Abstract
OBJECTIVE:
The expression of transcription factors involved in early pituitary development, such as PROP1 and POU1F1, has been detected in pituitary adenoma tissues. In this study, we sought to characterize the transcriptional profiles of PROP1, POU1F1, and TBX19 in functioning and nonfunctioning pituitary adenomas in an attempt to identify their roles in tumorigenesis and hormone hypersecretion.
METHODS:
RT-qPCR analyses were performed to assess the transcriptional pattern of PROP1, POU1F1, TBX19, and hormone-producing genes in tissue samples of corticotrophinomas (n = 10), somatotrophinomas (n = 8), and nonfunctioning adenomas (n = 6).
RESULTS:
Compared with normal pituitary tissue, POU1F1 was overexpressed in somatotrophinomas by 3-fold. PROP1 expression was 18-fold higher in corticotrophinomas, 10-fold higher in somatotrophinomas, and 3-fold higher in nonfunctioning adenomas. TBX19 expression was 27-fold higher in corticotrophinomas. Additionally, the level of TBX19 mRNA positively correlated with that of pro-opiomelanocortin (r = 0.49, p = 0.014).
CONCLUSIONS:
Our data demonstrate that PROP1 is overexpressed in pituitary adenomas, mainly in corticotrophinomas. Together with previously published data showing that patients who harbor PROP1 loss-of-function mutations present a progressive decline in corticotrope function, our results support a role for PROP1 in pituitary tumor development and in the maintenance of cell lineages committed to corticotrophic differentiation.
Keywords: Pituitary Neoplasms, PROP1, POU1F1, TBX19
INTRODUCTION
The expression of transcription factors involved in early pituitary development, such as PROP1 and POU1F1, has been detected in pituitary adenoma tissues (1-5). Mutations in these genes have been implicated in the etiology of combined pituitary hormone deficiency and are associated with pituitary hypoplasia or hyperplasia (6,7), based on Magnetic Resonance Imaging (MRI) findings. In adult mice, the constitutive expression of Prop1 results in delayed gonadotrophin production and pituitary tumor development (8). It has been shown that the mutated POU1F1 protein decreases GH and PRL hypersecretion in the rat pituitary tumor cell line GH4C1 in vitro. Additionally, reduced GH secretion and tumor growth is observed in xenografts obtained after the transplantation of GH4C1 cells expressing mutant POU1F1 (9).
In this study, we sought to characterize the transcriptional profiles of PROP1, POU1F1, and TBX19 in functioning and nonfunctioning pituitary adenomas in an attempt to identify their roles in tumorigenesis and hormone hypersecretion.
MATERIALS AND METHODS
Patients and tumor characterization – The cohort was composed of 24 patients (17 female, 7 male) with positive clinical, laboratory, and imaging tests for the diagnosis of pituitary tumors (Table 1). Ten patients had Cushing's disease (7 female, 3 male); 4 cases were due to microadenomas, 5 cases were due to macroadenomas, and 1 patient developed Nelson's syndrome. These patients were aged between 15 and 35 years at the time of surgery. Eight patients (4 female, 4 male) were diagnosed with acromegaly caused by macroadenomas and underwent surgery when they were between 33 and 71 years of age. Six patients were diagnosed with non-functional macroadenomas (3 female, 3 male) and were between 47 and 66 years of age at the time of surgery.
Table 1.
Clinical and pathological features of the tumor samples.
| Patient | Clinical features | Sex | Age (years) | Tumor size (cm) | Immunohistochemistry1) |
| 1 | Nelson's Syndrome | F | 33 | >1.0 | ACTH 3+ |
| 2 | Cushing's Disease | F | 35 | 1.8 | ACTH 3+ |
| 3 | Cushing's Disease | M | 16 | 0.9 | ACTH 2+ |
| 4 | Cushing's Disease | M | 18 | 1.0 | ACTH 3+ |
| 5 | Cushing's Disease | F | 27 | 2.0 | ACTH 3+ |
| 6 | Cushing's Disease | F | 24 | 1.0 | ACTH 3+ |
| 7 | Cushing's Disease | F | 34 | 0.6 | ACTH 2+ |
| 8 | Cushing's Disease | F | 34 | 1.0 | ACTH 2+ |
| 9 | Cushing's Disease | M | 15 | 0.4 | ACTH 2+ |
| 10 | Cushing's Disease | F | 19 | 0.6 | ACTH 2+ |
| 11 | Acromegaly | M | 71 | 1.7 | GH 3+, FSH 1+ |
| 12 | Acromegaly | M | 45 | 6.4 | GH 3+, PRL 3+ |
| 13 | Acromegaly | M | 33 | 1.2 | GH 3+, LH 2+, FSH 2+ |
| 14 | Acromegaly | M | 40 | 5.9 | GH 2+, PRL 1+ |
| 15 | Acromegaly | F | 37 | 2.2 | GH 3+, LH 1+, TSH 1+, PRL 3+ |
| 16 | Acromegaly | F | 59 | 1.1 | GH 3+ |
| 17 | Acromegaly | F | 53 | 2.2 | GH 3+, FSH 1+, TSH 1+ |
| 18 | Acromegaly | F | 54 | 7.0 | GH 3+, FSH 1+ |
| 19 | Nonfunctioning adenoma | M | 51 | 2.5 | TSH 1+ |
| 20 | Nonfunctioning adenoma | F | 50 | 2.6 | TSH 2+ |
| 21 | Nonfunctioning adenoma | M | 56 | 3.4 | LH 1+, FSH 1+ |
| 22 | Nonfunctioning adenoma | F | 66 | 3.3 | - |
| 23 | Nonfunctioning adenoma | F | 47 | 2.4 | - |
| 24 | Nonfunctioning adenoma | M | 62 | 6.0 | GH 1+, ACTH 1+ |
Immunostaining was performed using anti-pituitary hormone antibodies. Immunostaining was evaluated on each slide using a semiquantitative scale as follows: -, no immunostaining; 1+, 1 to 10% immunoreactive cells; 2+, 11 to 50% immunoreactive cells; 3+, >50% immunoreactive cells.
RT-qPCR – Following surgical resection of the tumors, total RNA was extracted using TRIzol (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's protocol. Total RNA pooled from 88 human pituitary glands (Clontech, Mountain View, CA, USA) was used as a control. We used 5 μg of total tumor RNA or total human pituitary gland RNA (Clontech) for cDNA synthesis using the RT2 First Strand Kit (SABiosciences/Qiagen, Frederick, MD, USA), including the genomic DNA decontamination step. Real-time PCR reactions were performed using the RT2 Profiler™ PCR Array (CAPH09297A; SABiosciences/Qiagen) and SYBR Green I as the DNA-binding dye to analyze the transcriptional profile of POU1F1 (NM_001122757) and PROP1 (NM_006261) and hormone producing genes (GH - NM_000515, POMC - NM_001035256, PRL - NM_000948, TSHB - 000549, FSHB - NM_000510, LH - NM_000894 and CGA - NM_000735). The first-strand cDNA was subjected to PCR under the following conditions: 95°C for 10 min and 40 amplification cycles of 95°C for 15 s and 60°C for 1 min, followed by a melting curve analysis. The expression levels of POUF1 and PROP1 relative to those in the normal pituitary gland were calculated by the 2-ΔΔCT method using the Ct geometric means of eight endogenous genes (ACTB, NM_001101; B2M, NM_004048; GAPDH, NM_002046; HPRT1, NM_000194; PGK1, NM_000291; PPIA, NM_021130; RPL13A, NM_012423; and TBP, NM_003194).
The transcriptional level of TBX19 (also known as TPIT) was also analyzed in the tumor samples using a TaqMan® Gene Expression Assay (TBX19 FAM, Hs01113611_m1; Applied Biosystems, Carlsbad, CA, USA). We used 2 μg of total tumor RNA or total human pituitary gland RNA (Clontech) for cDNA synthesis using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The PCR protocol consisted of 50°C for 2 min, denaturation at 95°C for 10 min, followed by 40 amplification cycles of 95°C for 15 s and 60°C for 60 s. The expression of TBX19 in the tumor samples relative to that in the normal pituitary gland was calculated using the 2-ΔΔCT method using the Ct geometric means of three endogenous genes (PPIA, Hs99999904_m1; PGK1, Hs99999906_m1; and HPRT1, Hs99999909_m1). The TaqMan® Gene Expression Assay consisted of hybridization probes that span the exon/exon boundaries. Genomic DNA was used as a negative control.
Genes with a fold change of ≥2.5 were considered to be differentially expressed. The PCR reactions were performed with an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA). Quantitative measurements were performed in quadruplicate.
Statistical analysis – The relative expression values of PROP1, POU1F1, and TBX19 were analyzed by one-way ANOVA. Bonferroni correction was applied to assess the significant difference between the tumoral groups, and the differences were considered statistically significant at a p-value of <2.5% (p<0.025). Pearson's correlation coefficient was applied to assess the relationship of PROP1, POU1F1, and TBX19 with the expression of the hormone-producing genes GH, ACTH, PRL, TSH, FSH, LH, and αGSU. The statistical analyses were performed using BioEstat 5.0 software (National Institute of Space Research – INPE, São José dos Campos/SP, Brazil).
ETHICS
All participants provided written informed consent, and the protocol was approved by the Human Research Ethics Committee of the Medical School of the University of São Paulo. The clinical investigations were conducted according to the principles in the Declaration of Helsinki.
RESULTS
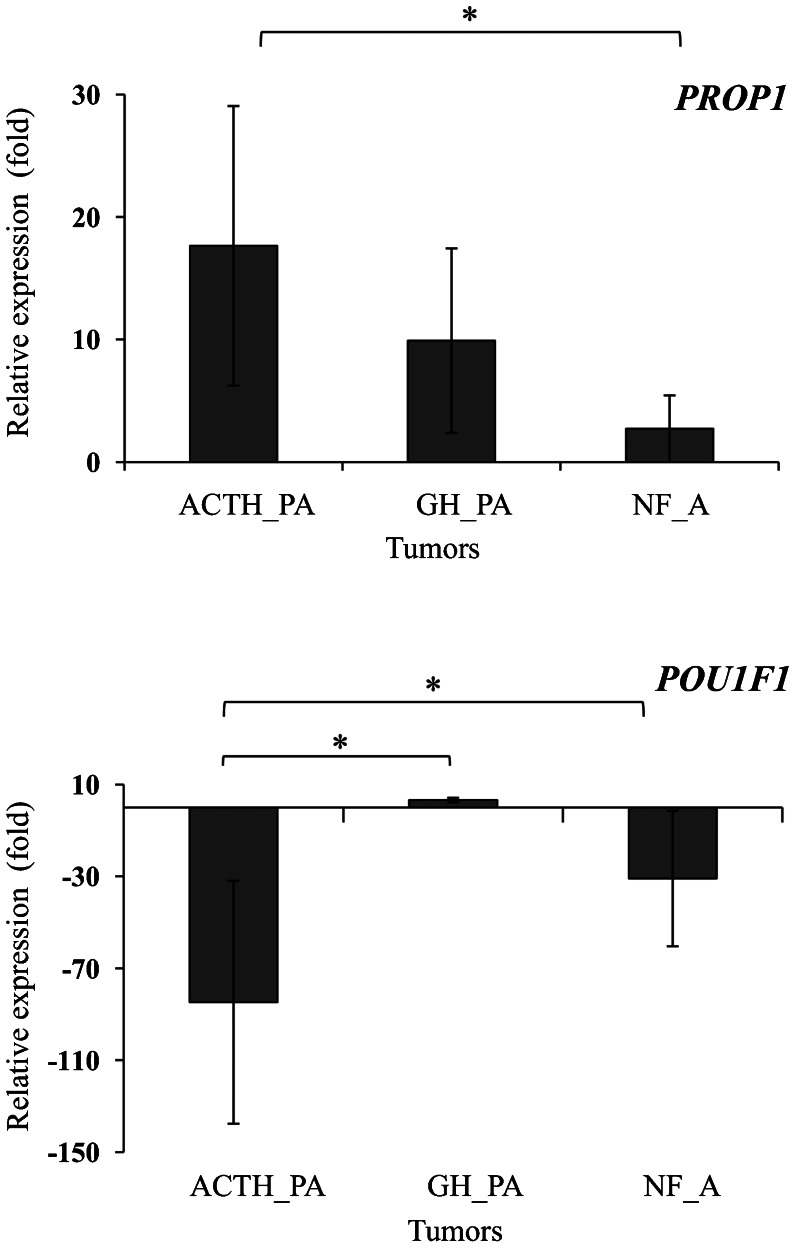
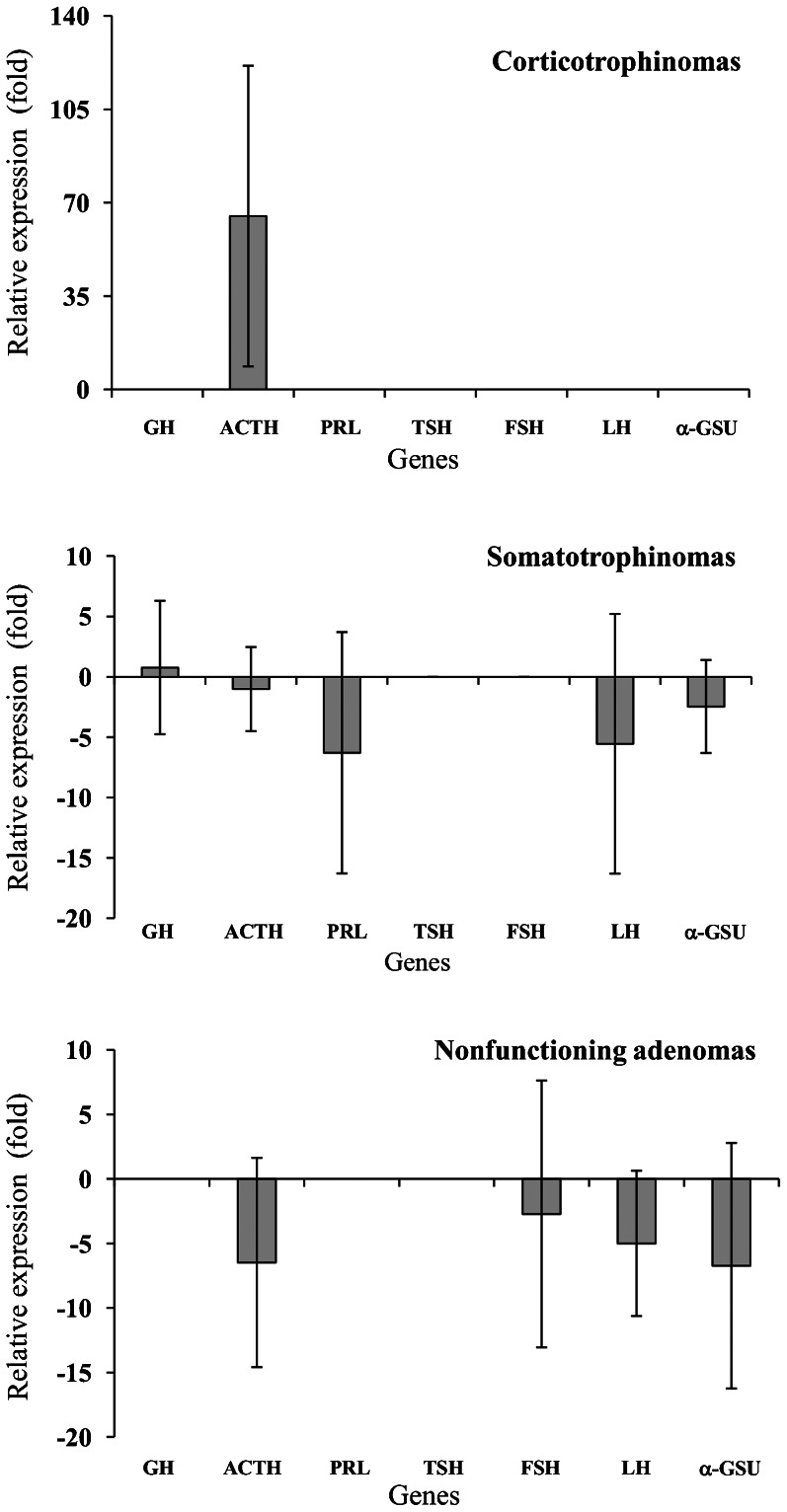
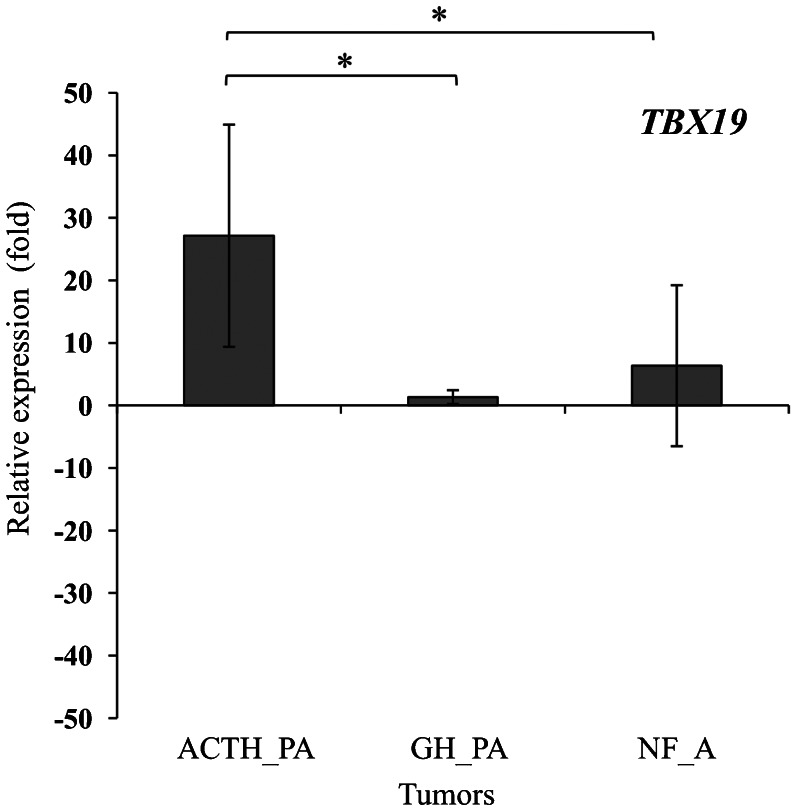
Analysis of the RT-qPCR array showed that PROP1 was overexpressed in the tumor samples compared to the control. The expression of PROP1 was approximately 18-fold higher in the corticotrophinomas, 10-fold higher in the somatotrophinomas, and 3-fold higher in the nonfunctioning adenomas (ANOVA: F = 6.8, p = 0.005). On the other hand, POUIF1 was overexpressed only in the somatotrophinomas (3-fold higher; ANOVA: F = 5.0, p = 0.016) (Figure 1). Pro-opiomelanocortin (POMC), the peptide precursor of adrenocorticotropin (ACTH), was overexpressed in the corticotrophinomas (65-fold higher), while GH was constitutively expressed in almost all of the somatotrophinoma samples. The expression of hormone-producing genes was downregulated in the nonfunctioning adenomas (Figure 2). Because PROP1 was upregulated in the corticotrophinomas, the mRNA level of TBX19 (which is only expressed in cells committed to corticotrope and melanotrope differentiation) was analyzed using a TaqMan® Gene Expression Assay in the same samples analyzed with the RT-qPCR array (Figure 3). As expected, the expression of TBX19 in the corticotrophinomas was, on average, 27-fold higher than in the control (ANOVA: F = 9.6, p = 0.001). The somatotrophinomas exhibited constitutive expression of TBX19, while the nonfunctioning adenomas presented a heterogeneous pattern of TBX19 expression. Additionally, the TBX19 mRNA levels positively correlated with POMC expression (r = 0.49, p = 0.014).
Figure 1.
Transcriptional profile of PROP1 and POU1F1 in pituitary adenomas. Analysis of the RT-qPCR array showed that PROP1 expression was 18-fold higher in the corticotrophinomas, 10-fold higher in the somatotrophinomas, and 3-fold higher in the nonfunctioning adenomas. POU1F1 was overexpressed only in the somatotrophinomas (3-fold increase). The results are expressed as the means ± SD of quadruplicate assays. *p<0.025. ACTH_PA, ACTH-producing adenomas; GH_PA, GH-producing adenomas; NF_A, nonfunctioning adenomas.
Figure 2.
Transcriptional profile of hormone-producing genes in pituitary adenomas. POMC expression was 65-fold higher in the corticotrophinomas. GH was constitutively expressed in almost all of the somatotrophinoma samples analyzed. The expression of hormone-producing genes was downregulated in the nonfunctioning adenomas. The results are expressed as the means ± SD of quadruplicate assays.
Figure 3.
Transcriptional profile of TBX19 in pituitary adenomas. RT-qPCR analyses showed that TBX19 expression was 27-fold higher in the corticotrophinomas. The results are expressed as the means ± SD of quadruplicate assays. *p<0.025. ACTH_PA, ACTH-producing adenomas; GH_PA, GH-producing adenomas; NF_A, nonfunctioning adenomas.
DISCUSSION
Although pituitary adenomas do not usually metastasize, these tumors result in high morbidity due to their intracranial localization and/or excessive hormone production. In this study, the transcriptional profile of genes involved in early pituitary embryogenesis was analyzed in pituitary adenomas, aiming to identify their role in tumorigenesis and hormone hypersecretion.
TBX19 is present in normal pituitary glands, POMC-expressing corticotropes that form in the anterior lobe, and melanotropes that form in the intermediate lobe (10-12). POU1F1 is essential for the development of the somatotrope, lactotrope, and thyrotroph cell lineages in the anterior pituitary and for the subsequent expression of GH, PRL, and α-TSH (13-15). In this study, TBX19 and POU1F1 were found to be overexpressed in corticotrophinomas and somatotrophinomas, respectively, as has been previously demonstrated (16-18).
The expression pattern of hormone-producing genes was related to the functional category of the adenomas. ACTH was overexpressed in the corticotrophinomas, and GH was constitutively expressed in most of the somatotrophinomas that were investigated in this study. The constitutive expression of GH in somatotrophinomas has previously been shown by microarray (4).
Interestingly, we found that the expression of PROP1 was higher in the corticotrophinomas than in the somatotrophinomas. This increased PROP1 expression in corticotrophinomas was unexpected because Prop1 is not associated with corticotrope differentiation, according to studies in murine models (15). However, since our first report in 1999 (7), a progressive decline in cortisol levels has been reported in a number of patients with PROP1 loss-of-function mutations (19).
Although PROP1 gene expression in human pituitary adenomas, including in corticotrophinomas (1), has been confirmed, these results were only qualitatively demonstrated using semiquantitative RT-PCR analysis. In contrast, our work is the first to demonstrate the differential expression of PROP1 in corticotrophinomas using quantitative RT-PCR.
The increased expression of PROP1 in corticotrophinomas that was observed in the present study supports a role for PROP1 in the maintenance of cells committed to corticotrophic differentiation.
In conclusion, our data show that PROP1 is overexpressed in pituitary adenomas, mainly in corticotrophinomas, supporting a role for this transcription factor in pituitary tumor development and also in the maintenance of cells committed to corticotrophic differentiation.
ACKNOWLEDGMENTS
We acknowledge Dr. Ericka B. Trarbarch, Beatriz M. P. Mariani, and Dr. Luciana P. Brito for their technical assistance with the tumor collection and RNA extractions. This study was supported by Fundação Faculdade de Medicina (FFM) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Nakamura S, Ohtsuru A, Takamura N, Kitange G, Tokunaga Y, Yasunaga A, et al. Prop-1 gene expression in human pituitary tumors. J Clin Endocrinol Metab. 1999;84:2581–4. doi: 10.1210/jcem.84.7.5974. [DOI] [PubMed] [Google Scholar]
- 2.Evans CO, Moreno CS, Zhan X, McCabe MT, Vertino PM, Desiderio DM, et al. Molecular pathogenesis of human prolactinomas identified by gene expression profiling, RT-qPCR, and proteomic analyses. Pituitary. 2008;11(3):231–45. doi: 10.1007/s11102-007-0082-2. [DOI] [PubMed] [Google Scholar]
- 3.Moreno CS, Evans CO, Zhan X, Okor M, Desiderio DM, Oyesiku NM. Novel molecular signaling and classification of human clinically nonfunctional pituitary adenomas identified by gene expression profiling and proteomic analyses. Cancer Res. 2005;65(22):10214–22. doi: 10.1158/0008-5472.CAN-05-0884. [DOI] [PubMed] [Google Scholar]
- 4.Evans CO, Young AN, Brown MR, Brat DJ, Parks JS, Neish AS, et al. Novel patterns of gene expression in pituitary adenomas identified by complementary deoxyribonucleic acid microarrays and quantitative reverse transcription-polymerase chain reaction. J Clin Endocrinol Metab. 2001;86(7):3097–107. doi: 10.1210/jcem.86.7.7616. [DOI] [PubMed] [Google Scholar]
- 5.Melmed S. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest. 2003;112(11):1603–18. doi: 10.1172/JCI20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turton JP, Mehta A, Raza J, Woods KS, Tiulpakov A, Cassar J, et al. Mutations within the transcription factor PROP1 are rare in a cohort of patients with sporadic combined pituitary hormone deficiency (CPHD) Clin Endocrinol (Oxf) 2005;63(1):10–8. doi: 10.1111/j.1365-2265.2005.02291.x. [DOI] [PubMed] [Google Scholar]
- 7.Mendonca BB, Osorio MG, Latronico AC, Estefan V, Lo LS, Arnhold IJ. Longitudinal hormonal and pituitary imaging changes in two females with combined pituitary hormone deficiency due to deletion of A301,G302 in the PROP1 gene. J Clin Endocrinol Metab. 1999;84(3):942–5. doi: 10.1210/jcem.84.3.5537. [DOI] [PubMed] [Google Scholar]
- 8.Cushman LJ, Watkins-Chow DE, Brinkmeier ML, Raetzman LT, Radak AL, Lloyd RV, et al. Persistent Prop1 expression delays gonadotrope differentiation and enhances pituitary tumor susceptibility. Hum Mol Genet. 2001;10(11):1141–53. doi: 10.1093/hmg/10.11.1141. [DOI] [PubMed] [Google Scholar]
- 9.Roche C, Rasolonjanahary R, Thirion S, Goddard I, Fusco A, Figarella-Branger D, et al. Inactivation of transcription factor pit-1 to target tumoral somatolactotroph cells. Hum Gene Ther. 2012;23(1):104–14. doi: 10.1089/hum.2011.105. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Lin C, Gleiberman A, Ohgi KA, Herman T, Huang HP, et al. Tbx19, a tissue-selective regulator of POMC gene expression. Proc Natl Acad Sci U S A. 2001;98:8674–9. doi: 10.1073/pnas.141234898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev. 2003;17(6):738–47. doi: 10.1101/gad.1065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen B, Rosenfeld MG. POU domain factors in the neuroendocrine system: lessons from developmental biology provide insights into human disease. Endocr Rev. 2001;22(1):2–35. doi: 10.1210/edrv.22.1.0421. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes SJ, DiMattia GE, Rosenfeld MG. Transcriptional mechanisms in anterior pituitary cell differentiation. Curr Opin Genet Dev. 1994;4(5):709–17. doi: 10.1016/0959-437x(94)90138-s. [DOI] [PubMed] [Google Scholar]
- 14.Vankelecom H. Pituitary stem/progenitor cells: embryonic players in the adult gland. Eur J Neurosci. 2010;32(12):2063–81. doi: 10.1111/j.1460-9568.2010.07523.x. [DOI] [PubMed] [Google Scholar]
- 15.Savage JJ, Yaden BC, Kiratipranon P, Rhodes SJ. Transcriptional control during mammalian anterior pituitary development. Gene. 2003;319:1–19. doi: 10.1016/s0378-1119(03)00804-7. [DOI] [PubMed] [Google Scholar]
- 16.Asa SL, Puy LA, Lew AM, Sundmark VC, Elsholtz HP. Cell type-specific expression of the pituitary transcription activator pit-1 in the human pituitary and pituitary adenomas. J Clin Endocrinol Metab. 1993;77(5):1275–80. doi: 10.1210/jcem.77.5.8077321. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd RV, Jin L, Chandler WF, Horvath E, Stefaneanu L, Kovacs K. Pituitary specific transcription factor messenger ribonucleic expression in adenomatous and nontumorous human pituitary tissues. Lab Invest. 1993;69(5):570–5. [PubMed] [Google Scholar]
- 18.Tateno T, Izumiyama H, Doi M, Yoshimoto T, Shichiri M, Inoshita N, et al. Differential gene expression in ACTH -secreting and non-functioning pituitary tumors. Eur J Endocrinol. 2007;157(6):717–24. doi: 10.1530/EJE-07-0428. [DOI] [PubMed] [Google Scholar]
- 19.Bottner A, Keller E, Kratzsch J, Stobbe H, Weigel JF, Keller A, et al. PROP1 mutations cause progressive deterioration of anterior pituitary function including adrenal insufficiency: a longitudinal analysis. J Clin Endocrinol Metab. 2004;89(10):5256–65. doi: 10.1210/jc.2004-0661. [DOI] [PubMed] [Google Scholar]