Abstract
Development of drugs targeting Bcl-2 relatives and caspases, for treating diseases including cancer and inflammatory disorders, often involves measuring interactions with recombinant target molecules, and/or monitoring cancer cell killing in vitro. Here, we present yeast-based methods for evaluating drug-mediated inhibition of Bcl-2 relatives or caspases. Active Bax and caspases kill Saccharomyces cerevisiae, and pro-survival Bcl-2 proteins can inhibit Bax-induced yeast death. By measuring the growth or adenosine triphosphate content of transformants co-expressing Bax with pro-survival Bcl-2 relatives, we found that the Bcl-2 antagonist drugs ABT-737 or ABT-263 abolished Bcl-2 or Bcl-xL function and reduced Bcl-w activity, but failed to inhibit Mcl-1, A1 or the poxvirus orthologs DPV022 and SPPV14. Using this technique, we also demonstrated that adenoviral E1B19K was resistant to these agents. The caspase inhibitor Q-VD-OPh suppressed yeast death induced by caspases 1 and 3. Yeast engineered to express human apoptotic regulators enable simple, automatable assessment of the activity and specificity of candidate drugs targeting Bcl-2 relatives or caspases.
Keywords: apoptosis, Bcl-2, caspase, cerevisiae, drug development, BH3-mimetic
Progress in understanding the molecular mechanisms governing apoptosis, and their perturbation during oncogenesis, has facilitated the development of anti-cancer drugs that directly activate apoptotic pathways. Many cancers overexpress pro-survival members of the Bcl-2 family.1 These proteins suppress the pro-apoptotic activity of other family members, Bax and Bak, either through direct interaction and/or by sequestering death promoting ‘Bcl-2 homology 3 (BH3)-only' relatives. Pro-survival Bcl-2 relatives prevent Bax/Bak-mediated release from the mitochondria of apoptogenic molecules including cytochrome c. Cytosolic cytochrome c complexes with Apaf-1, dATP and pro-caspase 9 to form the ‘apoptosome'. Caspase 9 becomes activated in this complex, empowering it to proteolytically activate caspases 3 and 7, which cleave numerous proteins to destroy the cell.2 Bcl-2 was initially identified through its overexpression in follicular lymphoma, but researchers have since discovered that Bcl-2 and its pro-survival relatives Bcl-xL, Bcl-w, A1, Mcl-1 and Bcl-B are upregulated in a wide variety of cancer types,1 presumably due to selection for cancer cells that withstand the pro-apoptotic pressures of oncogenic transformation and metastatic spread.3
Anti-cancer agents have been developed that inhibit or downregulate Bcl-2-like proteins. The BH3-mimetic drug ABT-263/Navitoclax4 and its pre-clinical precursor ABT-737,5 induce apoptosis in a Bax/Bak-dependent manner,6, 7, 8 implying that they induce apoptosis via antagonism of Bcl-2-like pro-survival proteins. Both agents strongly inhibited the binding of Bcl-2, Bcl-xL and Bcl-w to BH3 peptides in vitro (inhibition constant (Ki)=∼1 nM), but exhibited much weaker binding to Mcl-1 and A1 (half maximal inhibitory concentration (IC50) >350 nM).5, 9 The potency of these drugs can be influenced by the levels, localization and post-translational modifications of various Bcl-2 family members. ABT-737 and ABT-263 most effectively killed tumor cells whose survival depended upon Bcl-2 sequestration of BH3-only proteins such as Bim.10, 11 Cell-based studies confirmed the resistance of A1 and Mcl-1 to inhibition by ABT-737 and ABT-263, but demonstrated that Bcl-w conferred resistance to ABT-263/737.10, 12, 13 Platelet levels fell in patients treated with ABT-263,14 consistent with an established role for Bcl-xL in megakaryocyte survival.15 Enforced expression of Bcl-xL sensitized some cell types,13 but not others,11 to ABT-737.
Many lymphoma and leukemia patients responded well to ABT-263.14, 16 However, inhibition of Bcl-xL led to thrombocytopenia in patients treated with high doses of ABT-263.14, 16 Overexpression of ABT-263-resistant Bcl-2 relatives such as A1 and Mcl-1 rendered some tumors resistant to ABT-263.17 Hence, the ability of ABT-263 to promote regression of some tumors demonstrated the utility of targeting the Bcl-2 family for cancer treatment, but the drug's limitations highlight the need to develop other BH3-mimetics with distinct specificities.
Other agents have been proposed to antagonize different subsets of pro-survival Bcl-2 relatives from ABT737/263. Obatoclax mesylate (GX15-070) bound to all pro-survival Bcl-2 relatives in aqueous solution with similar low affinity (IC50=1–7 μM).18 TW-37 bound to purified Mcl-1 and Bcl-xL with intermediate affinity (Ki=260–290 nM) but with lower affinity to Bcl-2 (1.1 μM).19 HA14-1 could efficiently displace a Bak-derived BH3 peptide from Bcl-2, Bcl-xL and Bcl-w (Ki=169, 59 and 59 nM, respectively).20 To our knowledge, the ability of HA14-1 to interact with Mcl-1 and A1 has not been explored. Evidence suggests that these agents may not always act solely through antagonism of Bcl-2 and its close relatives.7, 8, 21, 22, 23
Diseases other than cancer also feature alterations in apoptotic signaling. Many viruses express inhibitors of pro-apoptotic host proteins to block defensive host cell death. Some DNA viruses encode Bcl-2-like inhibitors of the intrinsic apoptosis pathway,24, 25 and many poxviruses express cross-class serpins that inhibit caspase 1, caspase 8 and granzyme B.25 It is conceivable that drugs targeting these anti-apoptotic proteins may prevent viral propagation and may conceivably be useful prophylactic or therapeutic anti-viral agents.
Caspase-targeting drugs have also been developed recently. Ischemic events, neurodegeneration and liver disease could be ameliorated by suppression of apoptotic caspase activity.26 Inhibitors of apoptotic caspases may help minimize cell death during organ transplantation.27 Suppressing non-apoptotic caspases such as caspase 1, which catalyzes the maturation of interleukins-1β and -18, could be useful for treating inflammatory diseases.26, 28
Anti-cancer drugs are frequently identified via their ability to kill cancer cell lines. Compounds can also be assessed in vitro for binding to purified target molecules and/or disruption of biochemical interactions. Although these methods can undoubtedly yield useful drugs, they have limitations. Cancer cell lethality could arise through numerous mechanisms, so subsequent investigations must define molecular target(s). Proteins expressed in bacteria can lack important post-translational modifications and can be incorrectly folded.29 Yeast are genetically tractable eukaryotic microbes, which many researchers have employed for drug discovery.30 Yeast cell death researchers have reported putative yeast counterparts of mammalian apoptosis regulators.31 Although the equivalence of yeast cell death and classical mammalian apoptosis remains controversial,32 endogenous yeast pathways may one day facilitate the discovery of drugs that modulate mammalian apoptotic signaling. In this study, however, we took an alternative approach: exploiting the activity of reconstituted human apoptotic pathways in budding yeast to monitor the ability of drugs to inhibit members of the Bcl-2 and caspase families.
Enforced expression of Bax was found to provoke mitochondrial dysfunction and death of S. cerevisiae.33 Expression of active caspases also killed yeast.34 Yeast could be protected from Bax-induced lethality by co-expression of pro-survival Bcl-2 proteins,34, 35, 36, 37, 38 and this protection could be reversed by the introduction of BH3-only proteins.35, 38, 39 Similarly, caspase-mediated yeast lethality was blocked through co-expression of endogenous or viral caspase inhibitors.40, 41, 42, 43, 44, 45, 46, 47 The ability of cellular and viral proteins to interfere with the activity of Bcl-2 and caspase family members in yeast prompted us to use yeast to model the activity of drugs designed to inhibit these apoptotic regulators.
Results
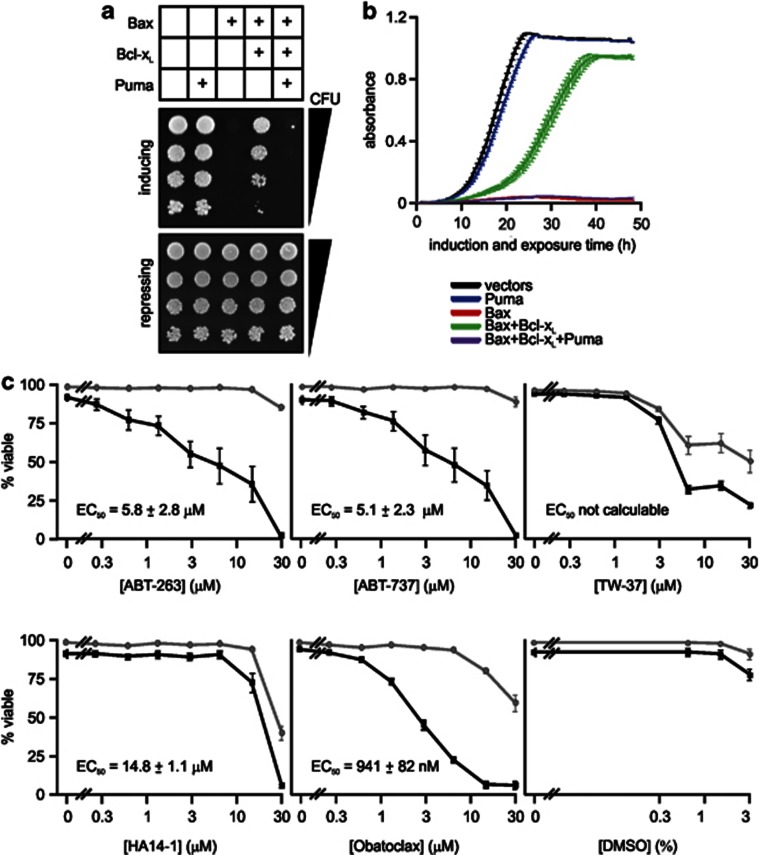
Inhibition of Bax-induced yeast lethality by Bcl-xL could be abolished by co-expression of the BH3-only proteins Puma (Figures 1a and b), Bim or Bid,35, 38, 39 demonstrating that yeast could be used to detect protein-mediated antagonism of pro-survival Bcl-2 family members. We chose to test the activity in yeast of five drugs developed to target Bcl-2 subfamily members, and first tested their activity and specificity in murine cells. The ability of ABT-263 and ABT-737 to kill factor-dependent myeloid (FDM) cells absolutely required Bax or Bak (Figure 1c), as previously reported.8, 22 In contrast, wild-type cells were only slightly more sensitive to HA14-1 and TW-37 than cells lacking Bax and Bak (Figure 1c). High doses of obatoclax killed some Bax/Bak knockout FDMs, but lower concentrations were only lethal to cells expressing Bax and Bak (Figure 1c).
Figure 1.
Antagonism of pro-survival Bcl-2 proteins by BH3-only proteins in yeast, and by drugs in mammalian cells. (a and b) FY1679-28C (wild-type) yeast were transformed with plasmids encoding the listed Bcl-2 family members. (a) Suspensions containing equivalent concentrations of each transformant were serially diluted and 5 μl of each dilution were spotted onto plates containing galactose (to induce transgene expression) or glucose (to repress transgene expression). Growth on inducing plates indicates survival and proliferation of yeast expressing the transgenes. (b) These clones and another two sets of independent transformants were grown in raffinose-containing medium then transferred into medium containing galactose. The absorbance of each culture was measured every 0.5 h for 48 h. Error bars indicate standard errors of the means from analyses of three independent clones of each type. (c) Factor-dependent myeloid cells from wild-type mice (black lines) or animals lacking Bax and Bak (gray lines) were treated with the indicated concentrations of each drug for 24 h. Apoptosis was measured by Annexin V binding for obatoclax mesylate, and by propidium iodide uptake assays for the remaining drugs. Cell death stimulated by the corresponding concentrations of the solvent (DMSO) was also quantitated. The drug doses that killed half of the wild-type cells are stated where they could be calculated. Error bars indicate the standard errors of the means from four separate experiments
We next assessed the ability of each drug to prevent growth of yeast that either lacked human transgenes or expressed FLAG-tagged Bax plus Bcl-xL, Bcl-2, Bcl-w, A1 or Mcl-1. To mimic a high-throughput screening methodology, small volumes of drugs (or solvent), were pipetted onto yeast embedded in agar. In this assay, the appearance of a dark circle indicates drug-mediated prevention of yeast growth. Wild-type yeast transformed with empty vectors were killed by application of 30–300 μM obatoclax (Figure 2). The other drugs did not markedly impair the growth of empty vector transformants. Yeast co-expressing Bax with either Bcl-xL or Bcl-2 were sensitive to 10 μM or more of ABT-263 or ABT-737 (Figure 2). No drugs (apart from obatoclax) inhibited the growth of yeast co-expressing Bax with Bcl-w, A1 or Mcl-1, and negligible activity was detected for HA14-1 or TW-37 (Figure 2).
Figure 2.
ABT-737 and ABT-263 prevent the growth in agar of yeast co-expressing Bax and either Bcl-xL or Bcl-2. The indicated yeast strains were transformed with plasmids encoding the specified human Bcl-2 family members. Yeast were embedded in inducing agar and the stated concentrations of each drug, or the DMSO carrier (‘No drug'), were pipetted onto the agar, then the yeast were allowed to grow for 3 days before being photographed. Dark circles reveal drug-mediated suppression of yeast growth
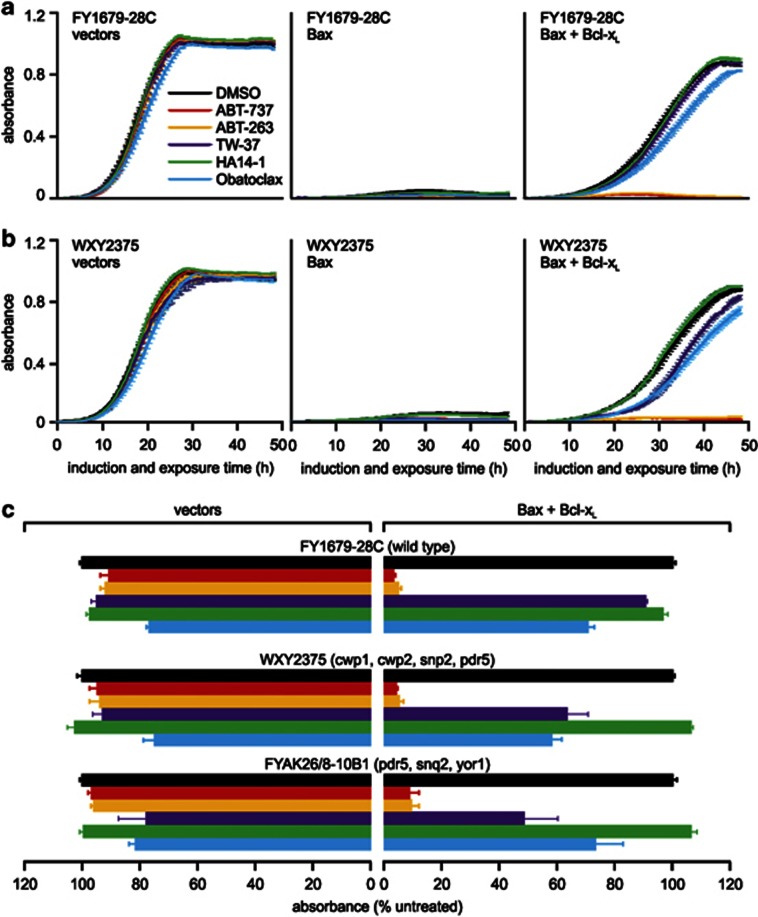
Previous research revealed that yeast could be sensitized to some compounds by either disabling ABC transporter efflux pumps,48 and/or by mutating the cwp1 and 2 genes that encode mannoprotein components of the yeast cell wall.49 We transformed plasmids encoding Bax and pro-survival proteins (or empty vectors) into four yeast strains bearing mutations in ABC transporter proteins (yor1, snq1 and/or pdq5), and a fifth that bore mutations in ABC transporter and cell wall proteins. The activities of the BH3-mimetic drugs were tested on these transformants embedded in agar. Interestingly, the mutations did not dramatically enhance sensitivity to this panel of BH3-mimetics, relative to the parental strain (Figure 2).
We also quantitatively monitored the activity and antagonism of pro-survival Bcl-2 relatives in yeast, by measuring growth in liquid medium. The parental yeast strain was transformed with plasmids encoding Bax with or without Bcl-xL and/or Puma. Growth was monitored by measuring absorbance over time, after transgene induction. The absorbance of yeast expressing Bax alone hardly changed (Figure 1b). Co-expression of Bcl-xL increased the proliferation of Bax-expressing yeast, and Puma completely antagonized this protection (Figure 1b). We used a similar assay to assess the impact of the BH3-mimetic drugs on viability of yeast bearing empty vectors or co-expressing Bax with cellular or viral pro-survival Bcl-2 relatives. These and subsequent experiments were conducted in the triple ABC-transporter mutant strain. Initially, we tested the drugs for nonspecific killing of yeast. Cultures of empty vector transformants incubated with 30 μM of each drug all achieved similar absorbances by 48 h of growth (Supplementary Figure 1), consistent with a previous report.21 However, when the untreated yeast were in log phase (absorbance=0.5), it was obvious that the growth of empty vector transformants in liquid media was dramatically slowed by 30 μM obatoclax or TW-37, and slightly impaired by 30 μM of ABT-263 and ABT-737 (Supplementary Figure 1). To minimize these nonspecific effects, we chose concentrations of 10 μM or lower for subsequent experiments.
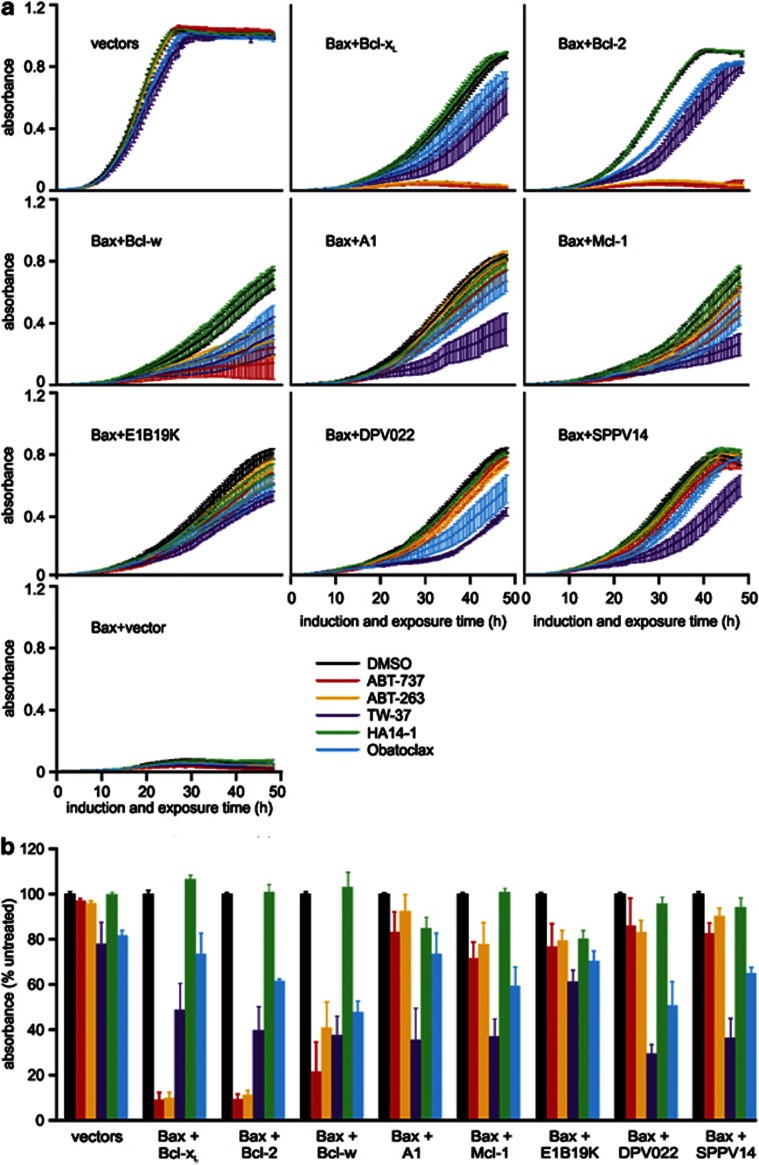
The pro-survival Bcl-2 relatives differed in their ability to inhibit Bax toxicity when spotted onto inducing plates (Supplementary Figure 2). A sub-set of anti-apoptotic Bcl-2 relatives were expressed in yeast as GFP-fusion proteins to directly compare their expression levels, their abilities to inhibit Bax-induced death and the strength of their binding to Bax (Supplementary Figure 3). Distinctive binding affinities and expression levels appeared to influence the ability of various pro-survival Bcl-2 relatives to inhibit Bax-induced yeast death. The different transformants also exhibited distinct growth rates in liquid media lacking drugs (Figure 3a, black lines). To accurately compare the impacts of drugs on yeast expressing different Bcl-2 family members (or none), we graphed the absorbance of each drug-treated culture when the corresponding untreated culture was in log phase growth (absorbance=0.5; Figure 3b). Ten micromolar ABT-263 or ABT-737 nearly abolished growth of the Bax/Bcl-2 and Bax/Bcl-xL transformants. Proliferation of Bax/Bcl-w transformants was less dramatically impaired. ABT-737 and ABT-263 only very slightly suppressed growth of yeast co-expressing Bax with either Mcl-1 or A1 (Figure 3). Yeast lacking Bcl-2 family members were only marginally affected by 10 μM of obatoclax or TW-37. This concentration of TW-37 reduced the growth rates of yeast co-expressing Bax and each of the pro-survival proteins by about half, suggesting that this agent is a weak pan-Bcl-2 inhibitor (Figure 3). Obatoclax weakly inhibited the growth of yeast co-expressing Bax and Bcl-2, Bcl-w or Mcl-1 (Figure 3). HA14-1 did not affect the growth of any transformants (Figure 3).
Figure 3.
Ten micromolar ABT-737 or ABT-263 antagonize Bcl-xL, Bcl-2 and Bcl-w in yeast. FYAK 26/8-10B1 yeast (mutant for pdr5, snq2 and yor1) were transformed with plasmids encoding the listed Bcl-2 family members or empty vectors. Suspensions containing equivalent concentrations of three independent transformants of each type were grown in raffinose-containing medium then transferred into media containing galactose plus 10 μM of the indicated drugs in 1% DMSO, or DMSO alone. (a) The absorbance of each culture was measured every 0.5 h for 48 h. (b) The graph depicts the average absorbance of yeast expressing Bax and each pro-survival protein and treated with each drug, at the time when the absorbance of each untreated clone (grown in DMSO alone) was closest to 0.5. (a and b) Error bars indicate standard errors of the means from analyses of three independent clones
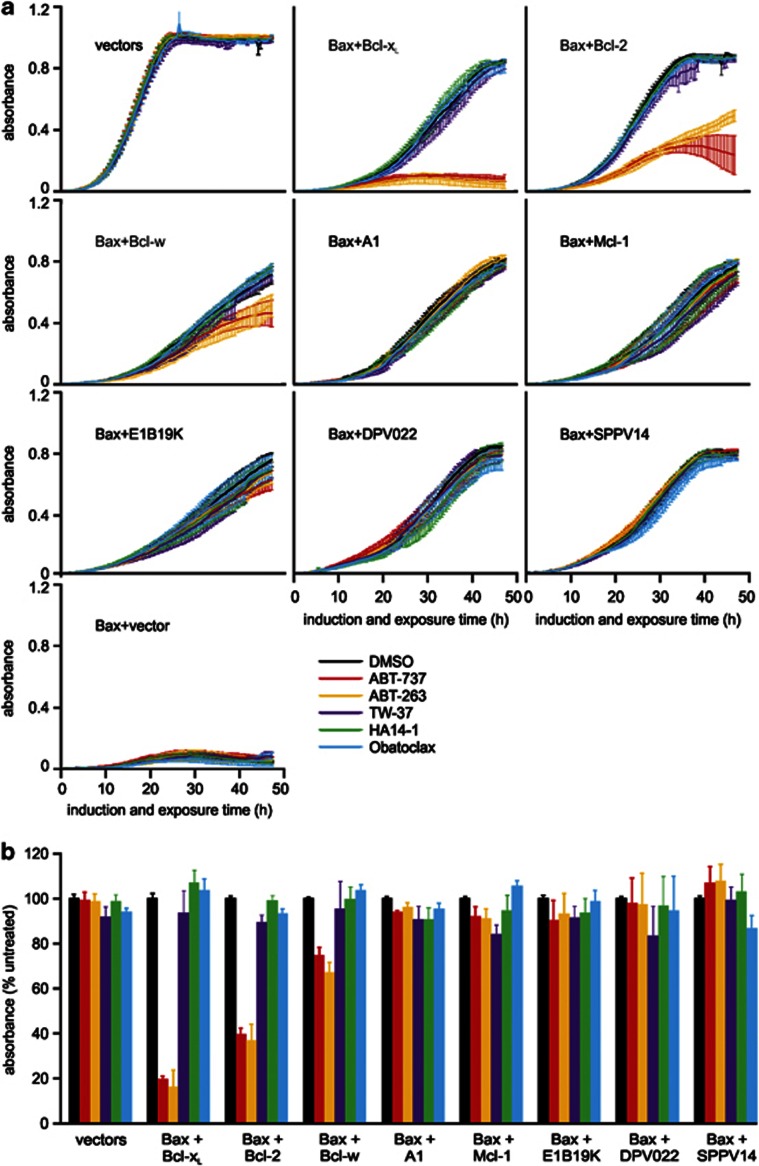
The activity of the panel of BH3-mimetics was also tested at 3 μM; approximately half the peak plasma concentration of ABT-26314 and around 10 times that of obatoclax.50 Only ABT-263 and ABT-737 had any impact on yeast growth when applied at this concentration (Figure 4). Both drugs negated Bcl-xL-mediated protection of Bax lethality. At this dose, these drugs partially impeded growth of the Bax/Bcl-2 transformants, only slightly slowed the proliferation of the Bax/Bcl-w yeast, and neither drug interfered with the inhibition of Bax toxicity by Mcl-1 or A1 proteins.
Figure 4.
Three micromolar ABT-737 or ABT-263 completely antagonize Bcl-xL in yeast. FYAK 26/8-10B1 yeast (mutant for pdr5, snq2 and yor1) were transformed with plasmids encoding the listed Bcl-2 family members or empty vectors. Suspensions containing equivalent concentrations of three independent transformants of each type were grown in raffinose-containing medium then transferred into media containing galactose plus 3 μM of the indicated drugs in 1% DMSO, or DMSO alone. (a) The absorbance of each culture was measured every 0.5 h for 48 h. (b) The graph depicts the average absorbance of yeast expressing Bax and each pro-survival protein and treated with each drug, at the time when the absorbance of each untreated clone (grown in DMSO alone) was closest to 0.5. (a and b) Error bars indicate standard errors of the means from analyses of three independent clones
We also employed this approach to examine the drug sensitivity of viral Bcl-2 proteins. The sensitivity of E1B19K to antagonism by BH3-mimetics has not been reported to date. In yeast, we demonstrated that neither ABT-737 nor ABT-263 could antagonize Bax inhibition by E1B19K (Figures 3 and 4). Deerpox DPV022 and SPPV14 were resistant to ABT-737 and ABT-263 (Figures 3 and 4), consistent with our previous observations.51, 52 The poxvirus Bcl-2 homologs displayed partial sensitivity to 10 μM of TW-37, but Bax/E1B19K yeast were less sensitive (Figure 3). Growth of transformants expressing Bax and the viral Bcl-2 homologues was unaffected by 3 μM of any of the drugs tested (Figure 4).
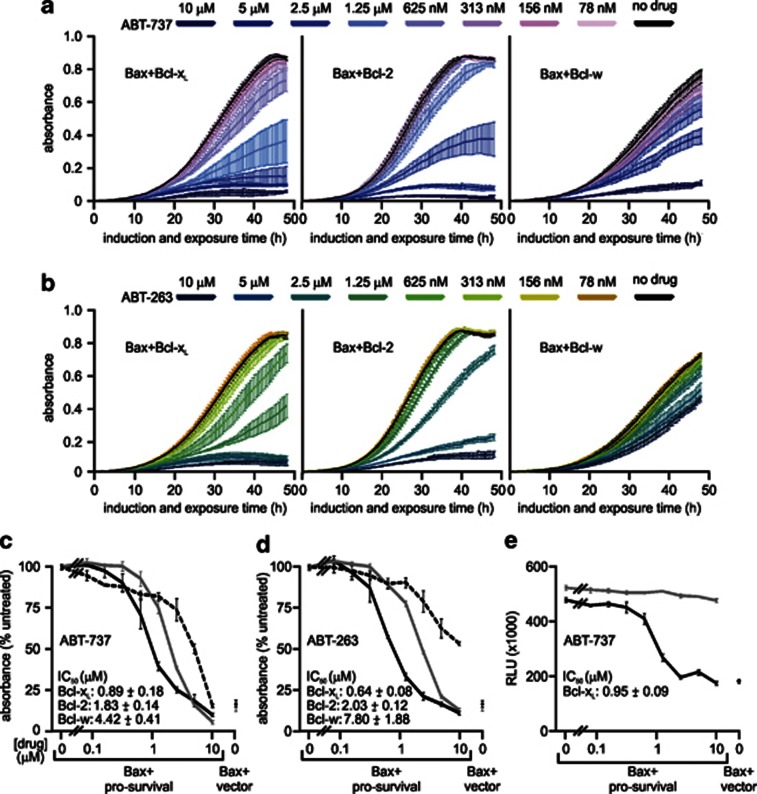
To further quantitate the sensitivity of Bcl-xL, Bcl-2 and Bcl-w to ABT-263 and ABT-737, we monitored the absorbance of yeast expressing Bax with these pro-survival proteins grown in a range of concentrations of the drugs (Figures 5a and b). Bcl-xL-expressing yeast were twice as sensitive as Bax/Bcl-2 transformants to ABT-737, and three times more sensitive to ABT-263 (Figures 5c and d). Bax transformants co-expressing Bcl-w were five times less sensitive to ABT-737 than those co-expressing Bcl-xL. Even 10 μM ABT-263 failed to completely suppress the growth of Bax/Bcl-w yeast (Figures 5c and d).
Figure 5.
Bcl-xL and Bcl-2 are more efficiently antagonized by ABT-737 and ABT-263 than Bcl-w in yeast. FYAK 26/8-10B1 yeast (mutant for pdr5, snq2 and yor1) were transformed with plasmids encoding the listed Bcl-2 family members or empty vectors. Suspensions containing equivalent concentrations of three independent transformants of each type were grown in raffinose-containing medium then transferred into media containing galactose plus 0–10 μM ABT-737 or ABT-263 in 1% DMSO, or DMSO alone. The absorbance of each culture was measured every 0.5 h after addition of galactose and either ABT-737 (a and c) or ABT-263 (b and d). (c and d) The graphs depict the average absorbance of yeast co-expressing Bax and either Bcl-xL (black solid lines), Bcl-2 (gray solid lines) or Bcl-w (black dotted lines), treated with each dose of ABT-737 (c) or ABT-263 (d), at the time when the absorbance of each untreated clone (grown in DMSO alone) was closest to 0.5. The absorbance of yeast expressing Bax without a pro-survival relative at the same time points is also shown. (e) FYAK 26/8-10B1 yeast bearing a Bax expression plasmid were transformed with plasmids encoding Bcl-xL (black line) or A1 (gray line), or empty vector (data point at right of graph). Suspensions containing equivalent concentrations of three independent transformants of each type were grown in raffinose-containing medium then transferred into media containing galactose plus 0–10 μM ABT-737 in 1% DMSO, or DMSO alone. ATP levels in each culture were assayed 30 h after addition of galactose and ABT-737, using a luciferase assay. Signals are expressed in arbitrary relative luminescence units (RLU). (a–e) Error bars indicate standard errors of the means from analyses of three independent clones of each type
Some robotic drug-screening applications detect luminescence rather than absorbance, so we tested a luminescence-based adenosine triphosphate (ATP) assay for detecting BH3-mimetic activity in yeast. Addition of up to 10 μM of ABT-737 had little effect on the ATP levels in cultures of Bax/A1 transformants, but around 1 μM of ABT-737 reduced the ATP content of Bax/Bcl-xL transformants by half (Figure 5e).
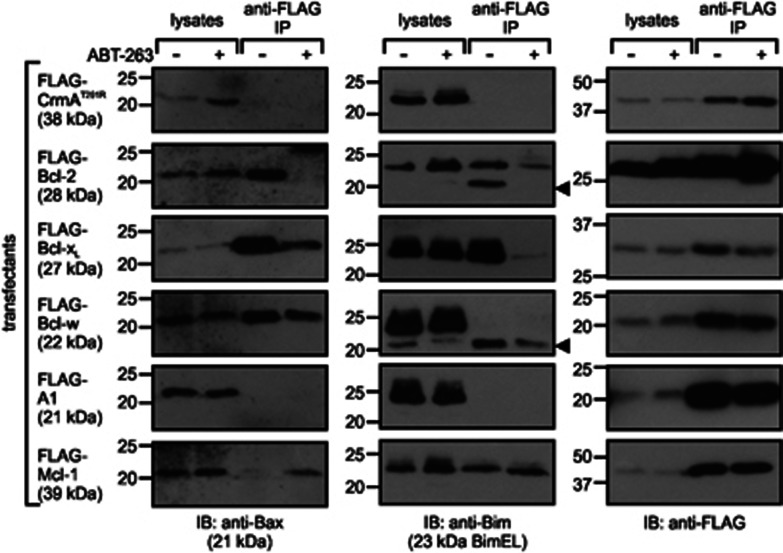
Using a co-immunoprecipitation approach, we explored whether the sensitivity of Bcl-2 relatives to ABT-263 in yeast reflected the drug's specificity in human cells (Figure 6). FLAG-tagged Bcl-xL, Bcl-2, Bcl-w and Mcl-1, but not A1 nor CrmA, could detectably co-immunoprecipitate Bax from untreated 293T cells lysed using Triton X-100 (which prompts formation of an active conformation of Bax and permits detection of stable interactions between members of the Bcl-2 subfamily and Bax53). FLAG-tagged Bcl-xL, Bcl-2 and Mcl-1 also interacted with Bim in lysates from untreated cells. Treatment with ABT-263 abolished or substantially diminished interactions between Bax or Bim with Bcl-2 or Bcl-xL, and very slightly reduced the association of Bax with Bcl-w. Interestingly, ABT-263 exposure prompted more Bax to bind to Mcl-1, presumably because the drug displaced Bax from endogenous Bcl-2 and/or Bcl-xL, so more was available to bind to Mcl-1. Mcl-1 binding to Bim was unaffected by exposure to ABT-263.
Figure 6.
Specificity of ABT-263 displacement of Bax and Bim from pro-survival Bcl-2 relatives in mammalian cell lysates. 293T cells were transfected with plasmids encoding the specified FLAG-tagged Bcl-2 relatives or a CrmA mutant, then treated with or without 10 μM ABT-263. Lysates were subjected to anti-FLAG immunoprecipitation (IP) and immunoblotting of lysates and eluates was performed using antibodies recognizing the FLAG tag, Bax and Bim. The arrow indicates residual anti-Bax signal still visible after anti-Bim re-probing in some lanes
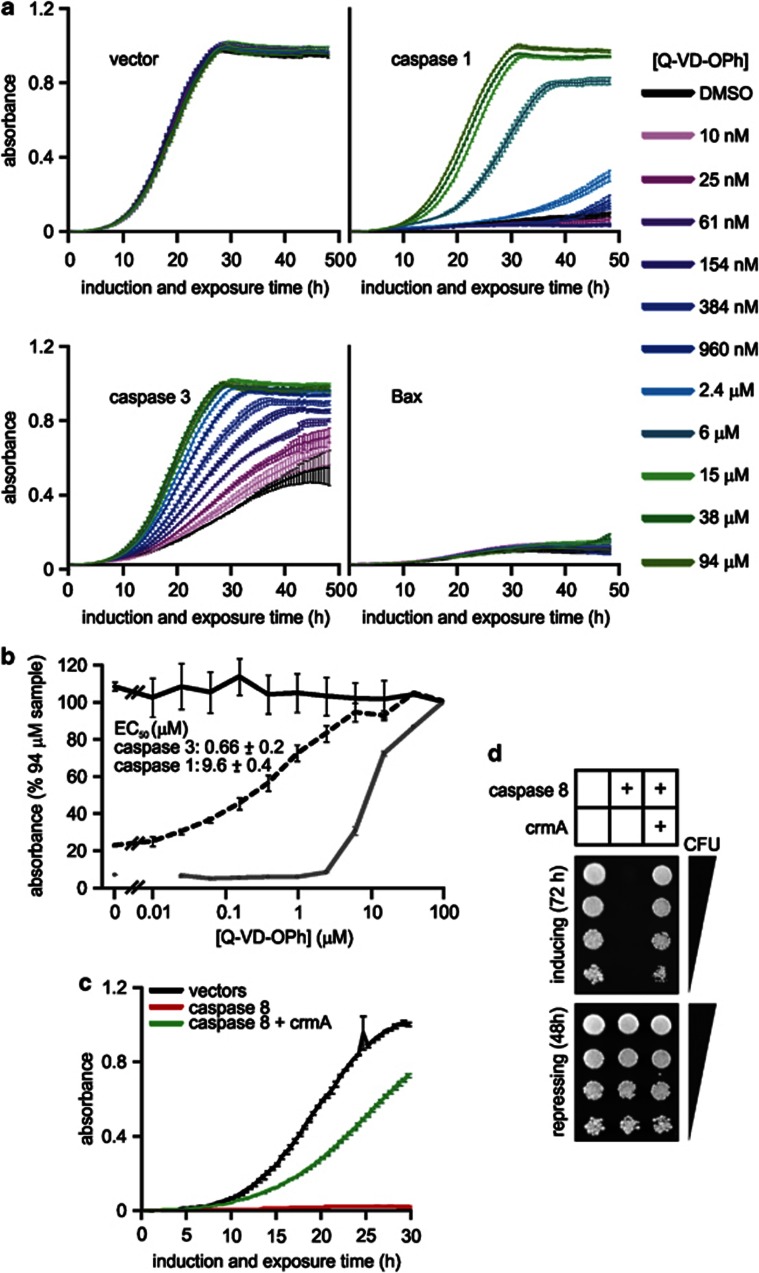
Targeting members of the caspase and viral serpin families may be clinically useful, so we investigated whether yeast expressing members of these families could be used to model drugs directed at these proteins. The pan-caspase inhibitor Q-VD-OPh was reported to efficiently inhibit caspases 1–10 in vitro, with IC50 values of less than 250 nM.54 Q-VD-OPh potently suppressed the relatively mild yeast toxicity provoked by expression of autoactivating caspase 3 (Figures 7a and b), but afforded weaker protection against the efficient death provoked by caspase 1 expression (Figures 7a and b). Consistent with a caspase-specific mode of action, concentrations of up to 94 μM failed to protect yeast against Bax-induced lethality (Figures 7a and b).
Figure 7.
Modeling caspase inhibition in yeast. (a and b) FYAK 26/8-10B1 yeast (mutant for pdr5, snq2 and yor1) were transformed with the stated expresson plasmids. Suspensions containing equivalent concentrations of three independent transformants of each type were grown in raffinose-containing medium then transferred into media containing galactose plus 0–94 μM Q-VD-OPh in 1% DMSO, or DMSO alone. The absorbance of each culture was measured every 0.5 h for 48 h after addition of galactose and drug or DMSO. (b) The graph depicts the average absorbance of empty vector transformants (black solid line) or yeast expressing caspase 3 (gray line) or caspase 1 (black dotted lines), treated with each drug dose, at the time when the absorbance of each untreated clone (grown in DMSO alone) was closest to 0.5. These data were used to calculate the concentrations of Q-VD-OPh that reduced the caspase-mediated lethality by half. (c and d) FYAK 26/8-10B1 yeast were transformed with the indicated expression plasmids or corresponding empty vectors. (c) Suspensions containing equivalent concentrations of three independent transformants of each type were grown in raffinose-containing medium then transferred into media containing galactose, and the absorbance of each culture was measured every 0.5 h for 30 h. (d) Suspensions containing equivalent concentrations of one transformant of each class were serially diluted and 5 μl of each dilution were spotted onto plates containing galactose (to induce transgene expression) or glucose (to repress transgene expression). Growth on inducing plates indicates survival and proliferation of yeast expressing the transgenes. Similar results were obtained with another two independent sets of transformants. (a–c) Error bars indicate standard errors of the means from analyses of three independent clones of each type
Some poxviruses encode inhibitors of caspases 1, 8 and granzyme B, such as crmA from cowpox virus.25 CrmA potently inhibited caspase 8-induced yeast death (Figures 7c and d). To date no drugs have been developed to target viral serpins, but this assay may enable identification of compounds that inhibit these proteins.
Discussion
The yeast S. cerevisiae is a well-characterized eukaryotic microbe that offers many attractive features for drug discovery. Rapid growth and ease of culturing facilitate high-throughput screening. Protein folding, post-translational modification and sub-cellular localization are often similar in yeast and human cells.55 Here, we present methods for detecting the drug-mediated inhibition of anti-apoptotic Bcl-2 relatives or caspases expressed in budding yeast.
The BH3-mimetics ABT-737 and ABT-263 specifically and potently impaired the growth of yeast expressing Bax plus either Bcl-xL or Bcl-2 in agar and in liquid media, and reduced the ATP levels of liquid cultures of these transformants. Previously published data suggested that ABT-737 and ABT-263 displaced BH3 peptides from bacterially expressed Bcl-xL, Bcl-2 or Bcl-w with similar efficiency.5, 9 However, Bcl-w was previously published not to be targeted by these drugs in mammalian cells.10, 12, 13 We found that ABT-263 dramatically reduced binding of Bax to Bcl-xL or Bcl-2 in human cell lysates, but had negligible impact on its association with Bcl-w. The conformation adopted by Bcl-w within its natural location of the mitochondrial outer membrane has been proposed to differ from that of the bacterially expressed protein.56, 57 Consistent with the proposed importance of correct folding and sub-cellular localization for Bcl-w activity, and the faithful modeling of these features in yeast, Bcl-w was 5-fold less sensitive to ABT-737 inhibition in yeast than Bcl-xL, and 12-fold less sensitive to inhibition by ABT-263. Only very high levels of ABT-263 or ABT-737 had any impact on A1 or Mcl-1 activity in yeast, confirming the previously reported resistance of these proteins to these drugs in vitro, in cells and in animals.
Three other proposed BH3-mimetic drugs were analyzed in this study. Obatoclax mesylate was toxic to yeast grown in agar and in liquid, whereas the lethality of TW-37 was only evident when yeast were grown in liquid media. Both drugs also exhibited nonspecific apoptotic activity in mammalian cells, although it is not clear whether similar mechanisms caused off-target toxicity in yeast and in mammalian cells. Despite nonspecific yeast lethality, high concentrations of TW-37 did appear to slightly suppress the Bax-inhibitory activity of all tested pro-survival family members in yeast. A high concentration of obatoclax impaired the growth of yeast containing Bax plus Bcl-2, Bcl-w or Mcl-1 slightly more than the empty vector transformants, consistent with its reported specificity in vitro.18 HA14-1 killed mammalian cells only at very high concentrations, and had no discernible effect on the growth of yeast containing or lacking human Bcl-2 family members. This inactivity may be due to the short half-life of this drug.23
The BH3-mimetic yeast assays presented here were less sensitive than in vitro affinity assays. IC50 values calculated from the yeast analyses of ABT-737 and ABT-263 were just under 1 μM for Bcl-xL and just over 1 μM for Bcl-2. Published Ki values for the disruption by ABT737/263 of BH3 peptide binding to these pro-survival proteins were around a thousand-fold lower.5, 9 Yeast whose survival depended on Bcl-xL or Bcl-2 were more sensitive than FDM cells to death induced by ABT-373 and -263 (half maximal effective concentration (EC50)=5–6 μM), but less responsive than chronic lymphocytic leukemia cells (EC50=7 nM).12 Likewise, although Q-VD-OPh specifically suppressed caspase-mediated yeast lethality, it functioned more efficiently in vitro. The pipeline of anti-apoptotic drug development sometimes starts with identification of small molecules that bind weakly to pro-survival target proteins, with subsequent affinity boosting via chemical modification and/or linking of multiple ligands. We suspect that the yeast-based methods reported here would be insufficiently sensitive to replace in vitro binding assays for ‘first hit' identification of lead compounds. However, these techniques could be useful in the refinement and optimization stages of drug development, providing a simple, cheap and automatable way to test chemical derivatives for desired specificity profiles and activity in eukaryotic cells.
These yeast techniques also offer an easy method for profiling the drug sensitivity of uncharacterized Bcl-2 orthologs, including Bcl-2 relatives that are refractory to bacterial expression. E1B19K was the first viral Bcl-2 homologue to be identified,58 but its drug sensitivity was unknown. In yeast, we found that E1B19K (like DPV022 and SPPV14) was resistant to ABT-737 and ABT-263. Cellular and poxviral Bcl-2 relatives were weakly sensitive to TW-37, but even high concentrations of this drug had negligible impact on the growth of yeast co-expressing Bax with E1B19K. This study focused on Bax-mediated yeast lethality, its inhibition by pro-survival Bcl-2 relatives and drug-based antagonism of that inhibition. However, we expect that yeast co-expressing Bak with pro-survival Bcl-2 family members could also be used to model the activity of drugs targeting members of this family.
Rapid efflux of some compounds by ABC transporter proteins can limit their intracellular concentrations within yeast59 and mammalian cells.60 The yeast cell wall can also impede uptake of some agents.49 We tested the sensitivity of yeast transformants bearing mutations in ABC transporters and/or cell wall mannoproteins to Bcl-2-targeting drugs. The different strains exhibited subtle variations in drug sensitivity (Figures 2 and 8), suggesting that these efflux pumps and cell wall components do not interfere markedly with the uptake of the agents tested in this study.
Figure 8.
Bcl-2 targeting drugs act similarly in wild-type and mutant yeast. FY1679-28C (wild type) (a) or WXY2375 (cwp1, cwp2, snp2, pdr5) (b) yeast were transformed with plasmids encoding the listed Bcl-2 family members or empty vectors. Suspensions containing equivalent concentrations of four (a) or three (b) independent transformants of each type were grown in raffinose-containing medium then transferred into media containing galactose plus 10 μM of the indicated drugs in 1% DMSO, or DMSO alone. (a and b) The absorbance of each culture was measured every 0.5 h for 48 h. (c) The graph depicts the average absorbance of transformants bearing empty vectors or co-expressing Bax and Bcl-xL, treated with each drug, at the time when the absorbance of each untreated clone (grown in DMSO alone) was around 0.5. Data from FYAK26/8-10B1 transformants from Figure 3 was included for comparison. Error bars indicate standard errors of the means
Like Bax and Bak, active human caspases are also lethal to yeast. This toxicity could be blocked by the pan-caspase inhibitor Q-VD-OPh. Yeast expressing human caspases could be used to identify agents with the appropriate anti-caspase specificity profiles for treating conditions including sepsis (inflammatory caspases) or liver disease (apoptotic caspases). Yeast can also be used to model the inhibition of caspases by disease-associated proteins such as the IAPs44 or viral serpins (this study). It is therefore possible that yeast co-expressing caspases with these inhibitors could be used to test candidate compounds for antagonism of IAPs or viral serpins, to develop agents for treating cancer or preventing viral infection.
Materials and Methods
Materials
The following drugs were purchased from Selleck Chemicals (Houston, TX, USA): ABT-263, ABT-737, TW-37, Obatoclax mesylate. HA14-1 was bought from Tocris (Bristol, UK). Q-VD-OPh was purchased from SM Biochemicals (Anaheim, CA, USA). Caspase substrates were bought from Enzo Life Sciences (Farmingdale, NY, USA). Anti-GFP (mixture of clones 7.1 and 13.1) was from Roche Applied Science (Castle Hill, New South Wales, Australia). Anti-FLAG M2 antibody, affinity resin and FLAG peptide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-Bax (NT) was bought from Merck Millipore (Billerica, MA, USA). Anti-Bim was purchased from Cell Signaling Technology (Danvers, MA, USA). Details of plasmids used in this study are provided as Supplementary Information.
Yeast strains and methods
The S. cerevisiae strains48, 49 were kindly provided by Andre Goffeau, Stan Ulaszewski, Wei Xiao and Anna Kolaczkowska. Published protocols were used for growth and transformation.42 The semi-quantitative (‘spotting') yeast viability assay was performed as previously reported.45
Assaying drug activity using yeast embedded in agar was performed using an adaptation of a published method.48 Overnight cultures of transformants in minimal media containing glucose (2%, w/v) were washed twice in 10 mM Tris-HCl (pH 8.0), 1 mM EDTA (TE) then resuspended in TE. OD600 was measured and used to introduce 5 × 107 colony-forming units of yeast into 7.5 ml of minimal media containing galactose, which was equilibrated to 37 °C. Each yeast suspension was mixed with 7.5 ml of minimal media agar containing galactose (2%, w/v) that had been pre-equilibrated to 50 °C, quickly mixed by inversion, then poured onto previously made 10 cm plates containing 15 ml of galactose-containing minimal media agar. The plates were set at room temperature for an hour. Drugs were diluted from 1 mM stocks (dissolved in DMSO) to yield the desired concentrations in 10% DMSO/90% TE. Five microliters of each concentration of each drug (or solvent) were pipetted onto plates containing embedded yeast, allowed to dry at room temperature, incubated at 30 °C for 3 days, then photographed.
The drug sensitivity of yeast in liquid media was assayed as follows: overnight cultures of transformants were washed twice in TE then inoculated into 150 μl minimal media containing raffinose (2%, w/v) to yield an absorbance at 620 nm of 0.1 (using a Multiskan Ascent; Thermo Fisher Scientific Australia, Scoresby, Victoria, Australia) and grown for 5 h. Drugs to be tested were diluted at the appropriate concentrations in 150 μl minimal media containing galactose (2%, w/v) in 1–3% DMSO (as indicated), per well in sterile 96-well plates (BD, Franklin Lakes, NJ, USA), then equilibrated at 30 °C for 0.5–1 h. Ten microliters of the yeast cultures were then pipetted into the wells containing drugs or solvent. Absorbance at 620 nm was measured using the Multiskan Ascent every 0.5 h, with continuous incubation at 30 °C, and shaking for 2 min at 960 r.p.m. immediately before each measurement.
The BacTiter-Glo reagent (Promega, Madison, WI, USA) was used to monitor ATP levels in yeast cultures. Yeast transformants were processed and grown as described for absorbance monitoring. After 30 h growth in media containing galactose and drugs (or solvent), 20 μl of each yeast/drug suspension was added to 50 μl BacTiter-Glo pre-mixed with 30 μl of TE in wells of a white 96-well microplate (Greiner Bio-One GmbH, Frickenhausen, Germany). Plates were shaken for 2 min at 960 r.p.m. to mix yeast with the BacTiter-Glo reagent. Five minutes later, luminescence was detected using a Spectramax M5 instrument (Molecular Devices, Sunnyvale, CA, USA) set for 1 ms signal integration.
For immunoprecipitation from yeast, transformants were grown overnight in minimal media containing glucose (2%, w/v), washed twice in TE then grown for 5 h in media containing raffinose (2%, w/v), pelleted and grown in media containing galactose (2%, w/v) for a further 8 h, then pelleted and frozen. The pellets were resuspended in lysis buffer (20 mM Tris–HCl, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100, pH 7.4) supplemented with a protease inhibitor cocktail (Roche), then lysed using matrix C in a Fastprep instrument (MP Biomedicals, Santa Ana, CA, USA) at 6 m/s for 40 s. Lysates were incubated with M2 anti-FLAG affinity resin (Sigma-Aldrich) for 3 h at 4 °C, then washed three times with lysis buffer. The beads were incubated with lysis buffer containing 200 μg/ml FLAG peptide (Sigma-Aldrich) for 15 min at 4 °C to elute the bound proteins.
Mammalian cell culture and assays
Cells were grown at 37 °C in air supplemented with 5% CO2, in Dulbecco's modified Eagle medium with high glucose (Invitrogen, Carlsbad, CA, USA) containing 10% fetal calf serum (Invitrogen), supplemented with 0.25 ng/ml IL3 (R&D systems; Minneapolis, MN, USA) for FDM cells. HoxB8-transformed FDM cells from wild-type or knockout mice were kindly provided by Anissa Jabbour and Paul Ekert. FDMs were harvested after 24 h treatment and pelleted at 750 × g for 5 min, washed in phosphate-buffered saline (Amresco, Solon, Ohio, USA) then resuspended for Annexin V assays in binding buffer (10 mM HEPES pH 7.4, 140 mM NaCl, 5 mM CaCl2) containing 1 : 1000 dilution of Annexin-V-FITC (kindly provided by Hamsa Puthalakath, La Trobe University). Alternatively, cells were resuspended in 1 mg/ml propidium iodide in phosphate-buffered saline. Stained cells were analyzed using a FACS-Canto (BD Biosciences; Franklin Lakes, NJ, USA), limiting the analysis to intact cells based on forward and side scatter parameters.
293T cells were seeded into 6-well plates (3 × 105 cells per well) and the following day were transfected using Lipofectamine 2000 (Life Technologies). Six wells were transfected with each of the FLAG-Bcl-xL-pEF and FLAG-CrmAT291R-pEF plasmids, and twenty wells were transfected with each of the plasmids encoding the other pro-survival proteins. The media was replaced 9 and 19 h after addition of the transfection mixture. Half of the wells transfected with each plasmid received fresh media and the other half received fresh media containing ABT-263 (10 μM). Cells were harvested 24 h after transfection and lysed in 200 mM NaCl, 50 mM Tris-HCl, 1% Triton X-100, 1 mM EDTA, pH 7.5, supplemented with a protease inhibitor cocktail (Roche). Insoluble material was pelleted by centrifuging at 16 100g for 15 min 4 °C. Anti-FLAG immunoprecipitations were performed as described above, using this lysis buffer for washing (without protease inhibitors) and for eluting.
Statistics
Drug potency in yeast and mammalian cells was assessed by calculating absolute IC50 or EC50 values using Graphpad Prism 5.0 (Graphpad Prism, La Jolla, CA, USA). IC50 values from yeast experiments were calculated using the absorbances of each treated transformant culture at the time when the corresponding untreated culture was closest to 0.5. The calculations included absorbances of untreated transformants expressing either only Bax, or co-expressing Bax plus pro-survival relatives, assigning drug doses of 1 M and 1 pM, respectively. IC50 calculations for Q-VD-OPh also included data from untreated cultures, which were attributed an artificial dose of 1 pM. Drug sensitivities (EC50) of wild-type FDMs were calculated similarly with untreated samples allocated a value of 1 pM and a survival value of zero designated as 1 M.
Acknowledgments
We thank Paul Ekert and Anissa Jabbour for cells, and Andre Goffeau, Stan Ulaszewski, Wei Xiao and Anna Kolaczkowska for yeast strains. This work was funded by a National Health and Medical Research Council project grant to CJH (#602525) and a fellowship to MK (#637372), an Australian Research Council Future Fellowship to CJH (#FT0991464), scholarships to TMS from La Trobe University and to TEB and LK from the Cooperative Research Centre for Biomarker Translation.
Glossary
- ATP
adenosine triphosphate
- BH
Bcl-2 homology
- FDM
factor-dependent myeloid
- EC50
half maximal effective concentration
- IC50
half maximal inhibitory concentration
- Ki
inhibition constant
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by M Piacentini
Supplementary Material
References
- Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–1424. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo M, Moore Vdel G, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Park CM, Bruncko M, Adickes J, Bauch J, Ding H, Kunzer A, et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J Med Chem. 2008;51:6902–6915. doi: 10.1021/jm800669s. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Vogler M, Furdas SD, Jung M, Kuwana T, Dyer MJ, Cohen GM. Diminished sensitivity of chronic lymphocytic leukemia cells to ABT-737 and ABT-263 due to albumin binding in blood. Clin Cancer Res. 2010;16:4217–4225. doi: 10.1158/1078-0432.CCR-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- Rooswinkel RW, van de Kooij B, Verheij M, Borst J. Bcl-2 is a better ABT-737 target than Bcl-xL or Bcl-w and only Noxa overcomes resistance mediated by Mcl-1, Bfl-1, or Bcl-B. Cell Death Dis. 2012;3:e366. doi: 10.1038/cddis.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-xL and Bcl-w are not equivalent targets of ABT-737 and Navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012;119:5807–5816. doi: 10.1182/blood-2011-12-400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- Whitecross KF, Alsop AE, Cluse LA, Wiegmans A, Banks KM, Coomans C, et al. Defining the target specificity of ABT-737 and synergistic antitumor activities in combination with histone deacetylase inhibitors. Blood. 2009;113:1982–1991. doi: 10.1182/blood-2008-05-156851. [DOI] [PubMed] [Google Scholar]
- Wilson WH, O'Connor OA, Czuczman MS, Lacasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamelos VA, Redman CW, Richardson A. Understanding sensitivity to BH3 mimetics: ABT-737 as a case study to foresee the complexities of personalized medicine. J Mol Signal. 2012;7:12. doi: 10.1186/1750-2187-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–1421. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, Guo J, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- Doshi JM, Tian D, Xing C. Structure-activity relationship studies of ethyl 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (HA 14-1), an antagonist for antiapoptotic Bcl-2 proteins to overcome drug resistance in cancer. J Med Chem. 2006;49:7731–7739. doi: 10.1021/jm060968r. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buron N, Porceddu M, Brabant M, Desgue D, Racoeur C, Lassalle M, et al. Use of human cancer cell lines mitochondria to explore the mechanisms of BH3 peptides and ABT-737-induced mitochondrial membrane permeabilization. PLoS One. 2010;5:e9924. doi: 10.1371/journal.pone.0009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi JM, Tian D, Xing C. Ethyl-2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H- chromene-3-carboxylate (HA 14-1), a prototype small-molecule antagonist against antiapoptotic Bcl-2 proteins, decomposes to generate reactive oxygen species that induce apoptosis. Mol Pharm. 2007;4:919–928. doi: 10.1021/mp7000846. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4:e1000018. doi: 10.1371/journal.ppat.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Barry M. Near death experiences: poxvirus regulation of apoptotic death. Virology. 2006;344:139–150. doi: 10.1016/j.virol.2005.09.032. [DOI] [PubMed] [Google Scholar]
- MacKenzie SH, Schipper JL, Clark AC. The potential for caspases in drug discovery. Curr Opin Drug Discov Devel. 2010;13:568–576. [PMC free article] [PubMed] [Google Scholar]
- Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, et al. Clinical Trial of the Pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am J Transplant. 2007;7:218–225. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- Cornelis S, Kersse K, Festjens N, Lamkanfi M, Vandenabeele P. Inflammatory caspases: targets for novel therapies. Curr Pharm Des. 2007;13:367–385. doi: 10.2174/138161207780163006. [DOI] [PubMed] [Google Scholar]
- Celik E, Calik P. Production of recombinant proteins by yeast cells. Biotechnol Adv. 2012;30:1108–1118. doi: 10.1016/j.biotechadv.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Menacho-Marquez M, Murguia JR. Yeast on drugs: Saccharomyces cerevisiae as a tool for anticancer drug research. Clin Transl Oncol. 2007;9:221–228. doi: 10.1007/s12094-007-0043-2. [DOI] [PubMed] [Google Scholar]
- Carmona-Gutierrez D, Eisenberg T, Buttner S, Meisinger C, Kroemer G, Madeo F. Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ. 2010;17:763–773. doi: 10.1038/cdd.2009.219. [DOI] [PubMed] [Google Scholar]
- Vachova L, Palkova Z. Caspases in yeast apoptosis-like death: facts and artefacts. FEMS Yeast Res. 2007;7:12–21. doi: 10.1111/j.1567-1364.2006.00137.x. [DOI] [PubMed] [Google Scholar]
- Sato T, Hanada M, Bodrug S, Irie SJ, Iwama N, Boise LH, et al. Interactions among members of the bcl-2 protein family analyzed with a yeast two-hybrid system. Proc Natl Acad Sci USA. 1994;91:9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puryer MA, Hawkins CJ. Human, insect and nematode caspases kill Saccharomyces cerevisiae independently of YCA1 and Aif1p. Apoptosis. 2006;11:509–517. doi: 10.1007/s10495-006-5114-2. [DOI] [PubMed] [Google Scholar]
- Jabbour AM, Puryer MA, Yu JY, Lithgow T, Riffkin CD, Ashley DM, et al. Human Bcl-2 cannot directly inhibit the Caenorhabditis elegans Apaf-1 homologue CED-4, but can interact with EGL-1. J Cell Sci. 2006;119:2572–2582. doi: 10.1242/jcs.02985. [DOI] [PubMed] [Google Scholar]
- Fletcher JI, Meusburger S, Hawkins CJ, Riglar DT, Lee EF, Fairlie WD, et al. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci USA. 2008;105:18081–18087. doi: 10.1073/pnas.0808691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manon S, Chaudhuri B, Guerin M. Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells, and prevention of these effects by coexpression of Bcl-xL. FEBS Lett. 1997;415:29–32. doi: 10.1016/s0014-5793(97)01087-9. [DOI] [PubMed] [Google Scholar]
- Juhasova B, Bhatia-Kissova I, Polcicova K, Mentel M, Forte M, Polcic P. Reconstitution of interactions of Murine gammaherpesvirus 68 M11 with Bcl-2 family proteins in yeast. Biochem Biophys Res Commun. 2011;407:783–787. doi: 10.1016/j.bbrc.2011.03.100. [DOI] [PubMed] [Google Scholar]
- Juhasova B, Mentel M, Bhatia-Kissova I, Zeman I, Kolarov J, Forte M, et al. BH3-only protein Bim inhibits activity of antiapoptotic members of Bcl-2 family when expressed in yeast. FEBS Lett. 2011;585:2709–2713. doi: 10.1016/j.febslet.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Hawkins CJ, Wang SL, Hay BA. A cloning method to identify caspases and their regulators in yeast: identification of Drosophila IAP1 as an inhibitor of the Drosophila caspase DCP-1. Proc Natl Acad Sci USA. 1999;96:2885–2890. doi: 10.1073/pnas.96.6.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by REAPER, HID and GRIM, which disrupt DIAP1-caspase interactions. Cell. 1999;98:453–463. doi: 10.1016/s0092-8674(00)81974-1. [DOI] [PubMed] [Google Scholar]
- Hawkins CJ, Wang SL, Hay BA. Monitoring activity of caspases and their regulators in yeast Saccharomyces cerevisiae. Methods Enzymol. 2000;322:162–174. doi: 10.1016/s0076-6879(00)22016-8. [DOI] [PubMed] [Google Scholar]
- Hawkins CJ, Yoo SJ, Petersen EP, Wang SL, Vernooy SY, Hay BA. The Drosophila caspase DRONC cleaves following glutamate or aspartate and is regulated by DIAP1, HID, and GRIM. J. Biol. Chem. 2000;275:27084–27093. doi: 10.1074/jbc.M000869200. [DOI] [PubMed] [Google Scholar]
- Hawkins CJ, Silke J, Verhagen AM, Foster R, Ekert PG, Ashley DM. Analysis of candidate antagonists of IAP-mediated caspase inhibition using yeast reconstituted with the mammalian Apaf-1-activated apoptosis mechanism. Apoptosis. 2001;6:331–338. doi: 10.1023/a:1011329917895. [DOI] [PubMed] [Google Scholar]
- Jabbour AM, Ekert PG, Coulson EJ, Knight MJ, Ashley DM, Hawkins CJ. The p35 relative, p49, inhibits mammalian and Drosophila caspases including DRONC and protects against apoptosis. Cell Death Differ. 2002;9:1311–1320. doi: 10.1038/sj.cdd.4401135. [DOI] [PubMed] [Google Scholar]
- Ho PK, Jabbour AM, Ekert PG, Hawkins CJ. Caspase-2 is resistant to inhibition by inhibitor of apoptosis proteins (IAPs) and can activate caspase-7. FEBS J. 2005;272:1401–1414. doi: 10.1111/j.1742-4658.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- Brand IL, Green MM, Civciristov S, Pantaki-Eimany D, George C, Gort TR, et al. Functional and biochemical characterization of the baculovirus caspase inhibitor MaviP35. Cell Death Dis. 2011;2:e242. doi: 10.1038/cddis.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowski M, Kolaczowska A, Luczynski J, Witek S, Goffeau A. In vivo characterization of the drug resistance profile of the major ABC transporters and other components of the yeast pleiotropic drug resistance network. Microb Drug Resist. 1998;4:143–158. doi: 10.1089/mdr.1998.4.143. [DOI] [PubMed] [Google Scholar]
- Zhang M, Hanna M, Li J, Butcher S, Dai H, Xiao W. Creation of a hyperpermeable yeast strain to genotoxic agents through combined inactivation of PDR and CWP genes. Toxicol Sci. 2010;113:401–411. doi: 10.1093/toxsci/kfp267. [DOI] [PubMed] [Google Scholar]
- Hwang JJ, Kuruvilla J, Mendelson D, Pishvaian MJ, Deeken JF, Siu LL, et al. Phase I dose finding studies of obatoclax (GX15-070), a small molecule pan-BCL-2 family antagonist, in patients with advanced solid tumors or lymphoma. Clin Cancer Res. 2010;16:4038–4045. doi: 10.1158/1078-0432.CCR-10-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Campbell S, Mehta N, Thibault J, Colman PM, Barry M, et al. Sheeppox virus SPPV14 encodes a Bcl-2-like cell death inhibitor that counters a distinct set of mammalian pro-apoptotic proteins. J Virol. 2012;86:11501–11511. doi: 10.1128/JVI.01115-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banadyga L, Lam SC, Okamoto T, Kvansakul M, Huang DC, Barry M. Deerpox virus encodes an inhibitor of apoptosis that regulates Bak and Bax. J Virol. 2011;85:1922–1934. doi: 10.1128/JVI.01959-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Mischak R. Apoptosis. ICN/ESP Caspase inhibitors: Applications in vivo and in vitro. ICN Bioconcepts. 2002;8:1–5. [Google Scholar]
- Gasser B, Saloheimo M, Rinas U, Dragosits M, Rodriguez-Carmona E, Baumann K, et al. Protein folding and conformational stress in microbial cells producing recombinant proteins: a host comparative overview. Microb Cell Fact. 2008;7:11. doi: 10.1186/1475-2859-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds MG, Lackmann M, Skea GL, Harrison PJ, Huang DC, Day CL. The structure of Bcl-w reveals a role for the C-terminal residues in modulating biological activity. EMBO J. 2003;22:1497–1507. doi: 10.1093/emboj/cdg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Annan J, O'Reilly LA, Crawford SA, Hausmann G, Beaumont JG, Parma LP, et al. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J Cell Biol. 2003;162:877–887. doi: 10.1083/jcb.200302144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E, Sabbatini P, Debbas M, Wold WS, Kusher DI, Gooding LR. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungwirth H, Kuchler K, Yeast ABC. transporters—a tale of sex, stress, drugs and aging. FEBS Lett. 2006;580:1131–1138. doi: 10.1016/j.febslet.2005.12.050. [DOI] [PubMed] [Google Scholar]
- DeGorter MK, Xia CQ, Yang JJ, Kim RB. Drug transporters in drug efficacy and toxicity. Annu Rev Pharmacol Toxicol. 2012;52:249–273. doi: 10.1146/annurev-pharmtox-010611-134529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.