Abstract
Metastasis is the leading cause of death by cancer. Non-small-cell lung cancer (NSCLC) represents nearly 85% of primary malignant lung tumours. Recent researches have demonstrated that epithelial-to-mesenchymal transition (EMT) plays a key role in the early process of metastasis of cancer cells. Transforming growth factor-β1 (TGF-β1) is the major inductor of EMT. The aim of this study is to investigate TGF-β1's effect on cancer stem cells (CSCs) identified as cells positive for CD133, side population (SP) and non-cancer stem cells (non-CSCs) identified as cells negative for CD133, and SP in the A549 cell line. We demonstrate that TGF-β1 induces EMT in both CSC and non-CSC A549 sublines, upregulating the expression of mesenchymal markers such as vimentin and Slug, and downregulating levels of epithelial markers such as e-cadherin and cytokeratins. CSC and non-CSC A549 sublines undergoing EMT show a strong migration and strong levels of MMP9 except for the CD133− cell fraction. OCT4 levels are strongly upregulated in all cell fractions except CD133− cells. On the contrary, wound size reveals that TGF-β1 enhances motility in wild-type A549 as well as CD133+ and SP+ cells. For CD133− and SP− cells, TGF-β1 exposure does not change the motility. Finally, assessment of growth kinetics reveals major colony-forming efficiency in CD133+ A549 cells. In particular, SP+ and SP− A549 cells show more efficiency to form colonies than untreated corresponding cells, while for CD133− cells no change in colony number was observable after TGF-β1 exposure. We conclude that it is possible to highlight different cell subpopulations with different grades of stemness. Each population seems to be involved in different biological mechanisms such as stemness maintenance, tumorigenicity, invasion and migration.
Keywords: epithelial–mesenchymal transition, cancer stem cells, TGF-β1, migration
Non-small-cell lung cancer (NSCLC) represents nearly 85% of primary malignant lung tumours,1 and is the leading cause of cancer deaths worldwide.2 Despite the potential benefits of improved diagnostic modalities, approximately 50% of patients with NSCLC present with advanced disease.3 Furthermore, the majority of cancer patients die of metastases rather than their primary tumours.
The process of metastatic dissemination remains poorly understood due to its complexity.4 It involves many steps: local invasion of cancer cells into the surrounding tissue, transport through the microvasculature of the lymph and blood systems, translocation, mainly through the bloodstream, to microvessels of distant tissues, exit from the bloodstream, survival and adaptation in the distant microenvironment, and, finally, formation of a secondary tumour.5, 6, 7
Recent researches have demonstrated that epithelial-to-mesenchymal transition (EMT) plays a key role in the early process of metastasis of cancer cells.4, 5 EMT is a process during which cells undergo a morphological switch from the epithelial polarized phenotype to a highly motile fibroblastic or mesenchymal phenotype. In the EMT process, epithelial cells lose their features, gain mesenchymal properties, and become motile and invasive.4 Transforming growth factor-β (TGF-β) is involved in many biological processes, including embryogenesis, wound healing, cell proliferation, differentiation, control of apoptosis and EMT.5, 8, 9, 10
In cancer, TGF-β works as a tumour suppressor in the early stages of tumorigenesis via inhibiting cell growth and inducing cell apoptosis. On the contrary, in the later stages of tumour progression, it acts as a tumour promoter, as tumour cells lose their ability to be growth arrested by TGF-β, undergoing EMT, which correlates to increasing invasiveness and metastasis.11
Cancer stem cells (CSCs) have been defined as ‘‘a cell within a tumour that possess the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumour''.12 These two definitive biological properties are what make the CSCs the prime candidate for initiation of relapse. These cells express stemness markers, are able to form floating spheres in serum-free medium, a property associated with stem cells, and are also able to differentiate in an aberrant cell phenotype constituting tumour heterogeneity.13 Experimentally, this population is identified by its ability to form new tumours through serial transplantations in immunodeficient hosts, re-establishing tumour heterogeneity.14 There are three distinct and main methodologies to isolate CSCs from solid tumours: (i) isolation of CSCs by flow cytometry according to CSC-specific cell surface markers such as CD13315, 16; (ii) detection of side population (SP) phenotype by Hoechst33342 exclusion17; (iii) sphere formation by cultivation of defined serum-free medium with growth factors that maintain the CSCs undifferentiated.18, 19
In our previous study,20 we demonstrated that TGF-β1 upregulated all characteristics of stemness by using the EMT reprogram starting from one primary lung cancer cell line obtained in our laboratory. The TGF-β1-treated LC31 cell line lost its epithelial morphology, assuming a fibroblast-like appearance, showing upregulation of vimentin, CD90, Slug, Twist and β-catenin, and downregulation of cytocheratin, e-cadherin and CD326. This cell line showed also overexpression of Oct4, Nanog, Sox2 and CD133, all genes of stemness, an increased pneumosphere-forming capacity and increased tumour-forming ability in NOD/SCID mice.
However, both in our and another studies, TGF-β1 treatment was performed on the total cell population and its effect was evaluated. In most cases, as mentioned above, it increased stemness characteristics and migration potential. Interestingly, it was to understand and evaluate what cell subpopulation is targeted from TGF-β1. Therefore, we sorted the A549 lung cancer cell line for CD133, CSC marker for lung cancer and SP profile. Then, we treated the cell fractions sorted with TGF-β1, investigating its effect both on CSCs identified as cells positive for CD133 and the SP, and on non-cancer stem cells (non-CSCs) identified as cells negative for CD133 and the SP in the A549 cell line. We have focused our attention on EMT and migration ability.
Results
Flow cytometry and SP analyses
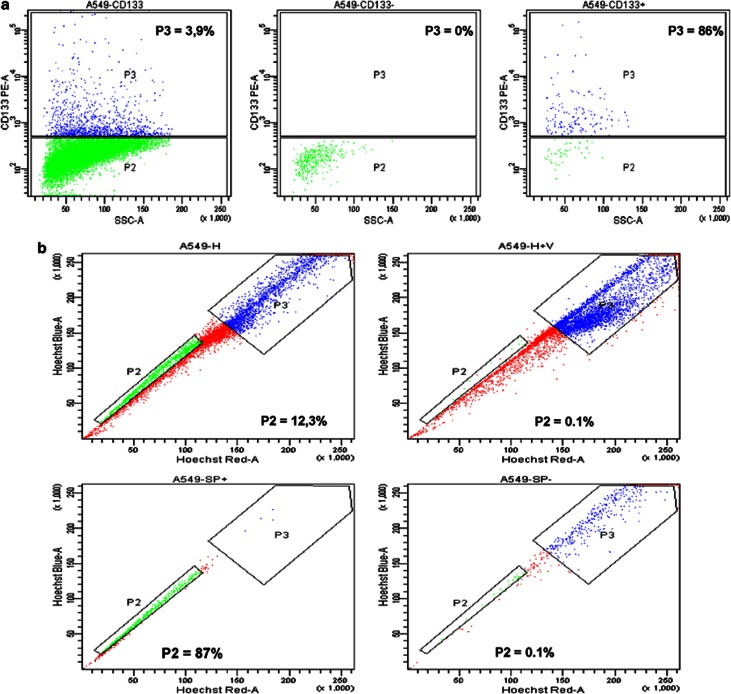
To isolate CSCs and non-CSCs starting from the A549 cell line, we sorted both for CD133 expression and SP phenotype. We found that the mean expression levels of CD133 were about 4% (Figure 1a). The presence of SP cells in the A549 cell line was examined by staining cells with Hoechst 33342 to generate a Hoechst blue–red profile. On the basis of the variance in Hoechst 33342 labeling, we gated the strongest dye efflux cells as SP cells, which were in the lower left quadrant of the FACS profile. As a control, the ATP-binding cassette (ABC) transporter inhibitor verapamil was added to reduce the capacity to exclude Hoechst 33342. SP cells were detected in A549 cells, with a mean percentage of about 15% of the total cell population, and the SP cell fraction was abolished in the presence of verapamil (Figure 1b).
Figure 1.
Cytometric analyses for CD133 expression and side population profile. (a) Expression levels of CD133 were ∼3.9%. The purity of sorted populations for CD133 was routinely 90%. (b) SP cells were detected in A549 cells, with a percentage of about 12.3% of the total cell population, and the SP cell fraction was abolished in the presence of verapamil. The purity of sorted populations for side population was routinely 90%
The purity of sorted populations was routinely 90% (Figures 1a and b) and sorted cells both for CD133 and for SP were used for TGF-β1 treatment assay, RT-PCR, immunofluorescence and in vitro migration assays.
TGF-β1 promotes morphological changes in both CSCs and non-CSCs
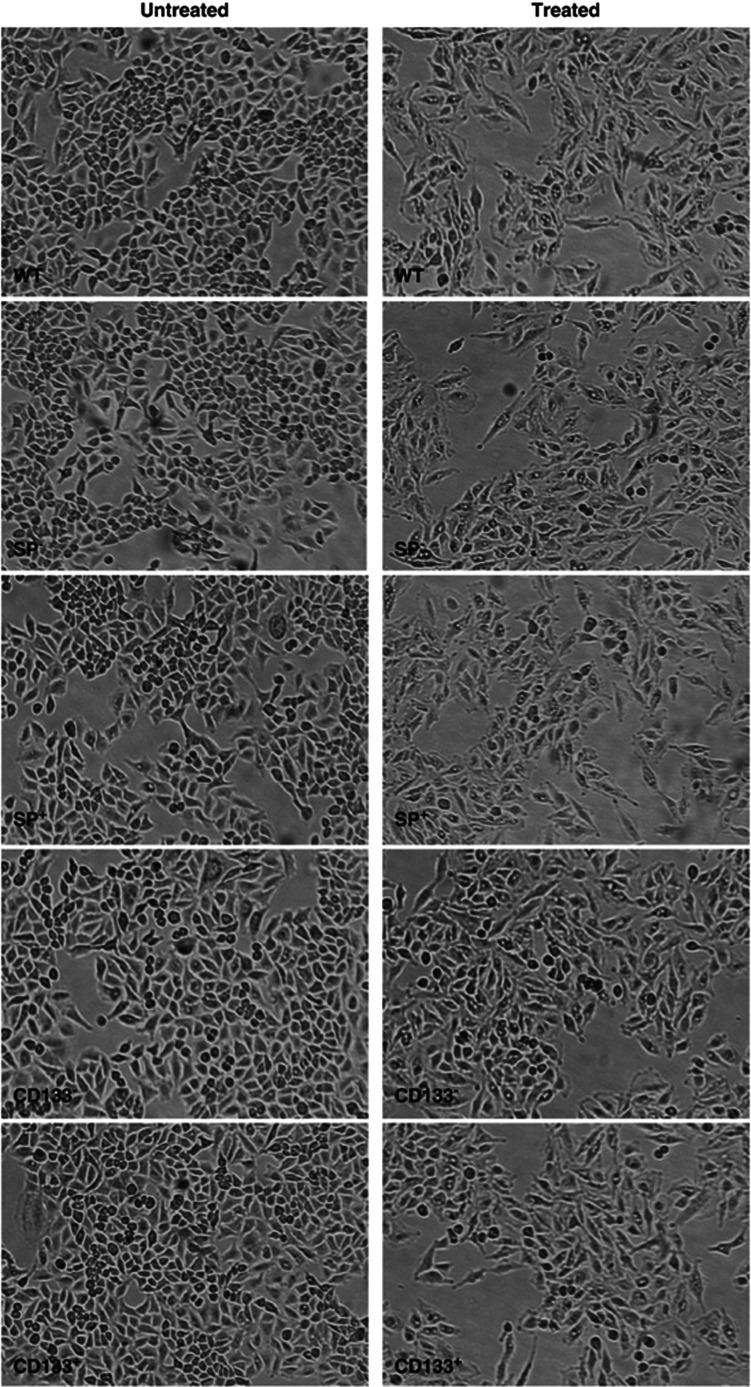
To evaluate the effect of TGF-β1 on wild-type (WT), CD133+, CD133−, SP+ and SP− A549 cells, we treated them with 2 ng/ml of TGF-β1 for 48 h. As already demonstrated by us,15 WT A549 cells treated with TGF-β1 lost their epithelial morphology after 48 h of treatment; they became dispersed and assumed a fibroblast-like appearance with long shape and a central nucleus. The same change was observed in all samples sorted already after 48 h of treatment independently from the stemness. Also, in this case, the cells lost their epithelial morphology and acquired mesenchymal traits with fibroblast-like shape (Figure 2).
Figure 2.
Light microscope analyses. Both WT and sorted A549 showed a morphological change with elongated, fibroblast-like cells after TGF-β1 treatment. Original magnification: × 100
TGF-β1 promotes EMT in CSCs and non-CSCs
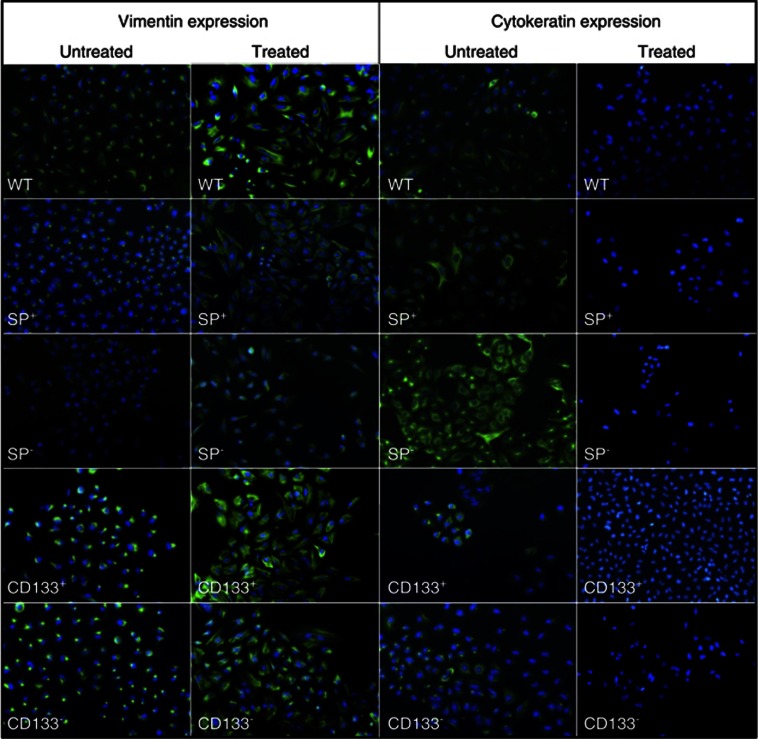
The morphological effect of TGF-β1 on WT A549 and sorted cell lines suggested that TGF-β1 promoted an EMT. The distinctive morphological changes of cells undergoing EMT are accompanied by a shift in expression from an epithelial to a mesenchymal repertoire. To determine whether TGF-β1 induced such shift, we used immunofluorescence and RT-PCR to examine the expression and distribution of vimentin, e-cadherin, cytokeratins and Slug. In immunofluorescence assay, before and after treatment, vimentin was expressed both in WT A549 and A549 sorted with a positivity of 100%. Interestingly, it was vimentin distribution. In all untreated cells, sorted and unsorted, vimentin was contained in perinuclear vesicles. After treatment, vimentin was uniformly distributed to form intermediate filaments of cytoskeleton, pointing out also the fibroblast shape of the cells. Both in CSCs and non-CSCs, this marker distribution was detectable (Figure 3). Regarding cytokeratin expression, before treatment it was weakly expressed in all cell fractions. In particular, in all samples tested, cytokeratins were localized in part in perinuclear vesicles and in part to form filaments of cell cytoskeleton. After treatment, all cell fractions became negative for this epithelial marker.
Figure 3.
Immunofluorescence analyses of EMT-related proteins. Both WT and sorted A549 showed an increase of vimentin and, in parallel, a decrease of cytokeratins after TGF-β1 treatment. Original magnification: × 100
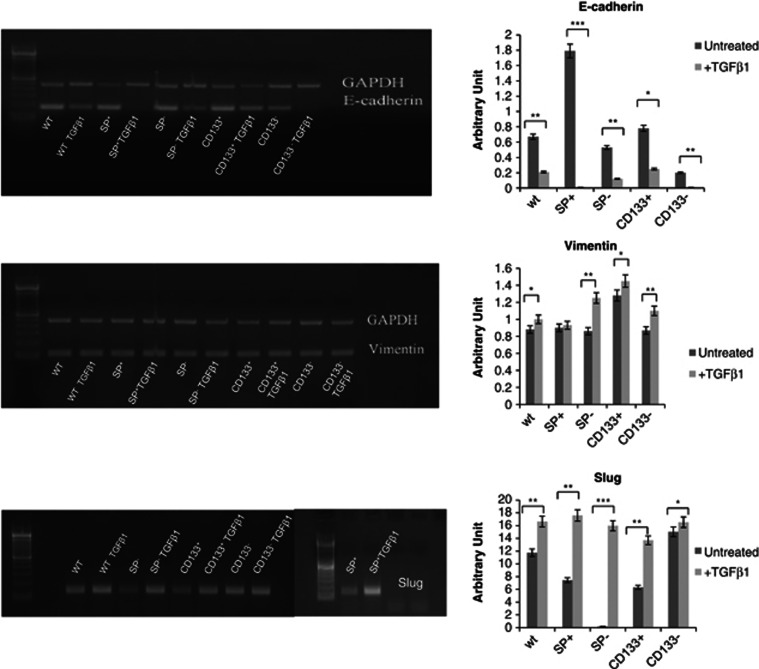
The RT-PCR data showed upregulation of vimentin and downregulation of e-cadherin both in treated WT A549 cells and in sorted treated cells, indicating a shift from epithelial to mesenchymal phenotype and confirming EMT phenotype. In particular, RT-PCR analyses showed a massive decrease of E-cadherin levels mainly in SP+ A549 cells and a strong increase of Slug gene expression in SP−, SP+ and CD133+ fractions. In contrast, vimentin increased slightly in all samples (Figure 4). RT-PCR data confirmed the results obtained by immunofluorescence for vimentin and, in summary, observation through a light microscope, immunofluorescence and RT-PCR analyses showed that CSCs and non-CSCs underwent EMT after TGF-β1 treatment. In particular, the response of SP+ A549 cells to TGF-β1 treatment was more efficient than all other sorted cell fractions and, instead, CD133− A549 cells were those that responded less to treatment.
Figure 4.
RT-PCR analyses and densitometry evaluation of EMT-related proteins. Both WT and sorted A549 showed an increase of vimentin and Slug and, in parallel, a decrease of e-cadherin after TGF-β1 treatment. *P<0.001, **P<0.0005, ***P<0.0001 compared to the parental cell line (day 0 of treatment)
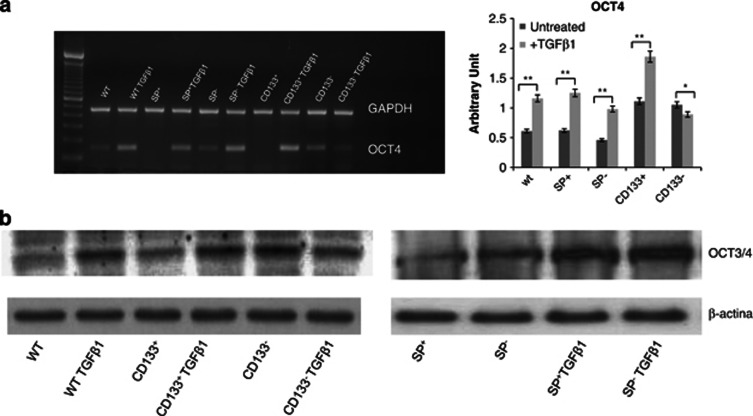
TGF-β1 increases the levels of stemness transcriptor factor OCT4
To investigate the genes regulating and maintaining the stem phenotype of cells having EMT signatures, we performed both RT-PCR (Figure 5a) and western blotting (Figure 5b) for OCT4. Interestingly, transcription factor OCT4, known to be sufficient to reprogram mouse or human somatic cells to undifferentiated, pluripotent stem cells, was found to be significantly increased in all samples treated with TGF-β1 compared to untreated corresponding controls. Amusingly, OCT4 levels were strongly upregulated in CD133+, SP+ cells as well as in SP− cells. In CD133− cells, OCT4 did not change after drug treatment both in RT-PCR and western blotting.
Figure 5.
RT-PCR, densitometry and western blot analyses of OCT4. (a) RT-PCR and densitometry evaluation showed an increase of OCT4 in all sorted and unsorted A549 cells except in CD133− A549 cells, after TGF-β1 treatment. (b) Western blot analyses showed an increase of OCT4 in all sorted and unsorted A549 cells except in CD133− A549 cells, after TGF-β1 treatment. β-Actin is used as loading control. *P<0.001, **P<0.0005, compared to the parental cell line (day 0 of treatment)
TGF-β1 increases the in vitro migration potential by EMT program
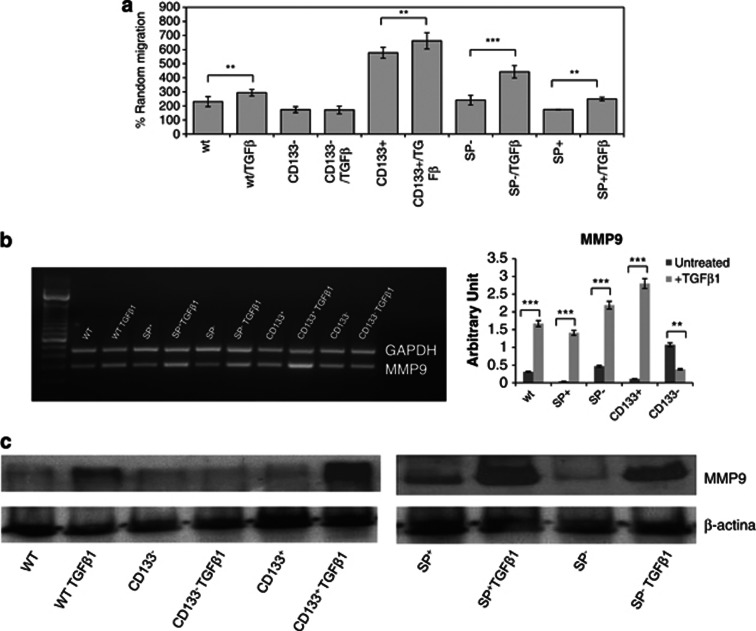
Another distinctive attribute of EMT is the acquisition of the ability to migrate. Once it was established that CSC and non-CSC A549 underwent EMT, we investigated their ability to migrate after 48 h of TGF-β1 treatment. The results showed that TGF-β1 significantly enhanced the migration of all samples compared with their corresponding untreated controls except for the CD133− fraction (Figure 6a). In fact, the two fractions showed the same percentage of random migration. Interestingly, after TGF-β1 treatment, the SP− fraction was the one that migrated more than all others in terms of fold increase (fold increase of 1.72 compared to 1.27, 1.14, 0.98 and 1.43 of WT, CD133+, CD133− and SP+ A549 cells, respectively). However, on comparing all fractions treated with TGF-β1, the CD133+ fraction showed a massive percentage of migration, as demonstrated also by Supplementary Table 1.
Figure 6.
Migration potential analyses. (a) Migration assay showed that TGF-β1 significantly enhanced the migration of all samples compared with their controls except for CD133− fraction. (b) RT-PCR and densitometry analyses showed that MMP-9 gene expression levels were increased in all sorted fractions except for the CD133− fraction. The CD133+ fraction showed higher levels of MMP-9 gene expression than other fractions. (c) Western blot analyses for MMP-9 confirmed RT-PCR results. β-Actin is used as loading control. **P<0.0005, ***P<0.0001 compared to the parental cell line (day 0 of treatment)
Finally, to investigate the ability to degrade the extracellular matrix, we also performed analyses of MMP-9 levels by RT-PCR. These data seemed to confirm the results obtained from migration assay. MMP-9 gene expression levels were increased in all sorted fractions except for the CD133− fraction, for which slightly decreased levels were observed compared to the untreated corresponding control. In addition, interestingly, the CD133+ fraction showed higher levels of MMP-9 gene expression than other fractions (Figure 6b). To confirm the data obtained by RT-PCR we analysed the levels of protein expression by western blot analysis. As shown in Figure 6c, the expression of MMP-9 was upregulated in all fractions treated with TGF-β1 except in the CD133− fraction. The cell subpopulation showing a higher level of metalloproteases was represented as CD133+; thus it is possible to conclude that this is precisely the fraction that best responds to treatment with TGF-β1.
Therefore, although all fractions underwent EMT, regarding migration, the results are different, with increased ability to migration observed for all sorted samples except for the CD133− fraction.
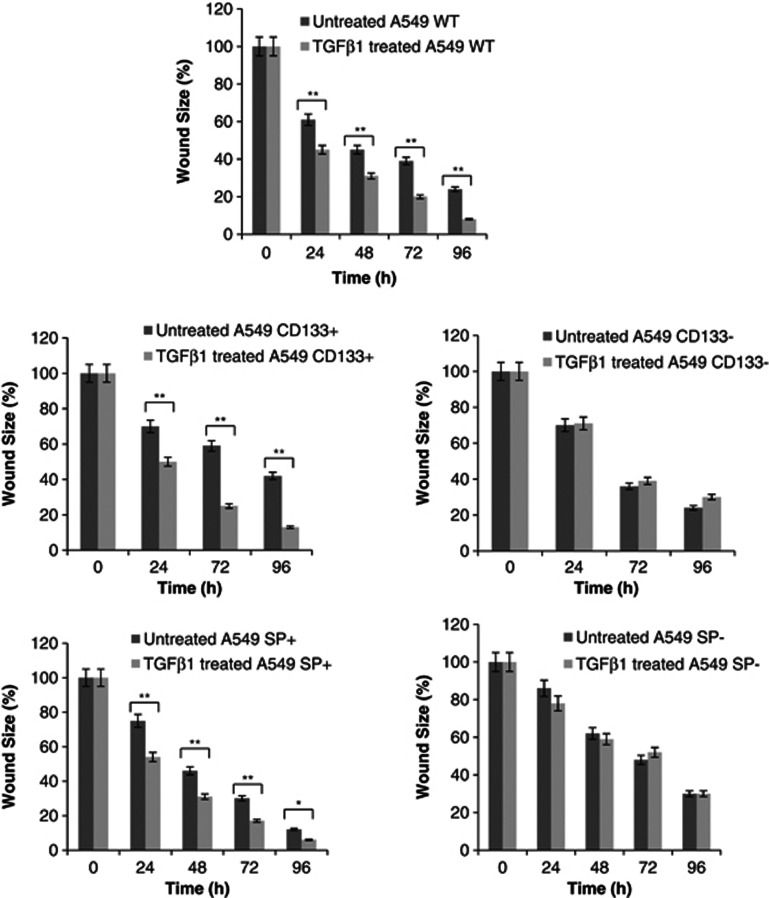
TGF-β1 enhances the in vitro motility potential by EMT program
The wound-healing assay was employed to measure the motility of A549 fractions, another characteristic of EMT. As shown in Figure 7, wound size analyses performed at 24, 48, 72 and 96 h, after 48 h of TGF-β1 treatment, revealed that TGF-β1 enhanced motility in WT A549 as well as CD133+ and SP+ cells compared to untreated corresponding controls. In this context, CD133+ cells showed higher motility than other cell fractions. For CD133− and SP− cells, TGF-β1 exposure did not change the motility.
Figure 7.
Wound healing analyses. Wound size analyses performed at 24, 48, 72 and 96 h, after TGF-β1 treatment, showed that TGF-β1 enhanced motility in WT A549 as well as CD133+ and SP+ cells compared to untreated controls. CD133+ cells showed higher motility than other cell fractions. For CD133− and SP− cells, TGF-β1 exposure did not change the motility
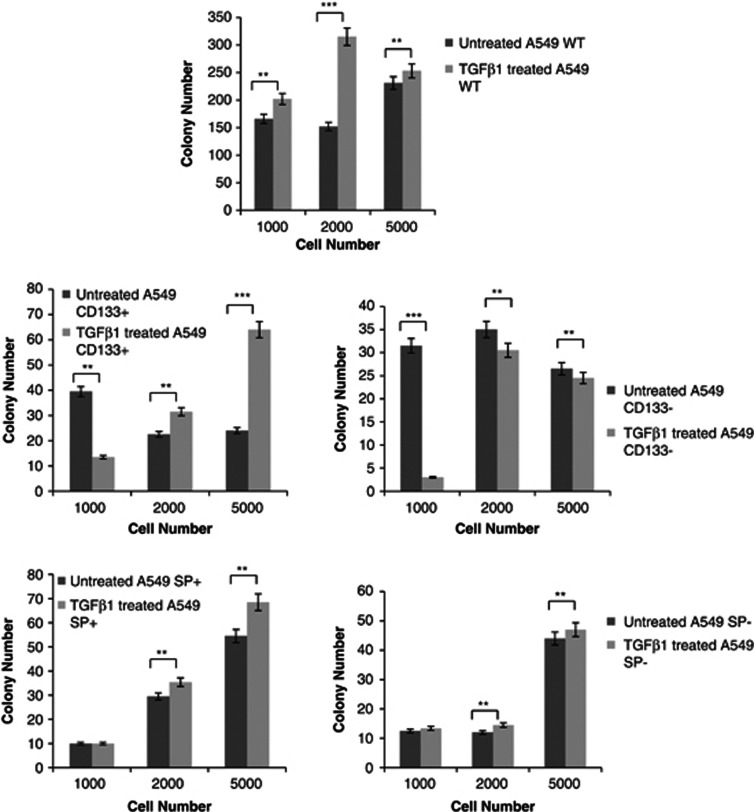
TGF-β1 increases the in vitro tumorigenicity potential
One method of analysing the tumorigenic potential is the soft agar assay, which measures anchorage-independent growth, which is an indicator for cell transformation. As reported in Figure 8, assessment of growth kinetics revealed major colony-forming efficiency in CD133+ A549 cells compared to both untreated corresponding cells and all cell fractions sorted, untreated and treated (Figure 8). In particular, SP+ and SP− A549 cells showed more efficiency to form colonies than the untreated corresponding cells, with a mean fold increase of 1,1 and 1,0, respectively. On the contrary, for CD133− cells, no change in colony number was observable after TGF-β1 exposure. When 1000 cells were seemed in soft agar, it was possible to observe a strong decrease in colony formation after TGF-β1 treatment.
Figure 8.
Soft agar analyses. Colony growth kinetics revealed major colony-forming efficiency in CD133+ A549 cells compared to both untreated cells and all cell fractions sorted. SP+ and SP− A549 cells showed more efficiency to form colonies than untreated cells. For CD133− cells, no change in colony number was observable after TGF-β1 exposure
Discussion
Tumour progression towards metastasis is a multistage process in which malignant cells spread from the tumour of origin to colonize distant organs.21, 22, 23 Prevention of later-arising metastasis has moved to the centre of clinical attention. Genes that allow transformed cells to invade the surrounding tissue and attract supportive stroma also facilitate the dispersion of cancer cells and probably continue to do so after cancer cells infiltrate distant tissues. The genes that determine these activities can be defined as metastasis initiation genes. These genes could promote cell motility, EMT, extracellular matrix degradation, bone marrow progenitor mobilization, angiogenesis or evasion of the immune system. EMT is mediated by developmental programs that are under the control of aberrantly regulated transcription factors, such as TWiST1, SNAi1 and SNAi2 (also known as SluG), and is defined as the transition of polarized epithelial cells to migratory mesenchymal cells. EMT is evidenced by changes in cell morphology, cell behavior and expression of EMT-related protein markers.23 In particular, reduction in E-cadherin level is considered as a hallmark of EMT.24, 25, 26 Concomitant with the loss of epithelial markers such as E-cadherin, cells undergoing EMT acquire mesenchymal markers such as vimentin and fibronectin.27 Recent studies provide evidence that EMT in various cell lines, including A549 cells, can be induced in vitro.28, 29, 30
In agreement with previous reports,31, 32 we showed that the A549 cell line underwent EMT in response to TGF-β1 stimulation. Change of morphology with cell scattering, decrease of cytokeratins and e-cadherin, and increase of Slug and vimentin were evident after TGF-β1 treatment. Recent researches,33 as well as our previous paper,20 showed that TGF-β1 upregulated both the stemness characteristics and migration. In these articles,20, 31, 32, 33 TGF-β1 treatment was performed on the total cell population. Here, after confirming that the A549 cell line underwent EMT, we investigated the role of TGF-β1 in different cell subpopulations in terms of stemness phenotype in order to identify the cell subset responding to TGF-β1 treatment. The questions that we try to answer in this manuscript are: does TGF-β1 work indiscriminately on all cell subpopulations or only on specific cell subpopulations? If it is true that TGF-β1 increases both stemness and migration characteristics, does TGF-β1 strengthen CSCs and/or induce also non-CSCs to become more stem and migrant?
For this purpose, we sorted the A549 cell line for CD133 marker and SP phenotype, obtaining CSC sublines with CD133+ and SP+ profile and non-CSC sublines with CD133− and SP− profile. After treatment with TGF-β1, both CSC and non-CSC sublines became more elongated and presented a spindle-shape, fibroblast-like phenotype compared with the untreated cells, with increase of vimentin and Slug and concomitant decrease of cytokeratins and e-cadherin. Consequently, the first important conclusion is that both CSC and non-CSC A549 sublines were prone to undergo a complete EMT.
EMT may contribute to greater motility and higher migration of tumour cells. Migration and motility are different steps undergoing to metastasis. Already Maeda et al. demonstrated that the upregulation of N-cadherin induced by TGF-β1 was indispensable for increasing cell motility,34 and that the downregulation of E-cadherin can predict invasiveness and metastasis in many forms of carcinoma, including invasive ductal breast carcinoma,35 esophageal adenocarcinoma36 and gastric adenocarcinoma.37 In our study, we observed that, interestingly and surprisingly, SP− A549 cells showed a stronger migration in terms of fold increase than other cell fractions sorted, although they did not display changes of motility after TGF-β1 treatment. CD133+ A549 cells showed a major efficiency in both migration and motility compared to the corresponding untreated cells and other cell fractions sorted, whereas SP+ cells were less migrant compared to all cell fractions sorted and unsorted, although they seemed to show more motility than the untreated control. The CD133− A549 subline did not show either migration potential or motility ability compared to both the corresponding untreated cells and other cell fractions sorted. MMP9 is a matrix metalloproteinase involved in extracellular matrix degradation and, in lung cancer, it promotes both tumour growth and metastasis by its angiogenic properties.38 MMP9 distribution reflected the data of migration and motility. In this context, we conclude for the metastasis process that, although it undergoes EMT, the CD133− cell subline is not able to migrate, move and degrade ECM as expected by us, because of not being stem. The CD133+ subline shows a very high basal percentage of migration although it is not strongly enhanced by TGF-β1 exposure. On the contrary, in terms of fold increase, the SP− subline is more migrant compared to others, although it does not move, as assumed in Supplementary Table 2. This seems to be a contradictory biological behavior, but motility and migration are two different mechanisms that undergo different steps of metastasis and they are not always directly proportional. Most probably, TGF-β1 stimulus, although induced by EMT, would not be sufficient to determine the motility characteristics of the SP− subline.
We could hypothesize that in vivo CD133+ cells will be those more prone to degrade ECM, migrate and move, as expected by us being CSCs. SP− cells could enhance CD133+ cells both in the degradation of ECM and in migration. Most probably, a longer exposure to TGF-β1 could induce also the motility phenotype.
Another characteristic of EMT is that of increasing both the efficiency of forming colonies and stemness. In our research, again the CD133+ A549 subline was able to form colonies with major efficiency compared to other cell sublines. However, also SP+ and SP− A549 sublines showed a stronger ability to form colonies than the untreated corresponding control, but their efficiency was shorter than the CD133+ A549 subline. On the contrary, the efficiency of forming colonies after TGF-β1 treatment did not change in the CD133− subline. OCT4 is a pluri/multipotent stemness transcriptor factor, involved in maintaining pluripotency and multipotency, and forming a complex with Nanog and Sox2. As already demonstrated by us, TGF-β1 induces an increase of such stemness factors. In this study, TGF-β1 led to an increase of OCT4 in all sublines except in the CD133− A549 subline. Interestingly, the SP− A549 subline, although it is not able to move, showed upregulation of OCT4, indicating that TGF-β1 induces stemness characteristics in cells that by definition are not stem. Once again, TGF-β1 has no effect on CD133− cells, as assumed in Supplementary Table 2. In this case, most probably, this subpopulation represents differentiated tumour cells, if characterizing the tumour in terms of size and not in terms of metastasis or invasion. In addition, TGF-β1 induces upregulation both of colony formation and of OCT4 in SP− cells although they are not stem.
In summary, we showed that the exposure of CSC and non-CSC A549 cell sublines to TGF-β1 resulted in EMT, marked by changes in cell morphology, and expression of EMT-related protein markers. Interestingly, TGF-β1 promotes migration and motility in the CD133+ cell subline, and not in CD133− or SP+ cell sublines. In the SP− cell sub-line, it promotes strong migration, ability to form colonies and muscular expression of OCT4.
Therefore, we hypothesize that TGF-β1 affects both CSCs and non-CSCs. Brabletz et al.39 proposed the existence of two CSC populations: (i) stationary CSC subpopulation and (ii) migratory CSC subpopulation. Mobile CSCs are located predominantly at the tumour–host interface and are derived from stationary CSCs through the acquisition of a transient EMT phenotype in addition to stemness. This model is further supported by the expression of EMT markers in CSCs from mammary carcinoma and, on the other hand, by the activation of stem cell markers in EMT-induced mammary epithelial cells.40 Therefore, we hypothesize that cells expressing CD133 could be a true migrating subpopulation in lung adenocarcinoma, while SP+ cells could be considered the stationary CSC subpopulation. In addition, TGF-β1 induces functional changes in SP− cells that migrate, form colonies and express OCT4, all markers of stemness, although they did not show motility. We hypothesize that this cell fraction could represent a potential stem subpopulation used by tumours as a reservoir of stem cells in certain conditions.
In conclusion, we can assume that it is possible to highlight different cell subpopulations with different grades of stemness. Each population seems to be involved in different biological mechanisms such as stemness maintenance, tumorigenicity, invasion and migration.
Further studies are required to reveal the mechanism of cell invasion in the process of EMT, which may provide novel therapeutic targets and strategies for cancer invasion and metastasis.
Materials and Methods
Flow cytometry and SP assay
A549 cell lines were sorted for CD133 surface expression and SP phenotype. For CD133 staining, 5 × 106 cells were stained with 2 μg/ml mouse anti-human CD133 PE (Miltenyi Biotec, Calderara di Reno, Bologna, Italy). The antibody was incubated for 30 min at 4 °C in the dark. After incubation, the samples were washed with PBS and analysed by FACSAriaII (Becton Dickinson, Franklin Lakes, NJ, USA).
To analyse the SP phenotype, A549 cells were stained according to the protocol of Goodell et al.41 Briefly, 1 × 106 cells/ml were suspended in pre-warmed RPMI1640 culture medium. Hoechst 33342 (Sigma, Milan, Italy) was added at a final concentration of 5 μg/ml in the presence and absence of 50 μM verapamil (Sigma), and the cell samples were incubated for 90 min at 37 °C with intermittent shaking. After incubation, the cells were washed with ice-cold PBS, re-suspended in ice-cold PBS, and analysed for Hoechst33342 efflux with a FACS Aria II (Becton Dickinson). The Hoechst 33342 dye was excited at 375 nm near-ultraviolet, and the resultant fluorescence was measured at two wavelengths using 450/40 BP and 530 LP filters for detection of Hoechst blue and red, respectively. All data were analysed by Diva 6.1 Software (Becton Dickinson).
CD133+ and CD133−, SP+ and SP− A549 cells were sorted and selected for experiments. The purity of sorted populations was routinely 90%.
Cell culture and TGF-β1 treatment
The A549 cell line was purchased from ATCC Cell Bank and was cultured in RPMI1640 (Lonza, Milan, Italy) with 10% fetal bovine serum (FBS) at 37 °C, 5% CO2. In order to induce the EMT process, WT, CD133+ and CD133−, SP+ and SP− A549 cells were treated with 2 ng/ml TGF-β1 (AbCAM, Milan, Italy) for 48 h. For experiments, cells were grown to 90% confluence.
Immunofluorescence assay
TGF-β1 untreated and treated WT, CD133+ and CD133−, SP+ and SP− A549 cells were plated in 24-well plates and were fixed with 70% ethanol/0.1% Triton for 30 min at 4 °C, washed with PBS, treated with 5% bovine serum albumin for 60 min at room temperature and then stained with primary antibodies at 4 °C overnight. The primary antibodies used were mouse anti-human vimentin (DAKO) and mouse anti-human citokeratins (DAKO). The secondary antibody, goat anti-mouse FITC (AbCAM) diluted 1 : 200 in PBS, was incubated for 60 min at 4 °C, and the DAPI (Sigma), used to stain the nucleus, was incubated for 7 min at room temperature. Cells were observed under the fluorescence microscope (Zeiss, Milan, Italy). Isotypes and non-probed cells were used as controls.
RT-PCR
Total RNA was extracted using TRIzol Reagent (Invitrogen, Milan, Italy) according to the manufacturer's protocol. RNA concentration and purity were determined by A260 and A260/A280 ratios, respectively. The integrity of total RNA was assessed on standard 1% agarose/formaldehyde gels. The RNA samples were treated with DNase I to remove residual traces of DNA. cDNA was obtained from 1 μg of total RNA, using reverse transcriptase (Promega Italia Srl, Milan, Italy) and random primers (Promega) in a final volume of 20 μl. cDNAs (1 μl for each sample) were amplified by PCR using the following primer sequences:
Slug: 5′-GAGCATTTGCAGACAGGTCA-3′ (sense) and 5′-CCTCATGTTTGTGCAGGAGA -3′ (antisense);
Vimentin: 5′-GACAATGCGTCTCTGGCACGTCT-3′(sense) and 5′ TCCGCCTCCTGCAGGTTCTT-3′ (antisense);
E-cadherin: 5′-GGTCACAGCCACAGACGCGG-3′ (sense) and 5′-GGAAACTCTCTCGGTCCAGCCCA-3′ (antisense);
MMP-9: 5′-TTGACAGCGACAAGAAGTGG-3′ (sense) and 5′-RCCCTCAGTGAAGCGGTACAT-3′ (antisense);
OCT4: 5′-ACATGTGTAAGCTGCGGCC-3′ (sense) and 5′- GTTGTGCATAGTCGCTGCTTG-3′ (antisense);
GAPDH: 5′-TGGACTCCACGACGTACTCAG-3′ (sense) and 5′-ACATGTTCCAATATGATTCCA-3′ (antisense), amplified as an internal control.
The RT-PCR products were separated by 2% agarose gel electrophoresis, stained with ethidium bromide, and photographed under UV illumination.
RT-PCR was performed on TGF-β1 treated and untreated, sorted and unsorted A549 cell line at 48 h after treatment. The densitometric analyses were performed using Image J software and were expressed as ratio of GAPDH densitometry versus tested gene densitometry.
Western blotting
Cells were lysed on ice by using mammalian protein extraction reagent (Qiagen, GmbH, Hilden, Germany) plus benzonase nuclease and protease inhibitors. After removing insoluble debris by centrifugation at 13 000 r.p.m. for 15 min at 4 °C, the supernatant was designated as whole-cell lysate. Protein concentrations were determined with Bradford method (Bio-Rad, Hercules, CA, USA). The protein for each cell lysate was separated by SDS-PAGE and elettrophoretically transferred to PVDF membranes (EMD Millipore, Milan, Italy). Membranes were blocked with 5% dry milk in PBS-Tween 20 and immunoblotted with primary antibodies as follows: MMP-9 (Abcam, Cambridge, MA, USA) and OCT3/4 (Santa Cruz, Heidelberg, Germany). HRP-conjugated secondary antibody was added for 2 h at room temperature. The detection was performed by chemo-luminescence using ECL kit (Roche, Milan, Italy). Cell results were normalized to β-actin (Cell Signalling, Danvers, MA, USA) as appropriate.
Migration assay
Cell migration assay was performed using Boyden chambers as previously described.42 At the end of the assay, cells in the upper chamber and on the upper filter surface were removed, whereas cells on the lower filter surface were fixed with ethanol and stained with haematoxylin. The number of migrating cells was determined by counting cells in 10 random fields/filter at × 200 magnification. Data were calculated as a percentage of migrated cells in the absence of chemoattractant, considered as 100%. All experiments were performed in triplicates.
Wound-healing assay
The wound-healing assay was performed to measure two-dimensional movement. All cell fractions (untreated or treated with TGF-β1) were cultured in six-well plates at a density of 50 000 cells/well until confluence. A wound was created in the centre of the cell monolayers by a sterile pipette tip. The phase contrast images were captured after 24, 48, 72 and 96 h. The analyses are performed considering 100% wound size at the time of culture. All experiments were performed in triplicates.
Soft agar
To measure in vitro tumorigenicity due to TGF-β1 treatment, treated and untreated WT, CD133+ and CD133−, SP+ and SP− A549 cells at a density of 1000, 2000, and 5000 cells per well in 24-well plates were plated in soft agar, in triplicate. The test was performed using 0.8% and 0.3% agar in RPMI1640 as the base and top layers, respectively. Cells were incubated for 21 days at 37 °C in a humidified atmosphere at 5% CO2 in air, and 50 ml of RPMI1640 culture medium was added twice a week. At the end of the incubation period, colonies were stained with nitroblue tetrazolium (NBT, Sigma) at a concentration of 1 mg/2 ml in PBS and counted using an inverted microscope (Nikon TS 100, Milan, Italy). The colony efficiency was calculated as proportion of colonies per total number of seeded cells. The data were analysed by Image Pro Plus software. All experiments were performed in triplicates.
Statistical analysis
Values are shown as the mean±S.E.M. of measurements of at least three independently performed experiments to avoid possible variation of cell cultures. Student's t test was employed, and P<0.05 was considered to be statistically significant.
Acknowledgments
We thank Dr. AlessandraTrocino and librarians of the National Cancer Institute, Naples, for providing excellent bibliographic services and assistance. This work was supported by grants from the Current Research of 2011/12 of the Italian Department of Health to G. Pirozzi.
Glossary
- NSCLC
non-small-cell lung cancer
- EMT
epithelial to mesenchymal transistion
- CSCs
cancer stem cells
- TGF-β1
transforming growth factor-β1
- ABC
ATP-binding cassette
- WT
wild type
- SP
side population
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Stephanou
Supplementary Material
References
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Cancer Report. IARC Press: Lyon; 2008. International Agency for Research on Cancer. [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Gavert N, Ben-Ze'ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199–209. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Nawshad A, Lagamba D, Polad A, Hay ED. Transforming growth factor-b signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179:11–23. doi: 10.1159/000084505. [DOI] [PubMed] [Google Scholar]
- Seton-Rogers S. Metastasis: signalling in transit. Nat Rev Cancer. 2011;12:4. doi: 10.1038/nrc3193. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Gomes LR, Terra LF, Sogayar MC, Labriola L. Epithelial-mesenchymal transition: implications in cancer progression and metastasis. Curr Pharm Biotechnol. 2011;12:1881–1890. doi: 10.2174/138920111798377102. [DOI] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Landstroöm M, Moustakas A. Mechanism of TGF-β signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21:166–716. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells-old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Tirino V, Camerlingo R, Franco R, Malanga D, La Rocca A, Viglietto G, et al. The role of CD133 in the identification and characterisation of tumour-initiating cells in non-small-cell lung cancer. Eur J Cardiothorac Surg. 2009;36:446–453. doi: 10.1016/j.ejcts.2009.03.063. [DOI] [PubMed] [Google Scholar]
- Yin S, Li J, Hu C, Chen X, Yao M, Yan M, et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- Hirschmann-Jax C, Foster AE, Wulf GG, Goodell MA, Brenner MK. A distinct ‘‘side population'' of cells in human tumor cells: implications for tumor biology and therapy. Cell Cycle. 2005;4:203–520. [PubMed] [Google Scholar]
- Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, La Noce M, et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J. 2013;27:13–24. doi: 10.1096/fj.12-218222. [DOI] [PubMed] [Google Scholar]
- Collura A, Marisa L, Trojan D, Buhard O, Lagrange A, Saget A, et al. Extensive characterization of sphere models established from colorectal cancer cell lines. Cell Mol Life Sci. 2012;70:729–742. doi: 10.1007/s00018-012-1160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozzi G, Tirino V, Camerlingo R, Franco R, La Rocca A, Liguori E, et al. Epithelial to mesenchymal transition by TGF-β1 induction increases stemness characteristics in primary non small cell lung cancer cell line. PLoS One. 2011;6:e21548. doi: 10.1371/journal.pone.0021548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavert N, Ben-Ze'ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199–209. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, et al. Differential expression of the epithelial- mesenchymal transition regulators Snail, SIP1, and Twist in gastric cancer. Am J Pathol. 2002;161:1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zhang X, Gang H, Li X, Li Z, Wang T, et al. Up-regulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and fibronectin expression. Biochem Biophys Res Commun. 2007;358:925–930. doi: 10.1016/j.bbrc.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KA, Aakre ME, Gorska AE, Price JO, Eltom SE, Pietenpol JA, et al. Induction by transforming growth factor-β1 of epithelial to mesenchymal transition is a rare event in vitro. Breast Cancer Res. 2004;6:R215–R231. doi: 10.1186/bcr778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-β signaling. Am J Respir Cell Mol Biol. 2008;38:95–104. doi: 10.1165/rcmb.2007-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui-Jun Zhang, He-Yong Wang, Hong-Tao Zhang, Jin-Mei Su, Jun Zhu, Hai-Bing Wang, et al. Transforming growth factor-b1 promotes lung adenocarcinoma invasion and metastasis by epithelial-to-mesenchymal transition. Mol Cell Biochem. 2011;355:309–314. doi: 10.1007/s11010-011-0869-3. [DOI] [PubMed] [Google Scholar]
- Ko H, So Y, Jeon H, Jeong MH, Choi HK, Ryu SH, Lee SW, Yoon HG, Choi KC.TGF-β1-induced epithelial-mesenchymal transition and acetylation of Smad2 and Smad3 are negatively regulated by EGCG in Human A549 lung cancer cells Cancer Lett 2013.. pii: S0304-3835(13)00130-4.doi: 10.1016/j.canlet.2013.02.018 [DOI] [PubMed]
- May CD, Sphyris N, Evans KW, Werden SJ, Guo W, Mani SA. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 2011;13:202. doi: 10.1186/bcr2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118:873–887. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- Harigopal M, Berger AJ, Camp RL, Rimm DL, Kluger HM. Automated quantitative analysis of E-cadherin expression in lymph node metastases is predictive of survival in invasive ductal breast cancer. Clin Cancer Res. 2005;11:4083–4089. doi: 10.1158/1078-0432.CCR-04-2191. [DOI] [PubMed] [Google Scholar]
- Rees JR, Onwuegbusi BA, Save VE, Alderson D, Fitzgerald RC. In vivo and in vitro evidence for transforming growth factor-b1-mediated epithelial to mesenchymal transition in esophageal adenocarcinoma. Cancer Res. 2006;66:9583–9590. doi: 10.1158/0008-5472.CAN-06-1842. [DOI] [PubMed] [Google Scholar]
- Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, et al. Differential expression of the epithelial- mesenchymal transition regulators Snail, SIP1, and Twist in gastric cancer. Am J Pathol. 2002;161:1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Chang Y, Hodges KB, Sun Y, Ma X, Xue Y, Williamson SR, Lopez-Beltran A, Montironi R, Cheng L. Expression of KISS1 and MMP-9 in non-small cell lung cancer and their relations to metastasis and survival. Anticancer Res. 2010;30:713–718. [PubMed] [Google Scholar]
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial– mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriero MV, Longanesi-Cattani I, Bifulco K, Maglio o, Lista L, Barbieri A, et al. Structure-based design of an urokinase-type plasminogen activator receptor-derived peptide inhibiting cell migration and lung metastasis. Mol Cancer Ther. 2009;8:2708–2717. doi: 10.1158/1535-7163.MCT-09-0174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.