Abstract
OBJECTIVE:
Ficus deltoidea leaves have been used in traditional medicine in Southeast Asia to treat diabetes, inflammation, diarrhea, and infections. The present study was conducted to assess the genotoxicity and acute and subchronic toxicity of a standardized methanol extract of F. deltoidea leaves.
METHODS:
Sprague Dawley rats were orally treated with five different single doses of the extract and screened for signs of toxicity for two weeks after administration. In the subchronic study, three different doses of the extract were administered for 28 days. Mortality, clinical signs, body weight changes, hematological and biochemical parameters, gross findings, organ weights, and histological parameters were monitored during the study. Genotoxicity was assessed using the Ames test with the TA98 and TA100 Salmonella typhimurium strains. Phytochemical standardization was performed using a colorimeter and high-performance liquid chromatography. Heavy metal detection was performed using an atomic absorption spectrometer.
RESULTS:
The acute toxicity study showed that the LD50 of the extract was greater than 5000 mg/kg. In the subchronic toxicity study, there were no significant adverse effects on food consumption, body weight, organ weights, mortality, clinical chemistry, hematology, gross pathology, or histopathology. However, a dose-dependent increase in the serum urea level was observed. The Ames test revealed that the extract did not have any potential to induce gene mutations in S. typhimurium, either in the presence or absence of S9 activation. Phytochemical analysis of the extract revealed high contents of phenolics, flavonoids, and tannins. High-performance liquid chromatography analysis revealed high levels of vitexin and isovitexin in the extract, and the levels of heavy metals were below the toxic levels.
CONCLUSION:
The no-observed adverse effect level of F. deltoidea in rats was determined to be 2500 mg/kg.
Keywords: Ficus deltoidea, Oral Toxicity, OECD, Genotoxicity, Isovitexin, Vitexin
INTRODUCTION
A number of studies have highlighted tremendous medical concerns through the systematic investigation of herbal remedies and their adverse effects on the vital organs of animals and humans (1,2). Anti-vitamins, anti-nutritional factors, immunomodulators, and heavy metals are among the potential toxic substances (3,4). Because of the absence of strict quality control and the complex mixture of the chemicals present in herbal medicines, there is limited knowledge available about the chemical compositions of these medicines and their effects on human physiology. This lack of data necessitates the thorough evaluation of the safety of medicinal herbs.
Ficus deltoidea (Moraceae), an epiphytic shrub, is widely distributed in Southeast Asian countries. In Malaysia, F. deltoidea is locally known as Mas cotek (5). Traditionally, this plant has been used in to treat inflammation and relieve pain. It is used to treat several diseases, including gout, high blood pressure, pneumonia, diarrhea, and skin infections (6). In addition, F. deltoidea has been used as an aphrodisiac, particularly to increase male fertility (7). Decoctions of the leaves of F. deltoidea have been extensively utilized in folk medicine to decrease the symptoms of diabetes mellitus, hyperlipidemia, and hypertension, and herbal healers often recommend the leaves of both male and female plants as libido boosters and postpartum treatments to strengthen the uterus (8). Studies have shown that F. deltoidea leaves possess antinociceptive, wound-healing, and anti-oxidant properties (6,9,10). The beneficial effects of F. deltoidea on hypertension, inflammation, and ulcers, its ability to inhibit carbohydrate-hydrolyzing enzymes, and its wound-healing, hepatoprotective, and antinociceptive activities have been verified (10-13). Despite the prevalent use of this plant as a food and medicine, the toxicity of F. deltoidea has not been fully explored. An aqueous extract of F. deltoidea leaves administered orally at 100 and 300 mg/kg/body weight has been shown not to cause any hematological or biochemical changes in rats (14). Although herbal medicines/dietary supplements are not covered under US-FDA drug-regulatory criteria because these products are considered safe, their safety profiles may not have been adequately documented. Hence, preclinical acute and subchronic toxicological evaluations using the Organisation for Economic Cooperation and Development (OECD) guidelines need to be undertaken to establish the safety profiles of drugs of herbal origin (15).
Few scientific data are available to validate the claims of folklore regarding the use of F. deltoidea as a remedy to treat various human ailments or to confirm the safety profile of repeated exposure to the extract of F. deltoidea leaves. To the best of our knowledge, there have been no genotoxicological studies to assess the safety of F. deltoidea. Thus, the present study was designed to evaluate the safety profile of a standardized methanol extract of F. deltoidea leaves (MEFL). Acute and 28-day subchronic oral toxicity tests were conducted in Sprague Dawley (SD) rats according to the OECD guidelines, and for the first time, the genotoxicity of MEFL was investigated using Salmonella typhimurium strains. In addition, qualitative and quantitative phytochemical analyses were performed colorimetrically. The quantitation of vitexin and isovitexin in MEFL was performed using HPLC. The detection of heavy metals in MEFL was conducted using atomic absorption spectrometry.
MATERIALS AND METHODS
Plant material and preparation of the extract
Leaves of F. deltoidea were purchased from HERBagus Sdn. Bhd., Malaysia. Taxonomical authentication was performed by a senior botanist, V. Shunmugam, and a voucher specimen (Ref. No. 11204) was deposited at the herbarium of the School of Biological Sciences, Universiti Sains Malaysia, Penang. The leaves of the plant were dried in an oven (37 °C) and powdered mechanically. The extract was prepared with 100 g of powdered material and 1 L of methanol using a Soxhlet extractor at 50 °C. The methanol extract (yield, 12% w/w) was filtered and evaporated to dryness under a vacuum. The residue was then lyophilized using a freeze drier (Labconco Cooperation, Denmark). The extract was stored at -80 °C until used.
High-performance liquid chromatography (HPLC)
Chemicals
HPLC-grade methanol and formic acid (Merck Chemicals, Germany) were used for the HPLC analysis. Two standards, vitexin and isovitexin (ChromaDex, USA), were used for the HPLC analysis.
HPLC analysis
The HPLC analysis of MEFL to determine the vitexin and isovitexin contents was performed according to the methodology of Fu et al. (16). This analysis was performed using an Agilent Technologies Series 1100 system equipped with a degasser, an autosampler, a column heater, a quaternary pump, and a UV detector. A reversed-phase Nucleosil C18 column (250 mm×4.6 mm, 5 μm) was maintained at 25 °C, and a 10 μl volume of injected sample was eluted using an isocratic mobile phase composed of methanol:water:formic acid (33:66.37:0.67 v/v/v) at a flow rate of 1 ml/min. The separation time was 30 min. The detection wavelength was 330 nm. Standard calibration curves were established by plotting the peak areas against different concentrations. The reference standards for vitexin and isovitexin were used to determine the retention times of these compounds and to spiked with the samples. The external standard method was used to quantify the bioactive markers in the sample of the extract.
Preparation of samples and standard solutions for HPLC analysis
A 100 mg portion of the methanol extract of F. deltoidea was dissolved in 25 ml of methanol and sonicated for 10-15 min. The contents were transferred to a 25 ml volumetric flask, and the volume was brought up to 25 ml. All samples were filtered through a 0.45 μm filter (Whatman). Similarly, the reference compounds were weighed (approximately 5 mg), each dissolved in 5 ml of methanol, and then filtered through a 0.45 μm filter (Whatman). The stock solution was used to prepare further dilutions. The samples were kept in a refrigerator at -20 °C prior to analysis.
Phytochemical screening and heavy metal analysis
The total contents of protein, polysaccharides, glycosaponins, phenolics, flavonoids, and tannins in MEFL were estimated colorimetrically (17,18). The total phenolic content was determined using the Folin-Ciocalteu reagent with gallic acid as a standard, and the results were expressed as mg of gallic acid equivalents. The total flavonoid content was determined using the AlCl3 colorimetric method with quercetin (QTN) as the standard, and the results were expressed as μg of QTN equivalents. The amount of total condensed tannins was expressed as (+)-catechin equivalents (CT, mg (+) catechin/g sample). The levels of lead (Pb), cadmium (Cd), arsenic (As), and mercury (Hg) in MEFL were determined using an atomic absorption spectrometer (Perkin Elmer, AAnalyst 800, Canada) according to the standard method of the British Pharmacopoeia 2008 (19).
Analysis of antimutagenic effects
The antimutagenic effects of MEFL at different concentrations (15.625 to 500 μg/well) were tested using the Salmonella typhimurium strains TA98 and TA100 for frameshift and base-pair substitution mutagenesis, respectively, with (indirect effect) and without (direct effect) metabolic activation. S. typhimurium TA100, TA98, TA1535, and TA1537 are the most commonly used strains for bacterial mutation assays within the pharmaceutical industry (20). 2-Nitrofluorene (2-NF) and 2-anthramine (2-AA, Chemtron, Singapore) were used as the indirect-acting mutagens in the metabolic activation system, and sodium azide phosphate (Chemtron, Singapore) was used as a direct-acting mutagen for TA98 or TA100. The broth (Oxoid, Malaysia) and reagents were prepared according to the method of Maron and Ames (21), and a preincubation mutagenicity test was performed (22). Moltox rat liver LS-9 (S9 mix, Chemtron, Singapore) was added in the indirect antimutagenic effect test to activate the metabolism of the mutagen. Incubated TA98 or TA100 cells (1×108 cells in 0.1 ml), the extract (100 μl), and the mutagen (10 μl) were mixed in a sterile test tube with a cap (12×75 mm). Sodium phosphate buffer (0.5 ml, 0.1 M, pH 7.4) was added to the direct mutagen–containing tubes, and 0.5 ml of 10% S9 mix was added to the indirect mutagen–containing tubes. After preincubation at 37 °C in a shaking water bath for 30 min, 2 ml of top agar containing 10% histidine/biotin solution was added and then spread on a minimal glucose agar plate. After the plates had been incubated at 37 °C for 48 h, the His+ revertant colonies were counted, and the percent inhibition induced by the extract treatment was calculated.
Experimental animals
SD rats of either sex (8 weeks of age) were obtained from the animal house of the School of Pharmaceutical Sciences, Universiti Sains Malaysia. The animals were housed under standard environmental conditions (temperature, 25 °C; humidity, 51%±10%) with a 12-h light–dark cycle and were provided a standard pellet diet (Gold Coin Holdings Sdn Bhd) and water ad libitum. The study was approved by the Animal Ethics Committee of Universiti Sains Malaysia, Penang, Malaysia [Protocol No: USM/Animal Ethics Approval/044/(58)].
Acute toxicity study in rats
Healthy adult female SD rats (200-225 g) were used in the acute toxicity study. The study was conducted according to the OECD guidelines for chemicals using a fixed-dose procedure (23). One group of rats was dosed by oral gavage with a single limit dose of 5,000 mg/kg MEFL dissolved in 0.5% carboxymethyl cellulose (CMC), and 0.5% CMC alone was administered to another group as a control. After this single administration, the animals were observed for signs of possible toxicity every hour for the first six hours and then every day for 14 days. All animals were weighed daily and monitored for any signs of toxicity and for mortality for up to 14 days. Food and water consumption were recorded daily. The rats were observed visually to identify the following: changes in the skin, fur, eyes, and mucous membranes; effects on the respiratory system, circulatory system, autonomic nervous system, and central nervous system; and changes in somatomotor activity and behavioral patterns. The animals were euthanized on the last day of experiment, and the LD50 values were estimated.
Subchronic toxicity study in rats
A subchronic repeated dose (28 days) study in rats was conducted according to the OECD testing guidelines (24). SD rats of both sexes were randomly distributed to four groups of six animals each. MEFL prepared in 0.5% CMC was orally administered daily for 28 days in single doses of 750 mg/kg (group I), 1250 mg/kg (group II), or 2500 mg/kg (group III). The control rats (group IV) received only vehicle (0.5% CMC). The body weight was recorded on days 0, 7, 14, and 28. Along with food and water consumption, signs of toxicity and mortality were also recorded daily throughout the study period. At the end of the experiment, all rats were anesthetized by carbon dioxide inhalation, and blood samples were collected via cardiac puncture into non-heparinized and EDTA-containing tubes for biochemical and hematological analyses. After blood collection, the animals were sacrificed by cervical dislocation, and their organs were isolated to assess histopathological changes. The liver, kidneys, adrenal glands, lungs, brain, spleen, heart, testes, ovaries, uterus, thymus, and gut were excised, weighed using an analytical lab balance (Mettler-Toledo AX-204, Japan), and examined macroscopically. These organs were then finally fixed in 10% buffered neutral formalin for histopathological examination.
Hematological and biochemical analyses
The following hematological parameters were analyzed using an automatic hematology analyzer (Sysmex-XT-1800 Germany): red blood cells (RBCs), white blood cells (WBCs), neutrophils, lymphocytes, eosinophils, monocytes, basophils, hemoglobin concentration (Hb), hematocrit (Ht), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelet count (Plt).
The following serum biochemical parameters were measured using a biochemistry autoanalyzer (Olympus 640 Japan): alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase, creatine phosphokinase, total protein, total albumin, albumin/globulin ratio, phosphorus, calcium, sodium, potassium, chloride, and total and conjugated bilirubin.
Histopathological analysis
For the histopathological analysis, three randomly selected rats in each experimental group were euthanized, and the organs listed above were harvested and fixed in 10% buffered neutral formalin for 48 hours and then in bovine solution for 6 hours. The fixed organs were processed for paraffin embedding. Sections (5 μm thick) were cut using a microtome, processed using an alcohol-xylene series, and stained with hematoxylin and eosin (25).
Statistical analysis
The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS 16.0 package). The data are given as the mean±S.E., and the analysis was performed using one-way analysis of variance (ANOVA). Significant differences between the control and treatment groups were identified using Dunnett's test. p-values of <0.05 and 0.01 were considered significant.
RESULTS
HPLC analysis of MEFL
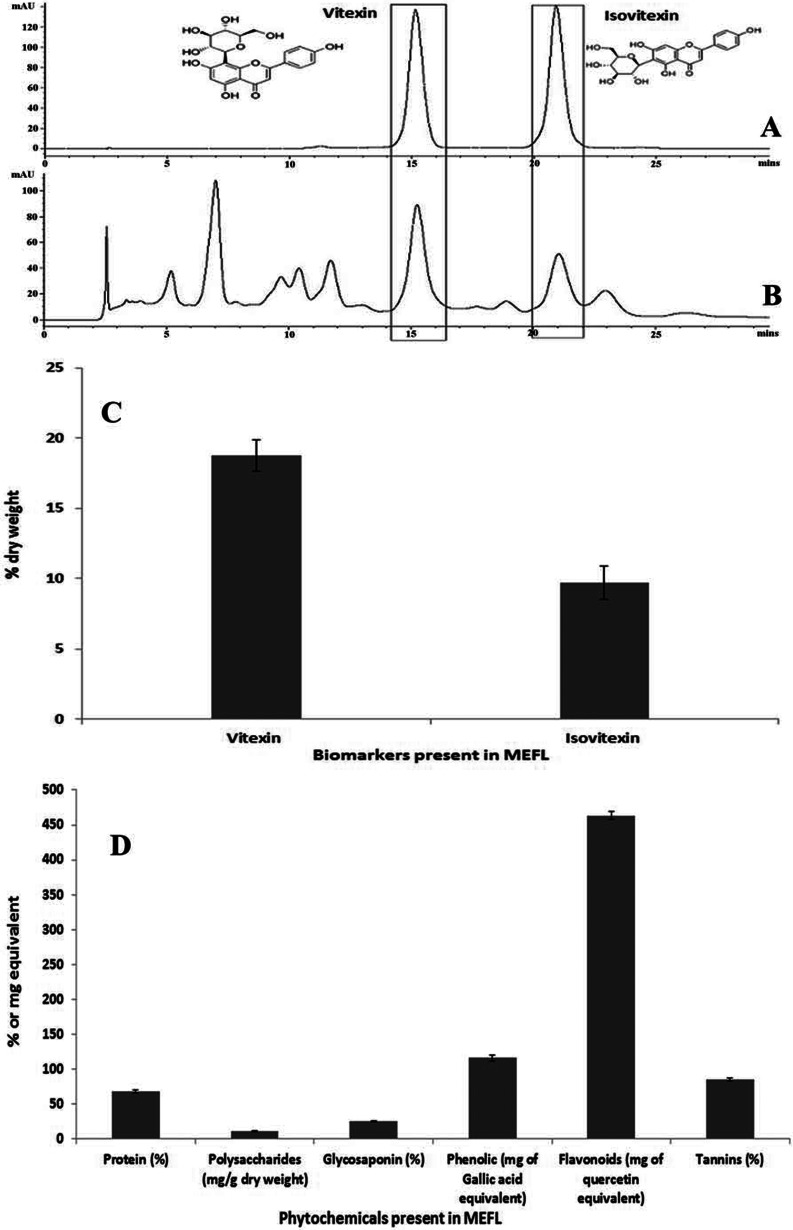
The HPLC chromatogram of the pure standards (Figure 1A) illustrated their Rf values and allowed the corresponding peaks in the MEFL chromatogram to be identified (Figure 1B). Vitexin accounted for 18.76%±1.12% of the dry weight of MEFL, and isovitexin accounted for 9.68%±1.18%. The results of this study are similar to those of previous studies suggesting that the flavone C-glycosides vitexin, and isovitexin are the major chemical constituents of MEFL along with other flavonoids (5). The chemical structures of the biomarkers used in this study are given in Figure 1. Good linearity and retention times and method validation using five-point calibration curves were obtained for all replicates. The quantitative results for the bioactive markers (% dry weight) are illustrated in Figure 1C. The concentrations in the samples were estimated based on the calibration curves for vitexin and isovitexin over the range of 5 to 200 μg/ml. The quantitative percentages of the dry weights of the standards were calculated using the formulas Y = 23.90X - 65.44 (R2 = 0.9992) and Y = 28.305X - 28.245 (R2 = 0.9982), respectively, where Y is the peak area for the analyte and X is the concentration of the analyte (μg/ml).
Figure 1.
HPLC chromatograms of MEFL and mixed standards of vitexin and isovitexin with detection at 330 nm. A) HPLC chromatogram of the standards (vitexin and isovitexin). B) HPLC chromatogram of MEFL highlighting the peaks corresponding to the standards at their respective Rf values. C) The contents of vitexin and isovitexin (% dry weight) present in the fractions of MEFL. D) Graphical representation of the phytochemical contents of MEFL. All values are expressed as the mean±S.E.M. (n = 6).
Phytochemical screening and heavy metal analysis of MEFL
The results of the quantitative analysis of the total contents of proteins, polysaccharides, glycosaponins, flavonoids, phenolics, and tannins present in MEFL are graphically depicted in Figure 1D. The results revealed that the levels of heavy metals such as cadmium (detected = 0.07 ppm, specification ≤0.1), mercury (not detected), and arsenic (detected = 0.4 ppm, Specification ≤0.4) in MEFL were below toxic levels (26). In contrast, lead had a level slightly higher (0.76 ppm) than the permitted limit (0.7 ppm).
Bacterial reverse mutation test
The Ames test was used to analyze the anti-mutagenic potential of MEFL. In this study, S. typhimurium strains TA98 and TA100 were used to measure the induction of frameshift and base-pair mutations, respectively. Mutagens make bacteria histidine independent, and thus, the mutated bacteria can form colonies on histidine-deficient medium. The mutagens used were either direct acting (NaN3 and 2-nitrofluorene) or required microsomal activation (2-AA). Adding antimutagenic agents considerably reduces the reverse mutation effects of mutagens.
The antimutagenic effects of MEFL were tested in S. typhimurium strains TA98 and TA100, both in the presence and absence of the S9 mix. The cytotoxicity of MEFL in S. typhimurium was preliminarily investigated in tests performed with TA100 using the plate pre-incubation method with or without the addition of the S9 mix. MEFL did not cause any decrease in the number of histidine+ revertant colonies compared with the negative control values obtained for the tester stains. Because MEFL exhibited no toxicity toward the tester strains, a concentration of 500 μg per plate was set as the upper limit of the concentration range tested. The test of the antimutagenic activity of MEFL was performed both in the absence of the S9 mix, in which NaN3 and 2-nitrofluorene were used as standard direct mutagens, and in the presence of the S9 mix, in which 2-AA was used as a standard indirect mutagen.
In both assays, no genotoxicity was noted at the tested concentrations. In the plate incorporation assay performed without rat liver S9 metabolic activation (Table 1), no biologically or statistically significant increase in the number of revertants was observed with the S. typhimurium TA98 or TA100 strain following treatment with MEFL at levels of 15.62 to 500 μg/well. In the pre-incubation test (Table 1), the assay with metabolic activation using the rat liver S9 fraction indicated that there was no statistically significant increase in the number of revertants for the S. typhimurium TA90 and TA100 strains. MEFL at concentrations up to 500 μg per plate did not increase the number of his+ revertant colonies over the negative control (Table 1). The results therefore indicated that MEFL was not mutagenic in the S. typhimurium mutagenicity assay.
Table 1.
Inhibitory effects of MEFL on direct mutagenicity induced by 2-nitrofluorene (NF) in TA98 cells or sodium azide phosphate (SA) in TA100 cells without the S9 mix.
| Direct | TA98 | TA100 | |||
| Concentration (μg/well)b | Number of revertants per plate±SD | % inhibition of mutation | Number of revertants per plate±SD | % inhibition of mutation | |
| 15.625 | 294±16 | 29 | 216±19 | 27 | |
| 31.25 | 322±8 | 18 | 247±6 | 15 | |
| 62.5 | 302±12 | 26 | 196±14 | 34 | |
| 125 | 228±25 | 56 | 238±8 | 19 | |
| 250 | 196±19 | 69 | 172±11 | 43 | |
| 500 | 163±7 | 82 | 133±31 | 58 | |
| SR | 119±21 | 19±5±8 | |||
| Sodium azide (0.5) | ---- | 288+7 | |||
| 2-Nitrofluorene | 366±41 | --- | |||
| Indirect | |||||
| 15.625 | 126±6 | 6 | 271±4 | 7 | |
| 31.25 | 121±9 | 12 | 279±16 | 3 | |
| 62.5 | 112±13 | 22 | 267±13 | 9 | |
| 125 | 86±24 | 49 | 224±11 | 31 | |
| 250 | 94±5 | 41 | 197±23 | 46 | |
| 500 | 79±7 | 57 | 164±8 | 63 | |
| SR | 39±5 | 93±2 | |||
| 2-anthramine (0.1) | 132±17 | 284±6 | |||
Values are the mean±S.E.M.
a Without the S9 mix.
b n = 3.
c With the S9 mix, n = 3.
Acute toxicity study
The acute toxicity study was performed according to OECD guideline 420, which specifies a limit test dose of 5000 mg/kg. No treatment-related mortality was observed at 5000 mg/kg, and throughout the 14-day observation period, there were no significant changes in behavior, such as apathy, hyperactivity, or morbidity, in any of the animals. No abnormal changes in body weight, respiration rate, or heart rate attributable to the treatment were noted. Ilyanie et al. (27) reported that no overt signs of acute toxicity or death were observed in mice and rats treated with a methanol extract of F. deltoidea up to the dose of 6400 mg/kg. In the present study, MEFL was found to be safe at a dose of 5000 mg/kg, and therefore, the LD50 value for oral toxicity was considered to be greater than 5000 mg/kg.
Subchronic toxicity study
Effects of 28 days of oral administration of MEFL on general behavior and hematological and biochemical parameters in rats.
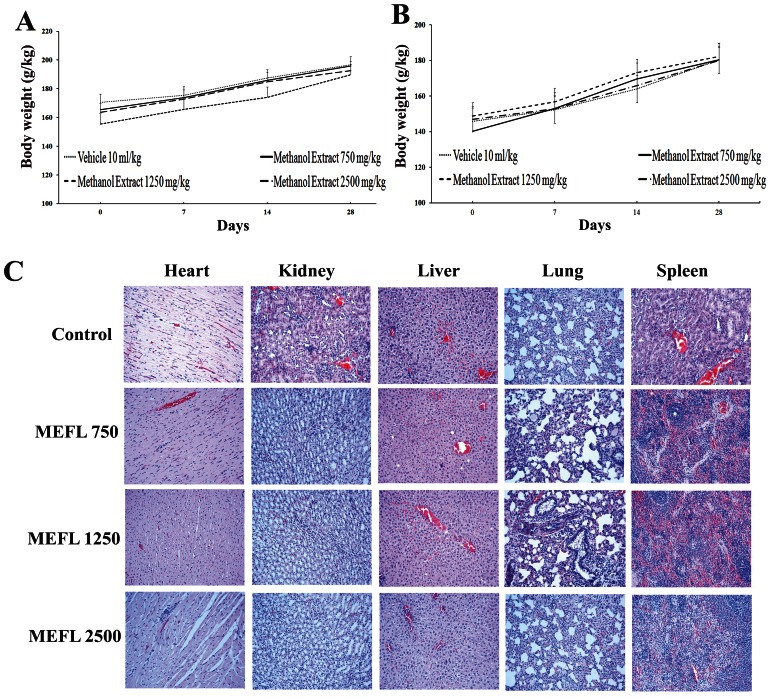
MEFL at doses of 750, 1250, and 2500 mg/kg administered orally every 24 hours for 28 days did not result in any mortality in the tested animals. No signs of observable toxicity were detected during the entire experimental period. The body weight gains in the treated groups were different from that in the control group, but the differences were not significant (Figures 2A and 2B). There were no differences in general behavior or food and water consumption between the treated groups of rats and the control group (data not shown). The effects of subchronic treatment on the hematological parameters are presented in Table 2). None of the parameters except the mean corpuscular hemoglobin (MCH) and packed cell volume (PCV) in female rats treated with 1250 mg/kg MEFL and the percentage of lymphocytes in female rats treated with 2500 mg/k showed a significant difference with respect to the untreated group. The changes in MCH and PCV were not dose dependent because they were only observed in the group treated with 1250 mg/kg, not in the group treated with the higher dose.
Figure 2.
Body weight changes of male (A) and female (B) SD rats during the 28-day toxicological assessment. The vehicle, 0.5% CMC (10 ml/kg/day), was administered to rats in the vehicle group. No significant differences were detected between the treated (750, 1250, 2500 mg/kg) and control (vehicle 10 ml/kg) groups. All values are expressed as the mean±S.E.M. (n = 5). Representative microscopic findings (C) for the heart, kidneys, liver, lungs, and spleen of SD rats treated orally with 750, 1250, or 2500 mg/kg MEFL or the vehicle for 28 days.
Table 2.
Effects of the subchronic oral administration of MEFL on hematological parameters in SD rats.
| Treatmenta | |||||
| Control | MEFL (mg/kg) | ||||
| 0 mg/kg | 750 | 1250 | 2500 | ||
| Male rats | |||||
| Hemoglobin | g/l | 145.25±2.21 | 140.8±0.20 | 141.16±3.55 | 140.40±2.70 |
| Total Red Blood Cells | 1012/l | 8.56±0.24 | 8.11±0.14 | 8.31±0.24 | 8.55±0.31 |
| Total White Blood Cells | 109/l | 7.25±4.81 | 6.97±1.16 | 4.45±1.69 | 5.96±2.05 |
| Neutrophils | % | 33.25±6.99 | 35.50±2.42 | 38.25±6.99 | 37.20±6.01 |
| Lymphocytes | % | 58.50±6.10 | 56.67±2.78 | 58.50±6.13 | 57.20±5.11 |
| Eosinophils | % | 2.75±0.50 | 3.00±0.37 | 3.50±0.15 | 2.55±0.25 |
| Monocytes | % | 6.00±20.10 | 5.00±0.48 | 6.33±2.50 | 4.80±1.40 |
| Basophils | % | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Packed Cell Volume | % | 45.00 ±0.81 | 44.65±0.99 | 41.00±0.86 | 43.80±0.99 |
| Mean Corpuscular Volume | fl | 54.50±0.57 | 55.17±0.65 | 56.83±0.40 | 57.80±0.80*) |
| Mean Corpuscular Hb | pg | 17.66±0.50 | 18.37±0.19 | 18.33±0.5 | 18.60±0.80*) |
| Mean Corpuscular Hb Conc | g/l | 328.75±6.65 | 326.9±0.5 | 325.66±7.99 | 321.00±4.47 |
| Platelet Count | 109/l | 851.25±146.64 | 775.5±83.7 | 678.5±125.27*) | 738.20±108.88 |
| Female rats | |||||
| Hemoglobin | g/l | 149.66±4.35 | 141.20±0.83 | 143.50±5.42 | 140.50±3.32 |
| Total Red Blood Cells | 1012/l | 8.18±0.7 | 7.81±0.20 | 8.14±0.40 | 8.04±0.50 |
| Total White Blood Cells | 109/l | 15.40±2.02 | 15.49±0.75 | 15.51±3.80 | 15.53±1.02 |
| Neutrophils | % | 19.56±3.28 | 23.16±2.47 | 28.66±2.83 | 23.40±2.08 |
| Lymphocytes | % | 75.50±3.39 | 67.17±2.03 | 65.33±9.10 | 63.80±4.10*) |
| Eosinophils | % | 3.80±0.07 | 3.00±0.51 | 5.80±0.50 | 3.80±0.07 |
| Monocytes | % | 6.16±0.31 | 6.3.00±1.53 | 6.80±0.48 | 6.70±0.45 |
| Basophils | % | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Packed Cell Volume | % | 48.16±0.30 | 45.25±0.33 | 45.00±0.53*) | 44.00±0.18*) |
| Mean Corpuscular Volume | fl | 59.00±0.17 | 57.67±0.42 | 56.00±0.14*) | 57.40±0.19 |
| Mean Corpuscular Hb | pg | 18.50±0.54 | 18.40±0.14 | 17.66±0.51*) | 18.20±0.44 |
| Mean Corpuscular Hb Conc | g/l | 310.16±5.49 | 313.0±4.30 | 315.83±4.26 | 313.00±2.54 |
| Platelet Count | 109/l | 980.16±25.49 | 860.3±24.4 | 977.50±20.26 | 772.60±21.50 |
Values are the mean ±S.E.M., a n = 6.
p<0.05.
The biochemical profiles of the treated and control groups are shown in Table 3). The oral administration of MEFL for up to 28 days did not cause significant changes in total protein, albumin, globulin, the albumin/globulin ratio, total bilirubin, alkaline phosphatase, AST, ALT, ALP, gamma glutamyl transferase, potassium, sodium, chloride, creatinine, or uric acid. However, a dose-dependent increase in the level of serum urea was observed in male rats. In a similar subchronic toxicity study (27), it was observed that the methanolic extract of F. deltoidea leaves at a dose of 200 mg/kg did not cause any abnormal changes as reflected by the liver and renal function tests, whereas in the present study, the higher doses (1250 and 2500 mg/kg) induced significant changes in the serum urea level.
Table 3.
Effects of the subchronic oral administration of MEFL on biochemical parameters in SD rats.
| a Treatment | |||||
| Control | MEFL (mg/kg) | ||||
| 0 mg/kg | 750 | 1250 | 2500 | ||
| Male rats | |||||
| Total Protein | g/l | 74.50±1.88 | 76.33±1.09 | 68.66±2.6 | 69.6±1.82 |
| Albumin | g/l | 31.75±1.58 | 33.33±0.95 | 33.33±0.95 | 32.33±1.58 |
| Globulin | g/l | 38.17±0.87 | 36.00±0.63 | 35.67±1.02 | 36.33±1.50 |
| Albumin/Globulin Ratio | 0.75±0.05 | 0.93±0.03 | 0.93±0.03 | 0.84±0.05 | |
| Total Bilirubin | μmol/l | <2 | <2 | <3 | <2 |
| Alkaline Phosphatase | U/l | 406.25±58.74 | 382.50±83.11 | 334.83±68.08 | 351.0±69.54 |
| Alanine Aminotransferase | U/l | 62.50±10.37 | 77.00±12.56 | 71.66±17.42 | 69.80±14.88 |
| Aspartate Aminotransferase | U/l | 248.00±10.42 | 241.50±12.45 | 239.50±8.45 | 252.40±9.82 |
| Gamma Glutamyl Transferase | U/l | <3 | <3 | <3 | <3 |
| Urea | mmol/l | 6.07±0.35 | 6.18±0.83*) | 7.12±0.27*) | 7.50±0.39**) |
| Potassium | mmol/l | 6.30± 0.06 | 5.08±0.05 | 5.90±0.03 | 6.26±0.02 |
| Sodium | mmol/l | 141.50±0.31 | 139.17±0.40 | 139.5±0.40 | 140.80±0.48 |
| Chloride | mmol/l | 98.75±5.49 | 100.83 ±4.31 | 101.00±6.63 | 101.25± 2.10 |
| Creatinine | μmol/l | 30.50±1.72 | 30.00±1.99 | 31.16± 1.16 | 26.20±1.27 |
| Uric Acid | μmol/l | 0.17±0.02 | 0.16±18.35 | 0.14±0.05 | 0.13±0.04 |
| Female rats | |||||
| Total Protein | g/l | 78.50±1.23 | 77.83±1.66 | 76.20±2.48 | 71.23±2.89 |
| Albumin | g/l | 33.83±0.98 | 35.00±0.68 | 34.00±1.39 | 33.17±0.60 |
| Globulin | g/l | 47.66±0.79 | 44.20±2.94 | 44.20±2.94 | 43.2±1.12 |
| Albumin/Globulin Ratio | 0.65±0.10 | 0.77±0.03 | 0.74±0.02 | 0.64±0.05 | |
| Total Bilirubin | μmol/l | <2 | <2 | <2 | <2 |
| Alkaline Phosphatase | IU/l | 334.33±5.05 | 415.83±4.44 | 339.80±11.50 | 416.8±21.25 |
| Alanine Aminotransferase | U/L | 102.33±2.67 | 118.50±3.29 | 101.83±4.45 | 111.33±2.17 |
| Aspartate Aminotransferase | U/L | 266.33± 13.94 | 266.83±4.09 | 267.17±14.86 | 270.6±15.19 |
| Gamma Glutamyl Transferase | U/L | <3 | <3 | <3 | <3 |
| Urea | mmol/l | 7.16±0.35 | 8.30±0.26*) | 10.30±0.28**) | 7.16±0.17 |
| Potassium | mmol/l | 4.48±0.10 | 4.42±0.07 | 4.57±0.08 | 4.33±0.11 |
| Sodium | mmol/l | 138.83±1.72 | 143.33±0.95 | 136.33±1.00 | 134.83±1.65 |
| Chloride | mmol/l | 101.83±1.45 | 99.60±1.67 | 100.00±0.52 | 98.00±1.06 |
| Creatinine | μmol/l | 29.50±2.24 | 26.33±1.50 | 27.20±2.68 | 23.20±2.38 |
| Uric Acid | μmol/l | 0.20±0.05 | 0.20±54.09 | 0.19±0.05 | 0.20±0.09 |
Values are the mean ±S.E.M., n = 6.
p<0.05,
p<0.01.
All the tested hematological parameters, including hemoglobin, total blood count, total white blood cells, neutrophils, lymphocytes, eosinophils, monocytes, basophils, packed cell volume, mean corpuscular volume, mean corpuscular Hb, mean corpuscular Hb concentration, and platelet count, were within the normal range.
Effects of 28 days of oral treatment with MEFL on histopathological parameters in rats
The results of the histopathological studies provided evidence supporting the findings of the biochemical analysis. No histopathological abnormalities were detected in the heart, liver, spleen, kidneys, or lungs of the control group. Histopathological sections of heart, liver, spleen, kidneys, and lungs are shown in Figure 2C. No lesions or pathological changes related to treatment with MEFL were observed in the organs of the animals from the treatment groups, except in the lungs, where there was evidence of mild inflammation. Nevertheless, the treatment-related results were very similar to those for the control group.
Effects of 28 days of oral treatment with MEFL on the organ weights of the rats
The weights of the organs of the control and treated rats are shown in Table 4). There were no significant differences in the organ weights between the treated groups and the control group.
Table 4.
Effects of the subchronic oral administration of MEFL on organ weights in SD rats.
| Organ weight g | a Treatment | |||
| Control | MEFL (mg/kg) | |||
| 0 mg/kg | 750 | 1250 | 2500 | |
| Male rats | ||||
| Brain | 0.49±0.02 | 0.51±0.04 | 0.51±0.03 | 0.51±0.02 |
| Heart | 0.98±0.06 | 0.82±0.01 | 0.79±0.04*) | 0.88±0.04 |
| Liver | 8.96±0.75 | 8.11±0.22 | 8.11±0.13 | 8.76±0.07 |
| Thymus | 0.27±0.10 | 0.23±0.03 | 0.23±0.01 | 0.27±0.02 |
| Spleen | 0.21±0.02 | 0.21±0.03 | 0.27±0.02 | 0.29±0.02 |
| Kidney (right) | 0.37±0.01 | 0.20±0.01 | 0.24±0.1 | 0.32±0.01 |
| Kidney (left) | 0.38±0.01 | 0.30±0.01 | 0.25±0.01 | 0.34±0.01 |
| Adrenal Gland (right) | 0.03±0.00 | 0.02±0.00 | 0.02±0.00 | 0.03±0.00 |
| Adrenal Gland (left) | 0.03±0.00 | 0.03±0.00 | 0.03±0.00 | 0.03±0.00 |
| Lungs | 1.41±0.02 | 1.44±0.02 | 1.21±0.02 | 1.34±0.03 |
| Testis (right) | 0.55±0.02 | 0.55±0.02 | 0.54±0.00 | 0.53±0.02 |
| Testis (left) | 0.56±0.01 | 0.52±0.02 | 0.56±0.01 | 0.54±0.02 |
| Stomach | 3.78±0.50 | 4.24±0.21 | 3.64±0.17 | 3.75±0.20 |
| Stomach (empty) | 1.32±0.01 | 1.513±0.01 | 1.28±0.02 | 1.38±0.01 |
| Gut | 11.50±0.47 | 12.52±0.51 | 11.59 ±.43 | 13.43 ±.53*) |
| Gut (empty) | 6.72±0.24 | 7.97±0.20 | 6.93±0.19 | 8.63 ±.24*) |
| Female rats | ||||
| Brain | 0.48±0.03 | 0.49±0.03 | 0.50±0.02 | 0.48±0.03 |
| Heart | 0.70±0.01 | 0.70±0.01 | 0.72±0.01 | 0.70±0.01 |
| Liver | 7.28±0.55 | 7.23±0.22 | 7.56±0.19 | 7.65±0.21 |
| Thymus | 0.27±0.02 | 0.23±0.01 | 0.24±0.01 | 0.20±0.01 |
| Spleen | 0.20±0.02 | 0.21±0.02 | 0.21± 0.02 | 0.23±0.02 |
| Kidney (right) | 0.39±0.01 | 0.31±0.01 | 0.39±0.01 | 0.34±0.01 |
| Kidney (left) | 0.29±0.02 | 0.30±0.01 | 0.29±0.01 | 0.28±0.01 |
| Adrenal Gland (right) | 0.03±0.001 | 0.03± 0.002 | 0.03±0.001 | 0.03±0.001 |
| Adrenal Gland (left) | 0.03±0.002 | 0.03±0.002 | 0.03±0.002 | 0.03±0.002 |
| Lungs | 1.87±0.02 | 1.59±0.05 | 1.69±0.03 | 1.85±0.02 |
| Ovary (right) | 0.06±0.01 | 0.06±0.002 | 0.06±0.004 | 0.05±0.006 |
| Ovary (left) | 0.06±0.02 | 0.05±0.04 | 0.05±0.03 | 0.05±0.02 |
| Uterus | 0.19±0.01 | 0.19±0.01 | 0.19±0.01 | 0.19±0.05 |
| Stomach | 3.85±0.32 | 3.34±0.17 | 3.28±0.17 | 2.72±0.26*) |
| Stomach (empty) | 1.29±0.07 | 1.33±0.03 | 1.37±0.03 | 1.33±0.03 |
| Gut | 10.17±0.48 | 11.15±0.41 | 11.50±0.17 | 10.46±0.26 |
| Gut (empty) | 5.99±0.18 | 4.64±0.20 | 5.30±0.031 | 6.44±0.03 |
Values are the mean ±S.E.M., a n = 6.
p<0.05.
DISCUSSION
Despite the popularity of medicinal plants, few scientific studies have been undertaken to determine the safety of traditional medicinal herbs. To determine the safety of medicines and plant products intended for human consumption, systematic toxicological studies must be performed using various experimental models to predict the toxicity and to set criteria for selecting a safe dose in humans. Most often, toxicity in animals and humans manifests in the form of adverse hematological, gastrointestinal or cardiovascular effects, and certain adverse health effects are correlated with structural rearrangements of the genome caused by different types of DNA damage. The evaluation of the adverse effects of single and repeated dosing in experimental animals and the study of mutagenicity using mutant strains of bacteria may be more relevant in determining the overall toxicity of plant preparations.
The pharmacological properties of F. deltoidea are widely known. Despite the widespread use of F. deltoidea in traditional medicine, there are insufficient data regarding its toxicity. Therefore, the objective of the present study was to assess the oral toxicity and genotoxicity of MEFL in rodents and mutant strains of S. typhimurium, respectively. In the acute toxicity assay, oral treatment with MEFL was well tolerated. A dose of 5000 mg/kg MEFL administered to female rats did not cause signs of toxicity, changes in behavior, or mortality. Any substance with an LD50 between 5000 and 15,000 mg/kg is considered non-toxic (28). Thus, in the present study, MEFL could be characterized as non-toxic because the LD50 for this extract was found to be greater than 5000 mg/kg. Although the LD50 does not predict the lethal dose in humans, it provides a guide for choosing a dose for use in subchronic studies. The daily administration of the lower dose in the toxicity study provides some indication of the long-term toxicity of MEFL. The results of the subchronic (28-day) toxicity study of MEFL demonstrated that there was no mortality and no change in the normal behavior or general condition of the treated rats. These results indicate that MEFL is safe even at the highest studied dose (2500 mg/kg). In the MEFL-treated animals, the body weight gain was not significantly different from that of the control group, suggesting that MEFL did not alter food intake through appetite suppression. The weights of the major organs did not significantly differ from those of the control group. This result implies that MEFL is non-toxic to these organs, even after 28 days of exposure.
Treatment with MEFL did not alter the hematological profile. Significant differences (p<0.05) were found in the lymphocyte count, MCV, and PVC in female animals treated with 1250 and 2500 mg/kg MEFL. Because no corresponding changes were observed in the other parameters, the significant changes in the MCV and PCV may be attributed to differences in the volumes of the collected blood samples. The number of lymphocytes was significantly (p<0.05) reduced in female rats treated with the dose of 2500 mg/kg, indicating that the defense mechanisms are likely altered at this dose in female rats. However, the differential leukocyte counts for eosinophils and monocytes remained within the reference value range (29), which strongly suggests that there is no relation to treatment with MEFL. Almost all biochemical parameters analyzed remained within the reference levels for the species (29). However, a dose-dependent increase in the serum urea level was observed in male rats; this increase could be related to renal overload. As an increase in the plasma level of urea is indicative of renal overload, acute renal failure or an increase in protein catabolism (30). A previous subchronic study (27) found that the oral administration of the extract at a lower dose (200 mg/kg) did not induce abnormal changes in the serum urea level. This result suggests that high doses of the extract may contribute to renal overload.
When the plasma membranes of liver cells are damaged, a variety of enzymes located in the cytosol are released into the bloodstream. The levels of these enzymes in the serum are quantitative measures of the extent and type of hepatocellular damage. The lack of alteration in the liver parameters (alkaline phosphatase, aspartate transaminase, alanine transaminase, lactate dehydrogenase, creatine phosphokinase, total protein, albumin/globulin ratio, and bilirubin) showed that the administration of MEFL for 28 days is not toxic to the liver. Furthermore, the results showed that the indicators of kidney function (creatinine, uric acid, phosphorus, calcium, sodium, potassium, and chloride) remained unaffected. Thus, it is reasonable to assume that the subchronic administration of MEFL did not cause any damage to the liver or the kidneys.
These results were confirmed by the histopathological examination of selected organs (heart, liver, lungs, spleen, and kidneys) harvested from treated and control animals. This analysis revealed normal architecture for all vital organs. In the liver parenchyma of animals treated with MEFL at doses up to 2500 mg/kg, normal-sized cells with a centrally located euchromatic nucleus and a very prominent nucleolus were observed. The hepatic vascular distribution was homogeneous when compared with that of the control group (Figure 2C, with a normal hepatic portal triad. All vital organs studied had a normal histological architecture except the lungs, which exhibited signs of an inflammatory state, with the infiltration of lymphocytes accompanied by enlarged alveolar macrophages in the air spaces for both the control and treated groups (Figure 2C). These morphological changes in the lungs were most likely caused by the daily oral gavage and not by MEFL itself because these alterations were also observed in the control group. The histological studies suggest that there are no obvious detrimental effects or morphological disturbances caused by the daily oral administration of MEFL for 28 days, even at the highest tested dose of 2500 mg/kg.
The results from the genotoxicity assay showed that, even at a very high concentration (5000 μg per plate), MEFL did not increase the number of histidine revertant colonies over the negative control in the tester strains TA100 and TA98, either in the presence or absence of S9 metabolic activation. Because the standard mutagens used in this study (2-NF, 2-AA, sodium azide phosphate) induced a clear positive response, the above results indicate that MEFL was not mutagenic in this assay. The absence of mutagenicity for MEFL in the tested S. typhimurium strains indicates that MEFL does not affect the structural integrity of DNA. In addition, no toxic effects associated with heavy metals in MEFL were expected because the contents of heavy metals were below the toxic ranges, with the exception of the lead content. The content of lead in MEFL was slightly higher than the acceptable limit. A high lead content can impair the normal functions of the brain and nervous system, and lead tends to displace vital minerals such as calcium in the body (31). Nevertheless, the administration of MEFL did not cause any lead-associated toxicity in rats. Signs or symptoms of toxicity manifest only when the level of lead is above 0.9 or 1 ppm (32). Therefore, the level of lead detected in MEFL can be considered the safe upper limit.
Phytochemical screening revealed the presence of phenolics, flavonoids, tannins, glycosaponins, and proteins in MEFL. The HPLC analysis further showed that in addition to these classes of chemical constituents, MEFL also contained remarkably high levels of isovitexin and vitexin. These two compounds are C-glycosyl flavones, which are known to be a rich source of biologically active antioxidants (33) and have received much attention recently because of their diverse pharmacological properties. Studies conducted to elucidate the mechanisms of protection against mutagens have found that the presence of phenolic and flavonoid compounds can suppress the toxicity and genotoxicity of toxins because phenolic and flavonoid compounds can readily scavenge free radicals or activate antioxidant enzyme cascades.
Based on our results, the oral administration of MEFL appears to be well tolerated by SD rats. MEFL seemed to have no discernible clinically significant toxic effects on the nervous system, respiratory system, or other physiological functions of animals of both sexes after acute and subchronic administration. MEFL treatment had inconsistent effects on body growth, organ weights, and hematological and biochemical parameters, and these effects failed to be supported by the gross and histopathologic assessments of the major organs.
The no-observed adverse effect level (NOAEL) for the 28-day study with MEFL was considered to be over 2500 mg/kg/day. This finding suggests that adverse health effects would not be expected at lower levels of daily MEFL exposure. Additionally, these findings could aid in the pharmacological evaluation of plant preparations using this route of administration in in vivo experimental models, and they provide reasonable and comprehensive preclinical evidence of the safety of MEFL, which is necessary to conduct phase I clinical trials on this standardized plant extract. However, it should be noted that this NOAEL was derived only from a subchronic study. Because the observed effects in animal studies alone cannot always be extrapolated to the effects in humans, clinical studies are necessary to precisely define the safe human dosage.
MEFL was not mutagenic in the AMES Salmonella/microsome assay. Furthermore, no heavy metals were detected in MEFL that could eventually be responsible for metal toxicity. Altogether, these results indicate that the mammalian toxicity of F. deltoidea extract is low and that its use in traditional medicine presents no genotoxic risks to humans.
To conduct a more reliable safety assessment based on the acceptable daily intake criteria, data on the long-term chronic toxicity, reproductive toxicity, and carcinogenicity of MEFL should also be collected.
The findings reported herein indicate that the acute and subchronic (28 day) oral administration of MEFL is safe at the doses (750, 1250, and 2500 mg/kg body weight/day in SD rats) tested in this study. In summary, the administration of MEFL for 28 days did not cause death or visible signs of toxicity in any animals. Moreover, MEFL did not have mutagenic effects even at extremely high concentrations in S. typhimurium strains. The HPLC analysis of MEFL revealed that vitexin and isovitexin were present at high levels. The heavy metal analysis of MEFL showed the absence of toxic levels of heavy metals. Cumulatively, these findings suggest that the standardized methanol extract of F. deltoidea can be considered devoid of acute and subchronic toxicity and genotoxicity. These data suggest that the consumption of F. deltoidea extract poses no threat of potential health risks. However, the increased level of serum urea suggests that a chronic administration study is necessary to evaluate the renal toxicity of F. deltoidea.
ACKNOWLEDGMENTS
The authors are grateful to the School of Pharmaceutical Sciences, Universiti Sains Malaysia, for providing financial and technical support.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Ernst E. Harmless herbs? A review of the recent literature. Am J Med. 1998;104(2):170–8. doi: 10.1016/s0002-9343(97)00397-5. [DOI] [PubMed] [Google Scholar]
- 2.Pak E, Esrason KT, Wu VH. Hepato-toxicity of herbal remedies: An emerging dilemma. Prog Transplant. 2004;14(2):91–6. doi: 10.1177/152692480401400203. [DOI] [PubMed] [Google Scholar]
- 3.Akueshi EU, Sabo AE, Ogugbuaja VO. Micronutrient and trace content of calyx and bud of Bomboax buonopozenes (P.Beauv.) Bioresearch. 2005;3:43–65. [Google Scholar]
- 4.Okafor PN, Okoronkwo CO, Maduagwu EN. Occupational and dietary exposure of humans to cyanide poisoning from large scale cassava processing and ingestion of cassava food. Food Chem Toxicol. 2002;40(7):1001–5. doi: 10.1016/s0278-6915(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 5.Zunoliza A, Hussain K, Zhari I, Rasadah MA, Mazura P, Jamaludin F, et al. Evaluation of extracts of leaf of three Ficus deltoidea varieties for antioxidant activities and secondary metabolites. Phcog Res. 2009;4(1):216–23. [Google Scholar]
- 6.Hakiman M, Mazziah M. Non enzymatic and enzymatic antioxidant activities in aqueous extract of different Ficus deltoidea accessions. J Med Plants Res. 2009;3(3):120–31. [Google Scholar]
- 7.Adam Z, Hamid M, Ismail A, Khamis S. Effect of Ficus deltoidea aqueous extract on blood glucose level in normal and mild diabetic rats. Malaysian J Health Sci. 2007;5(2):9–16. [Google Scholar]
- 8.Sulaiman MR, Hussain MK, Zakaria ZA, Somchit MN, Moin S, Mohamad AS, et al. Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia. 2008;79(7-8):557–61. doi: 10.1016/j.fitote.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Zahra MA, Mahmood AA, Hapipah MA, Suzita MN, Salmah I. Anti-ulcerogenic activity of aqueous extract of Ficus deltoidea against ethanol-induced gastric mucosal injury in rats. Res J Med Sci. 2009;3(2):42–6. [Google Scholar]
- 10.Abdulla MA, Khaled AA, Faisal MA, Mazin M. Role of Ficus deltoidea extract in the enhancement of wound healing in experimental rats. J Biomed Res. 2010;21(3):241–5. [Google Scholar]
- 11.Abdullah Z, Hussain K, Ismail Z, Ali RM. Anti-inflammatory activity of standardised extracts of leaves of three varieties of Ficus deltoidea. Asian J Pharm Clin Res. 2009;1(3):100–5. [Google Scholar]
- 12.Farsi E, Shafaei A, Hor SY, Ahamed MBK, Yam MF, Idress HA, et al. Correlation between enzymes inhibitory effects and antioxidant activities of standardized fractions of methanolic extract obtained from Ficus deltoidea leaves. Afr J Biotechnol. 2011;10(67):15184–94. [Google Scholar]
- 13.Adam Z, Hamid M, Ismail A, Khamis S. Effect of Ficus deltoidea extracts on hepatic basal and insulin-stimulated glucose uptake. J Biol Sci. 2009;9(8):796–803. [Google Scholar]
- 14.Fazliana MS, Muhajir H, Hazilawati H, Shafii K, Mazleha M. Effects of Ficus deltoidea aqueous extract on hematological and biochemical parameters in rats. Med J Malaysia. 2008;63(Supplement A):103–104. [PubMed] [Google Scholar]
- 15.Jadeja RN, Thounaojam MC, Ansarullah Snehal VJ, Mitul DP, Dipak KP, Sunita PS, et al. Toxicological evaluation and hepatoprotective potential of Clerodendron glandulosum Coleb leaf extract. Hum Exp Toxicol. 2011;30(1):63–70. doi: 10.1177/0960327110368420. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y, Zu Y, Liu W, Zhang L, Tong M, Efferth T, et al. Determination of vitexin and isovitexin in pigeonpea using ultrasonic extraction followed by LC-MS. J Sep Sci. 2008;31(2):268–75. doi: 10.1002/jssc.200700312. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui M, Hafizoh S, Ismail Z, Sahib H, Helal M, Abdul Majid A. Analysis of total proteins, polysaccharides and glycosaponins contents of orthosiphon stamineus Benth. in spray and freeze dried methanol: water (1: 1) extract and its contribution to cytotoxic and antiangiogenic activities. Pharmacogn Res. 2009;1(5):320–26. [Google Scholar]
- 18.Ahamed MB, Aisha AF, Nassar ZD, Siddiqui JM, Ismail Z, Omari SM, et al. Cat's whiskers tea (Orthosiphon stamineus) extract inhibits growth of colon tumor in nude mice and angiogenesis in endothelial cells via suppressing VEGFR phosphorylation. Nutr Cancer. 2012;64(1):89–99. doi: 10.1080/01635581.2012.630160. [DOI] [PubMed] [Google Scholar]
- 19.British Pharmacopoeia Commission. London: The Stationary Office; 2008. [Google Scholar]
- 20.Gatehouse D, Haworth S, Cebula T, Gocke E, Kier L, Matsushima T, et al. Recommendations for the performance of bacterial mutation assays. Mutat Res. 1994;312(3):217–33. doi: 10.1016/0165-1161(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 21.Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 22.Mastsushima T, Sugimura T, Nagao M, Yahagi T, Shirai A, Sawamura M. Factors modulating mutagenicity in microbial tests. In: Nor-poth K H, Gamer R C, editors. Short-Term System for Detecting Gzrcinogens. Berlin Verlag: Springer; 1980. pp. 273–85. [Google Scholar]
- 23.OECD: Acute oral toxicity, guideline 420, the OECD guideline for testing of chemical. 2001 doi: 10.1787/9789264070943-en. [DOI] [Google Scholar]
- 24.OECD: Acute oral toxicity, guideline 407, the OECD guideline for testing of chemical. 2006 doi: 10.1787/9789264070684-en. [DOI] [Google Scholar]
- 25.Galigher AE, Kayloff EN. Essential of practical microtechnique. Philadelphia: Lea and Febiger; 1971. [Google Scholar]
- 26.European pharmacopoeia. Council of Europe. 2002:3238–3239. [Google Scholar]
- 27.Ilyanie Y, Wong TW, Choo CY. Evaluation of hypoglycemic activity and toxicity profiles of the leaves of Ficus deltoidea in rodents. J Complement Integr Med. 2011;8(1) doi: 10.2202/1553-3840.1469. doi: 10.2202/1553-3840.1469. [DOI] [PubMed] [Google Scholar]
- 28.Zbinden G, Flury-Roversi M. Significance of the LD <sub>50</sub>-test for the toxicological evaluation of chemical substances. Arch Toxicol. 1981;47(2):77–99. doi: 10.1007/BF00332351. [DOI] [PubMed] [Google Scholar]
- 29.Harkness SE, Wagner JE. Biologia e Clínica de Coelhos Roedores. 3rd edition. São Paulo: Livraria Roca; 1993. pp. 48–55. [Google Scholar]
- 30.Adebayo JO, Yakubu MT, Egwim EC, Owoyele VB, Enaibe BU. Effect of ethanolic extract of Khaya senegalensis on some biochemical parameters of rat kidney. J Ethnopharmacol. 2003;88(1):69–72. doi: 10.1016/s0378-8741(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 31.Haider S, Naithani V, Barthwal J, Kakkar P. Heavy metal content in some therapeutically important medicinal plants. Bull Environ Contam Toxicol. 2004;72(1):119–27. doi: 10.1007/s00128-003-0249-0. [DOI] [PubMed] [Google Scholar]
- 32.Blagojevic N, Damjanović-Vratnica B, Vukašinović-Pešić V, Durovic D. Heavy Metals Content in Leaves and Extracts of Wild-Growing Salvia Officinalis from Montenegro. J Environ Studies. 2009;18(2):167–73. [Google Scholar]
- 33.Osawa T, Katsuzaki H, Hagiwara Y, Hagiwara H, Shibamoto T. A novel antioxidant isolated from young green barley leaves. J Agric Food Chem. 1992;40(7):1135–8. [Google Scholar]