Abstract
Context:
Associations between menstrual cycle length and chronic diseases are hypothesized to be due to differences in underlying hormonal patterns.
Objective:
The aim of the study was to evaluate the association between menstrual cycle length and the hormonal profile and anovulation.
Design and Setting:
We conducted a prospective cohort study at the University at Buffalo from 2005 to 2007.
Participants:
We recruited 259 healthy, regularly menstruating women aged 18–44 yr.
Main Outcome Measures:
Cycle length was observed for up to two cycles. Serum estradiol, progesterone, LH, and FSH were measured up to eight times per cycle for up to two cycles.
Results:
Women with short cycles (<26 d) had higher FSH concentrations during menses and in the late luteal phase, higher follicular estradiol concentrations, and lower LH concentrations across the cycle. Among women with longer cycles (>35 d), estradiol and LH peaks occurred on average about 3 d later, and FSH peaks about 1 d later compared to women with normal-length cycles. Both short and long cycles, compared with normal-length cycles, had an increased probability of anovulation. In general, per-cycle exposure to hormones was less in short cycles based on the area under the curve, although over time the cumulative exposure to estradiol would be greater for women with short cycles.
Conclusions:
Short ovulatory cycles were associated with higher follicular phase estradiol, an earlier rise in FSH, and an increased risk of anovulation. These results suggest that menstrual cycle length may be a relevant indicator of estradiol exposure and risk of anovulation among regularly cycling women.
Endogenous hormones are important for reproductive physiology, but they also play an important role in the development of chronic disease (1, 2). However, exposure to endogenous hormones is difficult to quantify in studies given the complex cyclical patterns of hormones, the need for timed collection, and the cost required for multiple sample collections. For these reasons, menstrual cycle characteristics such as cycle length are often used as proxies for hormone exposure because they can easily be assessed in population studies.
A woman's menstrual cycle length has been associated with her risk of chronic diseases such as breast cancer (3–5), ovarian cancer (6), cardiovascular disease (7), and osteoporosis (7). Cycle length is thought to be an indicator of cumulative exposure to ovarian steroids and to reflect underlying hormonal patterns. For example, it is hypothesized that short cycles are associated with an increased risk of breast cancer because short cycles are representative of greater cumulative exposure to estrogen over time (3–5). Along the same lines, long cycles and irregular cycles are thought to be associated with decreased estrogen exposure to explain their association with increased risk of osteoporosis (7), type 2 diabetes mellitus (8), cardiovascular disease (7), and decreased risk of ovarian cancer (6).
Although cycle length has been associated with several long-term outcomes, there is little evidence correlating cycle length with serum hormone concentrations across the menstrual cycle among healthy regularly menstruating women. Furthermore, the biological assumptions relating short cycles to greater cumulative hormone exposure have never been empirically evaluated in clinical or research settings, especially in the context of multiple sex hormones. If cycle length is to be used as a proxy for hormonal patterns or cumulative hormone exposure, it is important to understand what cycle length indicates in terms of endocrine function and whether or not it is an appropriate endpoint for future studies. Obtaining a more comprehensive understanding of cycle length as a marker of hormone variability will be critical to elucidate how a woman's endogenous hormone and gonadotropin concentrations impact her risk for menstrual dysfunction, chronic disease, and infertility. The objective of this study was to evaluate the association between cycle length and the hormonal profile during the normal menstrual cycle. To do this, we evaluated the shape, pattern, and cumulative exposure to multiple endogenous hormones, including estradiol, progesterone, LH, and FSH among regularly cycling women.
Subjects and Methods
Study population
This study used data from 259 regularly menstruating women enrolled in the BioCycle Study, a prospective study of the menstrual cycle (9). Participants were healthy premenopausal female volunteers aged 18 to 44 from the western New York region (51%, 18–24 yr; 15.7%, 25–29 yr; 7.6%, 30–34 yr; 12.9%, 35–39 yr; 12.9%, 40–44 yr). To be eligible for the study, women had to have a self-reported cycle length between 21 and 35 d for the past 6 months. Women were excluded if they used oral contraceptives during the past 3 months, were pregnant in the last 6 months, or had a chronic disease. The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. All participants provided written informed consent.
The study involved up to eight clinic visits per cycle for one (n = 9) or two (n = 250) cycles, with visits timed using calendars and assisted by the use of fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, MA) so that biospecimen collection occurred during specific phases of the cycle (10). Fertility monitors measured estrone-3-glucoronide and LH in urine, starting on calendar d 6 after menses and continuing for 10 to 20 d, depending on whether the woman reached peak levels on the monitor. Monitor indications of low, high, and peak fertility were used to time midcycle visits, with other visits scheduled according to an algorithm that took each woman's reported cycle length into consideration (11). If the monitor indicated “peak fertility” on a day without a scheduled visit, the women were asked to come in that morning and the following two mornings. The remaining visits were scheduled based on the algorithm previously described (11), after adjusting for self-reported cycle length. Visits were scheduled to correspond to biologically relevant windows including menstruation, mid and late follicular phase, LH and FSH surge, ovulation, and early, mid, and late luteal phases (approximately corresponding to d 2, 7, 12, 13, 14, 18, 22, and 27 of a standardized 28-d cycle), with collection dates adjusted for cycle length. For analysis, the date of the observed serum LH surge was used to classify visits into correct cycle phase categories (11). Participants were highly adherent with the study protocol, with 94% of all women completing seven or eight visits per cycle.
Hormone and ovulation assessment
Reproductive hormones were measured in fasting serum samples collected at each cycle visit by the Kaleida Health Center for Laboratory Medicine (Buffalo, NY). Estradiol was measured using a RIA. Progesterone, LH, and FSH were measured using solid-phase competitive chemiluminescent enzymatic immunoassays by Specialty Laboratories, Inc. (Valencia, CA) on the DPC Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics, Deerfield, IL). Across the study period, the coefficients of variation for these tests reported by the laboratory were less than 10% for estradiol, less than 5% for LH and FSH, and less than 14% for progesterone. Cycles were classified as anovulatory if the peak progesterone concentration across the cycle was 5 ng/ml or less and no serum LH peak was observed during the later cycle visits (42 of 509 cycles; 8.3%) (12).
Cycle length
Prospectively collected cycle length (number of days between menstrual bleeding) was observed for up to two cycles. Day 1 of the cycle was defined as menstruating by 1600 h on that day after confirming 2 consecutive days of bleeding, to differentiate from spotting. Day of ovulation among ovulatory women was assigned based on dates and levels of the LH peak using the fertility monitor compared with the observed LH maximum value in serum and the first day of progesterone rise. Follicular phase length was defined as d 1 of bleeding to the day of ovulation. Luteal phase length was defined as ovulation through the last day of the cycle.
Covariate assessment
Participants were interviewed regarding demographics and asked to complete questionnaires on lifestyle (smoking status), physical activity (13), dietary patterns via 24-h recall (Nutrition Data System for Research software, version 2005; Nutrition Coordinating Center of the University of Minnesota, Minneapolis, MN), and reproductive history. Physical and anthropometric measures were measured according to standardized protocols by trained study staff and included height and weight, which were used to calculate body mass index (BMI).
Statistical analysis
Descriptive statistics were computed for all study variables. Distributions of total cycle, follicular phase, and luteal phase length were evaluated. Exact χ2 tests and repeated measures ANOVA were used to test for associations between demographic, lifestyle, and cycle characteristics and category of cycle length. Geometric mean concentrations of hormones were calculated for each of the eight clinic visits. Repeated measures ANOVA was used to compute the P values for these comparisons, which take into account multiple cycles from the same woman. Hormone concentrations of ovulatory cycles were compared between categories of cycle length (short, <26 d; normal, 26 to 35 d; long, >35 d), and pairwise comparisons were evaluated at the 0.05 level of significance. Short cycles were defined as less than 26 d to provide a reasonable range for the normal cycles as well as to provide a sufficient number of short cycles to improve statistical power. Furthermore, linear mixed models were used to evaluate associations between continuous cycle length and reproductive hormone concentrations among women in the normal cycle length category.
To evaluate cumulative exposure to hormones, the area under the cycle-specific hormonal profile curve was calculated overall and by phase for each of the four hormones for each individual using log hormone concentrations. We assumed an approximately linear relationship between hormone values between visits and calculated the area under each trapezoid between visits. Mean areas under the curve (AUC) were then compared by cycle length category using repeated measures ANOVA and were restricted to ovulatory cycles. Sensitivity analyses were performed, which compared results restricting to cycles with no missing visits (n = 340 cycles with complete information).
Nonlinear mixed models with harmonic terms were used to assess the associations between cycle length and three aspects of hormonal patterns among the ovulatory cycles in this study: 1) mean differences in the hormone concentrations across the cycle; 2) differences in the peak hormone levels (i.e. difference between nadir and peak concentrations, also referred to as the amplitude); and 3) differences in the timing of the peak (i.e. the peak occurred earlier or later during the cycle, also referred to as the phase shift) (15). These models offer a flexible approach to study cyclic patterns over time while taking into account both between- and within-subject variation. Standardized time was derived by taking the calendar day of the clinic visit divided by the observed cycle length so that the start of the cycle is at time 0 and the end of the cycle is at time 1.0. We further centered on day of ovulation at a time of 0.5. To evaluate differences in the timing of the peak, we did not center on day of ovulation so that the results could be interpreted as differences in timing of the whole hormonal pattern, and not with respect to ovulation.
Generalized estimating equations were used to model the association between cycle length and the probability of incident anovulation. Unadjusted and adjusted odds ratios and confidence intervals (CI) were calculated. For all models, the choice of covariates was determined by a review of the prior literature and statistical testing for confounding identification. Harmonic models and models for anovulation were adjusted for age, race (white, black, other), and BMI. Other demographic characteristics, dietary intake, physical activity, smoking, and alcohol consumption were evaluated for confounding but did not appreciably alter the estimates. SAS version 9.2 (SAS Institute, Cary, NC) was used for all statistical analyses, and all analyses take the multiple cycles per woman into account.
Results
The average cycle length observed in this study was 28.8 d [±4.1 (sd)], with a range between 13 and 58 d and a median of 28 d. The average follicular phase length was 15.1 d (±3.9), and luteal phase length was 13.9 d (±2.4), with longer cycles associated with longer follicular and luteal phase lengths and shorter cycles with shorter follicular and luteal phase lengths (Table 1). As previously reported, the follicular phase was more variable than the luteal phase (16–18). The women in the BioCycle Study were on average 27.4 yr of age and consisted mainly of single, nulliparous, normal weight, white women with some postsecondary education (Table 1). Women with short cycles were more likely to be older, married, parous, and of white race. Women with short and long cycles had lower BMI and a larger percentage of anovulatory cycles. Cycle length was not associated with education, total energy intake, previous use of oral contraceptives, or age at menarche. Cycle length varied on average by 3.1 d (±3.0) within women from cycle 1 to cycle 2 (range, 0 to 21 d; median, 2 d; mode, 1 d). For the majority of women, both cycles were classified in the same cycle length category (71%); however, some women were also classified as having either a short and normal-length cycle or a long and normal-length cycle as well (28%). A very small percentage of women had a short and a long cycle during the study period (1%).
Table 1.
Demographic, lifestyle, and menstrual cycle characteristics of women enrolled in the BioCycle Study by cycle length category, short (<26 d), normal (26 to 35 d), long (>35 d)
| Overall cycles | Short cycle (<26 d) | Normal cycle (26 to 35 d) | Long cycle (>35 d) | P valueb | |
|---|---|---|---|---|---|
| n | 509a | 81 | 369 | 26 | |
| Demographics | |||||
| Age (yr) | 27.4 ± 8.2 | 31.0 ± 8.9 | 27.1 ± 8.0 | 24.5 ± 7.1 | 0.0002 |
| BMI (kg/m2) | 24.1 ± 3.9 | 23.3 ± 3.5 | 24.3 ± 4.0 | 23.1 ± 3.1 | 0.06 |
| Race | 0.003 | ||||
| White | 302 (59.3) | 53 (65.4) | 220 (59.6) | 12 (46.2) | |
| Black | 101 (19.8) | 22 (27.2) | 64 (17.3) | 6 (23.1) | |
| Other | 106 (20.8) | 6 (7.4) | 85 (23.0) | 8 (30.8) | |
| ≤ High school education | 65 (12.8) | 9 (11.1) | 48 (13.0) | 3 (11.5) | 0.9 |
| Married | 131 (25.7) | 31 (38.3) | 95 (25.8) | 2 (7.7) | 0.005 |
| Nulliparous | 367 (73.6) | 44 (55.0) | 274 (75.1) | 21 (87.5) | <0.0001 |
| Lifestyle characteristics | |||||
| Current smoker | 20 (3.9) | 3 (3.7) | 16 (4.3) | 1 (3.9) | 0.9 |
| Physical activity | 0.2 | ||||
| Low | 48 (9.4) | 5 (6.2) | 41 (11.1) | 1 (3.9) | |
| Moderate | 182 (35.8) | 37 (45.7) | 128 (34.7) | 12 (46.2) | |
| High | 279 (54.8) | 39 (48.2) | 200 (54.2) | 13 (50.0) | |
| Total energy (kcal) | 1608.1 ± 405.0 | 1634.3 ± 379.0 | 1612.5 ± 415.4 | 1576.2 ± 309.1 | 0.8 |
| Past OC use | 275 (54.7) | 52 (64.2) | 196 (53.4) | 11 (44.0) | 0.1 |
| Cycle characteristics | |||||
| Age at menarche (yr) | 12.4 ± 1.2 | 12.4 ± 1.1 | 12.5 ± 1.3 | 12.2 ± 1.2 | 0.4 |
| Cycle length (d) | 28.8 ± 4.1 | 23.9 ± 1.8 | 29.1 ± 2.4 | 39.5 ± 5.3 | <0.0001 |
| Follicular phase length (d) | 15.1 ± 3.9 | 11.3 ± 2.2 | 15.1 ± 2.9 | 23.3 ± 3.1 | <0.0001 |
| Luteal phase length (d) | 13.9 ± 2.4 | 12.7 ± 1.8 | 14.0 ± 2.0 | 16.3 ± 4.9 | <0.0001 |
| Anovulatory cycle | 42 (8.3) | 11 (13.6) | 19 (5.2) | 3 (11.5) | 0.01 |
Data are expressed as mean ± sd or number (percentage). OC, Oral contraceptive.
A total of 250 women in the BioCycle study were followed for two cycles, and nine women were followed for one cycle, for a total of 509 cycles.
Two-sided P values for continuous variables calculated using repeated measures ANOVA, and for categorical variables using Fisher's exact test. All comparisons take repeated measures and correlations between cycles into account.
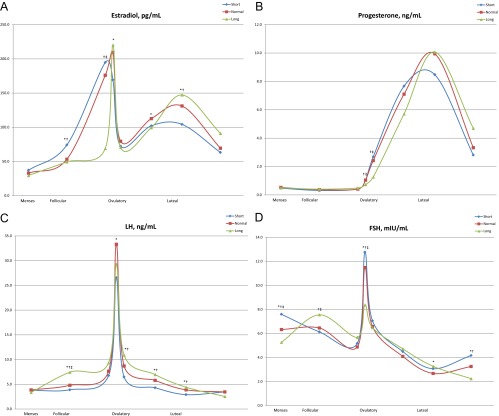
Cycle length category was also associated with concentrations of reproductive hormones at given visits. Figure 1 displays the unadjusted means of estradiol, progesterone, LH, and FSH concentrations at each clinic visit across the cycle. In women with short cycles, estradiol concentrations were higher in the mid to late follicular phase, and FSH concentrations were higher in the early follicular and late luteal phases. However, during the luteal phase, women with short cycles had lower estradiol concentrations and marginally lower progesterone concentrations. Among women in the normal cycle length category, each 1-d increase in cycle length was associated with a 3.2% increase in LH (P < 0.0001) and a 1.7% decrease in FSH concentration (P = 0.0002).
Fig. 1.
Hormonal profiles (realigned data) from ovulatory cycles (A, estradiol; B, progesterone; C, LH; D, FSH) of women enrolled in the BioCycle Study by cycle length category, short (<26 d), normal (26 to 35 d), long (>35 d). Serum measurements were collected at eight cycle visits timed to menstrual cycle phase using fertility monitors corresponding to the second day of menstruation, mid follicular, late follicular, 3 d around expected ovulation, and early, mid, and late luteal phase (approximately corresponding to d 2, 7, 12, 13, 14, 18, 22, and 27 of a standardized 28-d cycle), with collection dates adjusted for cycle length. *, Pairwise comparison for short vs. normal P < 0.05. †, Pairwise comparison for short vs. long P < 0.05. ‡, Pairwise comparison for normal vs. long P < 0.05.
Overall, short cycles were associated with reduced per-cycle exposure to estradiol, LH, and FSH as assessed by the AUC (Table 2). Higher estradiol concentrations observed during the follicular phase of women with short cycles resulted in higher per-cycle follicular phase exposure to estradiol among women with short cycles. However, because of overall shorter cycle lengths, per-cycle exposure to estradiol, based on the AUC, was decreased among women with short cycles. Long cycles were associated with lower area under the progesterone curve during the luteal phase and the cycle overall. Sensitivity analyses restricting to cycles with eight cycle visits yielded similar results (results not shown).
Table 2.
Area under the cycle-specific hormonal profile curvea by cycle length category overall and by phase among women in the BioCycle Study
| Short (<26 d) | Normal (26–35 d) | Long (>35 d) | P valueb | |
|---|---|---|---|---|
| No. of cycles | 70 | 350 | 23 | |
| Estradiol | ||||
| Overall | 2708.5 ± 937.8 | 3215.7 ± 1139.3 | 2948.9 ± 1021.8 | 0.002 |
| Follicular phase | 1413.9 ± 693.0 | 1470.9 ± 767.7 | 853.1 ± 638.5 | <0.0001 |
| Luteal phase | 1294.6 ± 622.5 | 1744.8 ± 758.9 | 2095.8 ± 758.7 | <0.0001 |
| Progesterone | ||||
| Overall | 97.6 ± 33.5 | 107.8 ± 39.5 | 65.1 ± 38.7 | <0.0001 |
| Follicular phase | 14.0 ± 15.7 | 12.3 ± 11.5 | 9.5 ± 4.6 | 0.8 |
| Luteal phase | 83.6 ± 34.5 | 95.5 ± 36.3 | 55.7 ± 37.0 | <0.0001 |
| LH | ||||
| Overall | 180.3 ± 71.2 | 252.6 ± 103.0 | 345.4 ± 118.2 | <0.0001 |
| Follicular phase | 105.9 ± 62.9 | 138.8 ± 91.1 | 120.6 ± 60.6 | 0.007 |
| Luteal phase | 74.4 ± 51.5 | 113.9 ± 72.3 | 224.9 ± 100.3 | <0.0001 |
| FSH | ||||
| Overall | 169.9 ± 66.9 | 180.8 ± 67.0 | 260.2 ± 139.9 | <0.0001 |
| Follicular phase | 106.1 ± 51.9 | 116.2 ± 52.6 | 147.7 ± 110.3 | 0.01 |
| Luteal phase | 63.8 ± 37.3 | 64.6 ± 31.6 | 112.5 ± 57.0 | <0.0001 |
To evaluate cumulative exposure to hormones, the area under the cycle-specific hormonal profile curve was calculated for each individual using log hormone concentrations. Anovulatory cycles were excluded from this analysis. An approximately linear relationship between hormone values between visits was assumed to calculate the area under each trapezoid.
Two-sided P values for continuous variables calculated using repeated measures ANOVA. All comparisons take repeated measures and correlations between cycles into account.
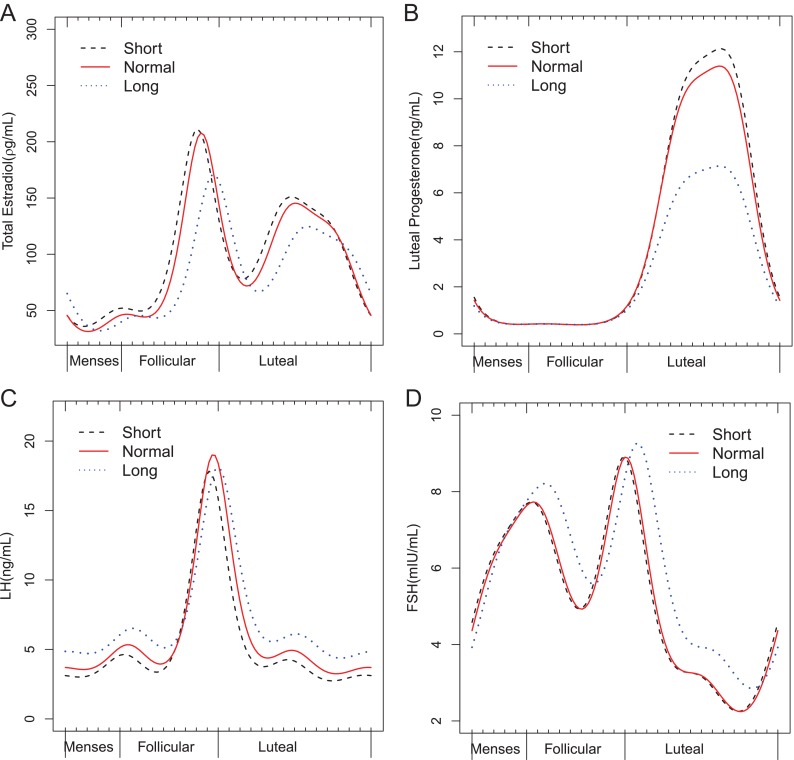
Nonlinear mixed models with harmonic terms (Table 3 and Fig. 2) confirmed the finding of higher estradiol concentrations across the cycle among women with short cycles compared with women with normal-length cycles, with estradiol concentrations increased by 8% (95% CI, 1.0, 15%; P = 0.02). These results were slightly attenuated after adjustment for age, race, and BMI (6%; 95% CI, −1.0, 13%; P = 0.09). LH concentrations were decreased by 12% (95% CI, 5, 19%) among women with short cycles. The follicular phase estradiol peak occurred on average 0.8 d earlier (95% CI, 0.4, 1.2), the FSH peak 0.5 d earlier (95% CI, 0.2, 0.9) and the LH peak (surge) 0.7 d earlier (95% CI, 0.4, 1.0) among women with short cycles compared with women with normal-length cycles after adjustment for age, race, and BMI.
Table 3.
Association between menstrual cycle length and hormonal patterns using nonlinear mixed models with harmonic terms in the BioCycle Study
| Mean (%)a |
Amplitudeb |
Phase Shift (days)c |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||||
| Estradiol | |||||||||
| Short (<26 d) | |||||||||
| Unadjusted | 7.84 | 1.03 | 15.11 | −0.04 | −0.08 | 0.01 | −0.88 | −1.29 | −0.49 |
| Adjustedd | 5.83 | −0.91 | 13.04 | −0.05 | −0.08 | 0.01 | −0.76 | −1.20 | −0.36 |
| Normal (26–35 d) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Long (>35 d) | |||||||||
| Unadjusted | −8.34 | −17.44 | 1.77 | −0.07 | −0.13 | 0.01 | 3.44 | 2.94 | 3.88 |
| Adjusted | −7.90 | −16.98 | 2.16 | −0.08 | −0.12 | 0.00 | 3.09 | 2.82 | 3.21 |
| Progesterone | |||||||||
| Short (<26 d) | |||||||||
| Unadjusted | 2.29 | −4.39 | 9.45 | 0.04 | −0.04 | 0.13 | −0.47 | −0.80 | −0.16 |
| Adjusted | 2.78 | −4.16 | 10.23 | −0.01 | −0.06 | 0.07 | −0.41 | −0.80 | −0.11 |
| Normal (26–35 d) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Long (>35 d) | |||||||||
| Unadjusted | −18.65 | −27.68 | −8.48 | −0.26 | −0.38 | −0.13 | 0.67 | 0.19 | 1.11 |
| Adjusted | −18.37 | −27.48 | −8.12 | −0.19 | −0.26 | −0.09 | 0.62 | 0.19 | 0.89 |
| LH | |||||||||
| Short (<26 d) | |||||||||
| Unadjusted | −13.21 | −20.05 | −5.79 | 0.03 | −0.02 | 0.09 | −1.02 | −1.42 | −0.62 |
| Adjusted | −12.49 | −19.39 | −5.00 | 0.01 | −0.01 | 0.06 | −0.71 | −1.04 | −0.39 |
| Normal (26–35 d) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Long (>35 d) | |||||||||
| Unadjusted | 22.29 | 7.35 | 39.32 | −0.11 | −0.17 | −0.02 | 3.32 | 2.68 | 3.92 |
| Adjusted | 20.43 | 5.99 | 36.83 | −0.06 | −0.09 | −0.002 | 2.66 | 2.23 | 2.96 |
| FSH | |||||||||
| Short (<26 d) | |||||||||
| Unadjusted | 0.11 | −6.27 | 6.94 | 0.00 | −0.04 | 0.05 | −0.65 | −1.00 | −0.28 |
| Adjusted | −3.09 | −9.23 | 3.47 | −0.01 | −0.02 | 0.03 | −0.54 | −0.89 | −0.15 |
| Normal (26–35 d) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Long (>35 d) | |||||||||
| Unadjusted | 13.78 | 2.11 | 26.79 | −0.07 | −0.15 | 0.04 | 0.92 | 0.29 | 1.58 |
| Adjusted | 15.60 | 4.02 | 28.48 | −0.04 | −0.07 | 0.03 | 1.13 | 0.46 | 1.81 |
Boldface indicates significance at the α = 0.05 level. All models take repeated measures and correlated cycles into account. Ref., Reference.
Ratios were obtained from the model to compare the mean hormone concentrations across the menstrual cycle for short and long cycles to the normal cycle length group. Estimates of the percentage difference in the hormone level and 95% CI were obtained by subtracting 1 from each ratio and multiplying by 100.
Amplitude differences are interpreted as the difference in peak hormone levels as compared to the normal length category.
Phase shift differences are interpreted as the difference in the number of days of a standardized 28-d cycle between the rise in hormone concentrations as compared to the normal length category (e.g. 1.12 = hormone rise is shifted 1.12 d later).
Models are adjusted for age, race, and BMI. Anovulatory cycles were excluded from this analysis (n = 42 cycles).
Fig. 2.
Harmonic model results for hormonal profiles during ovulatory cycles (A, estradiol; B, progesterone; C, LH; D, FSH) by cycle length category (short, <26 d; normal, 26–35 d; long, >35 d). The x-axis represents standardized time, which was derived by taking the calendar day of the clinic visit (up to eight per cycle) divided by the observed cycle length, with the cycles further centered on day of ovulation at a time of 0.5.
Women with long cycles had follicular phase estradiol, FSH, and LH peaks that occurred on average 3.1 (95% CI, 2.8, 3.2), 1.1 (95% CI, 0.5, 1.8), and 2.7 (95% CI, 2.2, 3.0) d later, respectively, than women with normal-length cycles. Compared with normal-length cycles, long cycles had decreased mean concentrations of progesterone by 18% (95% CI, −27, −87%) and marginally decreased mean estradiol concentrations by 8% (95% CI, −2, 17%), although LH concentrations were increased by 20% (95% CI, 4, 28%). There was also a slight reduction in the level of the LH peak by 0.06 (95% CI, −0.002, −0.09).
Using generalized estimating equations, both short and long cycles were associated with an increased odds of anovulation, compared with normal-length cycles. Specifically, the odds of anovulation comparing short cycles to normal-length cycles was 2.89 (95% CI, 1.27, 6.62), and comparing long cycles to normal-length cycles was 2.40 (95% CI, 0.64, 9.00). After adjustment for age, race, and BMI, the associations were similar: 4.53 (95% CI, 1.84, 11.16) and 1.88 (95% CI, 0.44, 8.01), respectively.
Discussion
Among regularly cycling women, we observed that shorter cycles were associated with higher late luteal and early follicular phase FSH concentrations and higher concentrations of estradiol, particularly during the follicular phase. Longer cycles were associated with a later rise in estradiol and later FSH and LH peaks. Women with longer cycles had higher LH concentrations but lower progesterone concentrations across the cycle. Short and long cycles were also associated with increased odds of anovulation. Per-cycle exposure to hormone concentrations, as measured by the AUC, was decreased during short cycles. However, because women with shorter cycles experience a greater number of cycles during their reproductive years, the cumulative exposure to estradiol would tend to be greater for women with shorter cycles.
Although several previous studies have evaluated the association between cycle length and estradiol concentrations, this is the first study, to our knowledge, to extend these findings to look at the impact on multiple hormone concentrations and detail the hormone patterns (mean concentrations, peak levels, and timing of the peaks) throughout the cycle. These findings are of interest because cycle length is often used as a proxy for hormonal variability and endocrine function in studies of reproductive and chronic disease outcomes. These results support the use of cycle length as a marker of exposure to hormones and risk of anovulation among women with regular cycles.
Our findings on higher estradiol concentrations in short cycles are consistent with three studies of comparable populations that evaluated hormonal patterns and cycle length. Landgren et al. (19) investigated the hormonal variability of 68 women through daily plasma samples. They found follicular phase length and cycle length to be inversely related to mean, peak, and baseline estradiol concentrations. Windham et al. (20) investigated daily urinary steroid metabolite concentrations and found that, compared with average-length cycles, cycles of women with a short follicular phase had elevations of 10–13% in baseline estrogen and average follicular phase estrogen metabolite concentrations (n = 411). Harlow et al. (21) examined daily urinary estrogen profiles for follicular phases of varying lengths (n = 167) and also found average follicular phase estrogen concentrations to be highest in cycles with short follicular phases (7–11 d). Similar associations between short cycle length and estrone conjugate concentrations were observed among perimenopausal women in the Study of Women's Health Across the Nation (SWAN) (22). Our work extends these previous findings on estradiol concentrations to a larger, healthy population of premenopausal women and to other reproductive hormones with details of the hormonal patterns.
Women with short cycles in our study had hormonal patterns classically associated with reproductive aging: elevated FSH concentrations in the early follicular phase, an earlier rise in follicular phase estradiol, and lower luteal phase progesterone concentrations (22). This hormonal pattern has also been attributed to lower serum inhibin concentrations observed with reproductive aging. It is uncertain as to whether the shortening of the follicular phase is due to a premature rise in FSH in the luteal phase, with advanced follicular development, a more rapid rise in FSH and faster follicular development, or increased ovarian responsiveness to FSH concentrations. Our data would suggest that shortened follicular phase and menstrual cycle lengths are due to a premature rise in FSH and advanced follicular development. Women with long cycles also had higher LH concentrations in the follicular phase with a delayed follicular FSH peak. It is possible that the elevated LH directly or indirectly leads to a delay in the rise in FSH concentrations, follicular development, and estradiol production.
Regarding the AUC, higher estradiol concentrations observed during the follicular phase resulted in higher per-cycle follicular phase exposure to estradiol among women with short cycles. However, because of overall shorter cycle lengths, per-cycle exposure to estradiol, based on the AUC, was decreased among women with short cycles. If we were to extend these findings to evaluate the cumulative exposure to estradiol over 10 yr, women with short- and normal-length cycles would have significantly higher exposure to estradiol compared with women with long cycles due to the greater number of cycles experienced over time. Although the AUC for progesterone was lower during long cycles, this could be due to the fact that the long cycles in our study had a higher probability of missing a late luteal phase measurement. Both of these factors would lead to artificially lower progesterone concentrations, which in turn would contribute to a reduced per-cycle exposure to progesterone.
Our finding of an increased odds of anovulation among short and long cycles is consistent with prior research (23). However, it is important to note that we observed this association even among a group of women who report regular cycles. Regular cycles have tended to serve as a surrogate for ovulatory cycles (24). Therefore, we demonstrated that even among regularly menstruating women, anovulation is more likely to occur among those with shorter or longer cycles, calling into question the use of regular cycles as a proxy for ovulatory cycles.
Collectively, prior research corresponds to our findings that shorter cycles are associated with higher concentrations of estrogen. However, the biological mechanisms through which variations in hormone concentrations influence cycle length have not been clearly identified. A better understanding of this relationship could lead to important determinants for some physiological phenomena; for instance, varying ovulatory function may be protective for some chronic diseases, but may also be risk factors for others. Further investigation and evaluation of the physiological mechanisms underlying hormone secretion is paramount to identifying prevention strategies for reproductive-age women and to classifying cyclic patterns that may be risk factors for infertility or for the development of chronic disease.
There are several strengths to the current study. In particular, we intensively monitored a large number of participants prospectively throughout two cycles. Clinic visits were timed using fertility monitors, which significantly improved the timing of sample collection. In addition, the prospective design and exclusion criteria at baseline strengthened our ability to draw inference among healthy regularly menstruating women. Collectively, these unique aspects of the study design allowed us to improve and expand upon previous studies of hormones and cycle length.
There are several limitations worth noting. First, there were a small number of long cycles observed in this study due to the strict exclusion criteria, which limits our conclusions regarding long and irregular cycles. However, there was considerable variability in cycle length even among this population of women (range, 13 to 58 d). There were also a small number of anovulatory cycles that may have limited our power to detect effects. Although using the fertility monitors to time visits was a significant improvement over previous studies, not all LH peaks were captured on the monitor. Because of this, there is the potential for some misclassification of cycle phases that could impact the distributions of hormone concentrations because timing was less accurate among women with irregular cycles, and long cycles in particular. Furthermore, our assessment of the AUC was limited because the curves were based on only eight measurements during each cycle. Although our study sample population is restricted to normally menstruating women to exclude potential confounders by design, it could also limit the generalizability of our findings. In addition, any observed effects are likely to be more modest than that observed among a population including women with irregular cycles.
In conclusion, we observed that short ovulatory cycles were associated with higher follicular phase estradiol and an earlier rise in FSH. Longer cycles were associated with higher LH concentrations and lower mean follicular phase estradiol. Both long and short cycles were associated with anovulation. Future studies may elucidate whether anovulatory short and long cycles have a follicular hormonal milieu similar or dissimilar to their ovulatory, cycle length counterparts. In the meantime, our data would suggest that menstrual cycle length may be a relevant indicator of estradiol exposure and risk of anovulation. Larger population studies including women with irregular cycles are needed to evaluate these patterns of variation and the dynamics of the menstrual cycle in regard to fertility or long-term risk of chronic disease.
Acknowledgments
We are indebted to all the investigators and staff at the Epidemiology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the University at Buffalo, as well as the BioCycle participants for their commitment to the study.
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Contract # HSN275200403394C).
Disclosure Summary: A.Z.S. has been a consultant for Roche diagnostics. The other authors have nothing to declare.
Footnotes
- AUC
- Area under the curve
- BMI
- body mass index
- CI
- confidence interval.
References
- 1. Goldman MB, Hatch MC. 2000. Women and health. San Diego: Academic Press [Google Scholar]
- 2. Strauss JF., III 2004. The synthesis and metabolism of steroid hormones. In: Strauss JF, Barbieri RL, eds. Yen and Jaffe's reproductive endocrinology: physiology, pathophysiology, and clinical management. 5th ed Philadelphia: Elsevier Inc; 125–154 [Google Scholar]
- 3. Parazzini F, La Vecchia C, Negri E, Franceschi S, Tozzi L. 1993. Lifelong menstrual pattern and risk of breast cancer. Oncology 50:222–225 [DOI] [PubMed] [Google Scholar]
- 4. Terry KL, Willett WC, Rich-Edwards JW, Hunter DJ, Michels KB. 2005. Menstrual cycle characteristics and incidence of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 14:1509–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whelan EA, Sandler DP, Root JL, Smith KR, Weinberg CR. 1994. Menstrual cycle patterns and risk of breast cancer. Am J Epidemiol 140:1081–1090 [DOI] [PubMed] [Google Scholar]
- 6. Parazzini F, La Vecchia C, Negri E, Gentile A. 1989. Menstrual factors and the risk of epithelial ovarian cancer. J Clin Epidemiol 42:443–448 [DOI] [PubMed] [Google Scholar]
- 7. Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. 2002. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab 87:2013–2017 [DOI] [PubMed] [Google Scholar]
- 8. Solomon CG, Hu FB, Dunaif A, Rich-Edwards J, Willett WC, Hunter DJ, Colditz GA, Speizer FE, Manson JE. 2001. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA 286:2421–2426 [DOI] [PubMed] [Google Scholar]
- 9. Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Liu A, Trevisan M. 2009. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol 23:171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. 2009. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol 169:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mumford SL, Schisterman EF, Gaskins AJ, Pollack AZ, Perkins NJ, Whitcomb BW, Ye A, Wactawski-Wende J. 2011. Realignment and multiple imputation of longitudinal data: an application to menstrual cycle data. Paediatr Perinat Epidemiol 25:448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaskins AJ, Mumford SL, Wactawski-Wende J, Schisterman EF. 2012. Effect of daily fiber intake on luteinizing hormone levels in reproductive-aged women. Eur J Nutr 51:249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. 2003. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395 [DOI] [PubMed] [Google Scholar]
- 14. Lohman TG, Roche AF, Martorell R. 1988. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books [Google Scholar]
- 15. Albert PS, Hunsberger S. 2005. On analyzing circadian rhythms data using nonlinear mixed models with harmonic terms. Biometrics 61:1115–1120; discussion 1120–1122 [DOI] [PubMed] [Google Scholar]
- 16. Fehring RJ, Schneider M, Raviele K. 2006. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs 35:376–384 [DOI] [PubMed] [Google Scholar]
- 17. Lenton EA, Landgren BM, Sexton L, Harper R. 1984. Normal variation in the length of the follicular phase of the menstrual cycle: effect of chronological age. Br J Obstet Gynaecol 91:681–684 [DOI] [PubMed] [Google Scholar]
- 18. Lenton EA, Landgren BM, Sexton L. 1984. Normal variation in the length of the luteal phase of the menstrual cycle: identification of the short luteal phase. Br J Obstet Gynaecol 91:685–689 [DOI] [PubMed] [Google Scholar]
- 19. Landgren BM, Undén AL, Diczfalusy E. 1980. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol (Copenh) 94:89–98 [DOI] [PubMed] [Google Scholar]
- 20. Windham GC, Elkin E, Fenster L, Waller K, Anderson M, Mitchell PR, Lasley B, Swan SH. 2002. Ovarian hormones in premenopausal women: variation by demographic, reproductive and menstrual cycle characteristics. Epidemiology 13:675–684 [DOI] [PubMed] [Google Scholar]
- 21. Harlow SD, Baird DD, Weinberg CR, Wilcox AJ. 2000. Urinary oestrogen patterns in long follicular phases. Hum Reprod 15:11–16 [DOI] [PubMed] [Google Scholar]
- 22. Meyer PM, Zeger SL, Harlow SD, Sowers M, Crawford S, Luborsky JL, Janssen I, McConnell DS, Randolph JF, Weiss G. 2007. Characterizing daily urinary hormone profiles for women at midlife using functional data analysis. Am J Epidemiol 165:936–945 [DOI] [PubMed] [Google Scholar]
- 23. Venturoli S, Porcu E, Fabbri R, Paradisi R, Gammi L, Passarini M, Orsini LF, Flamigni C. 1986. Ovarian multifollicularity, high LH and androgen plasma levels, and anovulation are frequent and strongly linked in adolescent irregular cycles. Acta Endocrinol (Copenh) 111:368–372 [DOI] [PubMed] [Google Scholar]
- 24. Magyar DM, Boyers SP, Marshall JR, Abraham GE. 1979. Regular menstrual cycles and premenstrual molimina as indicators of ovulation. Obstet Gynecol 53:411–414 [PubMed] [Google Scholar]