren Calhoun (Medstar Health Research Institute), Dr. Tauqeer Ali (University of Oklahoma), and Marcia O'Leary (Missouri Breaks Industries Research Inc.), and their staffs; and the physicians who performed the mortality and morbidity reviews. We also thank Rachel Schaperow (MedStar Health Research Institute) for editing the manuscript.
This work was supported by Cooperative Agreement Grants U01-HL-41642, HL-41652, HL-41654, and HL-65521 from the National Heart, Lung, and Blood Institute and, in part, by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. This project has been funded in whole or in part with federal funds (Grant UL1RR031975) from the National Center for Research Resources, and the National Center for Advancing Translational Sciences, National Institutes of Health, through the Clinical and Translational Science Awards Program, a trademark of the Department of Health and Human Services, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise.”
Disclosure Summary: B.V.H. has served on the advisory boards of Merck/Schering-Plough and has received research support in the form of drug donations from Merck/Schering-Plough. The other authors have nothing to declare.
Footnotes
- BMI
- Body mass index
- BP
- blood pressure
- CHD
- coronary heart disease
- CI
- confidence interval
- CVD
- cardiovascular disease
- DBP
- diastolic BP
- eGFR
- estimated glomerular filtration rate
- HDL
- high-density lipoprotein
- HDL-C
- HDL cholesterol
- HR
- hazard ratio
- IQR
- interquartile range
- LDL
- low-density lipoprotein
- LDL-C
- LDL cholesterol
- MI
- myocardial infarction
- SBP
- systolic BP
- SHS
- Strong Heart Study
- UACR
- urinary albumin/creatinine ratio.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. 2012. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125:e2–e220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klain M, Coulehan JL, Arena VC, Janett R. 1988. More frequent diagnosis of acute myocardial infarction among Navajo Indians. Am J Public Health 78:1351–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson RG, Sievers ML, Knowler WC, Swinburn BA, Pettitt DJ, Saad MF, Liebow IM, Howard BV, Bennett PH. 1990. Low incidence of fatal coronary heart disease in Pima Indians despite high prevalence of non-insulin-dependent diabetes. Circulation 81:987–995 [DOI] [PubMed] [Google Scholar]
- 4. Sievers ML, Fisher KR. 1979. Increasing rates of acute myocardial infarction in southwestern American Indians. Ariz Med 39:739–742 [PubMed] [Google Scholar]
- 5. Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, Howard WJ, Rhoades ER, Robbins DC, Sievers ML, Welty TK. 1999. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation 99:2389–2395 [DOI] [PubMed] [Google Scholar]
- 6. Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. 1990. The Strong Heart Study—a study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol 132:1141–1155 [DOI] [PubMed] [Google Scholar]
- 7. Jones DW, Chambless LE, Folsom AR, Heiss G, Hutchinson RG, Sharrett AR, Szklo M, Taylor HA., Jr 2002. Risk factors for coronary heart disease in African Americans: the Atherosclerosis Risk in Communities Study, 1987–1997. Arch Intern Med 162:2565–2571 [DOI] [PubMed] [Google Scholar]
- 8. Sekikawa A, Satoh T, Hayakawa T, Ueshima H, Kuller LH. 2001. Coronary heart disease mortality among men aged 35–44 years by prefecture in Japan in 1995–1999 compared with that among white men aged 35–44 by state in the United States in 1995–1998: vital statistics data in recent birth cohort. Jpn Circ J 65:887–892 [DOI] [PubMed] [Google Scholar]
- 9. Zhang XH, Lu ZL, Liu L. 2008. Coronary heart disease in China. Heart 94:1126–1131 [DOI] [PubMed] [Google Scholar]
- 10. Chasson AL, Grady HJ, Stanley MA. 1961. Determination of creatinine by means of automatic chemical analysis. Am J Clin Pathol 35:83–88 [PubMed] [Google Scholar]
- 11. Yurgalevitch SM, Kriska AM, Welty TK, Go O, Robbins DC, Howard BV. 1998. Physical activity and lipids and lipoproteins in American Indians ages 45–74. Med Sci Sports Exerc 30:543–549 [DOI] [PubMed] [Google Scholar]
- 12. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. 2003. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42:1206–1252 [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association 2010. Standards of medical care in diabetes–2010. Diabetes Care 33(Suppl 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. 2002. K/DOQI clinical practice guidelines for chronic kidney disease. Evaluation, classification, and stratification. Am J Kidney Dis 39(Suppl 1):S1–S266 [PubMed] [Google Scholar]
- 15. Lee ET, Cowan LD, Welty TK, Sievers M, Howard WJ, Oopik A, Wang W, Yeh J, Devereux RB, Rhoades ER, Fabsitz RR, Go O, Howard BV. 1998. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45–74 years, 1984–1988. The Strong Heart Study. Am J Epidemiol 147:995–1008 [DOI] [PubMed] [Google Scholar]
- 16. The National Heart, Lung and Blood Institute 1989. The Strong Heart Study Coordinating Center. Strong Heart Study operation manual. Oklahoma City, OK: University of Oklahoma Health Sciences Center [Google Scholar]
- 17. 1990. Census of Population, General Population Characteristics, United States. http://www.census.gov/prod/cen1990/cp1/cp-1-1.pdf
- 18. Rothman KJ, Greenland S, Lash TL. 2008. Modern epidemiology. 3rd ed Philadelphia: Lippincott Williams & Wilkins [Google Scholar]
- 19. Cox DR. 1972. Regression models and life tables. J R Stat Soc B 34:187–220 [Google Scholar]
- 20. Hoffmann K, Pischon T, Schulz M, Schulze MB, Ray J, Boeing H. 2008. A statistical test for the equality of differently adjusted incidence rate ratios. Am J Epidemiol 167:517–522 [DOI] [PubMed] [Google Scholar]
- 21. StataCorp 2009. Statistical software: release 10. College Station, TX: StataCorp LP [Google Scholar]
- 22. Folsom AR, Chambless LE, Duncan BB, Gilbert AC, Pankow JS. 2003. Prediction of coronary heart disease in middle-aged adults with diabetes. Diabetes Care 26:2777–2784 [DOI] [PubMed] [Google Scholar]
- 23. Hu D, Henderson JA, Welty TK, Lee ET, Jablonski KA, Magee MF, Robbins DC, Howard BV. 1999. Glycemic control in diabetic American Indians. Longitudinal data from the Strong Heart Study. Diabetes Care 22:1802–1807 [DOI] [PubMed] [Google Scholar]
- 24. Lee ET, Howard BV, Go O, Savage PJ, Fabsitz RR, Robbins DC, Welty TK. 2000. Prevalence of undiagnosed diabetes in three American Indian populations. A comparison of the 1997 American Diabetes Association diagnostic criteria and the 1985 World Health Organization diagnostic criteria: the Strong Heart Study. Diabetes Care 23:181–186 [DOI] [PubMed] [Google Scholar]
- 25. Eckel RH. 1997. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation 96:3248–3250 [DOI] [PubMed] [Google Scholar]
- 26. Pettitt DJ, Lisse JR, Knowler WC, Bennett PH. 1982. Mortality as a function of obesity and diabetes mellitus. Am J Epidemiol 115:359–366 [DOI] [PubMed] [Google Scholar]
- 27. Stevens J, Keil JE, Rust PF, Tyroler HA, Davis CE, Gazes PC. 1992. Body mass index and body girths as predictors of mortality in black and white women. Arch Intern Med 152:1257–1262 [PubMed] [Google Scholar]
- 28. Xu J, Knowler WC, Devereux RB, Yeh J, Umans JG, Begum M, Fabsitz RR, Lee ET. 2007. Albuminuria within the “normal” range and risk of cardiovascular disease and death in American Indians: the Strong Heart Study. Am J Kidney Dis 49:208–216 [DOI] [PubMed] [Google Scholar]
- 29. Shara NM, Resnick HE, Lu L, Xu J, Vupputuri S, Howard BV, Umans JG. 2009. Decreased GFR estimated by MDRD or Cockcroft-Gault equation predicts incident CVD: the Strong Heart Study. J Nephrol 22:373–380 [PMC free article] [PubMed] [Google Scholar]
- 30. Pavkov ME, Bennett PH, Sievers ML, Krakoff J, Williams DE, Knowler WC, Nelson RG. 2005. Predominant effect of kidney disease on mortality in Pima Indians with or without type 2 diabetes. Kidney Int 68:1267–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, Lee ET, Devereux RB, Yeh J, Best LG, Fabsitz RR, Howard BV. 2006. Prehypertension, diabetes, and cardiovascular disease risk in a population-based sample: the Strong Heart Study. Hypertension 47:410–414 [DOI] [PubMed] [Google Scholar]
- 32. Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. 2001. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 345:1291–1297 [DOI] [PubMed] [Google Scholar]
- 33. Lee ET, Howard BV, Wang W, Welty TK, Galloway JM, Best LG, Fabsitz RR, Zhang Y, Yeh J, Devereux RB. 2006. Prediction of coronary heart disease in a population with high prevalence of diabetes and albuminuria: the Strong Heart Study. Circulation 113:2897–2905 [DOI] [PubMed] [Google Scholar]
- 34. Gray RS, Robbins DC, Wang W, Yeh JL, Fabsitz RR, Cowan LD, Welty TK, Lee ET, Krauss RM, Howard BV. 1997. Relation of LDL size to the insulin resistance syndrome and coronary heart disease in American Indians. The Strong Heart Study. Arterioscler Thromb Vasc Biol 17:2713–2720 [DOI] [PubMed] [Google Scholar]
- 35. Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. 2008. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 31:811–822 [DOI] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention 2000. Prevalence of selected cardiovascular disease risk factors among American Indians and Alaska Natives-United States. MMWR Morb Mortal Wkly Rep 49:461–465 [PubMed] [Google Scholar]
- 37. Welty TK, Lee ET, Yeh J, Cowan LD, Go O, Fabsitz RR, Le NA, Oopik AJ, Robbins DC, Howard BV. 1995. Cardiovascular disease risk factors among American Indians. The Strong Heart Study. Am J Epidemiol 142:269–287 [DOI] [PubMed] [Google Scholar]
- 38. Devereux RB, Roman MJ, O'Grady MJ, Fabsitz RR, Rhoades ER, Crawford A, Howard BV, Lee ET, Welty TK. 2000. Differences in echocardiographic findings and systemic hemodynamics among non-diabetic American Indians in different regions: The Strong Heart Study. Ann Epidemiol 10:324–332 [DOI] [PubMed] [Google Scholar]
- 39. Welty TK, Rhoades DA, Yeh F, Lee ET, Cowan LD, Fabsitz RR, Robbins DC, Devereux RB, Henderson JA, Howard BV. 2002. Changes in cardiovascular disease risk factors among American Indians. The Strong Heart Study. Ann Epidemiol 12:97–106 [DOI] [PubMed] [Google Scholar]


