Abstract
Purpose:
Children with acute lymphoblastic leukemia (ALL) are at risk for impaired bone accrual. This peripheral quantitative computed tomography study assessed changes in bone mineral density (BMD) and structure after completion of ALL treatment.
Methods:
Fifty ALL participants, ages 5–22 yr, were enrolled within 2 yr (median 0.8 yr) after completing ALL therapy. Tibia peripheral quantitative computed tomography scans were performed at enrollment and 12 months later. Age-, sex-, and race-specific Z-scores for trabecular BMD (TrabBMD), cortical BMD (CortBMD), and cortical area (CortArea) were generated based on more than 650 reference participants. Multivariable linear regression models examined determinants of changes in Z-scores.
Results:
At enrollment, mean TrabBMD (−1.03 ± 1.34) and CortBMD (−0.84 ± 1.05) Z-scores were low (both P < 0.001) compared with reference participants. TrabBMD and CortBMD Z-scores increased to −0.58 ± 1.41 and −0.51 ± 0.91 over 1 yr, respectively (both P < 0.001). Changes in cortical outcomes varied according to the interval since completion of therapy. Among those enrolled less than 6 months after therapy, CortArea Z-scores increased and CortBMD Z-scores decreased (both P < 0.01). Among those enrolled 6 months or more after therapy, CortArea Z-scores did not change and CortBMD Z-scores increased (P < 0.01). Changes in CortArea and CortBMD Z-scores were inversely associated (r = −0.32, P < 0.001). Cumulative glucocorticoid exposure, leukemia risk status, and antimetabolite chemotherapy were not associated with outcomes.
Conclusion:
TrabBMD was low after completion of ALL therapy and improved significantly. Early increases in cortical dimensions were associated with declines in CortBMD; however, participants further from ALL therapy demonstrated stable cortical dimensions and increases in CortBMD, potentially reflecting the time necessary to mineralize newly formed bone.
Skeletal development in childhood is characterized by sex-, race-, and maturation-specific increases in trabecular and cortical bone mineral density (BMD) and cortical dimensions (1). Acute lymphoblastic leukemia (ALL) is the most common malignancy of childhood with survival rates exceeding 80% (2). ALL is associated with numerous risk factors for poor bone acquisition including the underlying disease (3), malnutrition (4), physical inactivity (5), reduced muscle strength (6), chemotherapy (7), radiation exposure (8), and endocrinopathies (9). Glucocorticoid and methotrexate therapy compound these effects (10). Bone metabolism in ALL has not been well characterized; however, small studies suggest reduced bone formation and variable alterations in bone resorption (11). The impact of abnormal bone accrual in ALL may be immediate, resulting in fragility fractures during treatment (12), or delayed due to suboptimal peak bone mass acquisition.
Previous dual-energy x-ray absorptiometry (DXA)-based studies documented bone deficits in childhood ALL (13–17). However, DXA is a two-dimensional projection technique that does not provide discrete measures of trabecular and cortical bone. In contrast, quantitative computed tomography (QCT) provides three-dimensional measures of trabecular and cortical volumetric BMD and geometry. Appendicular peripheral QCT (pQCT) measures are highly correlated with in vitro fracture load (18). The three previous QCT studies in ALL survivors included children with a history of cranial radiation (19, 20) at a widely variable interval off therapy (21).
This prospective pQCT study describes a cohort of children and young adults without a history of cranial radiation enrolled within 2 yr of completion of ALL therapy and followed for an additional year. The objectives were to characterize bone deficits compared with a large reference population and to assess the potential for recovery in survivors.
Patients and Methods
Study participants
Children and young adults, ages 5–25 yr, diagnosed and treated with ALL at the Children's Hospital of Philadelphia (CHOP) were eligible for participation if in complete remission. Exclusion criteria included cranial radiation, second malignancy, and Down's syndrome. Baseline study visits were completed within 24 months after completion of maintenance chemotherapy with a subsequent 12-month follow-up study visit. We established contact with a total of 86 eligible and enrolled 50 (58%) of these patients. Participant demographics and ALL disease characteristics did not differ between eligible patients who declined participation and those who enrolled. ALL participants were compared with 894 healthy reference participants at CHOP, ages 5–25 yr, as previously described (23–25). The study protocol was approved by the Institutional Review Board at CHOP. Informed consent was obtained directly from study participants at least 18 yr of age and assent along with parental consent from participants less than 18 yr of age.
Anthropometry and physical maturation
Weight was measured with a digital scale (Scaletronix, White Plains, NY) and height with a stadiometer (Holtain, Crymych, UK). Pubertal stage was determined according to the method of Tanner by pediatric endocrinologists (S.M.M. and J.B.) in the ALL participants and using a validated self-assessment questionnaire in reference participants (26). Tibia length was measured from the distal margin of the medial malleolus to the proximal border of the medial tibial condyle.
ALL disease and treatment characteristics
ALL participants received chemotherapy per the Children's Oncology Group ALL Consortium protocols. Medical charts were reviewed for date of diagnosis, leukemia risk group (standard vs. high risk) (27), duration of chemotherapy, and time since completion of chemotherapy. Cumulative (grams per square meter) glucocorticoid (prednisone and/or dexamethasone), methotrexate, and 6-mercaptopurine exposure over the treatment interval were tabulated. Medical (including endocrine abnormalities) and fracture history as well as calcium and vitamin D supplements were reviewed at each visit.
pQCT assessment
Tibia pQCT scans were obtained at 3, 38, and 66% of the tibia length proximal to the distal growth plate, as previously described (1, 24). Trabecular BMD was assessed at the 3% metaphyseal site, and cortical BMD (milligrams per cubic centimeter) and dimensions [periosteal and endosteal circumference (millimeters), cortical cross-sectional area (CSA) (square millimeters)] at the 38% diaphyseal sites. Cortical CSA is strongly associated with bone failure load (r = 0.71) (28). Muscle and fat CSA (square millimeters) were evaluated at the 66% site. The manufacturer's hydroxyapatite phantom was scanned daily. In our laboratory, the coefficient of variation for short-term precision ranged from 0.5–1.6% for pQCT outcomes in children and young adults.
Laboratory studies
Nonfasting blood samples were collected in healthy reference participants. Serum 25-hydroxyvitamin D [25(OH)D] concentration was analyzed using 125I-labeled RIA (Diasorin Corp., Stillwater MN) with an intraassay coefficient of variation of 2.2% (29).
Statistical analysis
Stata version 12.0 (Stata Corp., College Station, TX) was used for all analyses. A P value <0.05 was considered significant, and two-sided tests of hypotheses were used throughout. Group differences between ALL and healthy reference participants were tested using the t test or Wilcoxon rank sum test if indicated. Correlations between continuous variables were assessed by Pearson or Spearman's rank correlations, where appropriate.
Age- and sex-specific Z-scores (sd scores) for height and body mass index (BMI) were calculated using National Center for Health Statistics data (30). The pQCT outcomes were converted to Z-scores relative to age (24, 31, 32). All pQCT Z-scores were sex and race specific (Black vs. all others), and were generated using the LMS Chartmaker Program (Medical Research Council, United Kingdom) in the reference participants as previously described (24, 33). The LMS (power for the Box-Cox transformation, median, sd) method accounts for the nonlinearity, heteroscedasticity, and skew of bone data in growing children. The pQCT cortical geometry, muscle, and fat outcomes were highly correlated with tibia length (all P < 0.0001). Z-scores at these sites were subsequently adjusted for tibia length for age Z-scores based on the method of Zemel et al. (34).
Z-scores in ALL vs. reference participants were compared using t test or Wilcoxon rank sum test as indicated. To determine whether differences in cortical area Z-scores between ALL and reference participants were explained by differences in muscle CSA (i.e. the functional muscle-bone unit) (35), cortical CSA models were adjusted for muscle CSA.
The pQCT Z-score models within the ALL participants examined associations with demographics, disease characteristics (leukemia risk status, age at diagnosis, and duration of treatment), medications (cumulative glucocorticoids and antimetabolite chemotherapy), and interval since completion of therapy.
Changes in pQCT Z-scores within ALL participants over 12 months were assessed using a paired t test. The linear regression models for changes in Z-scores within ALL participants examined the impact of baseline Z-score, age, maturation, and interval since completion of ALL therapy at enrollment. Models examining changes in cortical BMD Z-scores were further adjusted for changes in cortical CSA Z-scores because newly formed bone may demonstrate delayed mineralization and lower cortical BMD (36).
Values for cumulative glucocorticoid exposure (grams per square meter) during ALL treatment were natural log transformed to achieve normal distributions. Vitamin D deficiency was defined as 25(OH)D level below 20 ng/ml (37). Multivariate logistic regression was used to examine the odds of vitamin D deficiency in ALL participants at each visit, compared with healthy reference participants, adjusted for age, race, winter season, and BMI (38).
Results
Participant and disease characteristics
A total of 50 participants with ALL, median age 7.9 (range 5.1–22.1) years, 19 (38%) male, and five (10%) Black, were enrolled (Table 1). These characteristics were consistent with ALL demographics (39). Participants were predominantly prepubertal (P < 0.001) with no delay in pubertal maturation.
Table 1.
Participant and disease characteristics at enrollment (n = 50)
| Value at enrollment | |
|---|---|
| Age [median (range) yr] | 7.9 (5.1–22.1) |
| Sex, male [n (%)] | 19 (38) |
| Race, Black [n (%)] | 5 (10) |
| Tanner stage [n (%)] | |
| Prepubertal (Tanner 1) | 32 (64) |
| Pubertal (Tanner 2–3) | 7 (14) |
| Postpubertal (Tanner 4–5) | 11 (22) |
| Age at leukemia diagnosis [median (range) yr] | 4.9 (1.4–19.7) |
| Duration of therapy [median (range) yr] | 2.3 (1.3–3.4) |
| Time since completion of therapy [median (range) yr] | 0.8 (0.0–2.2) |
| Diagnosis [n (%)] | |
| Precursor B ALL | 49 (98) |
| T-cell ALL | 1 (2) |
| Leukemia risk [n (%)] | |
| Standard | 33 (66) |
| High | 17 (34) |
| Cumulative Steroids [n (%), median (range)] | |
| Dexamethasone (g/m2) | 49 (98), 1.1 (0.1–1.6) |
| Prednisone (g/m2) | 15 (30), 4.3 (0.5–6.8) |
| Nonphalangeal fractures (n) | (10 fractures; 9 patients) |
| At leukemia diagnosis | 2 |
| During leukemia treatment | 7 |
| After leukemia treatment | 1 |
| Laboratory parameters [median (range)] | |
| Calcium (mg/dl) | 9.4 (8.9–10.2) |
| Intact PTH (pg/ml) | 29 (3–66) |
| 25(OH)D (ng/ml) | 30.9 (4.1–93.6) |
The majority of participants (98%) were diagnosed with precursor B cell ALL and classified as standard risk (66%). None had central nervous system leukemia or a diagnosis of endocrinopathies. All participants received glucocorticoids. Thirty-one participants (62%) received iv methotrexate at a median cumulative dose of 1 (0.2–21.1) g/m2, and all patients received oral methotrexate at 1.6 (0.6–2.8) g/m2. All but one participant received oral 6-mercaptopurine with a median cumulative dose of 47.3 (1.7–85.9) g/m2.
Forty-seven (94%) ALL participants completed the 12-month follow-up study visit. One experienced a bone marrow relapse, and two withdrew. The remainder had an uneventful medical course for the duration of study.
Growth
Height and BMI Z-scores
Height Z-scores were not significantly different in ALL participants at baseline compared with the reference group (ALL 0.43 ± 0.93 vs. reference participants 0.25 ± 0.92; P = 0.17). ALL participants had significantly higher baseline BMI Z-scores (0.97 ± 0.83 vs. 0.37 ± 1.00; P < 0.001) with no sex differences (P = 0.28). Greater glucocorticoid exposure was associated with higher BMI Z-scores in ALL participants at enrollment (P = 0.01) and follow-up (P = 0.02). The interval since completion of therapy was not associated with height or BMI Z-scores.
Musculoskeletal outcomes
Trabecular BMD
Mean trabecular BMD Z-scores were significantly lower in ALL participants at enrollment (Table 2), compared with reference participants. Z-scores were not associated with participant demographics, interval since completion of ALL therapy, or ALL treatment characteristics. Although trabecular BMD improved significantly over the study interval, Z-scores at the follow-up visit were lower than the reference group. In multivariable models of changes in trabecular BMD over the study interval, demographics, maturation, and interval since completion of ALL therapy at enrollment were not significant.
Table 2.
pQCT and anthropometric Z-scores at enrollment and 12 months in ALL participants
| Z-score |
||||||
|---|---|---|---|---|---|---|
| Baseline ALL vs. reference |
12-Month ALL vs. reference |
Changes within ALL (0–12 months) |
||||
| Mean ± sd | P value | Mean ± sd | P value | Mean (range) | P value | |
| Anthropometry | ||||||
| Height | 0.43 ± 0.93 | 0.17 | 0.42 ± 0.87 | 0.22 | 0.05 (−0.76–1.28) | 0.34 |
| BMI | 0.97 ± 0.83 | <0.001 | 0.89 ± 0.83 | <0.001 | −0.04 (−1.13–0.56) | 0.44 |
| BMD | ||||||
| Trabecular BMD | −1.03 ± 1.34 | <0.001 | −0.58 ± 1.41 | <0.001 | 0.41 (−1.03–2.30) | <0.001 |
| Cortical BMD | −0.84 ± 1.05 | <0.001 | −0.51 ± 0.91 | <0.001 | 0.26 (−2.66–1.80) | 0.06 |
| Cortical dimensions | ||||||
| Cortical area | 0.16 ± 1.18 | 0.23 | 0.63 ± 1.22 | <0.001 | 0.48 (−0.63–1.19) | <0.001 |
| Periosteal circ | 0.11 ± 0.92 | 0.36 | 0.45 ± 1.02 | <0.001 | 0.34 (−0.23–1.05) | <0.001 |
| Endosteal circ | −0.07 ± 0.95 | 0.64 | −0.11 ± 1.00 | 0.43 | −0.06 (−3.30–2.95) | 0.35 |
| Body composition | ||||||
| Muscle area | 0.24 ± 0.93 | 0.09 | 0.54 ± 0.92 | <0.001 | 0.27 (−0.62–1.70) | <0.001 |
| Fat area | 0.67 ± 1.00 | <0.001 | 0.51 ± 1.13 | <0.001 | −0.16 (−1.24–0.55) | <0.01 |
Cortical BMD
At enrollment, cortical BMD Z-scores were significantly lower in ALL participants. These deficits were not associated with demographics or treatment characteristics. However, a longer interval since completion of ALL therapy was associated with a lower cortical BMD Z-score at enrollment (r = −0.32; P = 0.03).
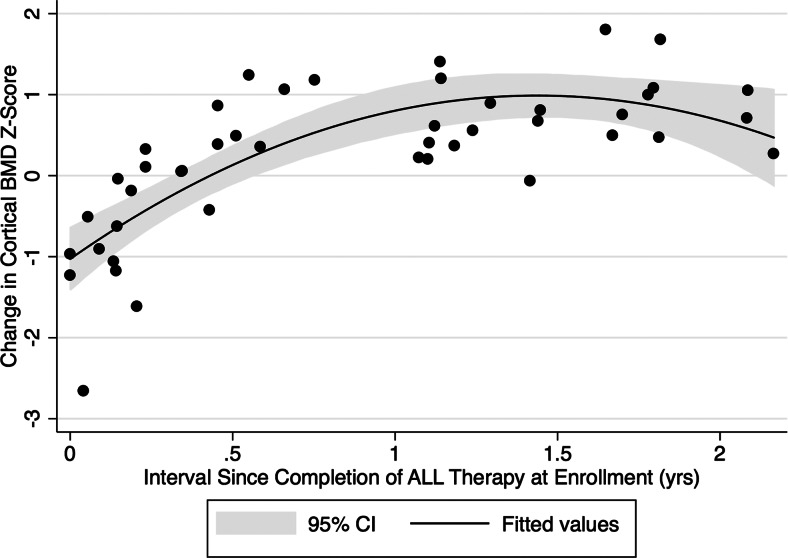
Although cortical BMD increased significantly over the study, it remained significantly lower at follow-up compared with the reference group. The change in cortical BMD Z-score over 12 months was significantly and positively associated with the interval since completion of therapy at enrollment (Fig. 1). Cortical BMD Z-scores decreased a mean of 0.54 during follow-up among those enrolled within 6 months of completing therapy, whereas cortical BMD Z-scores increased a mean of 0.77 during follow-up among those enrolled at least 6 months after completion of therapy. Therefore, data are presented stratified on early (<6 months) vs. late (≥6 months) enrollment (Table 3).
Fig. 1.
The change in cortical BMD (milligrams per cubic centimeter) Z-score over the 12-month study was significantly and positively associated with the interval since completion of ALL therapy at enrollment. Cortical BMD Z-scores decreased a mean of 0.54 during follow-up among those enrolled within 6 months of completing ALL therapy (P < 0.01) and increased a mean of 0.77 for those enrolled at least 6 months after completing ALL treatment (P < 0.01). CI, Confidence interval.
Table 3.
pQCT cortical Z-scores and changes in Z-scores from enrollment to 12 months in participants by interval since completion of ALL therapy
| Z-score |
P value | ||
|---|---|---|---|
| Early, n = 20 | Late, n = 29 | ||
| Cortical BMD | |||
| Baseline | −0.29 ± 0.93 (−2.22–1.24) | −1.17 ± 0.96 (−2.93–1.00) | <0.01 |
| Follow-up | −0.73 ± 0.87 (−2.51–0.70) | −0.36 ± 0.97 (−1.95–1.67) | 0.18 |
| Change | −0.54 ± 0.85 (−2.66–0.86) | 0.77 ± 0.46 (−0.07–1.80) | <0.001 |
| Cortical area | |||
| Baseline | 0.12 ± 0.81 (−0.94–1.86) | 0.26 ± 1.34 (−4.09–2.23) | 0.65 |
| Follow-up | 1.01 ± 0.96 (−0.90–2.39) | 0.54 ± 1.24 (−2.86–2.90) | 0.16 |
| Change | 0.82 ± 0.60 (−0.15–1.90) | 0.28 ± 0.51 (−0.63–1.26) | <0.01 |
| Periosteal circumference | |||
| Baseline | 0.04 ± 0.70 (−0.99–1.47) | 0.20 ± 1.04 (−1.46–2.01) | 0.56 |
| Follow-up | 0.53 ± 0.92 (−1.01–2.21) | 0.49 ± 1.06 (−1.27–2.62) | 0.91 |
| Change | 0.46 ± 0.39 (−0.14–1.05) | 0.27 ± 0.32 (−0.23–0.87) | 0.09 |
| Endosteal circumference | |||
| Baseline | −0.20 ± 0.89 (−2.46–1.25) | −0.01 ± 0.99 (−1.48–2.10) | 0.51 |
| Follow-up | −0.54 ± 0.81 (−2.61–0.71) | 0.11 ± 1.04 (−1.73–2.25) | 0.02 |
| Change | −0.61 ± 0.82 (−3.30–0.31) | −0.08 ± 1.02 (−2.78–2.95) | 0.06 |
| Muscle area | |||
| Baseline | 0.57 ± 0.82 (−0.72–2.30) | 0.11 ± 0.80 (−1.04–1.58) | 0.06 |
| Follow-up | 0.69 ± 0.87 (−0.78–2.63) | 0.53 ± 0.90 (−0.71–2.81) | 0.54 |
| Change | 0.08 ± 0.52 (−0.62–1.70) | 0.36 ± 0.35 (−0.22–1.20) | 0.06 |
| Fat area | |||
| Baseline | 0.77 ± 1.23 (−2.64–2.18) | 0.57 ± 0.82 (−1.22–2.13) | 0.53 |
| Follow-up | 0.37 ± 1.33 (−3.04–2.21) | 0.55 ± 0.97 (−1.20–2.90) | 0.62 |
| Change | −0.32 ± 0.45 (−1.24–0.32) | −0.07 ± 0.33 (−1.09–0.55) | 0.05 |
Early refers to participants with enrollment visit less than 6 months and late with enrollment visit more than 6 months after completion of ALL therapy. Data are presented as mean ± sd (range).
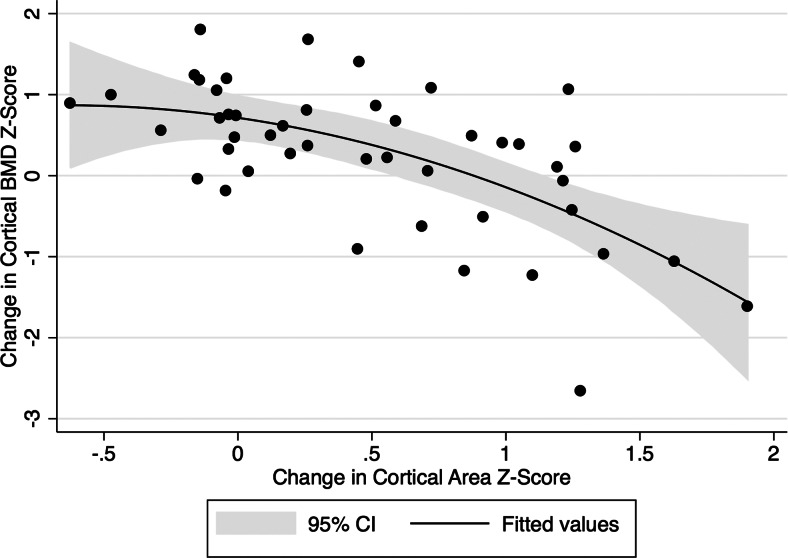
Multivariable regression models for changes in cortical BMD Z-scores demonstrated that increases in cortical area Z-scores were significantly associated with declines in cortical BMD, independent of interval since completion of ALL therapy (Fig. 2). Changes in tibia length over the study interval were not associated with changes in cortical BMD Z-scores. Further analyses revealed that increases in periosteal circumference Z-scores were associated with declines in cortical BMD Z-scores [β = −1.08 (95% confidence interval = −1.68–−0.49); P = 0.01], whereas changes in endosteal circumference Z-scores were not [β = 0.02 (−0.23–0.27); P = 0.86].
Fig. 2.
The change in cortical BMD (milligrams per cubic centimeter) Z-score is shown relative to the change in cortical area (square millimeter) Z-score over study interval. Increases in cortical area Z-scores were significantly associated with declines in cortical BMD Z-score, independent of the interval since completing ALL therapy. CI, Confidence interval.
Cortical dimensions
At the time of enrollment, cortical dimensions were not significantly different in ALL participants (Table 2) and were not associated with participant demographics, ALL treatment characteristics, or interval since completion of therapy.
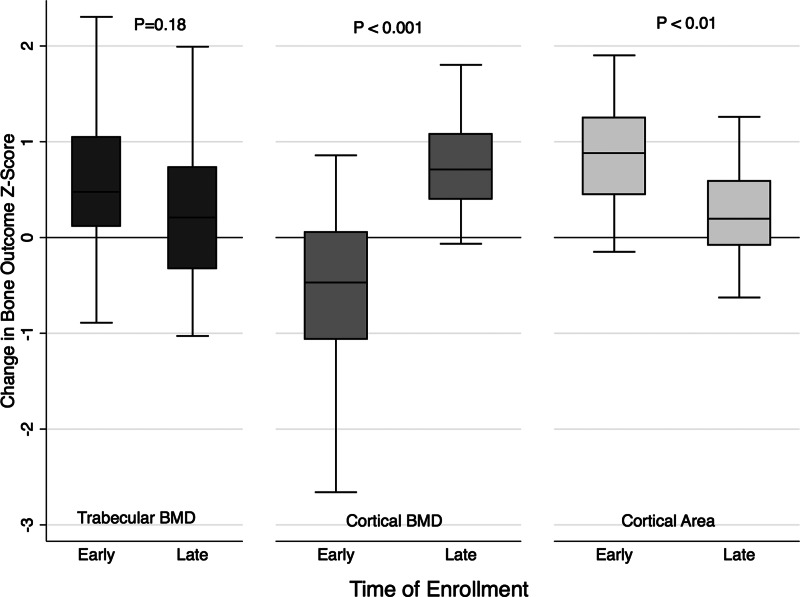
Over the 12 months after enrollment, cortical area Z-scores increased significantly with notable expansion of periosteal circumference Z-scores (Table 2). The greater periosteal circumference Z-scores in ALL participants at 12 months compared with reference participants were not significant after adjustment for the greater muscle CSA Z-scores (P = 0.11). Changes in cortical dimensions varied significantly according to the interval since completing ALL therapy. Among early enrollees, cortical area and periosteal circumference Z-scores increased, whereas, among late enrollees, cortical dimension Z-scores did not change.
The changes in trabecular BMD, cortical BMD and cortical area Z-scores in early vs. late enrollees are summarized in Fig. 3.
Fig. 3.
Changes in trabecular BMD (milligrams per cubic meter), cortical BMD (milligrams per cubic meter), and cortical area (square millimeters) Z-scores over the 12-month study interval in early (<6 months) vs. late (≥6 months) enrollees from the time of completing ALL therapy.
Muscle and fat CSA
In ALL participants, fat CSA was significantly higher at enrollment (P < 0.001). Muscle and fat CSA Z-scores at enrollment were not associated with participant or disease characteristics, medications, or the interval since completing therapy. Twelve months later, muscle and fat CSA Z-scores remained significantly higher in ALL participants.
Vitamin D levels and nutritional supplementation
At enrollment, 20% of ALL participants were vitamin D deficient. The odds of vitamin D deficiency in ALL participants was not significantly different compared with the reference group adjusted for age, race, and season (odds ratio = 0.71; 95% confidence interval = 0.30–1.70; P = 0.45). Adjustment for BMI Z-scores did not change the results. Serum 25(OH)D levels were not associated with bone or body composition Z-scores in ALL participants at either study visit.
Twenty ALL participants (40%) were taking a multivitamin with vitamin D or were prescribed a vitamin D supplement. The median dose was 400 IU/d (range 100-1000 IU). Supplementation was associated with higher levels (median 34.4 vs. 25.9 ng/dl, P = 0.08). Eleven (22%) were taking a multivitamin or supplement that contained calcium (eight ≤500 mg/d, two >500 to ≤1000 mg/d, and one >1000 mg/d). These numbers remained consistent at follow-up. Calcium supplementation was not associated with pQCT Z-scores at either visit.
Fracture summary
Nine ALL participants (18%) experienced a total of 10 nonphalangeal fractures (incidence rate 470 per 10,000 person-years), including two with vertebral fractures at diagnosis. Seven developed fractures during the maintenance phase of therapy and one after completion of therapy (one humerus, four radius/ulna, and three tibia/fibula). All fractures occurred during physical activity. Three participants experienced phalangeal fractures.
Discussion
This study is the first to examine changes in BMD and bone dimensions shortly after completion of ALL chemotherapy in children and young adults. Although ALL survivors had significantly reduced trabecular and cortical BMD, they demonstrated significant improvements over the study interval. The increases in cortical outcomes varied according to the interval since completion of ALL therapy, highlighting the need for longitudinal studies. Increases in cortical dimensions accompanied by a decline in cortical BMD occurred shortly after cessation of treatment followed by stabilization of dimensions and subsequent increases in cortical BMD. The early increases in cortical dimensions contributed to transient declines in cortical BMD, likely reflecting the longer time necessary to fully mineralize newly formed bone (36).
The majority of previous ALL BMD studies were limited by small sample size, subject heterogeneity, cross-sectional design, and inclusion of survivors treated with cranial radiation and DXA (7, 40, 41). Only three previous ALL studies used QCT (8, 20, 21). Gilsanz et al. (8) reported trabecular BMD deficits of the spine in a cohort of ALL patients shortly after completion of chemotherapy and cranial radiation. Cranial radiation is an established risk factor for impaired GH secretion, and GH plays a critical role in bone health (42). Moreover, current ALL protocols do not include cranial radiation in the majority of patients, reducing the generalizability of these findings. Brennan et al. (21) examined total BMD and cortical dimensions in a cohort of 53 children without cranial radiation a median of 4.6 (range 1.2–8.3) years after therapy. This study revealed reduced total and trabecular BMD but normal geometric properties at the distal radius. The study was limited by the cross-sectional design and wide interval since completion of therapy, and was thus unable to address determinants of skeletal recovery. More recently, Kaste et al. (20) conducted a retrospective study of lumbar trabecular and cortical BMD in 38 long-term ALL survivors (11.7–16.1 yr from diagnosis) compared with the QCT manufacturer's reference data. These survivors were predominantly postpubertal at the initial evaluation. Trabecular and cortical BMD deficits improved over a median interval of 5 yr. Male sex, Caucasian race, cranial radiation, hormone replacement, and alcohol use were negatively associated with bone accretion. Twenty-nine percent of participants exhibited a thyroid or GH abnormality related to radiation exposure, and the small sample size prohibited further analyses of this subset.
A previous study in children treated with GH demonstrated increases in periosteal dimensions with concurrent declines in cortical BMD Z-scores (36). Similarly, PTH (1–84) therapy in mature rhesus monkeys resulted in increases in cortical dimensions and the formation of new osteons with lower mineralization density (43). We hypothesize that accelerated increases in cortical dimensions with new, not yet fully mineralized bone during recovery from ALL chemotherapy explains the observed decline in cortical BMD.
The trabecular deficits were consistent with histomorphometry studies documenting that glucocorticoids result in preferential trabecular bone loss with reductions in bone volume fraction (44) and subsequent recovery after treatment (45). Failure to identify an association between trabecular BMD and previous glucocorticoid dose in ALL likely reflects the variable interval since completing therapy, rapid changes after completion of therapy, and inadequate pQCT resolution to assess trabecular microarchitecture. Similarly, leukemia risk status and antimetabolite chemotherapy were not associated with baseline pQCT results. Concurrent vitamin D levels were not associated with bone deficits.
BMI and pQCT fat and muscle CSA Z-scores were significantly higher in ALL participants, compared with the reference population. This is consistent with previous reports that greater fat mass (including glucocorticoid-induced obesity) (46) was associated with greater height and muscle Z-scores. These findings are also consistent with studies in children treated for childhood ALL without cranial radiation (21, 47), documenting increases in fat mass associated with glucocorticoid exposure.
Cortical dimensions expand during growth and development in response to biomechanical loading by muscle; i.e. there is a functional muscle-bone unit (35). The greater cortical CSA and periosteal circumference Z-scores in ALL participants at 12 months were not significantly different from reference participants after adjustment for muscle CSA Z-scores. In a previous study, we observed that greater gains in muscle were significantly associated with lesser declines in cortical dimensions and strength after diagnosis and treatment with glucocorticoids in children with Crohn's disease (31).
The limitations of this study include the absence of bone biopsies to assess bone microarchitecture, remodeling rates, and mineralization. An additional limitation was the modestly variable intervals since completion of therapy at enrollment; however, the variability was markedly lower than all previous ALL studies, and the mixed longitudinal measures provide insight into early years after therapy. The lack of measures of physical activity was likely not a significant limitation given the greater muscle area and cortical dimensions Z-scores at both study visits. The study had inadequate power to identify risk factors for fracture; however, the overall fracture rate was greater than observed among all age and gender distributions in a population-based study (22).
This study has several strengths. It is the first longitudinal pQCT study to enroll young survivors of childhood ALL without cranial radiation shortly after completion of chemotherapy, with subsequent assessment according to the interval since cessation of treatment. Second, the inclusion of a large, robust reference group facilitated adjustment for age, sex, race, tibia length, and muscle area in the assessment of bone outcomes and thus accounted for normal growth and developmental changes expected in bone density and cortical geometry.
These data suggest that ALL treatment in childhood without cranial radiation may not result in long-term detrimental effects on bone development. However, given the lack of complete normalization of trabecular and cortical BMD, future studies are needed to confirm complete BMD recovery in survivors of childhood ALL. Additional studies are necessary to relate changes in bone outcomes to short- and long-term increases in fracture rates.
Acknowledgments
The study was supported by National Institutes of Health Grants K24 DK076808 (to M.B.L.) and K12 HD000850 (to J.B.) and the Clinical and Translational Science Award from the Clinical and Translational Research Center (UL1-RR-024134).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALL
- Acute lymphoblastic leukemia
- BMD
- bone mineral density
- BMI
- body mass index
- CHOP
- Children's Hospital of Philadelphia
- CSA
- cross-sectional area
- DXA
- dual-energy x-ray absorptiometry
- 25(OH)D
- 25-hydroxyvitamin D
- pQCT
- peripheral QCT
- QCT
- quantitative computed tomography.
References
- 1. Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. 2010. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab 95:1681–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pui CH, Relling MV, Downing JR. 2004. Acute lymphoblastic leukemia. N Engl J Med 350:1535–1548 [DOI] [PubMed] [Google Scholar]
- 3. Halton JM, Atkinson SA, Fraher L, Webber CE, Cockshott WP, Tam C, Barr RD. 1995. Mineral homeostasis and bone mass at diagnosis in children with acute lymphoblastic leukemia. J Pediatr 126:557–564 [DOI] [PubMed] [Google Scholar]
- 4. Atkinson SA, Halton JM, Bradley C, Wu B, Barr RD. 1998. Bone and mineral abnormalities in childhood acute lymphoblastic leukemia: influence of disease, drugs and nutrition. Int J Cancer Suppl 11:35–39 [PubMed] [Google Scholar]
- 5. Heath JA, Ramzy JM, Donath SM. 2010. Physical activity in survivors of childhood acute lymphoblastic leukaemia. J Paediatr Child Health 46:149–153 [DOI] [PubMed] [Google Scholar]
- 6. Hartman A, van den Bos C, Stijnen T, Pieters R. 2008. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatr Blood Cancer 50:833–837 [DOI] [PubMed] [Google Scholar]
- 7. Nysom K, Holm K, Michaelsen KF, Hertz H, Müller J, Mølgaard C. 1998. Bone mass after treatment for acute lymphoblastic leukemia in childhood. J Clin Oncol 16:3752–3760 [DOI] [PubMed] [Google Scholar]
- 8. Gilsanz V, Carlson ME, Roe TF, Ortega JA. 1990. Osteoporosis after cranial irradiation for acute lymphoblastic leukemia. J Pediatr 117:238–244 [DOI] [PubMed] [Google Scholar]
- 9. Crofton PM, Ahmed SF, Wade JC, Stephen R, Elmlinger MW, Ranke MB, Kelnar CJ, Wallace WH. 1998. Effects of intensive chemotherapy on bone and collagen turnover and the growth hormone axis in children with acute lymphoblastic leukemia. J Clin Endocrinol Metab 83:3121–3129 [DOI] [PubMed] [Google Scholar]
- 10. Friedlaender GE, Tross RB, Doganis AC, Kirkwood JM, Baron R. 1984. Effects of chemotherapeutic agents on bone. I. Short-term methotrexate and doxorubicin (adriamycin) treatment in a rat model. J Bone Joint Surg Am 66:602–607 [PubMed] [Google Scholar]
- 11. Leeuw JA, Koudstaal J, Wiersema-Buist J, Kamps WA, Timens W. 2003. Bone histomorphometry in children with newly diagnosed acute lymphoblastic leukemia. Pediatr Res 54:814–818 [DOI] [PubMed] [Google Scholar]
- 12. Högler W, Wehl G, van Staa T, Meister B, Klein-Franke A, Kropshofer G. 2007. Incidence of skeletal complications during treatment of childhood acute lymphoblastic leukemia: comparison of fracture risk with the General Practice Research Database. Pediatr Blood Cancer 48:21–27 [DOI] [PubMed] [Google Scholar]
- 13. Maniadaki I, Stiakaki E, Germanakis I, Kalmanti M. 2006. Evaluation of bone mineral density at different phases of therapy of childhood all. Pediatr Hematol Oncol 23:11–18 [DOI] [PubMed] [Google Scholar]
- 14. Mandel K, Atkinson S, Barr RD, Pencharz P. 2004. Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol 22:1215–1221 [DOI] [PubMed] [Google Scholar]
- 15. Kelly KM, Thornton JC, Hughes D, Osunkwo I, Weiner M, Wang J, Horlick M. 2009. Total body bone measurements: a cross-sectional study in children with acute lymphoblastic leukemia during and following completion of therapy. Pediatr Blood Cancer 52:33–38 [DOI] [PubMed] [Google Scholar]
- 16. Davies JH, Evans BA, Jenney ME, Gregory JW. 2005. Skeletal morbidity in childhood acute lymphoblastic leukaemia. Clin Endocrinol (Oxf) 63:1–9 [DOI] [PubMed] [Google Scholar]
- 17. van der Sluis IM, van den Heuvel-Eibrink MM, Hählen K, Krenning EP, de Muinck Keizer-Schrama SM. 2002. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr 141:204–210 [DOI] [PubMed] [Google Scholar]
- 18. Liu D, Manske SL, Kontulainen SA, Tang C, Guy P, Oxland TR, McKay HA. 2007. Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos Int 18:991–997 [DOI] [PubMed] [Google Scholar]
- 19. Kaste SC, Jones-Wallace D, Rose SR, Boyett JM, Lustig RH, Rivera GK, Pui CH, Hudson MM. 2001. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: frequency of occurrence and risk factors for their development. Leukemia 15:728–734 [DOI] [PubMed] [Google Scholar]
- 20. Kaste SC, Rai SN, Fleming K, McCammon EA, Tylavsky FA, Danish RK, Rose SR, Sitter CD, Pui CH, Hudson MM. 2006. Changes in bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 46:77–87 [DOI] [PubMed] [Google Scholar]
- 21. Brennan BM, Mughal Z, Roberts SA, Ward K, Shalet SM, Eden TO, Will AM, Stevens RF, Adams JE. 2005. Bone mineral density in childhood survivors of acute lymphoblastic leukemia treated without cranial irradiation. J Clin Endocrinol Metab 90:689–694 [DOI] [PubMed] [Google Scholar]
- 22. Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. 2004. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res 19:1976–1981 [DOI] [PubMed] [Google Scholar]
- 23. Burnham JM, Shults J, Semeao E, Foster B, Zemel BS, Stallings VA, Leonard MB. 2004. Whole body BMC in pediatric Crohn disease: independent effects of altered growth, maturation, and body composition. J Bone Miner Res 19:1961–1968 [DOI] [PubMed] [Google Scholar]
- 24. Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel B, Shults J, Leonard MB. 2012. Bone density and structure in long-term survivors of pediatric allogeneic hematopoietic stem cell transplantation. J Bone Miner Res 27:760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thayu M, Denson LA, Shults J, Zemel BS, Burnham JM, Baldassano RN, Howard KM, Ryan A, Leonard MB. 2010. Determinants of changes in linear growth and body composition in incident pediatric Crohn's disease. Gastroenterology 139:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morris NM, Udry JR. 1980. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 9:271–280 [DOI] [PubMed] [Google Scholar]
- 27. Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, Gelber R, Heerema N, Korn EL, Link M, Murphy S, Pui CH, Pullen J, Reamon G, Sallan SE, Sather H, Shuster J, Simon R, Trigg M, Tubergen D, Uckun F, Ungerleider R. 1996. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol 14:18–24 [DOI] [PubMed] [Google Scholar]
- 28. Ashe MC, Khan KM, Kontulainen SA, Guy P, Liu D, Beck TJ, McKay HA. 2006. Accuracy of pQCT for evaluating the aged human radius: an ashing, histomorphometry and failure load investigation. Osteoporos Int 17:1241–1251 [DOI] [PubMed] [Google Scholar]
- 29. Hollis BW. 1997. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol 282:174–186 [DOI] [PubMed] [Google Scholar]
- 30. Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. 2002. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60 [DOI] [PubMed] [Google Scholar]
- 31. Dubner SE, Shults J, Baldassano RN, Zemel BS, Thayu M, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. 2009. Longitudinal assessment of trabecular and cortical bone density and structure in an incident cohort of children with Crohn disease. Gastroenterology 136:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. 2009. Divergent effects of glucocorticoids on cortical and trabecular compartment bone mineral density in childhood nephrotic syndrome. J Bone Miner Res 24:503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cole TJ, Green PJ. 1992. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 11:1305–1319 [DOI] [PubMed] [Google Scholar]
- 34. Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ. 2010. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab 95:1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schoenau E, Neu CM, Beck B, Manz F, Rauch F. 2002. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J Bone Miner Res 17:1095–1101 [DOI] [PubMed] [Google Scholar]
- 36. Schweizer R, Martin DD, Schwarze CP, Binder G, Georgiadou A, Ihle J, Ranke MB. 2003. Cortical bone density is normal in prepubertal children with growth hormone (GH) deficiency, but initially decreases during GH replacement due to early bone remodeling. J Clin Endocrinol Metab 88:5266–5272 [DOI] [PubMed] [Google Scholar]
- 37. Heaney RP. 2004. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr 80:1706S–1709S [DOI] [PubMed] [Google Scholar]
- 38. Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. 2007. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr 86:150–158 [DOI] [PubMed] [Google Scholar]
- 39. Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. 2012. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood 119:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kadan-Lottick N, Marshall JA, Barón AE, Krebs NF, Hambidge KM, Albano E. 2001. Normal bone mineral density after treatment for childhood acute lymphoblastic leukemia diagnosed between 1991 and 1998. J Pediatr 138:898–904 [DOI] [PubMed] [Google Scholar]
- 41. Halton JM, Atkinson SA, Fraher L, Webber C, Gill GJ, Dawson S, Barr RD. 1996. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J Bone Miner Res 11:1774–1783 [DOI] [PubMed] [Google Scholar]
- 42. O'Halloran DJ, Tsatsoulis A, Whitehouse RW, Holmes SJ, Adams JE, Shalet SM. 1993. Increased bone density after recombinant human growth hormone (GH) therapy in adults with isolated GH deficiency. J Clin Endocrinol Metab 76:1344–1348 [DOI] [PubMed] [Google Scholar]
- 43. Fox J, Miller MA, Newman MK, Recker RR, Turner CH, Smith SY. 2007. Effects of daily treatment with parathyroid hormone 1–84 for 16 months on density, architecture and biomechanical properties of cortical bone in adult ovariectomized rhesus monkeys. Bone 41:321–330 [DOI] [PubMed] [Google Scholar]
- 44. Dalle Carbonare L, Arlot ME, Chavassieux PM, Roux JP, Portero NR, Meunier PJ. 2001. Comparison of trabecular bone microarchitecture and remodeling in glucocorticoid-induced and postmenopausal osteoporosis. J Bone Miner Res 16:97–103 [DOI] [PubMed] [Google Scholar]
- 45. Gafni RI, McCarthy EF, Hatcher T, Meyers JL, Inoue N, Reddy C, Weise M, Barnes KM, Abad V, Baron J. 2002. Recovery from osteoporosis through skeletal growth: early bone mass acquisition has little effect on adult bone density. FASEB J 16:736–738 [DOI] [PubMed] [Google Scholar]
- 46. Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA. 2004. Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med 351:868–875 [DOI] [PubMed] [Google Scholar]
- 47. Reilly JJ, Ventham JC, Ralston JM, Donaldson M, Gibson B. 1998. Reduced energy expenditure in preobese children treated for acute lymphoblastic leukemia. Pediatr Res 44:557–562 [DOI] [PubMed] [Google Scholar]